Effect of Metal and Carbon Nanotube Additives on the Thermal Diffusivity of a Silica Gel-Based Adsorption Bed
Abstract
:1. Introduction
2. Methods and Materials
3. Results
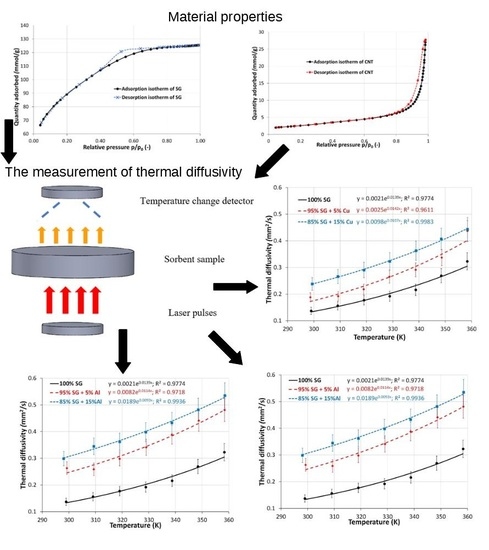
3.1. The Structural Analyses
3.2. The Influence of Metal Additives on the Thermal Diffusivity of the Adsorption Bed
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Symbols and Abbreviations
| adsorption capacity in pressure 0.1 MPa (mmol/g) | |
| Brunauer–Emett–Teller method | |
| CCVD | Catalytic Chemical Vapor Deposition method |
| CNT | carbon nanotubes |
| COP | coefficient of performance |
| ENG-TSA | expanded natural graphite treated with sulfuric acid |
| external surface area according to the t-plot model (m2/g) | |
| IUPAC | International Union of Pure and Applied Chemistry |
| LPNA | low-pressure nitrogen adsorption method |
| micropore area according to the t-plot model (m2/g) | |
| micropore volume according to the NLDFT model (cm3/g) | |
| MOF | metal–organic framework |
| NLDFT | non-localized density functional theory |
| total volume in pores according to the NLDFT model (cm3/g) | |
| p0 | saturation pressure of the adsorbate in the gas phase |
| SG | silica gel |
| specific surface area according to BET model (m2/g) | |
| STSA | Statistical Thickness Surface Area method |
| α | thermal diffusivity (mm2/s) |
| λ | thermal conductivity (W/m·K) |
| ρ | density, g/cm3 |
| Cp | specific heat capacity (J/kg·K) |
References
- Pan, Q.; Peng, J.; Wang, R. Experimental study of an adsorption chiller for extra low temperature waste heat utilization. Appl. Therm. Eng. 2019, 163, 114341. [Google Scholar] [CrossRef]
- Grabowska, K.; Krzywanski, J.; Nowak, W.; Wesolowska, M. Construction of an innovative adsorbent bed configuration in the adsorption chiller—Selection criteria for effective sorbent-glue pair. Energy 2018, 151, 317–323. [Google Scholar] [CrossRef]
- Grabowska, K.; Sosnowski, M.; Krzywanski, J.; Sztekler, K.; Kalawa, W.; Zylka, A.; Nowak, W. Analysis of heat transfer in a coated bed of an adsorption chiller. MATEC Web Conf. 2018, 240, 01010. [Google Scholar] [CrossRef] [Green Version]
- Krzywanski, J. A General Approach in Optimization of Heat Exchangers by Bio-Inspired Artificial Intelligence Methods. Energies 2019, 12, 4441. [Google Scholar] [CrossRef] [Green Version]
- Sosnowski, M. Evaluation of Heat Transfer Performance of a Multi-Disc Sorption Bed Dedicated for Adsorption Cooling Technology. Energies 2019, 12, 4660. [Google Scholar] [CrossRef] [Green Version]
- Sztekler, K.; Kalawa, W.; Mika, L.; Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Nowak, W.; Siwek, T.; Bieniek, A. Modeling of a Combined Cycle Gas Turbine Integrated with an Adsorption Chiller. Energies 2020, 13, 515. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, K.; Sosnowski, M.; Krzywanski, J.; Sztekler, K.; Kalawa, W.; Zylka, A.; Nowak, W. The Numerical Comparison of Heat Transfer in a Coated and Fixed Bed of an Adsorption Chiller. J. Therm. Sci. 2018, 27, 421–426. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Zylka, A.; Sztekler, K.; Kalawa, W.; Wojcik, T.; Nowak, W. An adaptive neuro-fuzzy model of a re-heat two-stage Adsorption Chiller. Therm. Sci. 2019, 23, S1053–S1063. [Google Scholar] [CrossRef] [Green Version]
- Younes, M.M.; El-sharkawy, I.I.; Kabeel, A.E.; Saha, B.B. A review on adsorbent-adsorbate pairs for cooling applications. App. Therm. Inż. 2017, 114, 394–414. [Google Scholar] [CrossRef]
- Saha, B.B.; Chakraborty, A.; Koyama, S.; Yoon, S.-H.; Mochida, I.; Kumja, M.; Yap, C.; Ng, K.C. Isotherms and thermodynamics for the adsorption of n-butane on pitch based activated carbon. Int. J. Heat Mass Transf. 2008, 51, 1582–1589. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Żyłka, A.; Sztekler, K.; Kalawa, W.; Wójcik, T.; Nowak, W. Modeling of a re-heat two-stage adsorption chiller by AI approach. MATEC Web Conf. 2018, 240, 05014. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Alam, K.C.A.; Saha, B.B.; Akisawa, A.; Kashiwagi, T. Study on a re-heat two-stage adsorption chiller—The influence of thermal capacitance ratio, overall thermal conductance ratio and adsorbent mass on system performance. Appl. Therm. Eng. 2007, 27, 1677–1685. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Alam, K.C.A.; Saha, B.B.; Hamamoto, Y.; Akisawa, A.; Kashiwagi, T. Parametric study of a two-stage adsorption chiller using re-heat—The effect of overall thermal conductance and adsorbent mass on system performance. Int. J. Therm. Sci. 2006, 45, 511–519. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.K.; Mahmoud, S.; Elsayed, A. Effects of contact resistance and metal additives in finned-tube adsorbent beds on the performance of silica gel/water adsorption chiller. Appl. Therm. Eng. 2013, 53, 278–284. [Google Scholar] [CrossRef]
- Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Zylka, A.; Kulakowska, A.; Czakiert, T.; Sztekler, K.; Wesolowska, M.; Nowak, W. Heat transfer in fluidized and fixed beds of adsorption chillers. E3S Web Conf. 2019, 128, 01003. [Google Scholar] [CrossRef]
- Sosnowski, M.; Krzywanski, J.; Grabowska, K.; Gnatowska, R. Polyhedral meshing in numerical analysis of conjugate heat transfer. EPJ Web Conf. 2018, 180, 02096. [Google Scholar] [CrossRef]
- Aristov, Y.I. Challenging offers of material science for adsorption heat transformation: A review. Appl. Therm. Eng. 2013, 50, 1610–1618. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Proverbio, E. Organosilanes functionalization of alumino-silica zeolites for water adsorption applications. Microporous Mesoporous Mater. 2016, 234, 113–119. [Google Scholar] [CrossRef]
- Bin, Z. Solid oxide fuel cell (SOFC) technical challenges and solutions from nano-aspects. Int. J. Energy Res. 2009, 31, 135–147. [Google Scholar]
- Solé, A.; Barreneche, C. Review on sorption materials and technologies for heat pumps and thermal energy storage. Renew. Energy 2017, 110, 3–39. [Google Scholar]
- Jeremias, F.; Khutia, A.; Henninger, S.K.; Janiak, C. MIL-100(Al, Fe) as water adsorbents for heat transformation purposes—A promising application. J. Mater. Chem. 2012, 22, 10148–10151. [Google Scholar]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Janiak, C. MOFs for use in adsorption heat pump processes. Eur. J. Inorg. Chem. 2012, 2012, 2625–2634. [Google Scholar]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Advancement of sorption-based heat transformation by a metal coating of highly-stable, hydrophilic aluminium fumarate MOF. RSC Adv. 2014, 4, 24073–24082. [Google Scholar]
- Solovyeva, M.V.; Gordeeva, L.G.; Krieger, T.A.; Aristov, Y.I. MOF-801 as a promising material for adsorption cooling: Equilibrium and dynamics of water adsorption. Energy Convers. Manag. 2018, 174, 356–363. [Google Scholar]
- Saha, B.B.; El-Sharkawy, I.I.; Shahzad, M.W.; Thu, K.; Ang, L.; Ng, K.C. Fundamental and application aspects of adsorption cooling and desalination. Appl. Therm. Eng. 2016, 97, 68–76. [Google Scholar]
- Henninger, S.K.; Ernst, S.-J.; Gordeeva, L.; Bendix, P.; Fröhlich, D.; Grekova, A.D.; Bonaccorsi, L.; Aristov, Y.; Jaenchen, J. New materials for adsorption heat transformation and storage. Renew. Energy 2017, 110, 59–68. [Google Scholar] [CrossRef]
- Towsif Abtab, S.M.; Alezi, D.; Bhatt, P.M.; Shkurenko, A.; Belmabkhout, Y.; Aggarwal, H.; Weseliński, Ł.J.; Alsadun, N.; Samin, U.; Hedhili, M.N. Reticular Chemistry in Action: A Hydrolytically Stable MOF Capturing Twice Its Weight in Adsorbed Water. Chem 2018, 4, 94–105. [Google Scholar]
- Whiting, G.T.; Grondin, D.; Stosic, D.; Bennici, S.; Auroux, A. Zeolite-MgCl2 composites as potential long-term heat storage materials: Influence of zeolite properties on heats of water sorption. Sol. Energy Mater. Sol. Cells 2014, 128, 289–295. [Google Scholar] [CrossRef]
- Palomba, V.; Vasta, S.; Freni, A. Experimental testing of AQSOA FAM Z02/water adsorption system for heat and cold storage. Appl. Therm. Eng. 2017, 124, 967–974. [Google Scholar]
- Younes, M.M.; El-sharkawy, I.I.; Kabeel, A.E.; Uddin, K.; Pal, A.; Mitra, S.; Thu, K.; Saha, B.B. Synthesis and characterization of silica gel composite with polymer binders for adsorption cooling applications. Int. J. Refrig. 2019, 98, 161–170. [Google Scholar]
- Brancato, V.; Gordeeva, L.G.; Grekova, A.D.; Sapienza, A.; Vasta, S.; Frazzica, A.; Aristov, Y.I. Water adsorption equilibrium and dynamics of LICL/MWCNT/PVA composite for adsorptive heat storage. Sol. Energy Mater. Sol. Cells 2019, 193, 133–140. [Google Scholar] [CrossRef]
- ul Qadir, N.; Said, S.A.M.; Mansour, R. Ben Modeling the performance of a two-bed solar adsorption chiller using a multi-walled carbon nanotube/MIL-100(Fe) composite adsorbent. Renew. Energy 2017, 109, 602–612. [Google Scholar] [CrossRef]
- Wang, R.Z.; Ge, T.S.; Chen, C.J.; Ma, Q.; Xiong, Z.Q. Solar sorption cooling systems for residential applications: Options and guidelines. Int. J. Refrig. 2009, 32, 638–660. [Google Scholar]
- Ng, K.C.; Chua, H.T.; Chung, C.Y.; Loke, C.H.; Kashiwagi, T.; Akisawa, A.; Saha, B.B. Experimental investigation of the silica gel–water adsorption isotherm characteristics. Appl. Therm. Eng. 2001, 21, 1631–1642. [Google Scholar] [CrossRef]
- Grushka, E. Porous silica its properties and use as support in column liquid chromatography. J. Chromatogr. A 1979, 179, 395. [Google Scholar]
- Boelman, E.C.; Saha, B.B.; Kashiwagi, T. Experimental investigation of a silica gel-water adsorption refrigeration cycle—The influence of operating conditions on cooling output and COP. Unkn. J. 1995, 101, 358–366. [Google Scholar]
- Choudhury, B.; Saha, B.B.; Chatterjee, P.K.; Sarkar, J.P. An overview of developments in adsorption refrigeration systems towards a sustainable way of cooling. Appl. Energy 2013, 104, 554–567. [Google Scholar]
- Sakoda, A.; Suzuki, M. Fundamental study on solar powered adsorption cooling system. J. Chem. Eng. Jpn. 1984, 17, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Chihara, K.; Suzuki, M. Air drying by pressure swing adsorption. J. Chem. Eng. Jpn. 1983, 16, 293–299. [Google Scholar]
- Wankat, P.C. Adsorption engineering: By Motoyuki Suzuk. React. Polym. 1991, 14, 269–270. [Google Scholar] [CrossRef]
- Myat, A.; Thu, K.; Choon, N.K. The experimental investigation on the performance of a low temperature waste heat-driven multi-bed desiccant dehumidifier (MBDD) and minimization of entropy generation. Appl. Therm. Eng. 2012, 39, 70–77. [Google Scholar] [CrossRef]
- Mitra, S.; Kumar, P.; Srinivasan, K.; Dutta, P. Development and performance studies of an air cooled two-stage multi-bed silica-gel + water adsorption system. Int. J. Refrig. 2016, 67, 174–189. [Google Scholar] [CrossRef]
- Ng, K.C.; Thu, K.; Kim, Y.; Chakraborty, A.; Amy, G. Adsorption desalination: An emerging low-cost thermal desalination method. Desalination 2013, 308, 161–179. [Google Scholar] [CrossRef]
- Thu, K.; Ng, K.C.; Saha, B.B.; Chakraborty, A.; Koyama, S. Operational strategy of adsorption desalination systems. Int. J. Heat Mass Transf. 2009, 52, 1811–1816. [Google Scholar] [CrossRef]
- Vodianitskaia, P.J.; Soares, J.J.; Melo, H.; Gurgel, J.M. Experimental chiller with silica gel: Adsorption kinetics analysis and performance evaluation. Energy Convers. Manag. 2017, 132, 172–179. [Google Scholar] [CrossRef]
- Chang, W.S.; Wang, C.C.; Shieh, C.C. Experimental study of a solid adsorption cooling system using flat-tube heat exchangers as adsorption bed. Appl. Therm. Eng. 2007, 27, 2195–2199. [Google Scholar] [CrossRef]
- Askalany, A.A.; Salem, M.; Ismael, I.M.; Ali, A.H.H.; Morsy, M.G.; Saha, B.B. An overview on adsorption pairs for cooling. Renew. Sustain. Energy Rev. 2013, 19, 565–572. [Google Scholar] [CrossRef]
- Uddin, K.; Amirul Islam, M.; Mitra, S.; Lee, J.; Thu, K.; Saha, B.B.; Koyama, S. Specific heat capacities of carbon-based adsorbents for adsorption heat pump application. Appl. Therm. Eng. 2018, 129, 117–126. [Google Scholar] [CrossRef]
- Islam, M.A.; Pal, A.; Saha, B.B. Experimental study on thermophysical and porous properties of silica gels. Int. J. Refrig. 2020, 110, 277–285. [Google Scholar] [CrossRef]
- Mohammed, R.H.; Mesalhy, O.; Elsayed, M.L.; Hou, S.; Su, M.; Chow, L.C. Physical properties and adsorption kinetics of silica-gel/water for adsorption chillers. Appl. Therm. Eng. 2018, 137, 368–376. [Google Scholar] [CrossRef]
- Maeda, H.; Mokuno, T.; Isu, N.; Kasuga, T. Thermal properties of silica-based hybrids with different alkyl chains. Ceram. Int. 2017, 43, 880–883. [Google Scholar] [CrossRef]
- Pajdak, A.; Walawska, B.; Szymanek, A. The effect of structure modification of sodium compounds on the SO2 and HCl removal efficiency from fumes in the conditions of circulating fluidised bed. Chem. Biochem. Eng. Q. 2017, 31, 261–273. [Google Scholar] [CrossRef]
- Pajdak, A. Purification of flue gases from combustion of solid fuels with sodium sorbents Oczyszczanie gazów ze spalania paliw stałych z SO2 sorbentami sodowymi. Przemysł. Chem. 2015, 1, 160–164. [Google Scholar] [CrossRef]
- Demir, H.; Mobedi, M.; Ülkü, S. The use of metal piece additives to enhance heat transfer rate through an unconsolidated adsorbent bed. Int. J. Refrig. 2010, 33, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Askalany, A.A.; Henninger, S.K.; Ghazy, M.; Saha, B.B. Effect of improving thermal conductivity of the adsorbent on performance of adsorption cooling system. Appl. Therm. Eng. 2017, 110, 695–702. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, L.W.; Wang, R.Z.; Ge, T.S.; Ishugah, T.F. Thermal conductivity, pore structure and adsorption performance of compact composite silica gel. Int. J. Heat Mass Transf. 2014, 68, 435–443. [Google Scholar] [CrossRef]
- Gurgel, J.M.; Filho, L.S.A.; Grenier, P.H.; Meunier, F. Thermal diffusivity and adsorption kinetics of silica-gel/water. Adsorption 2001, 7, 211–219. [Google Scholar] [CrossRef]
- Gurgel, J.M.; Klüppel, R.P. Thermal conductivity of hydrated silica-gel. Chem. Eng. J. Biochem. Eng. J. 1996, 61, 133–138. [Google Scholar] [CrossRef]
- Bahrehmand, H.; Khajehpour, M.; Bahrami, M. Finding optimal conductive additive content to enhance the performance of coated sorption beds: An experimental study. Appl. Therm. Eng. 2018, 143, 308–315. [Google Scholar] [CrossRef]
- Dasgupta, K.; Joshi, J.B.; Banerjee, S. Fluidized bed synthesis of carbon nanotubes—A review. Chem. Eng. J. 2011, 171, 841–869. [Google Scholar] [CrossRef]
- Tlili, I.; Alkanhal, T.A.; Barzinjy, A.A.; Dara, R.N.; Shafee, A.; Li, Z. Investigation of thermal characteristics of carbon nanotubes: Measurement and dependence. J. Mol. Liq. 2019, 294, 111564. [Google Scholar] [CrossRef]
- Wu, H.; Al-Rashed, A.A.A.A.; Barzinjy, A.A.; Shahsavar, A.; Karimi, A.; Talebizadehsardari, P. Curve-fitting on experimental thermal conductivity of motor oil under influence of hybrid nano additives containing multi-walled carbon nanotubes and zinc oxide. Phys. A Stat. Mechan. Appl. 2019, 535, 122128. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Motahari, K.; Sanatizadeh, E.; Afrand, M.; Rostamian, H.; Reza Hassani Ahangar, M. Estimation of thermal conductivity of CNTs-water in low temperature by artificial neural network and correlation. Int. Commun. Heat Mass Transf. 2016, 76, 376–381. [Google Scholar] [CrossRef]
- Aliev, A.E.; Guthy, C.; Zhang, M.; Fang, S.; Zakhidov, A.A.; Fischer, J.E.; Baughman, R.H. Thermal transport in MWCNT sheets and yarns. Carbon 2007, 45, 2880–2888. [Google Scholar] [CrossRef]
- Dellago, C.; Naor, M.M.; Hummer, G. Proton Transport through Water-Filled Carbon Nanotubes. Phys. Rev. Lett. 2003, 90, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Striolo, A. The mechanism of water diffusion in narrow carbon nanotubes. Nano Lett. 2006, 6, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Pajdak, A.; Skoczylas, N. A comparison of the kinetics of hydrogen sorption in a metallic LaNi5 alloy and in multiwall carbon nanotubes. Przem. Chem. 2018, 97, 959–962. [Google Scholar]
- Pajdak, A.; Kudasik, M.; Skoczylas, N.; Wierzbicki, M.; Teixeira Palla Braga, L. Studies on the competitive sorption of CO2 and CH4 on hard coal. Int. J. Greenh. Gas Control 2019, 90, 102789. [Google Scholar] [CrossRef]
- Skoulidas, A.I.; Ackerman, D.M.; Johnson, J.K.; Sholl, D.S. Rapid Transport of Gases in Carbon Nanotubes. Phys. Rev. Lett. 2002, 89, 13–16. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Hernadi, K.; Fonseca, A.; Nagy, J.B.; Bemaerts, D.; Fudala, A.; Lucas, A.A. Catalytic synthesis of carbon nanotubes using zeolite support. Zeolites 1996, 17, 416–423. [Google Scholar] [CrossRef]
- Inoue, S.; Ichikuni, N.; Suzuki, T.; Uematsu, T.; Kaneko, K. Capillary condensation of N2 on multiwall carbon nanotubes. J. Phys. Chem. B 1998, 102, 1–4. [Google Scholar] [CrossRef]
- Pajdak, A.; Skoczylas, N.; Dębski, A.; Grzegorek, J.; Maziarz, W.; Kudasik, M. CO2 and CH4 sorption on carbon nanomaterials and coals—Comparative characteristics. J. Nat. Gas Sci. Eng. 2019, 72, 103003. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Tarazona, P.; Marini Bettolo Marconi, U.; Evans, R.; Wills, H.H. Phase equilibria of fluid interfaces and conflned fluids non-local versus local density functionals. Mol. Phys. 1987, 60, 573–595. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the navy. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Win, K.K.; Nowak, W.; Matsuda, H.; Hasatani, M.; Bis, Z.; Krzywanski, J.; Gajewski, W. Transport Velocity of Coarse Particles in Multi-Solid Fluidized Bed. J. Chem. Eng. Jpn. 1995, 28, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.-C. Handbook of Fluidization and Fluid-Particle Systems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]







| Parent Material | Particle Diameter of Parent Material | Additive | Content | Thermal Conductivity | Improvement | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Thermal Conductivity | Cooling Capacity | Heating Capacity | COP | ||||||
| mm | % | W/mK | % | ||||||
| Silica gel | 3–5 | Al | 5 | 0.34 | 72 | [14] | |||
| 0.218 | 106 | [54] | |||||||
| 10 | 0.498 | 152 | [14] | ||||||
| 0.314 | 196 | [54] | |||||||
| 15 | 0.675 | 241 | 12.50 | 9.90 | 2.40 | [14] | |||
| 0.363 | 242 | [54] | |||||||
| Silica gel | 3–5 | Cu | 5 | 0.305 | 54 | [14] | |||
| 0.187 | 76 | [54] | |||||||
| 10 | 0.424 | 114 | [14] | ||||||
| 0.246 | 132 | [54] | |||||||
| 15 | 0.557 | 181 | 11.2 | 8.1 | 2.9 | [14] | |||
| 0.324 | 106 | [54] | |||||||
| Activated carbon | 1–2 | Al | 10 | 10 | 100 | 0.22 | [55] | ||
| 20 | 12 | 120 | 0.2 | ||||||
| 30 | 23 | 230 | 0.18 | ||||||
| Cu | 10 | 8 | 80 | 0.22 | |||||
| 20 | 12 | 120 | 0.21 | ||||||
| 30 | 16 | 160 | 0.19 | ||||||
| CaCl2 + silica gel | 150 µm × 1.3 mm *; 250–500 µm ** | Carbon flakes | 20 | 0.78 | 136 | 17 | [59] | ||
| Dimensions of Sample (ø/h) | Reaction Atmosphere | Gas Flow Rate | Reference Temperature | Measurement Temperatures |
|---|---|---|---|---|
| mm | cm3/min | K | K | |
| 13/4 | Ar | 50 | 293 | 298–358, every 10 |
| SG content in the sample (wt%) | 100 | 95 | 95 | 95 | 85 | 85 | 85 |
| An additive content in sample (wt%) | 0 | 5 | 5 | 5 | 15 | 15 | 15 |
| Additive | - | Al | Cu | CNTs | Al | Cu | CNTs |
| Material | Granulation | Thermal Conductivity | Thermal Diffusivity |
|---|---|---|---|
| µm | W/m·K | mm²/s | |
| Silica gel (SG) | 100–160 | 0.1760 [51] | 0.137 [51] |
| Aluminium (Al) | 45–425 | 237 [51] | 97.1 [54] |
| Copper (Cu) | <150 | 401 [51] | 117 [54] |
| Carbon nanotubes (CNTs) | Ø = 3 nm, l = 10 nm | 3000 [54] (by length) | 460 [61] |
| Sample | ||||||
|---|---|---|---|---|---|---|
| mmol/g | m2/g | m2/g | m2/g | cm3/g | cm3/g | |
| SG | 125.43 | 753 | 128 | 625 | 0.02 | 0.45 |
| CNTs | 27.74 | 208 | 9 | 199 | 0.01 | 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulakowska, A.; Pajdak, A.; Krzywanski, J.; Grabowska, K.; Zylka, A.; Sosnowski, M.; Wesolowska, M.; Sztekler, K.; Nowak, W. Effect of Metal and Carbon Nanotube Additives on the Thermal Diffusivity of a Silica Gel-Based Adsorption Bed. Energies 2020, 13, 1391. https://doi.org/10.3390/en13061391
Kulakowska A, Pajdak A, Krzywanski J, Grabowska K, Zylka A, Sosnowski M, Wesolowska M, Sztekler K, Nowak W. Effect of Metal and Carbon Nanotube Additives on the Thermal Diffusivity of a Silica Gel-Based Adsorption Bed. Energies. 2020; 13(6):1391. https://doi.org/10.3390/en13061391
Chicago/Turabian StyleKulakowska, Anna, Anna Pajdak, Jaroslaw Krzywanski, Karolina Grabowska, Anna Zylka, Marcin Sosnowski, Marta Wesolowska, Karol Sztekler, and Wojciech Nowak. 2020. "Effect of Metal and Carbon Nanotube Additives on the Thermal Diffusivity of a Silica Gel-Based Adsorption Bed" Energies 13, no. 6: 1391. https://doi.org/10.3390/en13061391