Reducing Energy Requirements in the Production of Acrylic Acid: Simulation and Design of a Multitubular Reactor Train
Abstract
:1. Introduction
2. Method Development
- Reaction kinetics
- Co-current or counter-current flow configurations
- Overall heat transfer coefficient
- Number of tubes, tube dimensions, reactor orientation
- Valid shell and tube side phases
- Catalyst bed voidage, particle density, particle diameter, particle shape factor
- Reactor length
- Number of tubes
- Heat transfer fluid flow rate
- Heat transfer fluid heat exchange circuit
- Catalyst physical properties
- Conversion of propylene above 95%
- Complete conversion of acrolein
- Tube side pressure drop below 10%
- Shell side pressure drop
- Temperature of propylene oxidation reactor between 633 and 703 K as per the selected reaction kinetics
- Temperature of acrolein oxidation reactor between 533 and 573 K as per the selected reaction kinetics
- Removal of hotspots and subsequent limitation of susceptibility to thermal runaway
- Minimization of heat transfer fluid usage
- Heat integration
3. Results and Discussion
3.1. Reaction Method Selection
3.2. Reactor Configuration
3.3. Heat Transfer Fluid Selection
3.4. Catalyst Selection
3.5. Propylene Partial Oxidation Reaction Kinetics Validation
3.6. Acrolein Partial Oxidation Reaction Kinetics Validation
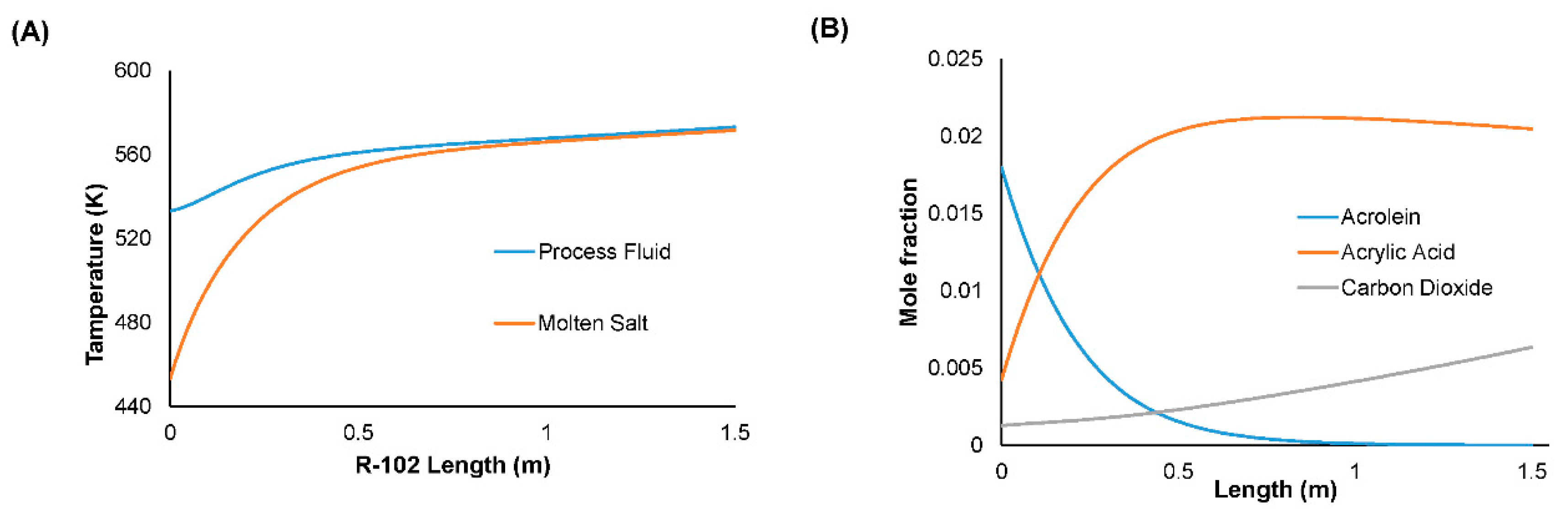
3.7. Assumptions and Simulation
4. Energy Analysis
5. Future Work
- Rigorous thermodynamic model selection for the reactant and product components via experimental data regression.
- The Aspen Plus® (AspenTech, Bedford, MA, USA) simulation model operation in this work is at steady state. A dynamic simulation can be developed to understand the impact of common operational changes, upsets and abnormal conditions; and thereafter develop a control procedure for the intensified process presented.
- The extent of the transferability of the optimized gas phase oxidation process in this work, to other highly exothermic, gas phase reactions.
- The optimized process in this work assumed negligible interphase heat and mass transfer resistances, axial dispersion and radial gradients. Future work can delve into the impacts of these non-idealities on the optimized process.
- Future work can explore the impact of catalyst deactivation on the optimized process, and the available levers to enable the optimized process until the catalyst end of run conditions.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaelwitz, J.A. Analysis, Synthesis and Design of Chemical Processes, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009; p. B.9. [Google Scholar]
- Sou, M.; Zhang, H.; Ye, Q.; Dai, X.; Yu, H.; Li, R. Design and control of an improved acrylic acid process. Chem. Eng. Res. Des. 2015, 104, 346–356. [Google Scholar]
- Jiang, B.; Hao, L.; Zhang, L.; Sun, Y.; Xiao, X. Numerical investigation of flow and heat transfer in a novel configuration multi-tubular fixed bed reactor for propylene to acrolein process. Heat Mass Trans. 2015, 51, 67–84. [Google Scholar] [CrossRef]
- Tripodi, A.; Compagnoni, M.; Martinazzo, R.; Ramis, G.; Rossetti, I. Process simulation for the design and scale up of heterogeneous catalytic process: Kinetic modelling issues. Catalysts 2017, 7, 159. [Google Scholar] [CrossRef]
- Luyben, W. Economic Trade-offs in acrylic acid production. Comp. Chem. Eng. 2017, 93, 118–127. [Google Scholar] [CrossRef]
- Redlingshofer, H.; Fischer, A.; Weckbecker, K.H.; Emig, G. Kinetic modelling of the heterogeneously catalyzed oxidation of propene to acrolein in a catalytic wall reactor. Ind. Eng. Chem. Res. 2003, 42, 5482–5488. [Google Scholar] [CrossRef]
- Estenfelder, M.; Lintz, H.G. Simultaneous determination of reaction kinetics and oxygen activity in single-phase oxidic catalysts and their mixture during partial oxidations. J Catal. 2002, 209, 177–185. [Google Scholar] [CrossRef]
- Stankiewicz, A. Advances in modelling and design of multitubular fixed-bed reactors. Chem. Eng. Tech. 1989, 12, 113–130. [Google Scholar] [CrossRef]
- Sinnott, R.K. Chemical Engineering Design; Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Darvishi, A.; Rashidi, A.M. Fixed-bed multi-tubular reactors for oxidative dehydrogenation in ethylene process. Chem. Eng. Tech. 2013, 36, 1691–1700. [Google Scholar] [CrossRef]
- Borio, D.O.; Gatica, J.E.; Porras, J.A. Wall cooled fixed bed reactors: Parametric sensitivity as a design criterion. AIChE J. 1989, 35, 287–292. [Google Scholar] [CrossRef]
- Kawakami, M.; Suzuki, K.; Yokoyama, S.; Takenaka, T. Heat Capacity Measurement of Molten NaNO3-NaNO2-KNO3 by Drop Calorimetry. In Proceedings of the VII International Conference on Molten Slags Fluxes and Salts, Cape Town, South Africa, 25–28 January 2004; The South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2004; pp. 201–208. [Google Scholar]
- Serrano-Lopez, R.; Fradera, J.; Cuesta-Lopez, S. Molten salts database for energy applications. Chem. Eng. Process. 2013, 73, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Grasselli, R.K. Fundamental principles of selective heterogeneous oxidation catalysis. Top. Catal. 2002, 2, 1–3. [Google Scholar] [CrossRef]
- Snyder, T.P.; Hill, C.G. The mechanism for the partial oxidation of propylene over bismuth molybdate catalysts. Catal. Rev. 1989, 31, 43–95. [Google Scholar] [CrossRef]
- Redingshofer, H.; Krocher, O.; Bock, W.; Huthmacher, K.; Emig, G. Catalytic wall reactor as a tool for isothermal investigations in the heterogeneously catalyzed oxidation of propene to acrolein. Ind. Eng. Chem. Res. 2002, 41, 1445–1453. [Google Scholar] [CrossRef]
- Zhu, J.; Araya, S.S.; Cui, X.; Sahlin, S.L.; Kaer, S.K. Modeling and design of a multi-tubular packed-bed reactor for methanol steam reforming over Cu/ZnO/Al2O3 catalyst. Energies 2020, 13, 610. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.K.; Sekulic, D.P. Fundamentals of Heat Exchanger Design; John Wiley and Sons: New York, NY, USA, 2003. [Google Scholar]
- Rase, H.F. Fixed Bed Reactor Design and Diagnostics: Gas-Phase Reactions; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Asif, M. Conversion enhancement of fixed-bed reactors using two-dimensional hollow cylindrical catalyst pellet. Int. J. Chem. React. Eng. 2013, 11, 159–168. [Google Scholar] [CrossRef]
- Afandizadeh, S.; Foumeny, E.A. Design of packed bed reactors: Guides to catalyst shape, size and loading selection. Appl. Thermal Eng. 2001, 21, 669–682. [Google Scholar] [CrossRef]
- Schüler, C.; Wolf, M.; Hinrichsen, O. Contactless temperature measurements under static and dynamic reaction conditions in a single-pass fixed bed reactor for CO2 methanation. J. CO2 Util. 2018, 25, 158–169. [Google Scholar] [CrossRef]
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process Advantages of Direct CO2 to Methanol Synthesis. Front. Chem. 2018, 6, 446. [Google Scholar] [CrossRef] [PubMed]
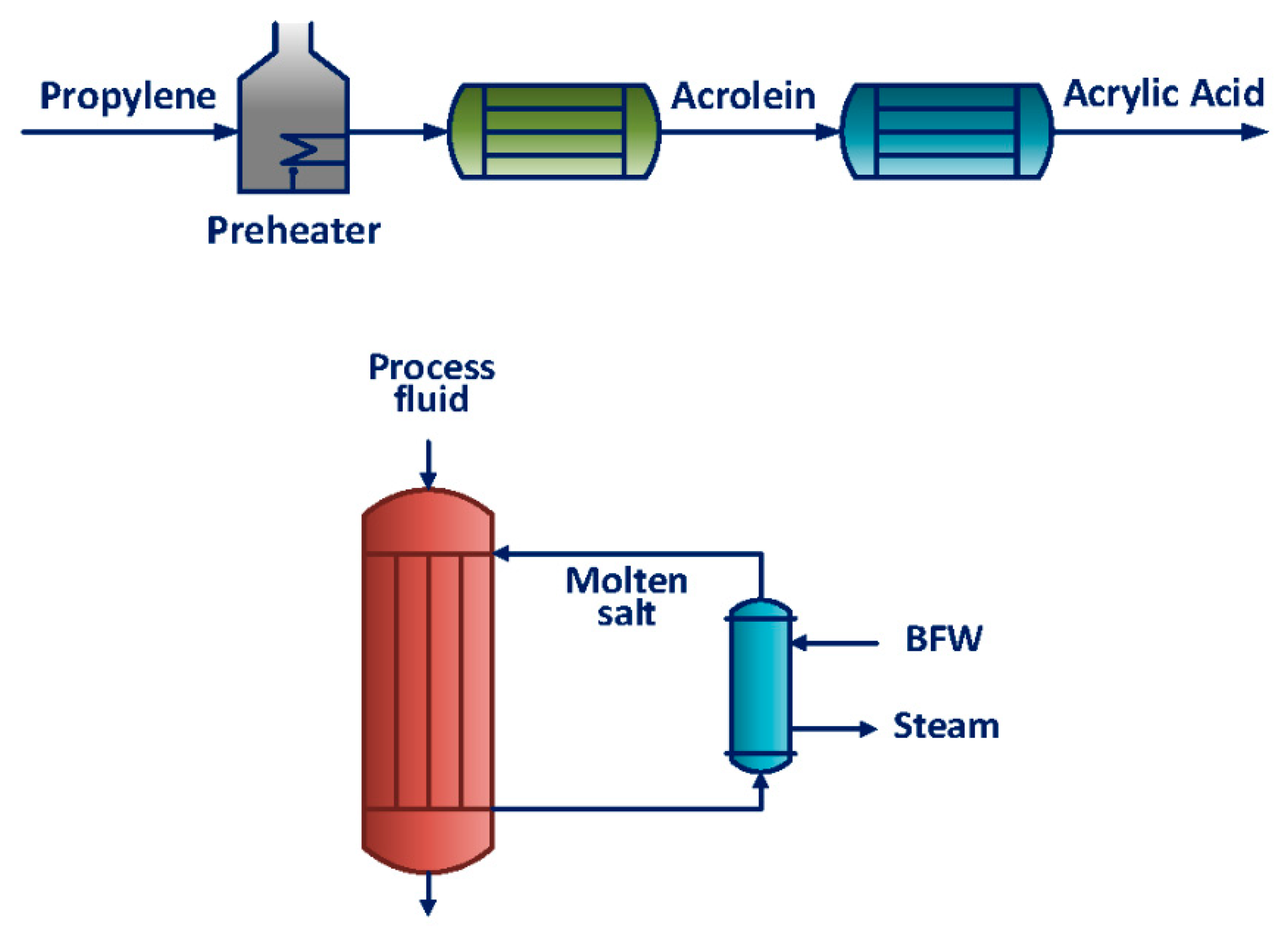

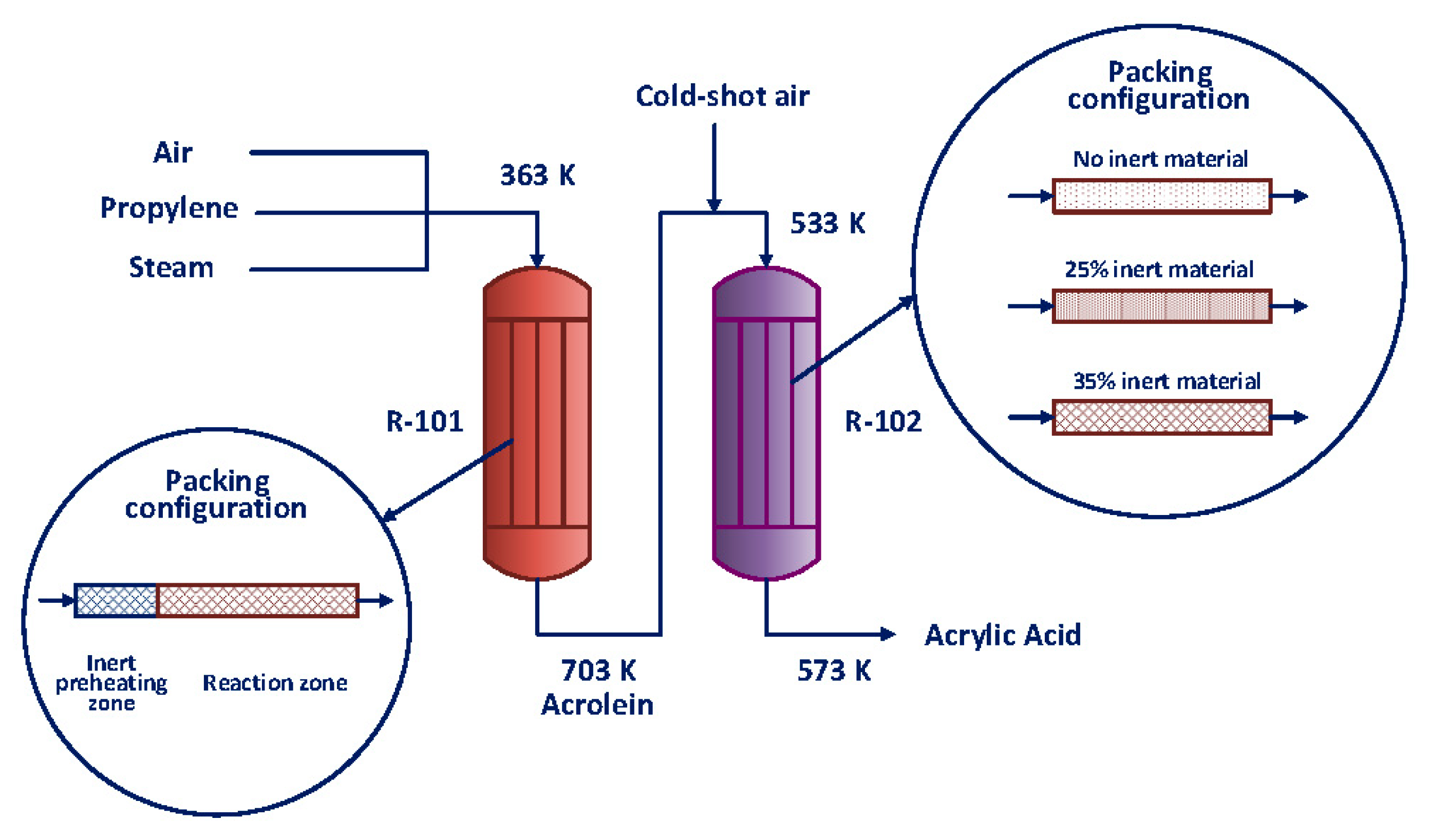
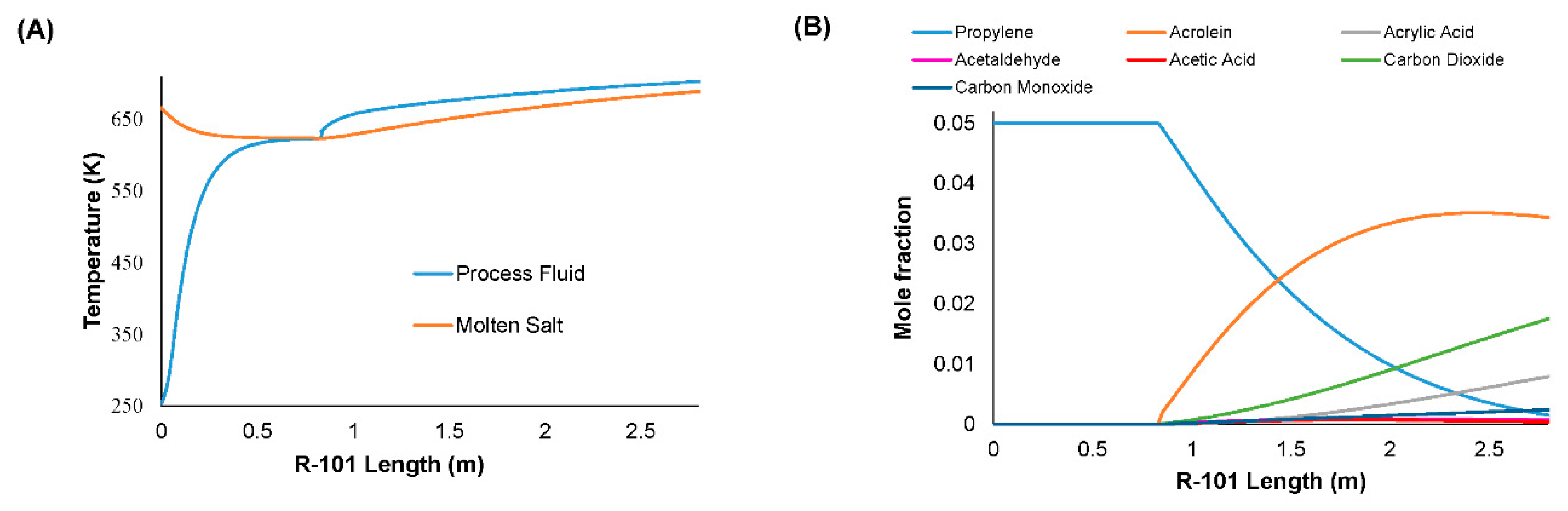
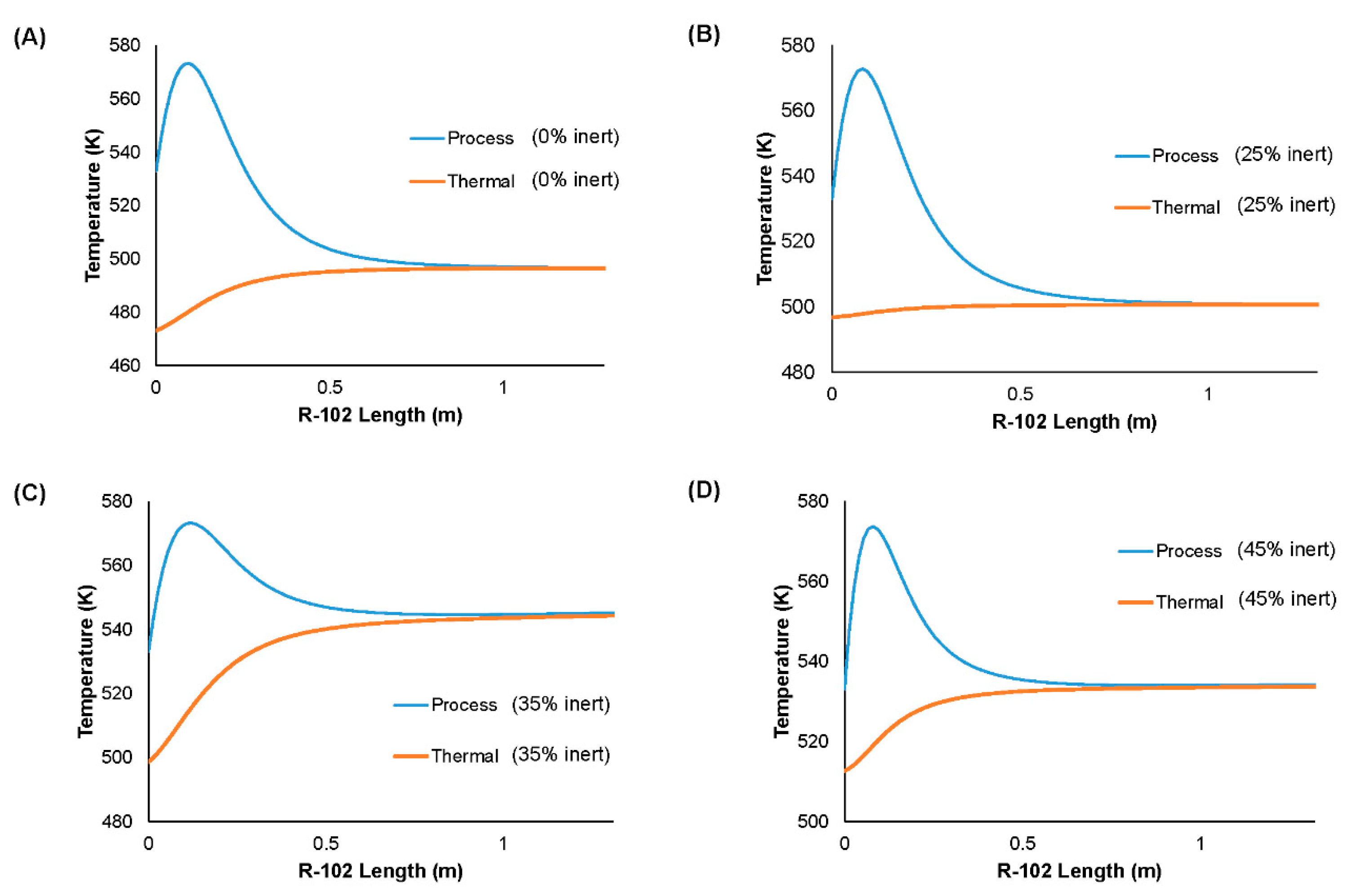
| Value | Conversion of Propylene (%) | Selectivity to Acrolein (%) |
|---|---|---|
| Maximum | 61.4 | 89.7 |
| Minimum | 59.6 | 89.1 |
| Mean value | 60.3 | 89.5 |
| Number of Tubes | 1 Selectivity of Acrylic Acid (%) | 1 Yield of Acrylic Acid (%) |
|---|---|---|
| 500 | 96 | 97.92 |
| 5000 | 96 | 97.96 |
| 10,000 | 96 | 98.03 |
| 15,000 | 96 | 97.98 |
| 20,000 | 96 | 97.91 |
| Recommended Ranges/Estimates | Reference |
|---|---|
| Shell side velocity ranges: 0.3–1 m/s 0.9–1.5 m/s 0.25–0.65 m/s | [9] [18] |
| Catalyst particle diameter should be 10% of tube diameter to prevent channeling. | [19] |
| Liquids with viscosities more than 1 mPa·s are restricted to a maximum shell-side pressure drop within the range 50–70 kPa. | [9] |
| Ratio of the tube length to catalyst particle diameter should exceed 50 for negligible axial dispersion. | [19] |
| Reactor tube-side pressure drop that is less than 10%. | [19] |
| Ratio of the tube diameter to the catalyst particle diameter less than 30 for a flat velocity profile. | [19] |
| Acrolein Production (kmol/h) | 1 Temperature Range (K) | Temperature of Heat Transfer Fluid (K) | Flow Rate of Heat Transfer Fluid (kmol/s) |
|---|---|---|---|
| 172 | 613–693 | 578 | 3 |
| 169 | 623–703 | 588 | 3 |
| 162 | 624–731 | 588 | 2.5 |
| 158 | 631–747 | 598 | 2.5 |
| 155 | 632–767 | 598 | 2.25 |
| 162 | 633–724 | 600.5 | 3 |
| 166 | 633–705 | 600.5 | 3.5 |
| 169 | 633–697 | 600.5 | 3.75 |
| Length (m) | Parameters | Value |
|---|---|---|
| 1.5 | Number of tubes: | 11,717 |
| Overall heat transfer coefficient (W/m2K) | 409 | |
| Coolant mole flow rate (kmol/s): | 3.75 | |
| Coolant inlet temperature (K): | 652 | |
| Tube-side pressure drop (Pa): | 20,161 | |
| Shell-side pressure drop (Pa): | 9938 | |
| Heat transfer surface area (m2) | 1844 | |
| 1.75 | Number of tubes: | 9952 |
| Overall heat transfer coefficient (W/m2K) | 449 | |
| Coolant mole flow rate (kmol/s): | 3.75 | |
| Coolant inlet temperature (K): | 624 | |
| Tube-side pressure drop (Pa): | 32,908 | |
| Shell-side pressure drop (Pa): | 14,803 | |
| Heat transfer surface area (m2) | 1827 | |
| 2 | Number of tubes: | 9028 |
| Overall heat transfer coefficient (W/m2K) | 471 | |
| Coolant mole flow rate (kmol/s): | 3.75 | |
| Coolant inlet temperature (K): | 623 | |
| Tube-side pressure drop (Pa): | 45,185 | |
| Shell-side pressure drop (Pa): | 19,439 | |
| Heat transfer surface area (m2) | 1805 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premlall, K.C.; Lokhat, D. Reducing Energy Requirements in the Production of Acrylic Acid: Simulation and Design of a Multitubular Reactor Train. Energies 2020, 13, 1971. https://doi.org/10.3390/en13081971
Premlall KC, Lokhat D. Reducing Energy Requirements in the Production of Acrylic Acid: Simulation and Design of a Multitubular Reactor Train. Energies. 2020; 13(8):1971. https://doi.org/10.3390/en13081971
Chicago/Turabian StylePremlall, Kiara Capreece, and David Lokhat. 2020. "Reducing Energy Requirements in the Production of Acrylic Acid: Simulation and Design of a Multitubular Reactor Train" Energies 13, no. 8: 1971. https://doi.org/10.3390/en13081971





