Abstract
Copper coordination complexes have emerged as a group of transition metal complexes that play important roles in solar energy conversion, utilization and storage, and have the potential to replace the quintessential commonly used transition metals, like Co, Pt, Ir and Ru as light sensitizers, redox mediators, electron donors and catalytic centers. The applications of copper coordination compounds in chemistry and energy related technologies are many and demonstrate their rightful place as sustainable, low toxicity and Earth-abundant alternative materials. In this perspective we show the most recent impact made by copper coordination complexes in dye-sensitized solar cells and other energy relevant applications.
1. Introduction
Environmental and energy issues will likely dominate science and society for the next several decades as climate change threatens Earth’s well-being. The predicted future energy demand by humankind can only be met if energy conversion and consumption is explored through renewable energy sources and sustainable energy practices in every aspect.
A tremendous research effort has been undertaken to develop green energy devices using organic optoelectronics since the 1980s. Compared to conventional inorganic semiconductors, organic/metalorganic small molecules have a higher degree of versatility and can be synthesized and fabricated by using different strategies to offer low-cost mass production over a span of applications, including organic light-emitting diodes (OLEDs) [1,2,3,4], energy storage (batteries) [5,6,7,8], hybrid photovoltaic cells [9,10,11,12] and artificial photosynthesis (catalysis, solar fuels) [13,14,15,16,17]. These properties triggered the fast-growing development of the field that involves the synthesis of new transition metal complexes, which have generated tremendous interest for different energy-related applications [18,19].
From a sustainability perspective, metal coordination complexes used in energy conversion (photovoltaics and solar fuels), storage (batteries) and consumption (OLEDs) have to be made from sustainable and Earth-abundant materials. To enter mass market applications, cost-efficient metals with a d10 orbital configuration—such as Cu(I)—have to be, and are currently, the focus of numerous research studies [20,21,22].
Recent advancements in copper coordination complexes have posed them as viable and better-performing alternatives in comparison to other transition metal coordination complexes, due to their success in photophysical and photoelectrochemical applications. Copper complexes are easy to synthesize, they are made from Earth-abundant sources and have low toxicity, especially in comparison with commonly used transition metals, such as Co(II), Pt(II), Ir(III) and Ru(II) [23,24], which are expensive, toxic and of low abundance. Coordination complexes of copper(I), copper(II), and copper(III) have been extensively studied and implemented as photosensitizers, triplet emitters, and catalysts in a variety of the aforementioned photochemical applications [21].
In this perspective, we will present an overview of copper coordination complexes that were used in various energy-relevant applications, from energy conversion to storage and lighting.
2. Fundamental Aspects of Copper Coordination Complexes
Copper was one of the first widely used metals, as it is among the 25 most abundant elements in the Earth’s crust. Copper complexes have both a well-defined coordination chemistry and an extensive redox chemistry: their oxidation states range from 0 to +4, although the most dominant ones are the +2 (cupric) and the +1 (cuprous). The coordination and geometry of copper complexes depend on their oxidation state. The closed shell d10 Cu(I) ions have the most prevailing coordination of four with tetrahedral geometry, but linear (coordination of two) and trigonal (coordination of three) geometries are common as well. The resulting coordination complexes are diamagnetic and colourless, unless the color stems from charge transfer through chelating ligands or the influence of counter-ions. The d9 Cu(II) ion is generally in a tetragonal coordination, with four short equatorial bonds and another one or two longer axial bonds, although complexes with other structures are known, including tetrahedral or square planar (Figure 1). With regards to photophysical properties at room temperature, the luminescence of [Cu(dmp)2]2+ (dmp = 2,9-dimethyl-1,10-phenanthroline) was first discovered and studied by Sauvage and McMillin [25,26] and attributed to the strong metal-metal interaction and to the corresponding ligand-to-metal-to-metal charge transfer (LMMCT). The development of sensitizers/dyes based on copper complexes faces several challenges as Sn → T1 (with n ≥ 1) and T1 → S0 transitions are highly spin-forbidden in Cu(I) complexes, resulting in recombination processes and device instabilities due to photochemical reactions [27]. Apart from the undesired recombination, fast ligand exchange and the ease of oxidation from the Cu(I) to the Cu(II) state also challenge the development of energy-related applications for copper complexes [21].
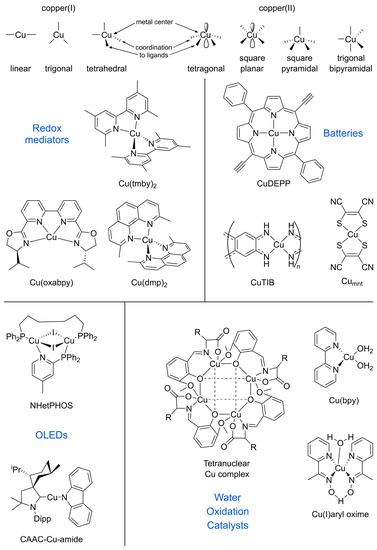
Figure 1.
Geometrical arrangements of Cu(I) and Cu(II) complexes, and structures of key molecules in this perspective.
The electrochemical properties of copper complexes strongly rely on the chelating ligands, since the coordination sphere impacts the redox potentials and the reorganisation between Cu(I) and Cu(II) ions [28]. For example, incorporation of bulky groups into neighboring sites of the chelating atoms exerts a remarkable steric hindrance effect to the Cu(I)/Cu(II) redox shuttle for Cu(I) complexes [11,29], which lowers the reorganisation energies and therefore the structural changes between copper I and II, which subsequently leads to more efficient charge transfer between the sensitizer and the redox species [30]. The emission quantum yield can also be boosted by the presence of a bulky alkyl group due to the increase in rigidity. As a result, it is important to consider the strategies for both structural modification of Cu(I) complexes and device fabrication in order to meet application requirements [28].
3. Dye-Sensitized Solar Cells
Dye-sensitized solar cells (DSCs) belong to the class of n-i-p (or p-i-n, depending) heterojunctions. Briefly, a light-absorbing dye, called intrinsic (i) layer, is placed between an electron (n) and a hole (p) selective layer. Upon electron excitation thanks to the absorption of a photon, the dye injects the excited electron in the n layer, which transports the charge to the device anode. The dye is regenerated by the p layer, which transports the resulting hole to the device cathode (Figure 2).
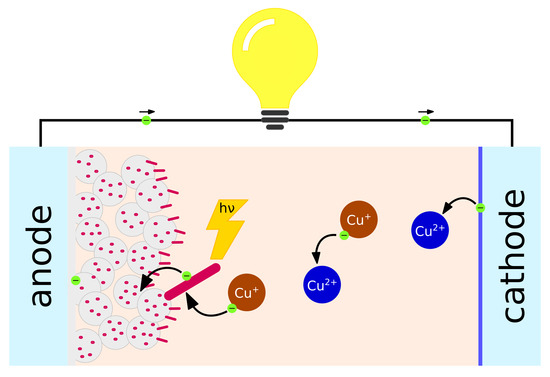
Figure 2.
Electron flow in a dye-sensitized solar cell upon excitation.
Dye-sensitized solar cells are divided in two categories: liquid (DSC) and solid-state (ssDSC). In DSCs, the p layer is comprised of an electrolyte solution containing a redox couple. The reduced species of the redox couple regenerates the dye and the resulting hole is transferred to the cathode via diffusion. DSCs are generally built by first sealing the anode and the cathode together with a spacer (a common occurrence is a thermoplastic film) and then filling the empty space between the electrodes with the electrolyte solution, followed by the sealing of the hole(s) left open for electrolyte injection. In ssDSCs, the liquid electrolyte is replaced with a solid hole transporting material (HTM), which is also usually comprised of a redox couple. Since the HTM is solid, after dye regeneration the transport of the hole towards the cathode happens via inter-molecular charge hopping from an oxidized species to the nearest reduced one, rather than via diffusion. Solid-state DSCs exist in two different architectures: monolithic and sandwich. In the monolithic architecture, each layer is built on top of the previous one. The cathode is usually comprised of an evaporated metal electrode, or a screen-printed carbon electrode. The sandwich architecture instead is identical to that of liquid DSCs, with the exception that a solid HTM replaces the liquid electrolyte (Figure 3).
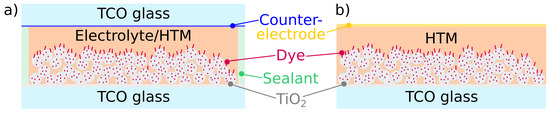
Figure 3.
Schematic diagram of (a) sandwich and (b) monolithic DSC structures. TCO = thin conductive oxide.
Dye-sensitized solar cells have struggled in the past to find an application market, given their relatively low efficiency compared to other technologies. However, technological advancements in the IT field have now opened a commercialization opportunity for DSCs—that of energy source for indoor low-power electronic devices for the Internet of Things (IoT). Copper coordination complexes are a key enabling factor for this new application. As it is explained in the next section, in fact, they can produce ssDSCs with high efficiency in low lighting conditions, while retaining the high operation voltages required to operate electronic devices with a small amount of solar cells connected in series. As an example, Freitag and co-workers have recently demonstrated how DSCs can power a self-sufficient neural network of intelligent sensors [31].
3.1. Redox Mediators and Hole Transporting Materials
Copper coordination complexes have given a new impulse to DSC research in recent years. This class of materials has proven to possess suitable redox potentials, excellent dye regeneration properties with an unprecedented small driving force requirement (as little as 100 mV), and the ability to provide good performance in the solid state and at very low lighting conditions. Freitag et al. have pioneered the work on copper complexes in DSCs in 2015, with a work reporting on a complex based on a phenantroline ligand (Cu(dmp)2, Figure 1) in conjunction with the LEG4 dye [32]. Not only did a ssDSC based on this HTM display a very high efficiency of 8.2% but—for the first time—a ssDSC proved to be more efficient than its liquid counterpart (6.0%). Most importantly, both solar cells have a VOC above 1 V, which is among the highest reported to date. This exceptional result is achieved thanks to the ability of copper complexes to “zombify”, i.e., to form an efficient solid-state HTM layer from an electrolyte solution, once the electrolyte solvent is fully evaporated. After this initial report, copper complexes have outclassed every other material in ssDSCs, while providing good performance (although not record-breaking) in liquid DSCs as well. Two years later, Cao et al. [33] and Freitag et al. [11] present a new copper complex, this time based on a substituted bipyridine (tmby, Figure 1), for both outdoor and indoor (with artificial light of low intensity) use. At full sun illumination, ssDSCs based on the Cu(tmby)2 HTM in combination with the Y123 dye reached a new record efficiency of 11%, overcoming for the first time the 10% mark. At low artificial light intensities, DSCs with Cu(tmby)2 and a mixture of XY1 and D35 dyes reached an efficiency of 29%, while retaining a VOC of 800 mV. A similar cell at full light intensity had an efficiency of 11% with a VOC over 1 V. The efficiency record for copper complex-based DSCs at full sun is currently held by Zhang et al., who fabricated DSCs with Cu(tmby)2 and the WS-72 dye with 11.7% (solid-state) and 11.6% (liquid) power conversion efficiencies (PCEs) [34]. For what concerns indoor artificial illumination, the record belongs to Michaels et al., with a device based on Cu(tmby)2 and a XY1:L1 dye mixture featuring a 34% PCE at 1000 lux [31].
The high VOC and little driving force requirements achieved by copper complex-based DSCs are due to the much smaller energy losses of this class of materials compared to others, such as cobalt complexes and the iodine/iodide redox couple [12]. However, copper complexes are not free from reorganization energy losses. In particular, Cu(I) favors a tetrahedral geometry, while Cu(II) prefers a tetragonal/square planar configuration. To overcome this issue, a steric hindrance approach was already taken by Freitag et al. in their first report [32], where they add methyl groups to the phenantroline in position 2,9 to reduce twisting. Hagashino et al. took a step further and studied five different steric groups in position 2,9 on the phenantroline [35]. A stronger approach is to avoid the use of bidentate ligands altogether and complexate the copper cation with tetradentate ligands instead. As all four coordination centers are covalently bonded, these ligands will force the same geometry on both copper oxidation states, with very minor rearrangements. Michaels et al. reported a first instance of this approach [18]. Although DSCs fabricated with the newly synthesized complex Cu(oxabpy) (Figure 1) were not as efficient as those with Cu(tmby)2, the reason was due to a much lower redox potential of the former compared to the latter. However, looking at VOC values, it can be noted that the difference between the two is much smaller compared to the difference of their redox potentials. This discrepancy can be attributed to a reduced loss of reorganization energy thanks to the tetradentate ligand. Rodrigues et al. also presented a series of tetradentate ligand-based copper complexes, with a behavior in terms of VOC compared to bidentate ligands similar to what has been discussed previously [19]. In the future, the identification of a tetradentate copper complex with an ideal redox potential could pave the way to unprecedented device efficiencies.
3.2. Dyes
Copper coordination complexes have been employed in DSCs not only as redox mediators, but also as dyes, although not with the same success in this role. The most active research group for what concerns copper complex-based dyes is that of Housecroft, who has published several articles in recent years, focusing mostly on heteroleptic complexes [36,37,38,39,40,41,42,43,44,45,46,47]. Their best device is comprised of a Cu(I) complex with a (6,6-dimethyl-[2,2-bipyridine]-4,4-diyl)bis(4,1-phenylene))bis(phosphonic acid) ligand attached to the mesoporous titania and an ancillary ligand with a 2,9-dimethyl-1,10-phenanthroline metal-binding domain substituted in 4,7-positions with hole-transporting 4-(diphenylamino)phenyl groups ([Cu(1)(4)]+) [40]. The best efficiency of 3.7% is obtained with this dye when coupled with a Co(bpy)3 electrolyte in 3-methoxypropionitrile. Housecroft and co-workers also presented the concept of a full-copper DSC, where a copper-based dye is coupled with a copper-based redox mediator [47]. In this case, the maximum achieved efficiency was 2.1%. Another research group very active in full-copper DSCs is that of Colombo, in collaboration with Biagini [48,49,50]. They report DSC efficiencies up to 2.5% when their D1 dye is coupled with a substituted phenantroline-based copper electrolyte. This result is only slighty worse compared to the use of the I−/I redox couple with the same dye (3.0%).
A class of ligands that has gained attention for copper-based dyes is that of phthalocyanines. While the aforementioned dyes were all based on Cu(I), phthalocyanine-based dyes have a Cu(II) metal center instead. Alamin Ali et al. presented two phthalocyanine dyes substituted with 2-phenylphenoxy moieties in different positions [51]. Their best solar cell with a I−/I redox couple attained a maximum efficiency of 6.8%. Solğun et al. introduced a 4-tritylphenoxy group as a substituent for their phthalocyanine [52]. When coupled with polysulfide electrolyte, their device reached an efficiency of 1.8%. The last phthalocyanine-based dye is presented by Gigli and co-workers [53] and in this case the anchoring group, rather than being attached directly to the phthalocyanine, is comprised of a benzoic acid linked to the phthalocyanine via an alkine bridge. For them, the maximum DSC efficiency was 1.7% when using a I−/I redox couple. It should be noted that phthalocyanines are efficient chromophores even without a metal center. Indeed, DSCs fabricated with the ligand alone, without the Cu(II) center, were only slightly less efficient than their metal-containing counterparts.
Wills et al. built upon Housecroft’s work and presented a homoleptic dye based on a thiophene-substituted bipiridyl ligand [54]. Device efficiency for this dye coupled with a I−/I redox couple was 1.4%. Yadav et al. synthesized a copper complex based on a dithiocarbamate ligand with ferrocene pendants which, together with I−/I, resulted in a solar cell efficiency of 2.6% [55]. Raithby and co-workers reported on a series of dinuclear copper complexes with substituted pyridinyl-based ligands [56]. The best-performing dye of this series achieved a power conversion efficiency of 1.6% in a DSC with I−/I. Finally, Zhong and co-workers take a very unique approach to dyes, presenting two polymeric dyes with repeating units based on copper complexes bearing titania anchoring groups [57]. The two dyes, both mediated by a I−/I redox couple, achieved efficiencies of 3.5 and 5.3%, respectively.
4. Organic Light-Emitting Diodes
Organic light-emitting diodes (OLEDs) are electronic devices that use electricity to produce light and that consume less power compared to older technologies such as liquid crystals [1]. The architecture of OLEDs consists in a layer-by-layer deposition of thin films. The simplest device is comprised of a monolayer emission layer (EML) sandwiched between two injection electrodes. These mono-layered structures show low quantum efficiency due to the fast electron-hole recombination in the emission layer. This structure was further modified by the introduction of a multi-layer configuration of organic compounds with specific charge transport properties, so that the electron-hole recombination region becomes more ordered within the emissive layer, and distant from the electrodes. Figure 4 depicts the structural scheme of a mono- and a multi-layered OLED.
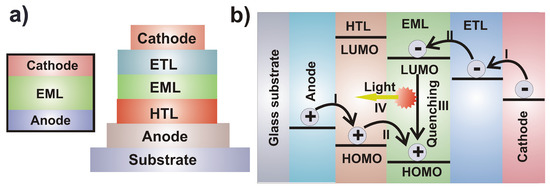
Figure 4.
(a) Structure of an organic light-emitting diode with monolayer (left) and multilayer (right) configuration. (b) Working principle of OLEDs: (I) injection of charge carriers; (II) transport of charge carriers; (III) exciton quenching; (IV) light emission.
OLEDs (Figure 4) work on four electronic processes: (i) charge injection, (ii) charge transport, (iii) electron-hole recombination (i.e., exciton quenching), and (iv) light emission. When an external voltage is applied, electrons (e) are injected through the cathode into the LUMO of its adjacent layer material and holes (h+) are injected through the anode into the HOMO of the adjacent layer material. The injected electrons and holes then migrate into the active layer (EML) where they recombine with each other in a region known as “recombination zone” to form a neutral state called exciton. This relaxes from the excited state to the ground state with emission of light.
Organometallic luminescent emitters based on Ir, Pt or Os are widely used for OLEDs because of their color tunability, high efficiencies [3,4] and long-lived triplet excited states [58]. However, to meet the mass market production demand, these expensive noble metals have been replaced by luminescent complexes based on more abundant, non-toxic and cost effective metal complexes of copper. Moreover, the thermally-activated delayed fluorescence (TADF) process to harvest both singlet and triplet excitons in third generation OLEDs make copper complexes very good candidates. A number of copper complexes have already been tested in OLEDs [59,60,61].
Copper Complexes as Light Emitters in OLEDs
Several new advancements have been made during the past few years to establish copper complexes as the third generation emitters that will replace iridium phosphors in electroluminescent devices. In particular, the TADF mechanism observed in some Cu(I) complexes resulted in unprecedented fluorescence quantum efficiencies [62]. The very first copper complex-based OLED reported by Che and Ma [63] showed very low device efficiency and limited brightness due to a relatively long-lived excited state. Since then, several efforts have been put forward in order to increase efficiency. Mononuclear four-coordinate Cu(I) complexes have been studied since late 1970s and quite a good number has been addressed by McMillin [64,65,66]. To minimize the flattening disorder upon photoexcitation, McMillin and co-workers attempted to increase the steric bulk on the phenanthroline moiety [65]. Later, a more sterically demanding ligand such as triphenyl phosphine (PPh3) was introduced [67,68]. Despite these improvements, the complex still underwent exciplex quenching in methanol. Much later in 2012, Adachi et al. [5] re-investigated the complexes reported by McMillin [68] and obtained a maximum external quantum efficiency (EQEmax) of 15% by tuning the electron blocking layer (EBL) of the device. Another class of neutral Cu(I) complexes containing bis(pyrazolyl)borate-based ligands were reported by Osawa and co-workers [69]. The best device showed an EQEmax of 17.7%. The improved thermal stability of the complexes allowed easy fabrication of their OLED devices with a vacuum deposition approach. In one of their previous reports, Osawa et al. [70] developed green OLEDs that exhibited an extraordinary EQEmax of 21.3% for three-coordinate Cu(I) complexes with a terminal halide group. The efficiency was further increased to 22.5% upon introducing steric bulk at the ortho positions of peripheral phenyl groups of the phosphine ligand [71]. A frontier orbital study showed that the HOMO was partially located on the Cu(I) and on the halogen atoms, whereas the LUMO was confined to the o-phenylene group in the phosphine ligand. Several bis-halide-bridged copper(I) complexes have been efficiently used in OLEDs, fabricated by solution-processing techniques [72]. The OLED fabricated with an iodide-bridged dinuclear Cu(I) emitter based on an N-heterocyclic phosphine (NHetPHOS, Figure 1) ligand showed an outstanding EQEmax (23%) and a very high PLQY (close to unity) with a short emission decay (several s). The rigidity imposed on the Cu(I) center by the bridging units was a successful attempt to increase performance. This complex gave the highest efficiency among the Cu(I)-based green-emitting OLEDs so far. Very recently, the pioneering work of Hamze et al. [62] has been published in Science that reports a series of two-coordinate CAAC-Cu-amide complexes (CAAC = cyclic(alkyl)(amino)carbene, Figure 1). The paper reports photoluminescence efficiencies greater than 99% and microsecond lifetimes (2 to 3 s), which lead to an efficient blue-emitting OLED.
5. Batteries
Unlike in DSCs and OLEDs, copper coordination complexes have not been widely employed in batteries yet. The few reports available in the literature make use of these materials either as electrodes for lithium ion batteries (LIB), or as redox couples in redox flow batteries (RFB).
5.1. Lithium Ion Batteries
Lithium ion batteries have revolutionised the world of portable electronic devices, thanks to their high energy density (100–265 W h kg for commercial batteries). However, they suffer from low charge-discharge rates (their advised charge rate is usually 0.5–1C). Moreover, electrode materials are usually comprised of scarce and/or toxic materials, which makes them non-environmentally friendly and raises concerns for their sourcing in the future. Copper coordination complexes are starting to being studied to address both concerns, as they do not provide environmental concerns and they are able to provide high rates without sacrificing capacity and energy density. One of the major challenges for these electrode materials remains their cyclability and their solubility in the electrolyte solvent.
The first report on copper complexes for LIBs came from Fichtner and co-workers, who used a copper porphyrin with two alkyne side groups (CuDEPP, Figure 1) [73]. Thanks to the high pi-stacking of CuDEPP, the resulting electrode was insoluble in the electrolyte solvent. A Li-ion battery using CuDEPP as cathode and Li metal as anode with a capacity of 186 mA h g was cycled at rates varying from 5C to 53C and back, with 100 cycles per rate constant for a total of 900 cycles. Although capacities decreased at increasing rates, the final capacity at 5C was mostly restored compared to the initial one, indicating good cyclability. At 53C the battery retained a capacity of 115 mA h g with a coulombic efficiency of 99%, which corresponds to an energy density of 345 W h kg and a specific power of 29 kW kg. In a long-term cycling test for 8000 cycles at 21C, the cell retained 60% of its initial capacity, with a coulombic efficiency close to 100%. The same copper porphyrin was used as anodic material in a Li-free battery with a graphite cathode and an ionic liquid electrolyte. An initial capacity of 94 mA h g was recorded at 10C, which decreased (and was retained for 200 cycles) to 44 mA h g at 53C. In a different communication, a similar Li-free battery was studied by the same research group with Raman spectroscopy, to elucidate its electrochemical energy storage processes [74]. Chen et al. continued their colleagues’ work on Li-free battery by studying a copper porphyrin with four alkyne side groups (CuTEP), which presented three different morphologies depending on synthetic conditions [75]. The best battery fabricated with CuTEP showed a stable capacity of 81 mA h g at 6C, which decreased to 41 mA h g at 174C. Such a battery also retained a capacity of 55 mA h g for 1000 cycles at 44C, with a coulombic efficiency of 98%.
Wu et al. have studied copper rhodizonate complexes grown in situ on reduced graphene oxide as anodic material for LIBs [76]. After 100 cycles at 0.1C, this battery showed a capacity of 937 mA h g, which decreased to 503 mA h g at 5C. At higher rate cycling, the battery showed a capacity of 510 mA h g at 1C after 250 cycles, with a coulombic efficiency close to 100%. Kapaev et al. built their LIBs with a copper coordination polymer derived from 1,2,4,5-tetraaminobenzene (CuTIB, Figure 1) used either as anode or as cathode [77]. In anode configuration, the battery had a capacity of 98 mA h g at 0.5C with a coulombic efficiency close to 100%. The battery also maintained a capacity of 55 mA h g after 20,000 cycles at 51C. In cathode configuration, the battery capacity reached 262 mA h g at 0.2C, corresponding to a specific energy density of 616 W h kg. However, the capacity dropped to 25 mA h g after only 25 cycles. Wang et al. reported on a series of copper phtalocyanines for dual ion batteries. Their best compound, CuTAPc, electropolymerized in situ, was used as either anode of cathode material in asymmetric batteries, or as both in symmetric devices [78]. Used as cathode coupled with a Li anode, the battery with CuTAPc had a capacity of 300 mA h g at 0.2C, with a coulombic efficiency of about 79%. This capacity was reduced down to 109 mA h g at 67C. For this battery, the energy density was 454 W h kg, while the specific power was 38 kW kg. When used in a symmetric system, the battery with CuTAPc registered a capacity of 133 mA h g at 1.5C, with a coulombic efficiency of 83%. The capacity was lowered to 60 mA h g after 200 cycles. For this battery, the energy density was 239 W h kg, while the specific power was 12 kW kg.
5.2. Redox Flow Batteries
Redox flow batteries are emerging as a promising technology for large-scale energy storage. Their design makes them unsuitable for mobile applications, while they can find a role as an affordable energy storage for grid stabilization when intermittent renewable energy sources are present. The main concept behind RFBs is to physically separate power and capacity. In these batteries, power generation/accumulation takes place at the interface of an ion-exchange membrane, where different redox species interact with each other. The power and rate of the battery is dependent on the potential difference between the two redox species, and on the charge and ion diffusion through the membrane. The capacity instead is solely based on the size of the tank of liquid electrolyte that compose the positive and negative electrodes of the battery, and on the concentration of the redox species dissolved within (Figure 5).
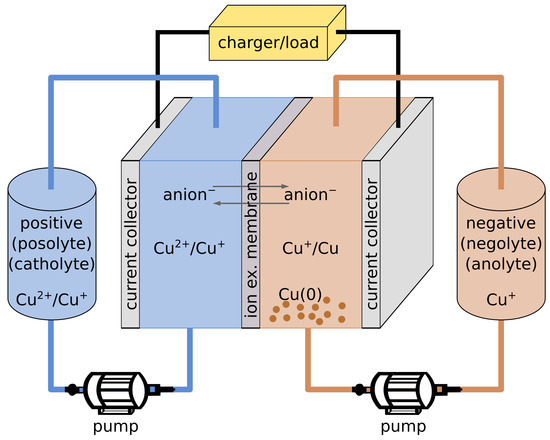
Figure 5.
Schematic diagram of a copper-based redox flow battery.
The most common RFBs are based on water solutions of vanadium salts. Vanadium is particularly suitable for this application because it has a suitable redox potential and three stable oxidation states, allowing the fabrication of an all-vanadium battery. RFBs based on a single element are particularly desirable, because they avoid self-poisoning (with the consequent loss of capacity) over time, when unwanted ions manage to diffuse through the exchange membrane. Despite having reached high capacity, water-based vanadium RFBs present a major drawback, given by the limited operational window of water in terms of applied potential, which limits the power of these devices. To overcome the limitation of water, organic-solvent based RFBs are being studied, with acetonitrile as the solvent of preference, given its stability even at high potential. This gain in potential, however, comes at a cost. From a safety point of view, in fact, we are substituting water with a flammable and more volatile solvent. From a specific capacity point of view, furthermore, we have to take into account the lower solubility of redox species in organic solvents, compared to the solubility of some inorganic salts in water. Finally, efficient ion exchange membranes for organic solvents are currently not available. For what concerns copper coordination complexes in RFBs, the two relevant oxidation processes are Cu → Cu+ and Cu+ → Cu.
Peljo et al. proposed the interesting concept of a copper-based RFB charged by low-grade heat (which is generated in many industrial processes), rather than electricity derived from renewable energy sources [79]. This battery takes advantage of the peculiar interaction of copper with acetonitrile, given by the following equation:
Shortly, when mixing solid copper and copper(II) with enough acetonitrile, the two oxidation states undergo a comproportionation process, forming a copper(I) complex with acetonitrile. Upon removal of acetonitrile by distillation the copper(I) complex disproportionates, yielding the two original oxidation states. In the RFB design proposed by Peljo, a suspension of Cu powder and a water solution with CuSO4, H2SO4 and acetonitrile would comprise the two electrodes of the battery. After power generation, the two liquids containing the copper(I) complex would be mixed together, then acetonitrile would be distilled thanks to the low-grade heat and the two copper species obtained separated again in their original electrodes. The actual battery used for laboratory experiments was of a simpler design, but based on the same concept. In such battery, a 7.6 mA h capacity was achieved at 0.2C, with cell potentials of 0.62 V and 0.40 V after charging and discharging, respectively, and an energy efficiency of 90%. Li et al. also reported on a RFB based on [Cu(MeCN)4]+ with a TFSI− counter-ion, this time in a full acetonitrile environment [80]. The use of TFSI ensured a high maximum solubility of 1.68 M for the copper at the cost of a lower conductivity through the membrane, given its size. The charge potential for the cell was 1.25–1.40 V, and the discharge potential was 0.85–1.05 V. The coulombic efficiency was 82%, while the energy efficiency was 49% at a current density of 2.5 mA cm. Given the complex solubility, the maximum theoretical energy density for this cell is 28 W h L, corresponding to a cell potential of 1.24 V. Finally, Toghill and co-workers investigated a RFB battery based on a copper dithiolene complex (Cumnt, Figure 1) [8]. This complex has a maximum solubility in acetonitrile of 0.91 M, for a maximum theoretical energy density of 14 W h L. In the laboratory battery, charge and discharge potentials were 1.1–1.3 V and 1.1–0.85 V, respectively, over 100 cycles. The corresponding coulombic and energy efficiency are 60–68% and 51–58%. The cell reached a capacity of 0.36 mA h (134%) at a current density of 0.48 mA cm, indicating self-discharge during charging.
6. Solar Fuels
One of the major challenges of today’s society is the urgent need to find alternatives to fossil fuels by designing and developing new methods and techniques to restore safe operating environments for humans. Solar fuels, a potential holy grail of clean energy, have been a focus for researchers around the world in the past few years. Solar fuels are synthetic chemical fuels produced when and where sunlight is available, and stored and transported for future use. Sunlight is the most appropriate energy source, which can initiate chemical reactions that make fuels and store solar energy in the form of chemical bonds. Solar fuels, in particular hydrogen, are considered as an alternative source of energy for replacing fossil fuels especially where storage is essential. The wide availability of H2 and ease of production from water makes it highly appealing. Water (11% H2) as a sole source of H2 is desirable as it is cheap and readily available. A number of processes are known to produce H2, which include (i) electrocatalytic processes that employ electricity and (ii) photolytic processes that use light energy or most simply sunlight, to split water to produce hydrogen. Under acidic conditions, water electrolysis proceeds via two half-cell reactions: (i) water oxidation and (ii) proton reduction:
However, in alkaline environments, the water splitting proceeds via the following equations:
6.1. Working Principles of Solar Fuels
Solar fuels are based on the principles of artificial photosynthesis. Natural photosynthesis begins with the absorption of sunlight, which is then transported and used to split charges. These charges are further transferred to catalytic sites to perform the fuel forming reactions. Figure 6 represents a general scheme for photo-electrocatalytic splitting of water.
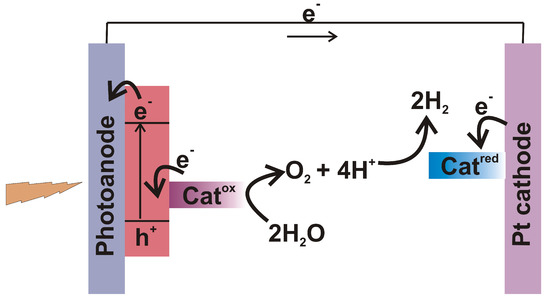
Figure 6.
General scheme representing water splitting in photoelectrochemical catalysis.
6.2. Copper Complexes as Water Oxidation Catalysts
Water oxidation catalysts (WOCs) have been a very important topic for researchers during the past few decades and copper-based catalysts have attracted increased attention due to their properties, which have been discussed earlier in this perspective. Besides, copper can exist in various oxidation states ranging from 0 to +3 and are considered good electrocatalysts [14,15,81].
The first molecular copper electrochemical WOC based on a bipyridine ligand (Figure 1) was reported by Mayer and co-workers in 2012 [16]. The reported copper complex showed remarkable self-assembling properties from simple copper salts and bipyridine under alkaline conditions (pH 11.8–13.3). In this high pH range, the copper complex showed large and irreversible currents indicative of catalysis, which occurred at an over-potential of 750 mV. This copper complex is among the most efficient catalyst for water oxidation with a very high turn over frequency (TOF) of 100 s. Lin and co-workers [82] reported a similar copper complex with a hydroxy-substituted bipyridine ligand (6,6-dihydroxy-2,2-bipyridine). The introduction of the hydroxy groups enabled the complex to catalyze the oxidation of water at over-potentials of 510–560 mV, which were much lower than those of its unsubstituted analogue [16]. However, the TOF was found to be 0.4 s. Further, Stott et al. replaced one of the pyridine rings in the parent bipyridine ligand with an imidazole moiety [83]. The strong -donor effect of the imidazole group resulted in an electron-rich copper center, leading to a reduced potential of 728 mV. The measured TOF at 150 M concentration was 35 s.
In 2016, Sun and co-workers reported on a Cu[(TMC)(H2O)](NO2)2 complex (TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane) and studied the role of its counter-ion in the catalytic performance of WOCs. Interestingly, the catalytic efficiency was found to increase dramatically for the nitrate counter-ion (TOF = 30 s at neutral pH) as compared to the perchlorate [84] counterpart. The potential for catalysis was 610 mV. Llobet and co-workers [17] reported on a series of Cu complexes bearing tetra-anionic tetradentate amidate ligands as WOCs. The reported Cu complexes were active electrocatalysts for water oxidation at high pH. The study showed that the water oxidation over-potential decreased tremendously to a value as low as 170 mV upon increasing the electron donating ability of the aromatic ring. To further investigate the effect of the electron donor group, Cau et al. synthesized a water-soluble Cu-based complex with a dianionic tridentate ligand, namely, N,N-2,6-di-methylphenyl-2,6-pyridinedicarboxamidate [85]. The electron density at the metal center was further increased due to the presence of dimethyl groups. Water oxidation happened at 650 mV and yielded a TOF value of 20.1 s at pH 10. More recently, Kuilya et al. reported on a very efficient electrocatalyst with a redox active aryl oxime ligand (Figure 1) for water oxidation that presented a remarkably high TOF of 100 s [86]. The complex also showed a low over-potential of 675 mV at neutral pH.
In 2013, Meyer and co-workers [87] reported on a self-assembled copper polypeptide complex with a triglycylglycine macrocyclic ligand, which efficiently catalyzed water oxidation at pH 11. The catalyst displayed high stability and superior activity toward water oxidation with a high TOF value of 33 s. In 2015, Pap et al. [88] developed mononuclear Cu complexes with two different l-2,3-diaminopropionic acid-based peptides, which formed stable 1:1 complexes with Cu(II) in alkaline solutions. TOFs of 24 and 53 s were recorded for the complexes. Cao and co-workers in 2019 reported on a very interesting water soluble Cu complex based on a porphyrin backbone (tetrakis(4-N-methylpyridine)porphyrin) that was found to catalyze water oxidation under much milder conditions [89]. At neutral pH and a low over-potential of 310 mV, the complex yielded a TOF value of 30 s. Apart from mononuclear Cu complexes, multinuclear Cu complexes have also been tested as potential catalysts for water oxidation. In 2015, Zhan et al. [90] synthesized a dinuclear Cu complex with an N,N-bis(3-aminopropyl)oxamido ligand. The complex showed a TOF of just 6.7 s, yet a very low over-potential value of 289 mV. Another dinuclear Cu complex that was reported by Kieber Emmons’ group [91] showed a moderate TOF value of 33 s.
Inspired by the tetranuclear cubic Mn4CaO5 cluster of the natural oxygen-evolving complex (OEC) in photosystem II, several tetranuclear Cu complexes have been developed in recent times. One of the most important works was reported by Wu and co-workers [92] in 2018 (Figure 1). The bio-inspired cubanes ([(Cu(LGly)4] and [(Cu(LGlu)4]) used by them were effective molecular WOCs in aqueous solutions (pH 12). With regard to their catalytic activity, TOF values of 267 s and 105 s, respectively, were obtained for the complexes.
7. Summary
Copper—being one of the cheapest and more highly abundant elements on Earth—is a good choice to develop inexpensive and efficient coordination complexes for all energy-related applications from energy conversion, to energy storage and lighting. These compounds can and will successfully replace other unsustainable and toxic transition metals (Co, Pt, Ir and Ru). There is a strong need for more development in copper coordination complexes to increase the performance of each energy relevant application.
For example, novel redox mediators and HTMs will be of the utmost importance for further improvement of DSCs, where copper complexes have been used both as redox mediators/HTMs and as dyes. They provide very good performance in liquid DSCs as redox mediators and they are currently the most efficient hole conductors for ssDSCs, thanks to their ability to “zombify”. In order to break efficiency records, redox mediators need to be further adapted towards the HOMO of dyes to optimize regeneration in terms of driving force, and to reduce recombinations with the TCO and TiO2. Research should therefore stir in the near future in the direction of complexes capable of generating greater currents and of being integrated into devices through scalable deposition methods. As dyes, copper complexes do not perform very well, and those with higher efficiency owe their results mostly to their ligands, rather than to the complex as a whole. Given the availability of highly efficient organic dyes, which avoid the presence of transition metals altogether, copper complexes probably will not have a bright future as dyes for DSCs.
Copper complexes and clusters have proven to be promising materials to replace OLEDs based on noble metals. With the progresses made during the past several years on the development of structurally and thermally stable copper-based luminescent systems, especially those of copper coordination complexes, this old dream is getting closer to be a reality. By modifying the ligands it is possible to precisely modulate key properties such as color and solubility. Subtle modification of the structure and their corresponding electronic properties of the ligands fine-tune the color of the light being generated across the entire visible spectrum, from red to green to blue. Although the advantages of Cu(I) complexes are directly related to their low cost, absence of emission self-quenching and low energy consumption, not all of them have comparable device performances as Ir(III) or Pt(II)-based devices. However, device performance can be further boosted by careful molecular engineering and by fine-tuning device structures. As a result, a comprehensive investigation and development of Cu(I)-based TADF/dual emissive complexes will be a part of the research for future generation OLEDs.
In lithium ion batteries, copper complexes as electrode materials have enabled very fast charge/discharge rates. They address environmental and ethical issues that arise with current electrode materials, while retaining good capacity and energy density compared to current commercial solutions. Their cyclability, although good, is not in par yet with other technologies, and their relatively large size limits their energy density compared to other technologies currently in development. It must be noted that very few reports exist to date about the use of copper complexes in lithium ion batteries, but the characteristics displayed so far make them a good class of materials for this application, especially where high power flows are required. In redox flow batteries, copper complexes perform better than complexes of other transition metals, mostly thanks to their greater solubility. However, the advantage of using organic solvent-based RFBs is not fully exploited, as cell potentials are similar to those water-based. This is mostly due to the lack of an ion exchange membrane tailored for organic solvents, and the development of such membrane could change the current scenario. However, capacity issues remain due to their limited solubility, especially when compared to that of inorganic salts in water.
Electrocatalytic or photocatalytic water splitting is a critical reaction due to its thermodynamically uphill and kinetically sluggish nature. Therefore, several efforts have been made towards the development of highly efficient and low-cost WOCs that are intended to lower the energy barrier and therefore enhance the catalytic water splitting efficiency. In this perspective, some of the most important and recent advancements of Cu-based WOCs have been discussed. Currently, the majority of the applied substances have been known to the inorganic/bioinorganic community and it is likely that other known, but untested systems will bring similarly significant achievements. The mononuclear copper catalysts reported so far lack any direct evidence of the nature of active intermediate/high-valent Cu-O species involved in the water oxidation reaction mechanism. In order to understand the involvement of the actual high-valent species in the catalytic process, development of new techniques is essential and remain a challenge for future research. Moreover, current studies on multinuclear Cu-based molecular catalysts and mimics of Mn4Ca in PS II are still at a very initial stage and we believe that this field can boom with future technological developments.
Funding
This research was funded by the Royal Society University Fellowship (URF\R1\191286), Swedish Energy Agency (43294-1), Swedish Research Council (2018-04570), Göran Gustafsson Young Researcher Prize 2019 and Olle Engkvist Byggmästare Stiftelsen (184-482).
Acknowledgments
We would like to thank the government for adequate measures towards the COVID-19 outbreak, giving us peace and time to focus on this work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OLED | Organic light-emitting diode |
| LMMCT | Ligand-to-metal-to-metal charge transfer |
| DSC | Dye-sensitized solar cell |
| ssDSC | Solid-state dye-sensitized solar cell |
| HTM | Hole transporting material |
| IoT | Internet of things |
| TCO | Thin conductive oxide |
| VOC | Open-circuit voltage |
| PCE | Power conversion efficiency |
| EML | Emission layer |
| LUMO | Lowest unoccupied molecular orbital |
| HOMO | Highest occupied molecular orbital |
| TADF | Thermally-activated delayed fluorescence |
| EBL | Electron blocking layer |
| EQE | External quantum efficiency |
| LIB | Lithium ion battery |
| RFB | Redox flow battery |
| WOC | Water oxidation catalyst |
| TOF | Turn over frequency |
| OEC | Oxygen-evolving complex |
References
- Zanoni, K.P.S.; Coppo, R.L.; Amaral, R.C.; Murakami Iha, N.Y. Ir(III) complexes designed for light-emitting devices: Beyond the luminescence color array. Dalton Trans. 2015, 44, 14559–14573. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- He, L.; Qiao, J.; Duan, L.; Dong, G.; Zhang, D.; Wang, L.; Qiu, Y. Toward highly efficient solid-state white light-emitting electrochemical cells: Blue-green to red emitting cationic iridium complexes with imidazole-type ancillary ligands. Adv. Funct. Mater. 2009, 19, 2950–2960. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Tokito, S. Highly efficient, low-voltage phosphorescent organic light-emitting diodes using an iridium complex as the host material. Adv. Mater. 2007, 19, 276–280. [Google Scholar] [CrossRef]
- Zhang, Q.; Komino, T.; Huang, S.; Matsunami, S.; Goushi, K.; Adachi, C. Triplet exciton confinement in green organic light-emitting diodes containing luminescent charge-transfer Cu(I) complexes. Adv. Funct. Mater. 2012, 22, 2327–2336. [Google Scholar] [CrossRef]
- Suttil, J.A.; Kucharyson, J.F.; Escalante-Garcia, I.L.; Cabrera, P.J.; James, B.R.; Savinell, R.F.; Sanford, M.S.; Thompson, L.T. Metal acetylacetonate complexes for high energy density non-aqueous redox flow batteries. J. Mater. Chem. A 2015, 3, 7929–7938. [Google Scholar] [CrossRef]
- Lloyd, D.; Vainikka, T.; Kontturi, K. The development of an all copper hybrid redox flow battery using deep eutectic solvents. Electrochim. Acta 2013, 100, 18–23. [Google Scholar] [CrossRef]
- Hogue, R.W.; Armstrong, C.G.; Toghill, K.E. Dithiolene Complexes of First-Row Transition Metals for Symmetric Nonaqueous Redox Flow Batteries. ChemSusChem 2019, 12, 4506–4515. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Pradhan, S.C.; Hagfeldt, A.; Soman, S. Resurgence of DSCs with copper electrolyte: A detailed investigation of interfacial charge dynamics with cobalt and iodine based electrolytes. J. Mater. Chem. A 2018, 6, 22204–22214. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, L. Artificial photosynthesis: Opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Wu, X.; Sun, L. Copper-based homogeneous and heterogeneous catalysts for electrochemical water oxidation. Nanoscale 2020, 12, 4187–4218. [Google Scholar] [CrossRef]
- Lei, H.; Li, X.; Meng, J.; Zheng, H.; Zhang, W.; Cao, R. Structure Effects of Metal Corroles on Energy-Related Small Molecule Activation Reactions. ACS Catal. 2019, 9, 4320–4344. [Google Scholar] [CrossRef]
- Barnett, S.M.; Goldberg, K.I.; Mayer, J.M. A soluble copper-bipyridine water-oxidation electrocatalyst. Nat. Chem. 2012, 4, 498–502. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Funes-Ardoiz, I.; Drouet, S.; Benet-Buchholz, J.; Maseras, F.; Llobet, A. Redox non-innocent ligand controls water oxidation overpotential in a new family of mononuclear cu-based efficient catalysts. J. Am. Chem. Soc. 2015, 137, 6758–6761. [Google Scholar] [CrossRef]
- Michaels, H.; Benesperi, I.; Edvinsson, T.; Muñoz-Garcia, A.B.; Pavone, M.; Boschloo, G.; Freitag, M. Copper Complexes with Tetradentate Ligands for Enhanced Charge Transport in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 53. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Lee, J.M.; Taylor, N.S.; Cheema, H.; Chen, L.; Fortenberry, R.C.; Delcamp, J.H.; Jurss, J.W. Copper-based redox shuttles supported by preorganized tetradentate ligands for dye-sensitized solar cells. Dalton Trans. 2020, 49, 343–355. [Google Scholar] [CrossRef]
- Mathews, I.; Kantareddy, S.N.; Buonassisi, T.; Peters, I.M. Technology and Market Perspective for Indoor Photovoltaic Cells. Joule 2019, 3, 1415–1426. [Google Scholar] [CrossRef]
- Liu, Y.; Yiu, S.C.; Ho, C.L.; Wong, W.Y. Recent advances in copper complexes for electrical/light energy conversion. Coord. Chem. Rev. 2018, 375, 514–557. [Google Scholar] [CrossRef]
- Fernández, M.R.; Casanova, E.Z.; Alonso, I.G. Review of Display Technologies Focusing on Power Consumption. Sustainability 2015, 7, 10854–10875. [Google Scholar] [CrossRef]
- Bella, F.; Galliano, S.; Gerbaldi, C.; Viscardi, G. Cobalt-Based Electrolytes for Dye-Sensitized Solar Cells: Recent Advances towards Stable Devices. Energies 2016, 9, 384. [Google Scholar] [CrossRef]
- Sprengard, R.; Bonrad, K.; Daeubler, T.K.; Frank, T.; Hagemann, V.; Koehler, I.; Pommerehne, J.; Ottermann, C.R.; Voges, F.; Vingerling, B. OLED devices for signage applications: A review of recent advances and remaining challenges. In Organic Light-Emitting Materials and Devices VIII; International Society for Optics and Photonics: Bellingham, WA, USA, 2004; Volume 5519, pp. 173–183. [Google Scholar] [CrossRef]
- McMillin, D.R.; Kirchhoff, J.R.; Goodwin, K.V. Exciplex quenching of photo-excitd copper complexes. Coord. Chem. Rev. 1985, 64, 83–92. [Google Scholar] [CrossRef]
- Gushurst, A.K.I.; McMillin, D.R.; Dietrich-Buchecker, C.O.; Sauvage, J.P. Comparative studies of the photophysical properties of copper phenanthrolines: From Cu(dmp)2+ to the copper(I) catenates. Inorg. Chem. 1989, 28, 4070–4072. [Google Scholar] [CrossRef]
- Freitag, M.; Giordano, F.; Yang, W.; Pazoki, M.; Hao, Y.; Zietz, B.; Grätzel, M.; Hagfeldt, A.; Boschloo, G. Copper Phenanthroline as a Fast and High-Performance Redox Mediator for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 9595–9603. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Michaels, H.; Tiepelt, J.; Teuscher, J.; Massaro, A.; Pavone, M.; Giordano, F.; Zakeeruddin, S.M.; Boschloo, G.; et al. Effect of Coordination Sphere Geometry of Copper Redox Mediators on Regeneration and Recombination Behavior in Dye-Sensitized Solar Cell Applications. ACS Appl. Energy Mater. 2018, 1, 4950–4962. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Flores-Díaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal Coordination Complexes as Redox Mediators in Regenerative Dye-Sensitized Solar Cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The emergence of copper(i)-based dye sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8386–8398. [Google Scholar] [CrossRef]
- Michaels, H.; Rinderle, M.; Freitag, R.; Benesperi, I.; Edvinsson, T.; Socher, R.; Gagliardi, A.; Freitag, M. Dye-sensitized solar cells under ambient light powering machine learning: Towards autonomous smart sensors for the internet of things. Chem. Sci. 2020, 11, 2895–2906. [Google Scholar] [CrossRef]
- Freitag, M.; Daniel, Q.; Pazoki, M.; Sveinbjörnsson, K.; Zhang, J.; Sun, L.; Hagfeldt, A.; Boschloo, G. High-efficiency dye-sensitized solar cells with molecular copper phenanthroline as solid hole conductor. Energy Environ. Sci. 2015, 8, 2634–2637. [Google Scholar] [CrossRef]
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Bahng, H.W.; Cao, Y.; Yi, C.; Saygili, Y.; Luo, J.; Liu, Y.; Kavan, L.; Moser, J.E.; et al. Comprehensive control of voltage loss enables 11.7% efficient solid-state dye-sensitized solar cells. Energy Environ. Sci. 2018, 11, 1779–1787. [Google Scholar] [CrossRef]
- Higashino, T.; Iiyama, H.; Nimura, S.; Kurumisawa, Y.; Imahori, H. Effect of Ligand Structures of Copper Redox Shuttles on Photovoltaic Performance of Dye-Sensitized Solar Cells. Inorg. Chem. 2020, 59, 452–459. [Google Scholar] [CrossRef]
- Fürer, S.O.; Bozic-Weber, B.; Neuburger, M.; Constable, E.C.; Housecroft, C.E. Heteroleptic copper(I) sensitizers with one versus two hole-transporting units in functionalized 2,9-dimethyl-1,10-phenanthroline ancillary ligands. RSC Adv. 2015, 5, 69430–69440. [Google Scholar] [CrossRef]
- Büttner, A.; Brauchli, S.Y.; Vogt, R.; Constable, E.C.; Housecroft, C.E. Combining phosphonic acid-functionalized anchoring ligands with asymmetric ancillary ligands in bis(diimine)copper(I) dyes for dye-sensitized solar cells. RSC Adv. 2016, 6, 5205–5213. [Google Scholar] [CrossRef]
- Klein, Y.M.; Willgert, M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Positional isomerism makes a difference: Phosphonic acid anchoring ligands with thienyl spacers in copper(I)-based dye-sensitized solar cells. Dalton Trans. 2016, 45, 4659–4672. [Google Scholar] [CrossRef]
- Fürer, S.O.; Luu, L.Y.N.; Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Improving performance of copper(I)-based dye sensitized solar cells through I3-/I- electrolyte manipulation. Dyes Pigments 2016, 132, 72–78. [Google Scholar] [CrossRef]
- Fürer, S.O.; Bozic-Weber, B.; Schefer, T.; Wobill, C.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Understanding why replacing I3-/I- by cobalt(II)/(III) electrolytes in bis(diimine)copper(I)-based dye-sensitized solar cells improves performance. J. Mater. Chem. A 2016, 4, 12995–13004. [Google Scholar] [CrossRef]
- Baumgartner, Y.; Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Cyanoacrylic- and (1-cyanovinyl)phosphonic acid anchoring ligands for application in copper-based dye-sensitized solar cells. RSC Adv. 2016, 6, 86220–86231. [Google Scholar] [CrossRef]
- Malzner, F.J.; Willgert, M.; Constable, E.C.; Housecroft, C.E. The way to panchromatic copper(I)-based dye-sensitized solar cells: Co-sensitization with the organic dye SQ2. J. Mater. Chem. A 2017, 5, 13717–13729. [Google Scholar] [CrossRef]
- Malzner, F.J.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Exploring simple ancillary ligands in copper-based dye-sensitized solar cells: Effects of a heteroatom switch and of co-sensitization. J. Mater. Chem. A 2017, 5, 4671–4685. [Google Scholar] [CrossRef]
- Stephens, A.J.; Malzner, F.J.; Constable, E.C.; Housecroft, C.E. The influence of phosphonic acid protonation state on the efficiency of bis(diimine)copper(I) dye-sensitized solar cells. Sustain. Energy Fuels 2018, 2, 786–794. [Google Scholar] [CrossRef]
- Büttner, A.; Brauchli, S.Y.; Constable, E.C.; Housecroft, C.E. Effects of Introducing Methoxy Groups into the Ancillary Ligands in Bis(diimine) Copper(I) Dyes for Dye-Sensitized Solar Cells. Inorganics 2018, 6, 40. [Google Scholar] [CrossRef]
- Malzner, F.J.; Housecroft, C.E.; Constable, E.C. The Versatile SALSAC Approach to Heteroleptic Copper(I) Dye Assembly in Dye-Sensitized Solar Cells. Inorganics 2018, 6, 57. [Google Scholar] [CrossRef]
- Karpacheva, M.; Malzner, F.J.; Wobill, C.; Büttner, A.; Constable, E.C.; Housecroft, C.E. Cuprophilia: Dye-sensitized solar cells with copper(I) dyes and copper(I)/(II) redox shuttles. Dyes Pigments 2018, 156, 410–416. [Google Scholar] [CrossRef]
- Dragonetti, C.; Magni, M.; Colombo, A.; Melchiorre, F.; Biagini, P.; Roberto, D. Coupling of a Copper Dye with a Copper Electrolyte: A Fascinating Springboard for Sustainable Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2018, 1, 751–756. [Google Scholar] [CrossRef]
- Dragonetti, C.; Magni, M.; Colombo, A.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P.; Fantacci, S. Towards efficient sustainable full-copper dye-sensitized solar cells. Dalton Trans. 2019, 48, 9703–9711. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Melchiorre, F.; Biagini, P. Improving the efficiency of copper-dye-sensitized solar cells by manipulating the electrolyte solution. Dalton Trans. 2019, 48, 9818–9823. [Google Scholar] [CrossRef]
- Alamin Ali, H.E.; Altındal, A.; Altun, S.; Odabaş, Z. Highly efficient dye-sensitized solar cells based on metal-free and copper(II) phthalocyanine bearing 2-phenylphenoxy moiety. Dyes Pigments 2016, 124, 180–187. [Google Scholar] [CrossRef]
- Solğun, D.G.; Horoz, S.; Ağırtaş, M.S. Synthesis of novel tetra (4-tritylphenoxy) substituted metallophthalocyanines and investigation of their aggregation, photovoltaic, solar cell properties. Inorg. Nano-Metal Chem. 2018, 48, 508–514. [Google Scholar] [CrossRef]
- Zanotti, G.; Angelini, N.; Mattioli, G.; Notarantonio, S.; Paoletti, A.M.; Pennesi, G.; Rossi, G.; Caschera, D.; De Marco, L.; Gigli, G. Modifications of an unsymmetrical phthalocyanine: Towards stable blue dyes for dye-sensitized solar cells. J. Porphyrins Phthalocyanines 2016, 20, 1207–1216. [Google Scholar] [CrossRef]
- Wills, K.A.; Mandujano-Ramírez, H.J.; Merino, G.; Oskam, G.; Cowper, P.; Jones, M.D.; Cameron, P.J.; Lewis, S.E. What difference does a thiophene make? Evaluation of a 4,4’-bis(thiophene) functionalised 2,2’-bipyridyl copper(I) complex in a dye-sensitized solar cell. Dyes Pigments 2016, 134, 419–426. [Google Scholar] [CrossRef]
- Yadav, R.; Waghadkar, Y.; Kociok-Köhn, G.; Kumar, A.; Rane, S.B.; Chauhan, R. Transition metal ferrocenyl dithiocarbamates functionalized dye-sensitized solar cells with hydroxy as an anchoring group. Opt. Mater. 2016, 62, 176–183. [Google Scholar] [CrossRef]
- Jayapal, M.; Haque, A.; Al-Busaidi, I.J.; Al-Rasbi, N.; Al-Suti, M.K.; Khan, M.S.; Al-Balushi, R.; Islam, S.M.; Xin, C.; Wu, W.; et al. Dicopper(I) Complexes Incorporating Acetylide-Functionalized Pyridinyl-Based Ligands: Synthesis, Structural, and Photovoltaic Studies. Inorg. Chem. 2018, 57, 12113–12124. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Y.; Liu, Y.; Zhu, C.; Chen, T.; Zhong, C. Dye sensitizers of polymer using the complex of Cd (II) or Cu (II) with imidazole as auxiliary electron acceptor for dye-sensitized solar cells. Dyes Pigments 2017, 139, 420–430. [Google Scholar] [CrossRef]
- Asatkar, A.K.; Bedi, A.; Zade, S.S. Metallo-organic conjugated systems for organic electronics. Isr. J. Chem. 2014, 54, 467–495. [Google Scholar] [CrossRef]
- Zink, D.M.; Volz, D.; Baumann, T.; Mydlak, M.; Flügge, H.; Friedrichs, J.; Nieger, M.; Bräse, S. Heteroleptic, dinuclear copper(i) complexes for application in organic light-emitting diodes. Chem. Mater. 2013, 25, 4471–4486. [Google Scholar] [CrossRef]
- Zink, D.M.; Grab, T.; Baumann, T.; Nieger, M.; Barnes, E.C.; Klopper, W.; Bräse, S. Experimental and theoretical study of novel luminescent Di-, Tri-, and tetranuclear copper triazole complexes. Organometallics 2011, 30, 3275–3283. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Su, Z. Realization of high-energy emission from [Cu(N-N)(P-P)] +complexes for organic light-emitting diode applications. J. Phys. Chem. C 2009, 113, 13968–13973. [Google Scholar] [CrossRef]
- Hamze, R.; Peltier, J.L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhavoup, M.; Jazzar, R.; Djurovich, P.I.; Bertrand, G.; et al. Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime. Science 2019, 363, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.G.; Chan, W.H.; Zhoub, X.M.; Che, C.M. Letter Light-emitting diode device from a luminescent organocopper(I) compound. New J. Chem. 1999, 23, 263–265. [Google Scholar] [CrossRef]
- Blaskie, M.W.; Mcmillin, D.R. Photostudies of Copper(I) Systems. 6. Room-Temperature Emission and Quenching Studies of [Cu(dmp)2]+. Inorg. Chem 1980, 19, 47907. [Google Scholar] [CrossRef]
- A Palmer, C.E.; McMillin, D.R.; Kirmaier, C.; Holten, D. Flash Photolysis and Quenching Studies of Copper(I) Systems in the Presence of Lewis Bases: Inorganic Exciplexes? Inorg. Chem 1987, 26, 3167–3170. [Google Scholar] [CrossRef]
- Buckner, M.T.; McMillin, D.R. Photoluminescence from copper(I) complexes with low-lying metal-to-ligand charge transfer excited states. J. Chem. Soc. Chem. Commun. 1978, 17, 759–761. [Google Scholar] [CrossRef]
- Kuang, S.M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A. Synthesis and structural characterization of Cu(I) and Ni(II) complexes that contain the bis[2-(diphenylphosphino)phenyl]ether ligand. Novel emission properties for the Cu(I) species. Inorg. Chem. 2002, 41, 3313–3322. [Google Scholar] [CrossRef]
- Cuttell, D.G.; Kuang, S.M.; Fanwick, P.E.; McMillin, D.R.; Walton, R.A. Simple Cu(I) complexes with unprecedented excited-state lifetimes. J. Am. Chem. Soc. 2002, 124, 6–7. [Google Scholar] [CrossRef]
- Igawa, S.; Hashimoto, M.; Kawata, I.; Yashima, M.; Hoshino, M.; Osawa, M. Highly efficient green organic light-emitting diodes containing luminescent tetrahedral copper(i) complexes. J. Mater. Chem. C 2013, 1, 542–551. [Google Scholar] [CrossRef]
- Hashimoto, M.; Igawa, S.; Yashima, M.; Kawata, I.; Hoshino, M.; Osawa, M. Highly efficient green organic light-emitting diodes containing luminescent three-coordinate copper(I) complexes. J. Am. Chem. Soc. 2011, 133, 10348–10351. [Google Scholar] [CrossRef]
- Osawa, M.; Hoshino, M.; Hashimoto, M.; Kawata, I.; Igawa, S.; Yashima, M. Application of three-coordinate copper(i) complexes with halide ligands in organic light-emitting diodes that exhibit delayed fluorescence. Dalton Trans. 2015, 44, 8369–8378. [Google Scholar] [CrossRef]
- Volz, D.; Chen, Y.; Wallesch, M.; Liu, R.; Fléchon, C.; Zink, D.M.; Friedrichs, J.; Flügge, H.; Steininger, R.; Göttlicher, J.; et al. Bridging the efficiency gap: Fully bridged dinuclear Cu(I)-complexes for singlet harvesting in high-efficiency OLEDs. Adv. Mater. 2015, 27, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, Z.; Zhao-Karger, Z.; Mueller, J.E.; Jung, C.; Klyatskaya, S.; Diemant, T.; Fuhr, O.; Jacob, T.; Behm, R.J.; et al. A Porphyrin Complex as a Self-Conditioned Electrode Material for High-Performance Energy Storage. Angew. Chem. Int. Ed. 2017, 56, 10341–10346. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Wu, D.Y.; Gao, P.; Chen, Z.; Ruben, M.; Fichtner, M. Monitoring the Electrochemical Energy Storage Processes of an Organic Full Rechargeable Battery via Operando Raman Spectroscopy: A Mechanistic Study. Chem. Mater. 2019, 31, 3239–3247. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, P.; Wang, W.; Klyatskaya, S.; Zhao-Karger, Z.; Wang, D.; Kübel, C.; Fuhr, O.; Fichtner, M.; Ruben, M. A Lithium-Free Energy-Storage Device Based on an Alkyne-Substituted-Porphyrin Complex. ChemSusChem 2019, 12, 3737–3741. [Google Scholar] [CrossRef]
- Wu, D.; Li, H.; Li, R.; Hu, Y.; Hu, X. In situ growth of copper rhodizonate complexes on reduced graphene oxide for high-performance organic lithium-ion batteries. Chem. Commun. 2018, 54, 11415–11418. [Google Scholar] [CrossRef]
- Kapaev, R.R.; Olthof, S.; Zhidkov, I.S.; Kurmaev, E.Z.; Stevenson, K.J.; Meerholz, K.; Troshin, P.A. Nickel(II) and Copper(II) Coordination Polymers Derived from 1,2,4,5-Tetraaminobenzene for Lithium-Ion Batteries. Chem. Mater. 2019, 31, 5197–5205. [Google Scholar] [CrossRef]
- Wang, H.G.; Wang, H.; Si, Z.; Li, Q.; Wu, Q.; Shao, Q.; Wu, L.; Liu, Y.; Wang, Y.; Song, S.; et al. A Bipolar and Self-Polymerized Phthalocyanine Complex for Fast and Tunable Energy Storage in Dual-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 10204–10208. [Google Scholar] [CrossRef]
- Peljo, P.; Lloyd, D.; Doan, N.; Majaneva, M.; Kontturi, K. Towards a thermally regenerative all-copper redox flow battery. Phys. Chem. Chem. Phys. 2014, 16, 2831–2835. [Google Scholar] [CrossRef]
- Li, Y.; Sniekers, J.; Malaquias, J.; Li, X.; Schaltin, S.; Stappers, L.; Binnemans, K.; Fransaer, J.; Vankelecom, I.F.J. A non-aqueous all-copper redox flow battery with highly soluble active species. Electrochim. Acta 2017, 236, 116–121. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Liu, S.; Wang, J.L.; Lin, W. A biomimetic copper water oxidation catalyst with low overpotential. J. Am. Chem. Soc. 2014, 136, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Stott, L.A.; Prosser, K.E.; Berdichevsky, E.K.; Walsby, C.J.; Warren, J.J. Lowering water oxidation overpotentials using the ionisable imidazole of copper(2-(2’-pyridyl)imidazole). Chem. Commun. 2017, 53, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Prevedello, A.; Bazzan, I.; Dalle Carbonare, N.; Giuliani, A.; Bhardwaj, S.; Africh, C.; Cepek, C.; Argazzi, R.; Bonchio, M.; Caramori, S.; et al. Heterogeneous and Homogeneous Routes in Water Oxidation Catalysis Starting from CuII Complexes with Tetraaza Macrocyclic Ligands. Chem. Asian J. 2016, 11, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, N.; Lei, H.; Guo, D.; Liu, H.; Zhang, Z.; Zhang, W.; Lai, W.; Cao, R. Electrocatalytic Water Oxidation by a Water-Soluble Copper(II) Complex with a Copper-Bound Carbonate Group Acting as a Potential Proton Shuttle. Inorg. Chem. 2017, 56, 13368–13375. [Google Scholar] [CrossRef]
- Kuilya, H.; Alam, N.; Sarma, D.; Choudhury, D.; Kalita, A. Ligand assisted electrocatalytic water oxidation by a copper(ii) complex in neutral phosphate buffer. Chem. Comm. 2019, 55, 5483–5486. [Google Scholar] [CrossRef]
- Zhang, M.T.; Chen, Z.; Kang, P.; Meyer, T.J. Electrocatalytic water oxidation with a copper(II) polypeptide complex. J. Am. Chem. Soc. 2013, 135, 2048–2051. [Google Scholar] [CrossRef]
- Pap, J.S.; Szyrwiel, Ł; Srankó, D.; Kerner, Z.; Setner, B.; Szewczuk, Z.; Malinka, W. Electrocatalytic water oxidation by CuII complexes with branched peptides. Chem. Comm. 2015, 51, 6322–6324. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Zhang, Z.; Zhang, W.; Lai, W.; Wang, Y.; Cao, R. Low overpotential water oxidation at neutral pH catalyzed by a copper(ii) porphyrin. Chem. Sci. 2019, 10, 2613–2622. [Google Scholar] [CrossRef]
- Fang, T.; Fu, L.Z.; Zhou, L.L.; Zhan, S.Z. A water-soluble dinuclear copper electrocatalyst, [Cu(oxpn)Cu(OH)2] for both water reduction and oxidation. Electrochim. Acta 2015, 161, 388–394. [Google Scholar] [CrossRef]
- Koepke, S.J.; Light, K.M.; Vannatta, P.E.; Wiley, K.M.; Kieber-Emmons, M.T. Electrocatalytic Water Oxidation by a Homogeneous Copper Catalyst Disfavors Single-Site Mechanisms. J. Am. Chem. Soc. 2017, 139, 8586–8600. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Yang, B.; Wei, X.Z.; Dong, B.W.; Kao, Y.; Huang, M.Y.; Tung, C.H.; Wu, L.Z. A Bio-inspired Cu4O4 Cubane: Effective Molecular Catalysts for Electrocatalytic Water Oxidation in Aqueous Solution. Angew. Chem. Int. Ed. 2018, 57, 7850–7854. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).