Chemical Looping Co-Gasification Characteristics of Cyanobacterial/Coal Blends
Abstract
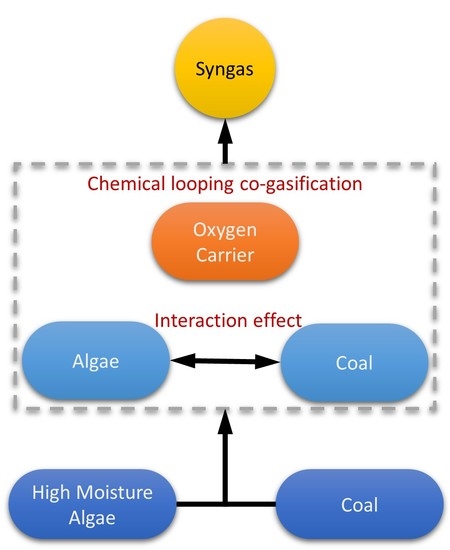
:1. Introduction
2. Experiment
2.1. Material
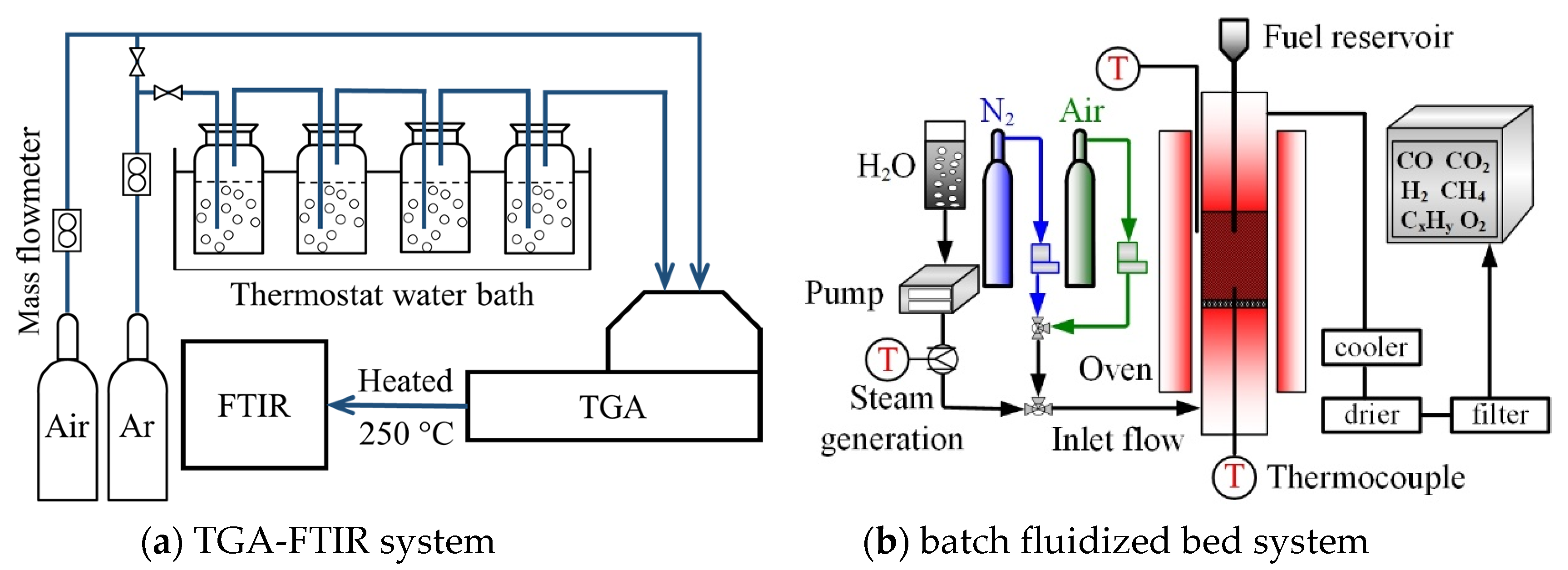
2.2. Experimental Procedure
2.3. The Interaction Index MR
2.4. Kinetic Models
2.5. Data Evaluation
2.5.1. Gas Yield
2.5.2. Carbon Conversion Efficiency
2.5.3. Oxygen Carrier-to-Fuel Ratio
3. Results and Discussion
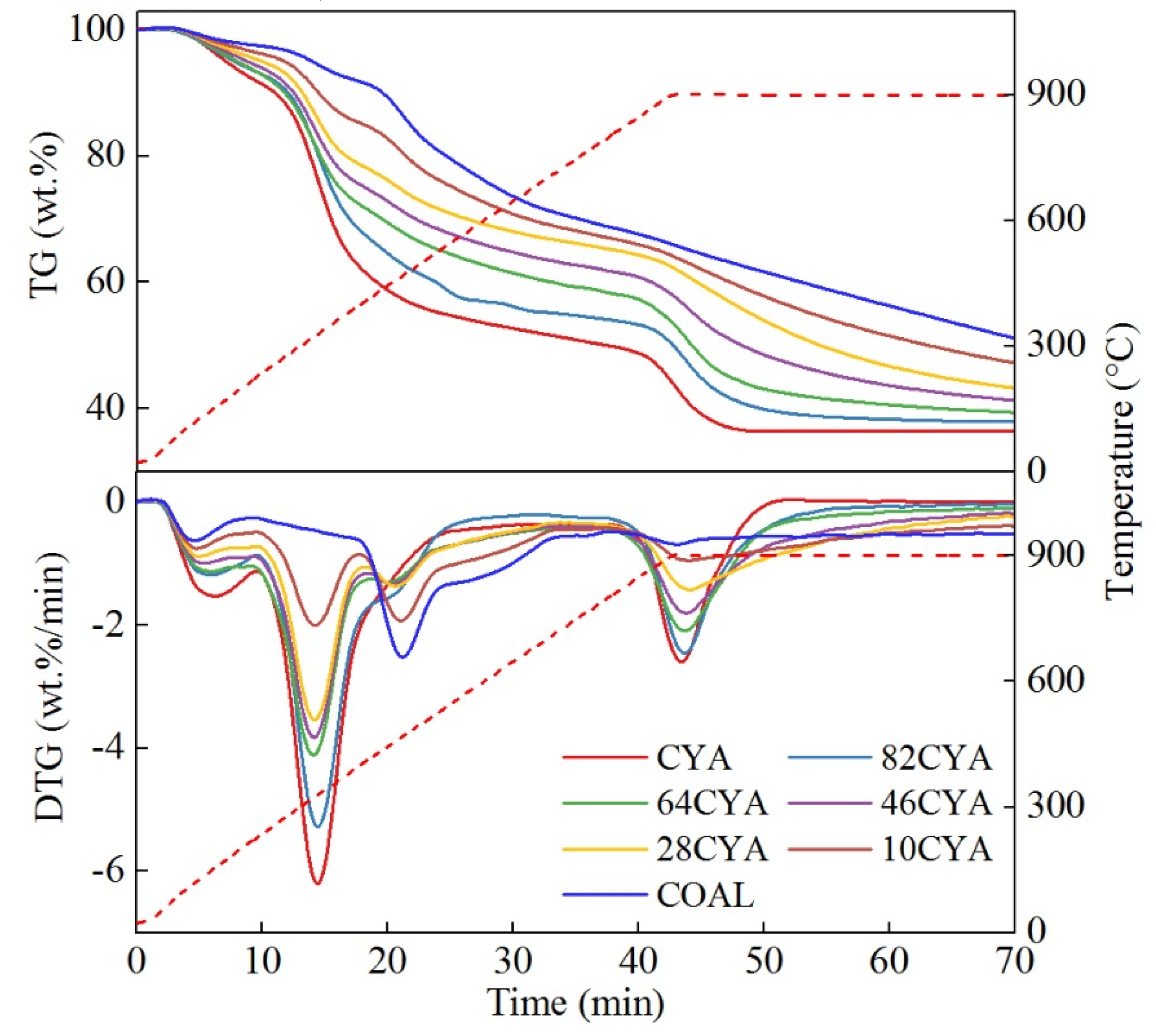
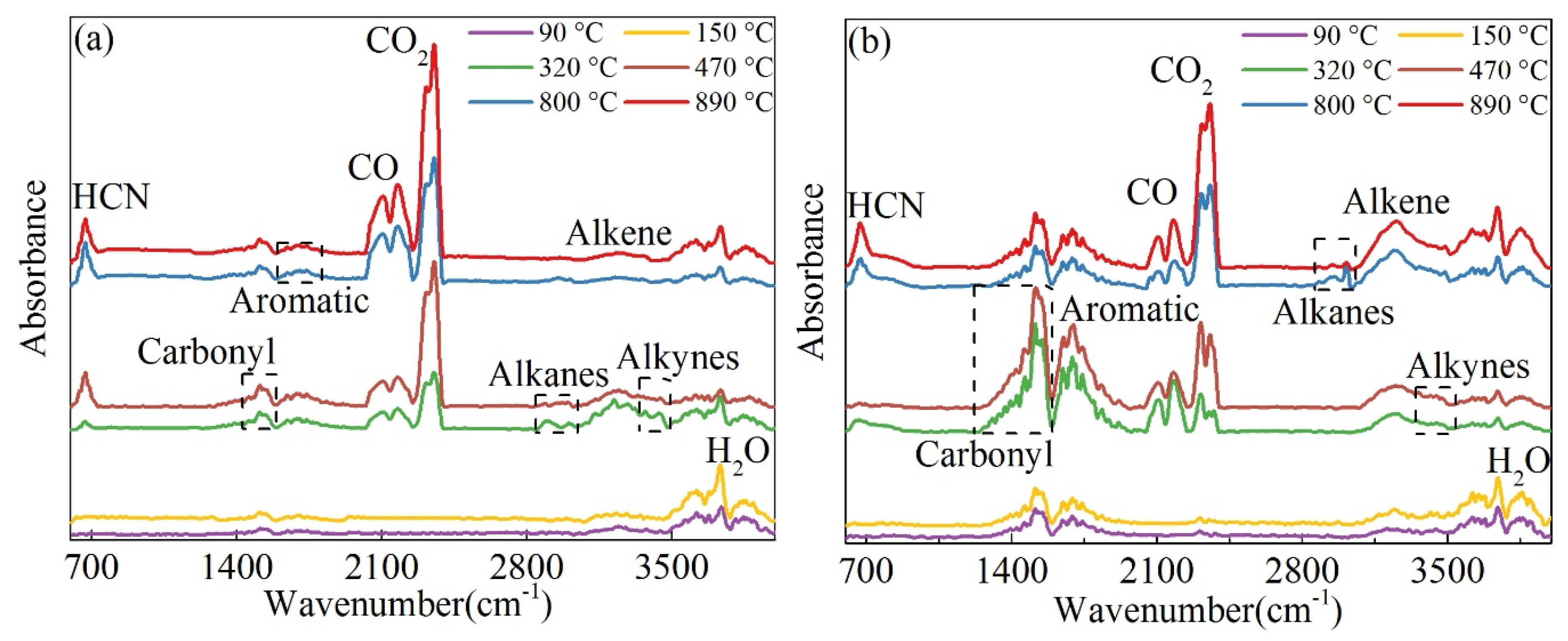
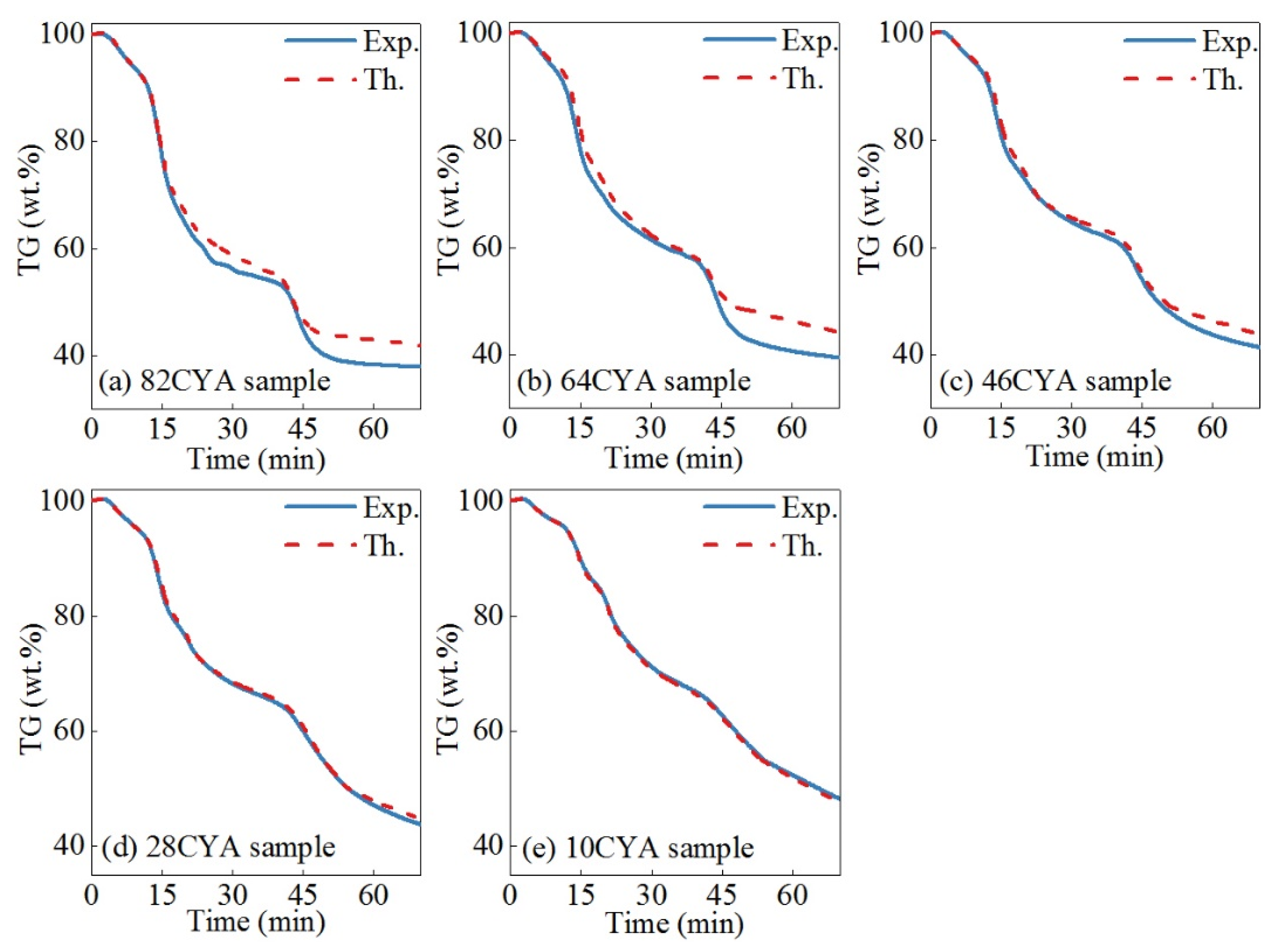
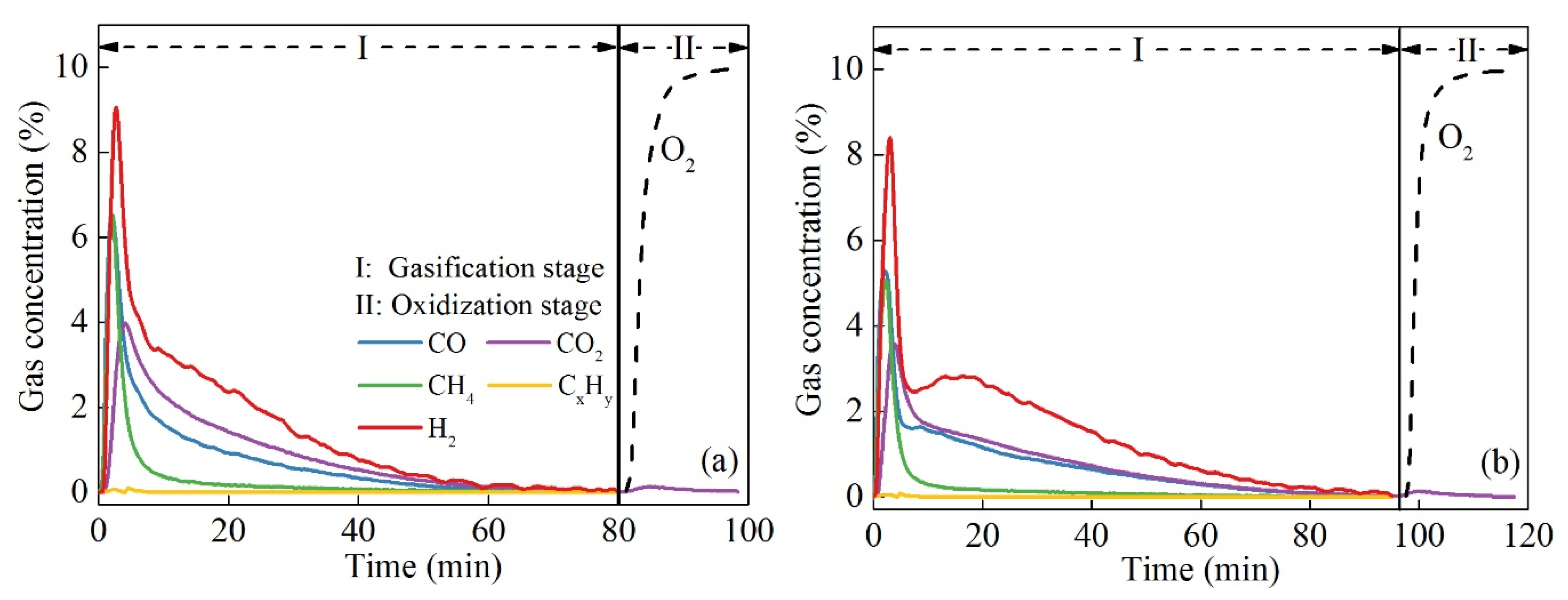
3.1. Thermogravimetric Characteristics and Gas Evolution
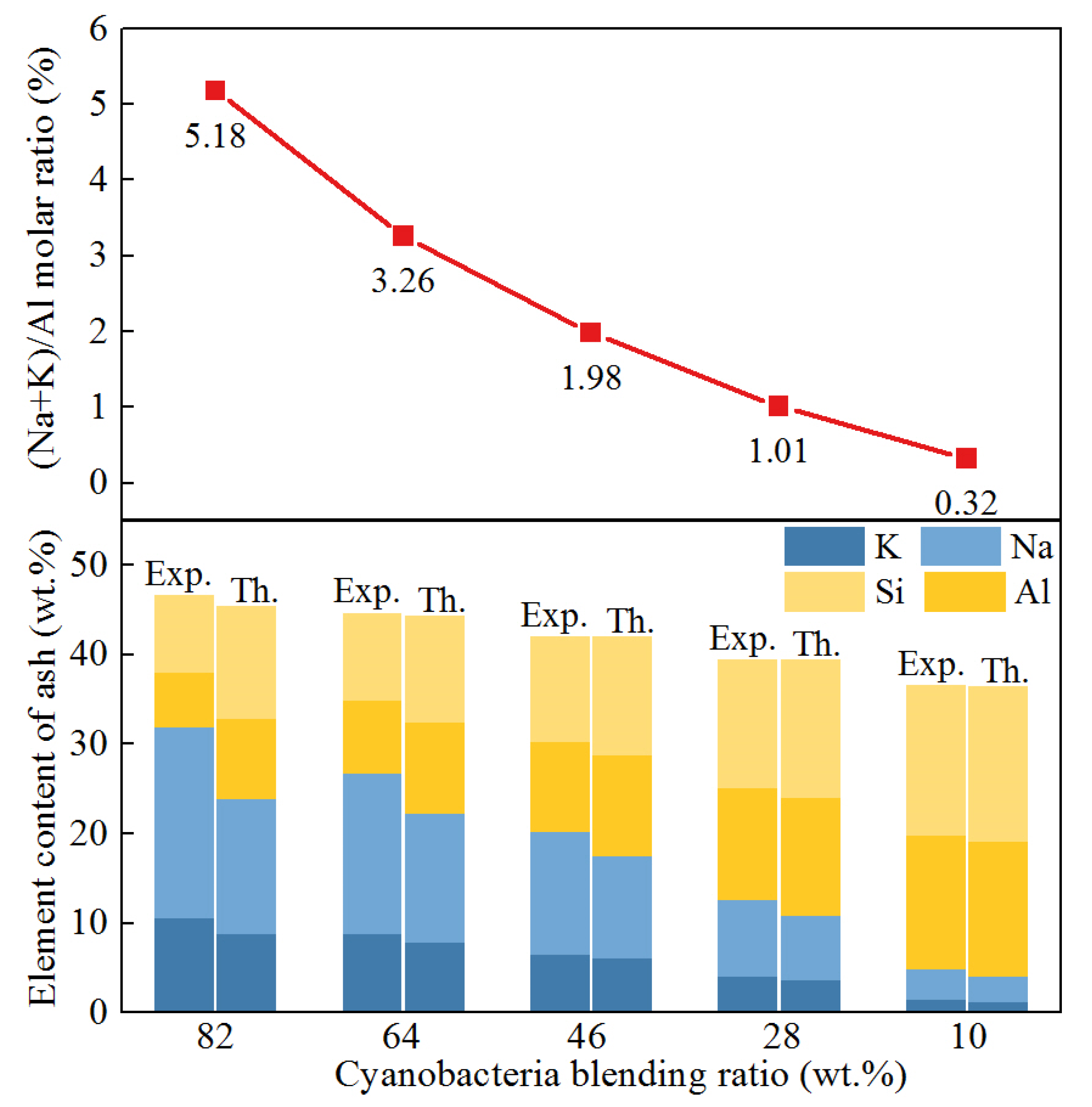
3.2. Interaction Effect Analysis
3.3. Kinetic Parameters
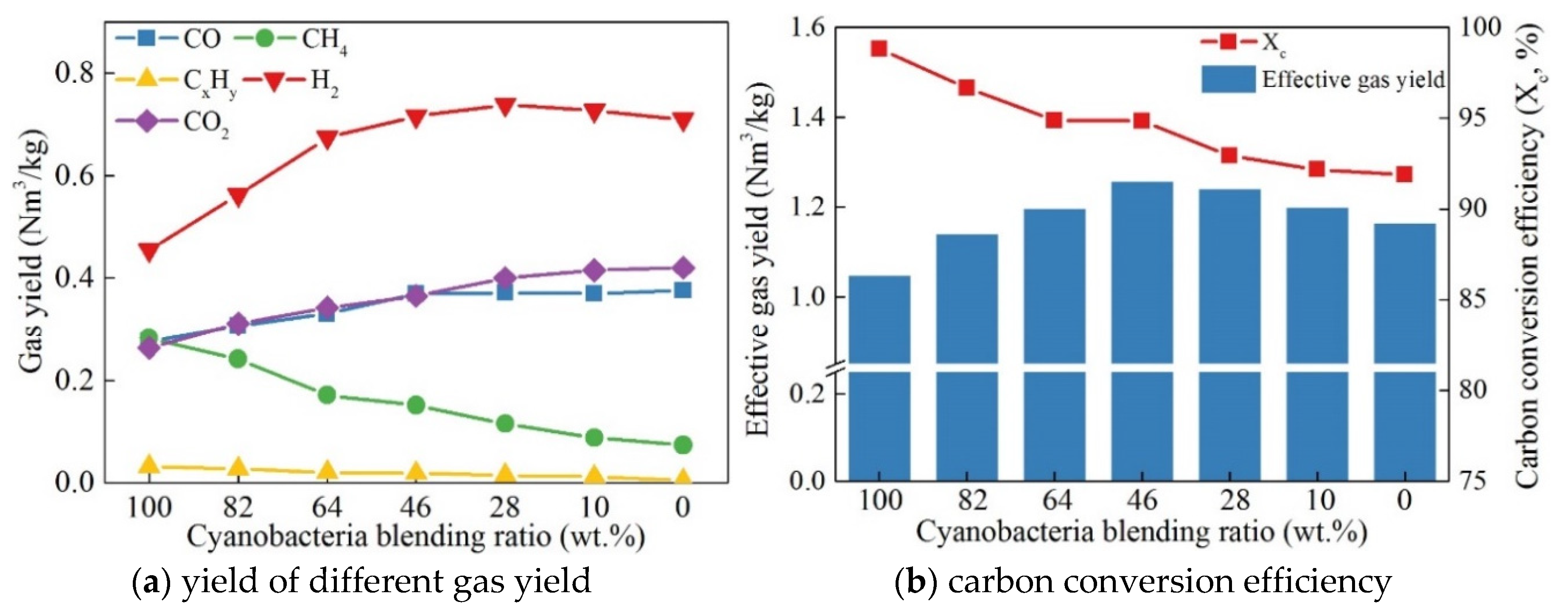
3.4. Fuel Blending Ratio
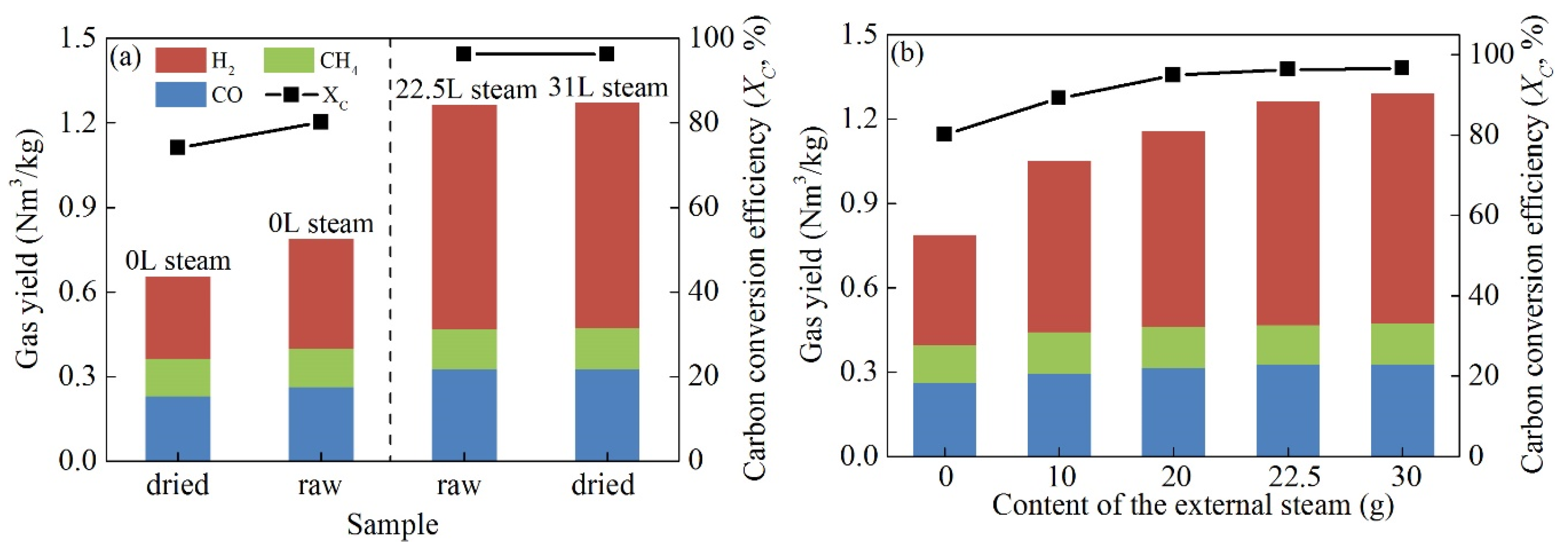
3.5. Cyanobacteria Residual Moisture
4. Conclusions
- ◼
- Cyanobacteria pyrolysis could improve the syngas quality by providing abundant hydrocarbons and hydrogen-rich gases. The highest syngas yield of 1.26 Nm3/kg was obtained in the mixture fuel of 46 wt.% cyanobacteria and 54 wt.% coal. Increasing the cyanobacteria blending ratio could increase the carbon conversion efficiency and CH4 yield, but decrease CO and H2 yields. The 0.3 oxygen carrier-to-fuel ratio was determined as the optimum OC demand in terms of the highest syngas yield and the acceptable carbon conversion efficiency.
- ◼
- The residual moisture of raw cyanobacteria not only could replace part of the injecting steam as the gasifying agent of coal, but also showed a better gasification effect than the free water. Compared with the injecting steam, the cyanobacteria moisture had an enhanced gas-solid contact, uniform distribution, and prolonged contact time.
- ◼
- The reactivity of coal gasification was enhanced by blending cyanobacteria because of the interaction effect. The interaction effect of co-gasification was highly relevant to the (Na + K)/Al molar ratio of ash. The dominant motive for the interaction effect was the catalytic effect of alkali metals on char gasification. However, the formation of aluminosilicates would deactivate alkali metals and inhibit char gasification. The most prominent interaction effect occurred for the sample with 82 wt.% cyanobacteria and 28 wt.% coal, but a negative interaction effect was observed in the sample with 28 wt.% cyanobacteria and 72 wt.% coal.
- ◼
- Both homogeneous reaction and shrinking core models showed excellent performance in fitting the char gasification process. However, these two models were not applicable to the initial pyrolysis stage because of the intricate mechanisms. The homogeneous reaction model was more suitable in fitting cyanobacteria behaviors, and the shrinking core model was applicable to the sample with high coal content.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Janssen, E.M.L. Cyanobacterial peptides beyond microcystins–a review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006, 51, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, J. Occurrence and potential risks of harmful algal blooms in the East China Sea. Sci. Total Environ. 2009, 407, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, W.; Liu, H. The resource utilization of algae—Preparing coal slurry with algae. Fuel 2010, 89, 965–970. [Google Scholar] [CrossRef]
- Aziz, M. Integrated supercritical water gasification and a combined cycle for microalgal utilization. Energy Convers. Manag. 2015, 91, 140–148. [Google Scholar] [CrossRef]
- Thiruvenkadam, S.; Izhar, S.; Yoshida, H.; Danquah, K.; Harun, R. Process application of Subcritical Water Extraction (SWE) for algal bio-products and biofuels production. Appl. Energy 2015, 154, 815–828. [Google Scholar] [CrossRef]
- Huynh, C.; Kong, S. Performance characteristics of a pilot-scale biomass gasifier using oxygen-enriched air and steam. Fuel 2013, 103, 987–996. [Google Scholar] [CrossRef]
- Doherty, W.; Reynolds, A.; Kennedy, D. The effect of air preheating in a biomass CFB gasifier using ASPEN Plus simulation. Biomass Bioenergy 2009, 33, 1158–1167. [Google Scholar] [CrossRef] [Green Version]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef] [Green Version]
- Zaini, I.N.; Nurdiawati, A.; Aziz, M. Cogeneration of power and H2 by steam gasification and syngas chemical looping of macroalgae. Appl. Energy 2017, 207, 134–145. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Zhong, Z.; Niu, X.; Ge, H.; Zhou, Y.; Xiao, S.; Jiang, S. NO release during chemical looping combustion with iron ore as an oxygen carrier. Chem. Eng. J. 2015, 264, 211–220. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Solid fuels in chemical-looping combustion. Int. J. Greenh. Gas Control 2008, 2, 180–193. [Google Scholar] [CrossRef]
- Sjoström, K.; Chen, G.; Yu, Q.; Rosén, B.C. Promoted reactivity of char in co-gasification of biomass and coal: Synergies in the thermochemical process. Fuel 1999, 78, 1189–1194. [Google Scholar] [CrossRef]
- McKee, D.W. Gasification of graphite in carbon dioxide and water vapor—The catalytic effects of alkali metal salts. Carbon 1982, 20, 59–66. [Google Scholar] [CrossRef]
- Masnadi-Shirazi, M.S. Biomass/Fossil Fuel co-Gasification with and without Integrated CO2 Capture; University of British Columbia: Vancouver, BC, Cananda, 2014. [Google Scholar]
- Wang, Q.; Zhao, W.Z.; Liu, H.P.; Jia, C.X.; Li, S.H. Interactions and kinetic analysis of oil shale semi-coke with cornstalk during co-combustion. Appl. Energy 2011, 88, 2080–2087. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, W.; Tian, X.; Yang, B. Synergistic effects from co-pyrolysis of low-rank coal and model components of microalgae biomass. Energy Convers. Manag. 2017, 135, 212–225. [Google Scholar] [CrossRef]
- Fermoso, J.; Stevanov, C.; Moghtaderi, B.; Arias, B.; Pevida, C.; Plaza, M.G.; Rubiera, F.; Pis, J.J. High-pressure gasification reactivity of biomass chars produced at different temperatures. J. Anal. Appl. Pyrolysis 2009, 85, 287–293. [Google Scholar] [CrossRef]
- Ishida, M.; Wen, C.Y. Comparison of zone-reaction model and unreacted-core shrinking model in solid—gas reactions—I isothermal analysis. Chem. Eng. Sci. 1971, 26, 1031–1041. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-gasification of coal and biomass blends: Chemistry and engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Szekely, J.; Evans, J.W. A structural model for gas—solid reactions with a moving boundary. Chem. Eng. Sci. 1970, 25, 1091–1107. [Google Scholar] [CrossRef]
- Yin, S.; Shen, L.H.; Dosta, M.; Hartge, E.-U.; Heinrich, S.; Liu, P.; Werther, J.; Song, T. Chemical looping gasification of biomass pellet with a Manganese ore as oxygen carrier in the fluidized bed. Energy Fuels 2018, 32, 11674–11682. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Marcilla, A.; Gomez-Siurana, A.; Gomis, C.; Gomis, G.; Chápuli, E.; Catalá, M.C.; Valdé, F.J. Characterization of microalgal species through TGA/FTIR analysis: Application to nannochloropsis sp. Thermochim. Acta 2009, 484, 41–47. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Sun, L.S.; Xiang, J.; Chen, Q.-Q.; Yang, T.; Zhang, J.-Y. Release characteristics and formation mechanism of gas products during rice straw and maize stalk pyrolysis. Proc. CSEE 2009, 29, 113–118. [Google Scholar]
- Puig-Gamero, M.; Lara-Diaz, J.; Valverde, J.L.; Sanchez-Silva, L.; Sánchez, P. Dolomite effect on steam co-gasification of olive pomace, coal and petcoke: TGA-MS analysis, reactivity and synergistic effect. Fuel 2018, 234, 142–150. [Google Scholar] [CrossRef]
- Habibi, R.; Kopyscinski, J.; Masnadi, M.S.; Lam, J.; Grace, J.R.; Mims, C.A.; Hill, J.M. Co-gasification of biomass and non-biomass feedstocks: Synergistic and inhibition effects of switchgrass mixed with sub-bituminous coal and fluid coke during CO2 gasification. Energy Fuels 2013, 27, 494–500. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Q.; Hu, H.; Li, A.; Yao, H. Influence of residual moisture on deep dewatered sludge pyrolysis. Int. J. Hydrog. Energy 2014, 39, 1253–1261. [Google Scholar] [CrossRef]
| Sample | Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Proximate Analysis | Ultimate Analysis | ||||||||
| Moisturear | Volatile Matterd | Fixed Carbond | Ashd | C | H | Oa | N | S | |
| Coal | 15 | 42.3 | 40.3 | 17.4 | 64.4 | 2.9 | 30.2 | 1.0 | 1.5 |
| Cyanobacteria | 84.7 | 81.2 | 8.7 | 10.1 | 44.7 | 6.7 | 38.3 | 10.0 | 0.3 |
| Ash Analysis | |||||||||
| SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | P2O5 | Others | |
| Coal | 47.0 | 39.1 | 4.8 | 5.2 | 1.0 | 0.9 | 0.3 | 0.1 | 1.6 |
| Cyanobacteria | 3.0 | 0.6 | 2.7 | 0.8 | 28.9 | 8.0 | 14.3 | 40.6 | 1.1 |
| Sample | 82CYA | 64CYA | 46CYA | 28CYA | 10CYA |
|---|---|---|---|---|---|
| Pyrolysis | 0.0417 | 0.0322 | 0.0303 | 0.0150 | −0.0105 |
| Gasification | 0.2423 | 0.2229 | 0.1016 | 0.0396 | −0.0229 |
| Region | Model | Kinetic | CYA | 82CYA | 64CYA | 46CYA | 28CYA | 10CYA | Coal |
|---|---|---|---|---|---|---|---|---|---|
| Fast pyrolysis region | HRM | E (kJ/mol) | 35.84 | 38.63 | 42.08 | 42.76 | 43.73 | 48.04 | 48.91 |
| A (min−1) | 117 | 319 | 930 | 1027 | 1197 | 2893 | 3835 | ||
| R2 | 0.991 | 0.979 | 0.981 | 0.984 | 0.986 | 0.983 | 0.989 | ||
| SCM | E (kJ/mol) | 30.96 | 32.61 | 37.48 | 38.09 | 38.98 | 40.21 | 41.01 | |
| A (min−1) | 35.39 | 71.98 | 282.83 | 309.94 | 357.04 | 446.28 | 579.7 | ||
| R2 | 0.990 | 0.981 | 0.983 | 0.977 | 0.988 | 0.987 | 0.992 | ||
| Second pyrolysis region | HRM | E (kJ/mol) | 62.49 | 63.28 | 63.76 | 63.43 | 63.77 | 64.05 | 66.18 |
| A (min−1) | 1.47 × 103 | 1.61 × 103 | 1.59 × 103 | 1.58 × 103 | 1.61 × 103 | 1.75 × 103 | 2.43 × 103 | ||
| R2 | 0.993 | 0.992 | 0.996 | 0.991 | 0.993 | 0.992 | 0.991 | ||
| SCM | E (kJ/mol) | 52.98 | 53.89 | 53.25 | 53.57 | 53.09 | 53.96 | 54.84 | |
| A (min−1) | 221.99 | 300.64 | 251.72 | 274.99 | 259.32 | 295.19 | 331.71 | ||
| R2 | 0.992 | 0.989 | 0.993 | 0.992 | 0.991 | 0.991 | 0.992 | ||
| Char gasification region | HRM | E (kJ/mol) | 136.43 | 137.79 | 142.24 | 144.54 | 149.41 | 160.28 | 162.47 |
| A (min−1) | 7.98 × 105 | 8.01 × 105 | 1.11 × 106 | 1.22 × 106 | 1.90 × 106 | 5.69 × 106 | 7.00 × 106 | ||
| R2 | 0.999 | 0.997 | 0.996 | 0.995 | 0.994 | 0.992 | 0.988 | ||
| SCM | E (kJ/mol) | 116.62 | 119.33 | 125.19 | 129.49 | 135.93 | 147.89 | 150.95 | |
| A (min−1) | 6.51 × 104 | 7.85 × 104 | 1.33 × 105 | 1.90 × 105 | 3.59 × 105 | 1.24 × 106 | 1.70 × 106 | ||
| R2 | 0.991 | 0.988 | 0.99 | 0.991 | 0.993 | 0.994 | 0.995 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, T.; Zhang, J.; Shen, L.; Bai, L.; Yan, J. Chemical Looping Co-Gasification Characteristics of Cyanobacterial/Coal Blends. Energies 2020, 13, 2352. https://doi.org/10.3390/en13092352
Shen T, Zhang J, Shen L, Bai L, Yan J. Chemical Looping Co-Gasification Characteristics of Cyanobacterial/Coal Blends. Energies. 2020; 13(9):2352. https://doi.org/10.3390/en13092352
Chicago/Turabian StyleShen, Tianxu, Jiang Zhang, Laihong Shen, Lei Bai, and Jingchun Yan. 2020. "Chemical Looping Co-Gasification Characteristics of Cyanobacterial/Coal Blends" Energies 13, no. 9: 2352. https://doi.org/10.3390/en13092352
APA StyleShen, T., Zhang, J., Shen, L., Bai, L., & Yan, J. (2020). Chemical Looping Co-Gasification Characteristics of Cyanobacterial/Coal Blends. Energies, 13(9), 2352. https://doi.org/10.3390/en13092352