Characteristics of Pyrolysis and Low Oxygen Combustion of Long Flame Coal and Reburning of Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coal Samples
2.2. Thermal Analysis Experiment
3. Results and Discussion
3.1. Characteristics of Coal Pyrolysis and Low Oxygen Combustion
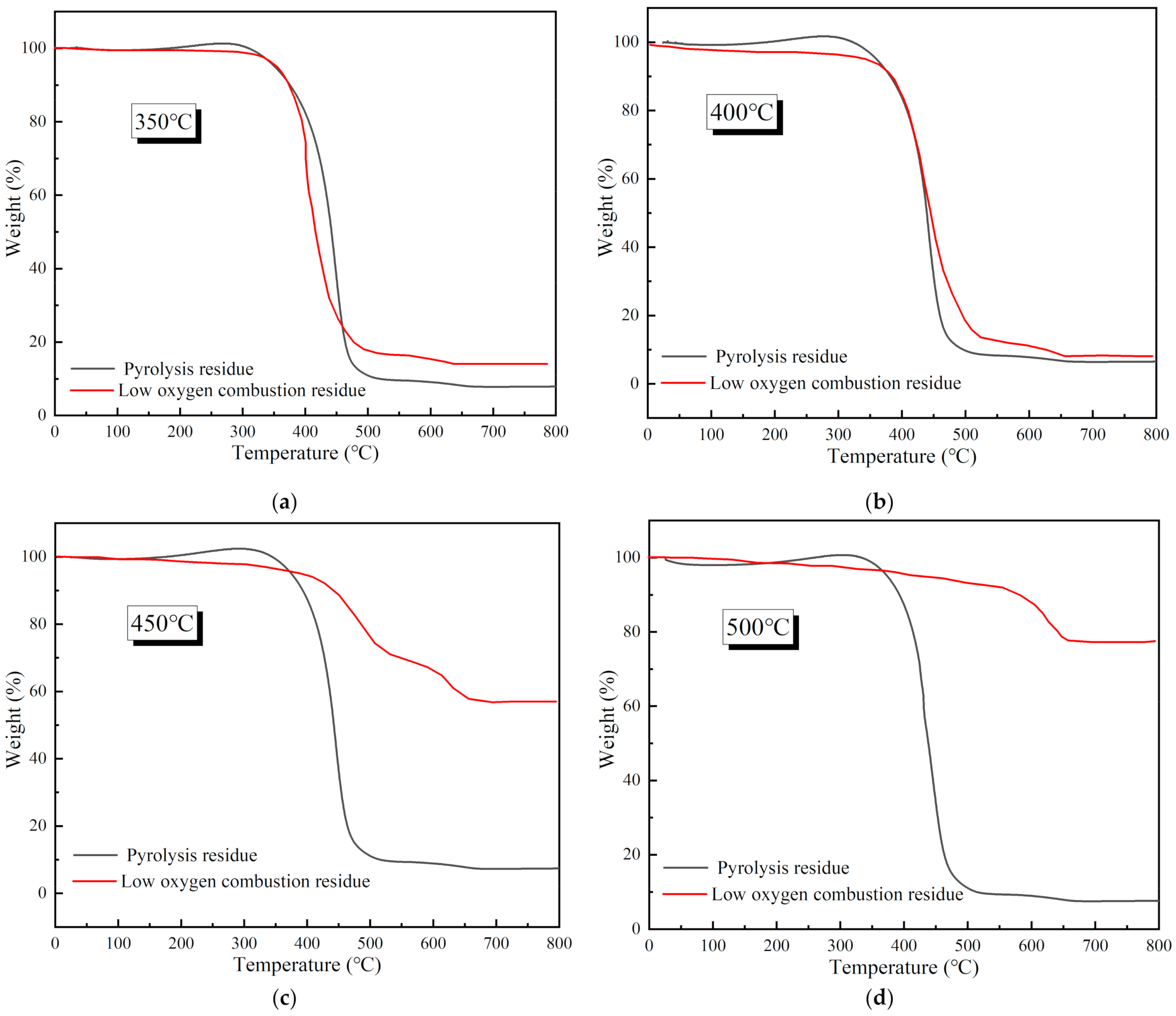
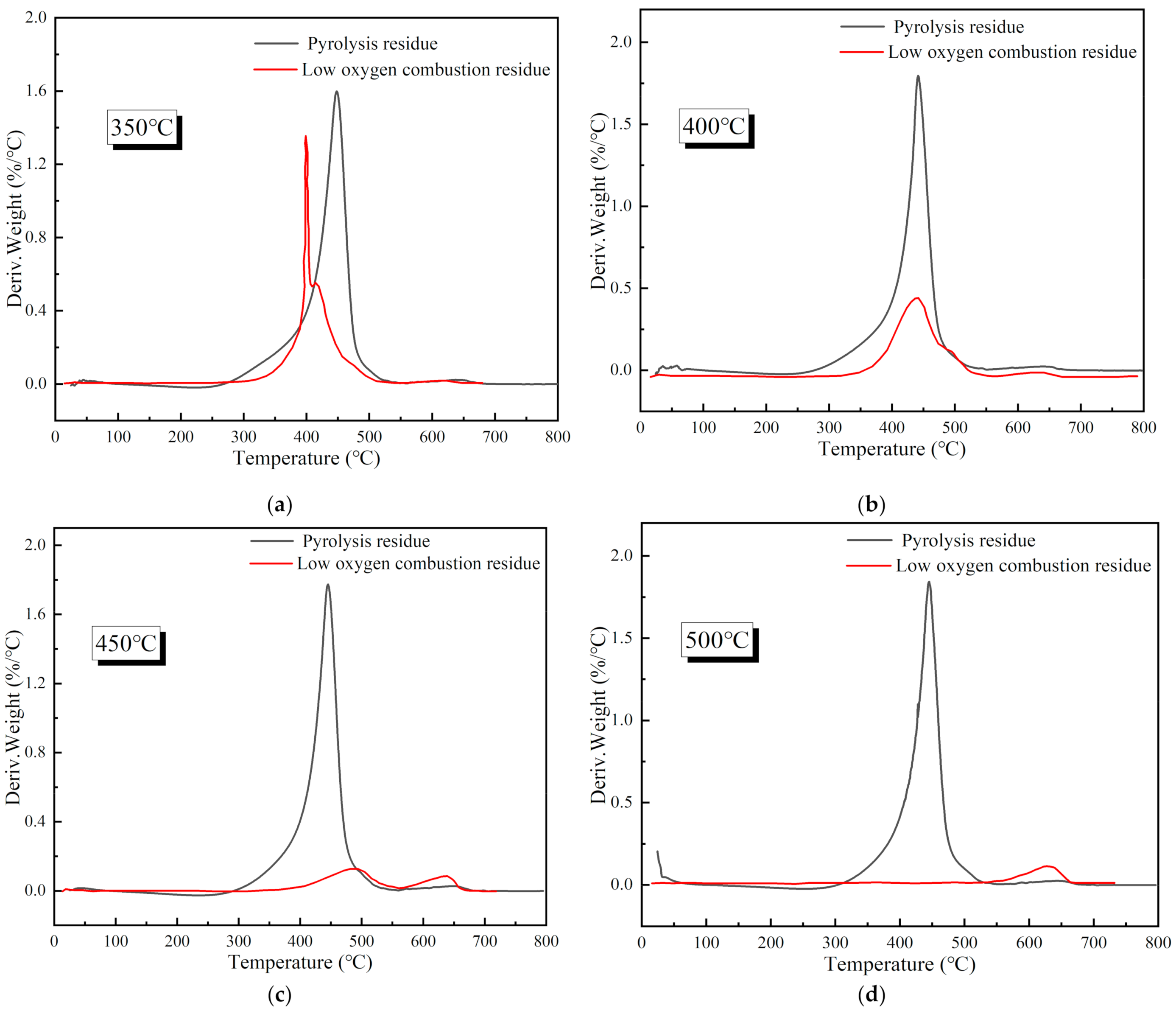
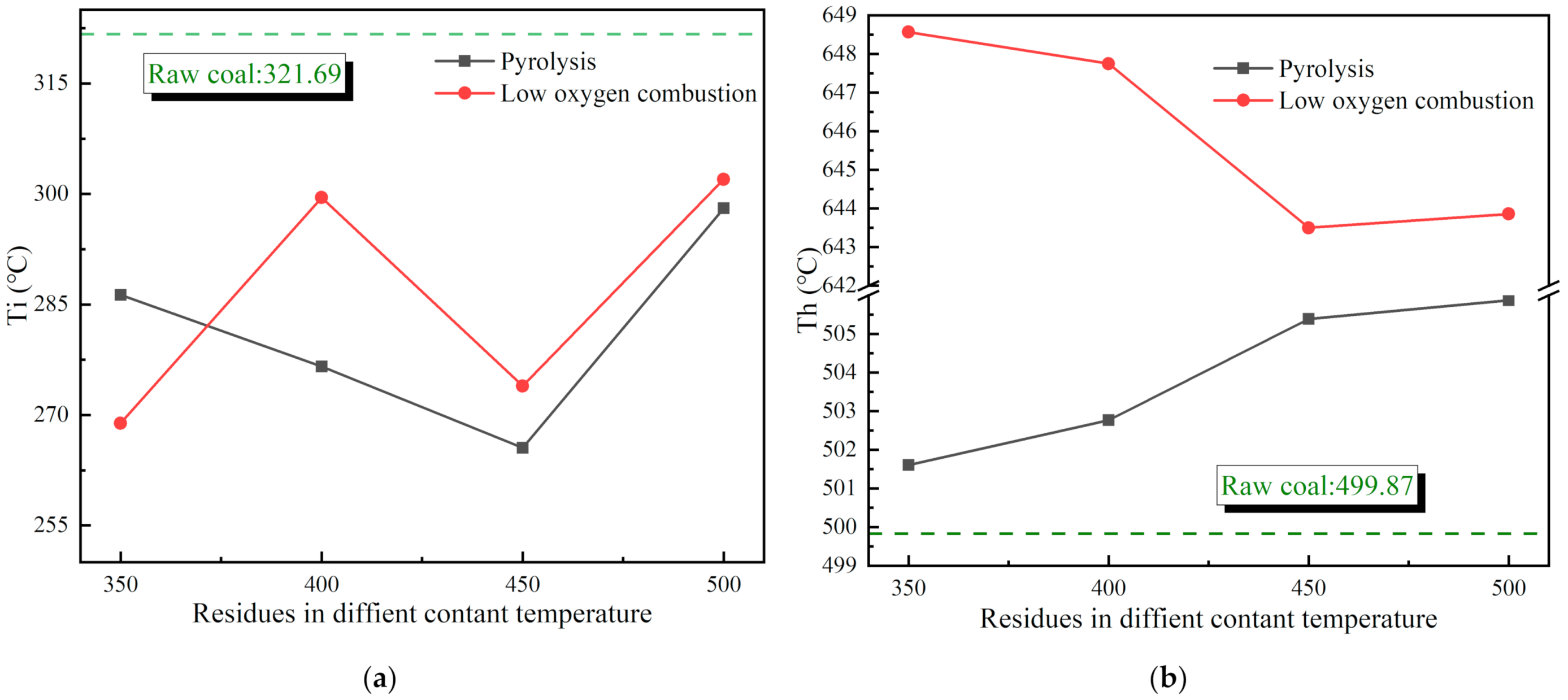
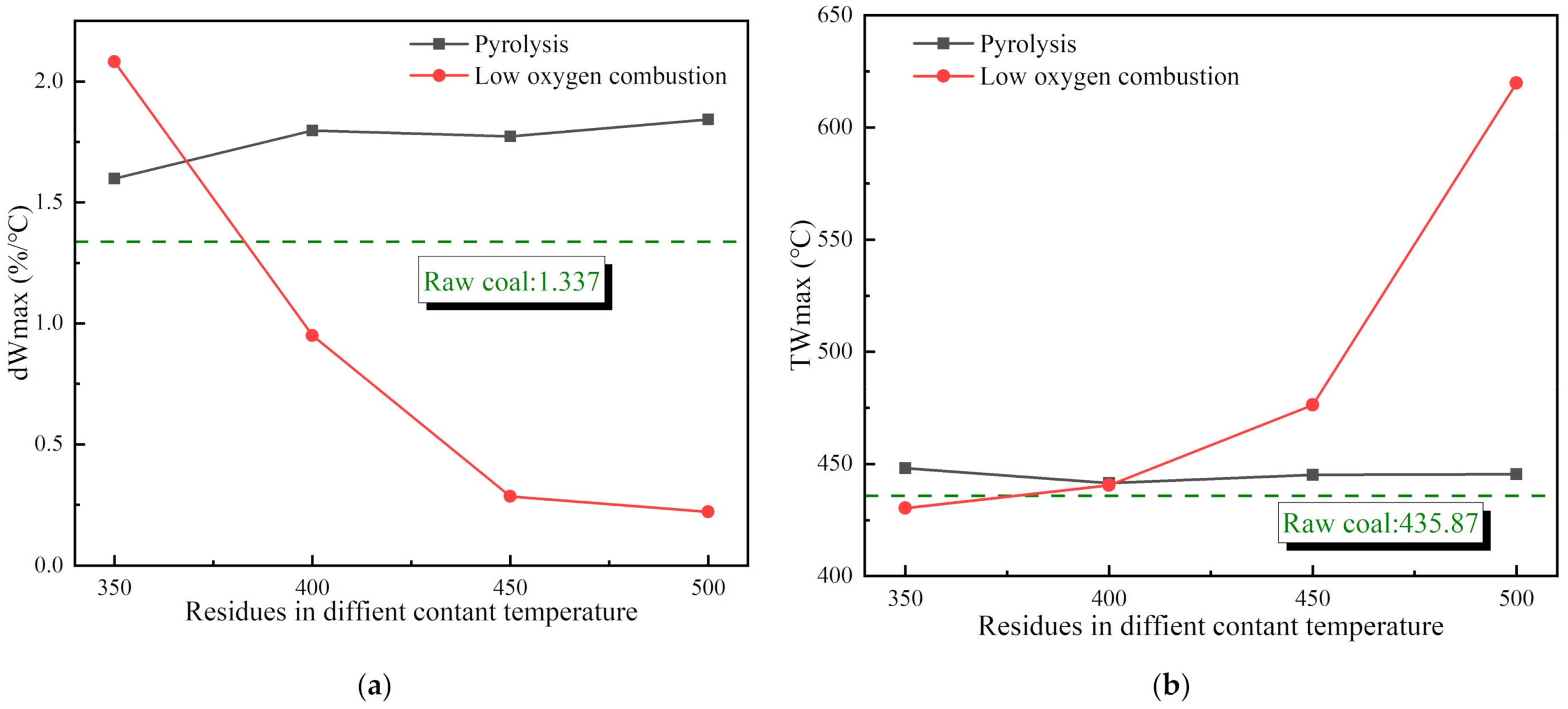
3.2. Reaction Characteristics of Reignition Oxidation of Residues
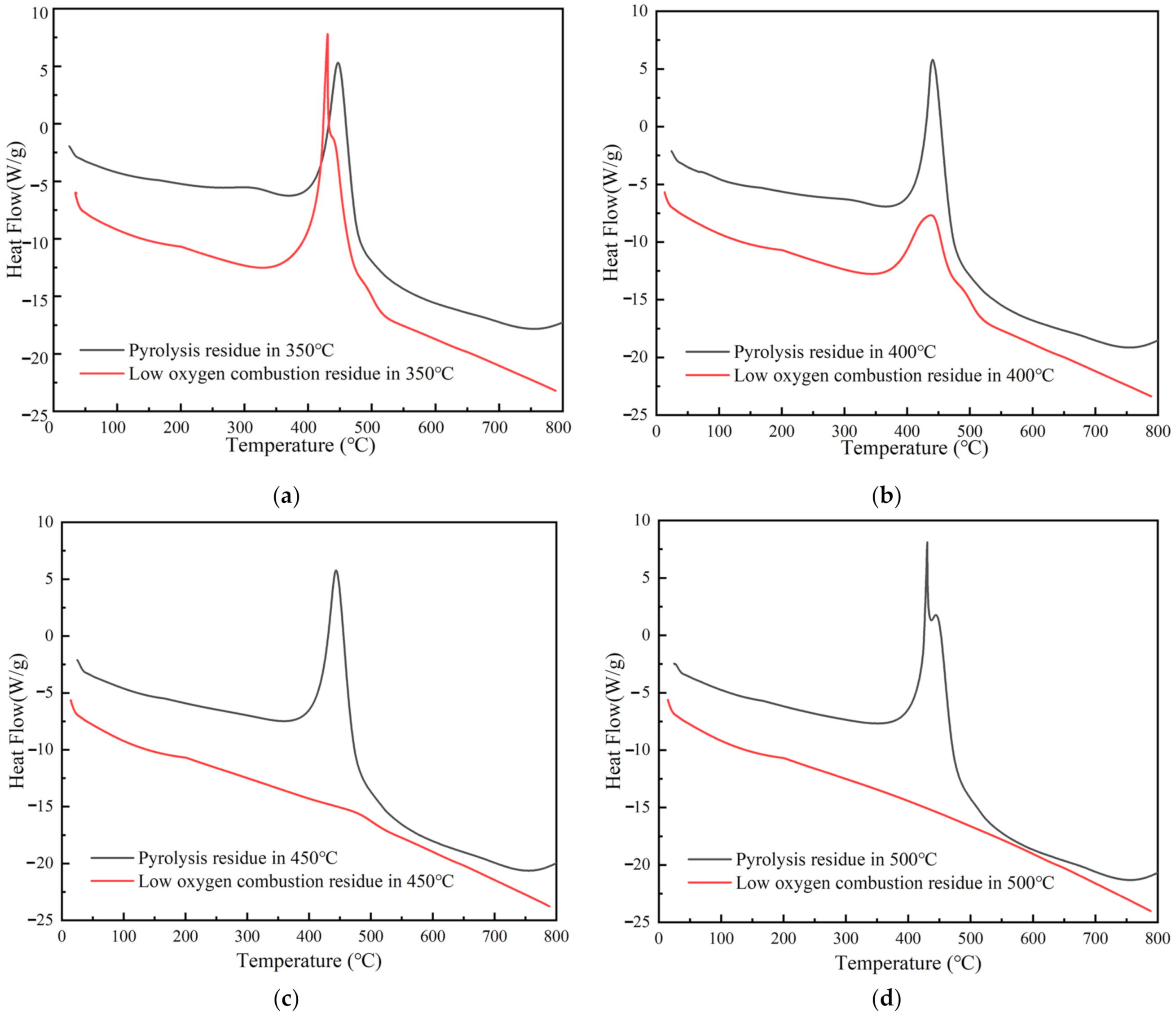
3.2.1. Thermogravimetric Characteristics of Residues
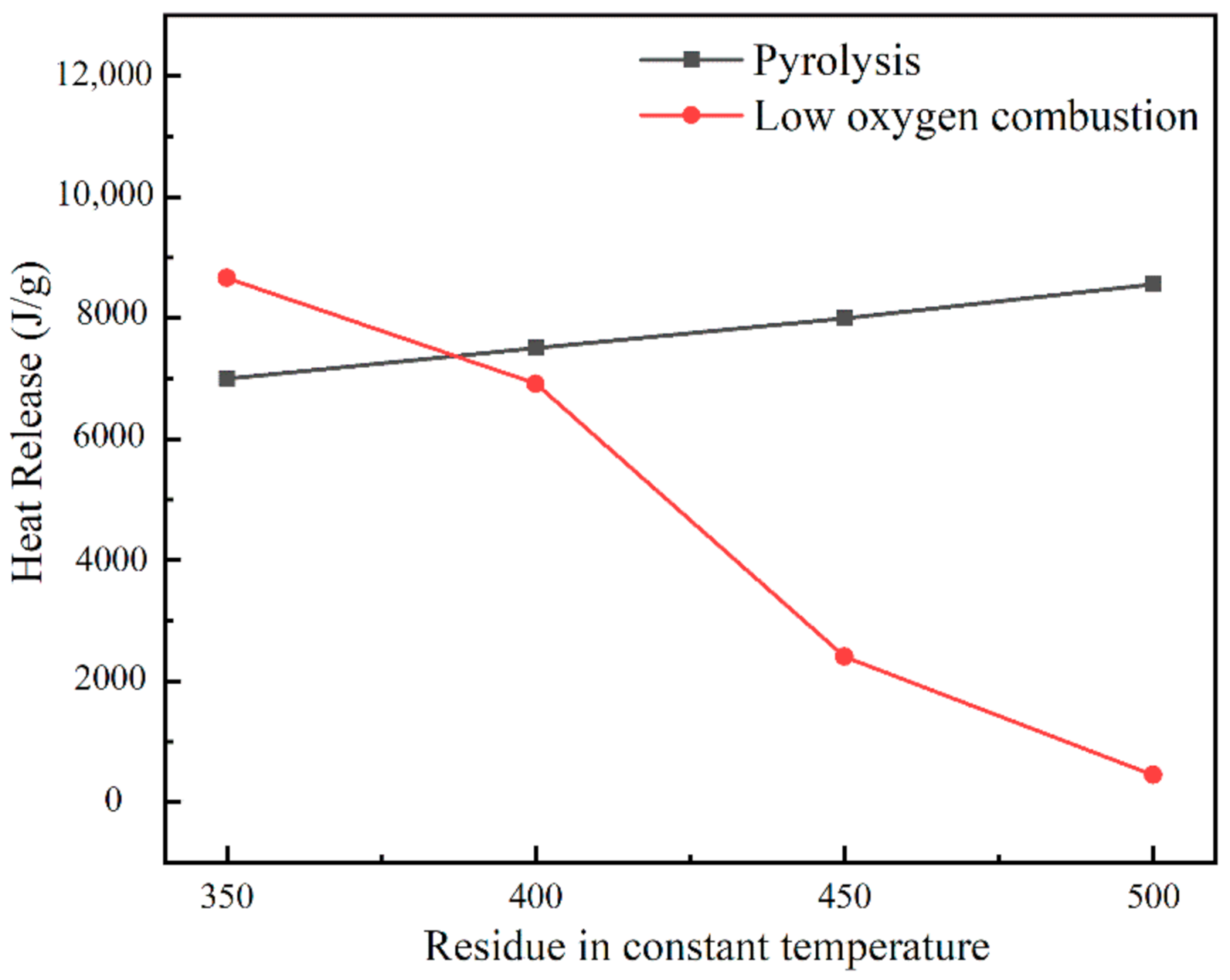
3.2.2. Heat Release Law of Residual Reignition Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gamiy, Y.; Kostenko, V.; Zavialova, O.; Kostenko, T.; Zhurbynskyi, D. Identifying sources of coal spontaneous heating in mine workings using aerogas control automatic systems. Min. Miner. Depos. 2020, 14, 120–127. [Google Scholar] [CrossRef]
- Jian, K.; Fu, X.; Wang, K.; Zhang, Y. Physical Characteristics and CBM Resources Potential of Long Flame Coal in China. Adv. Earth Sci. 2014, 29, 1065–1074. [Google Scholar] [CrossRef]
- Qian, W.; Sun, K.; Zhang, Q.; Huang, Y. Study of resolution capability of pyrolysis characteristic index of low-rank bituminous coal. J. China Univ. Min. Technol. 2012, 41, 256–261. [Google Scholar]
- Fu, X.; Peng, J. Numerical simulation of three level seepage of coalbed methane on flame coal reservoirs in Tiefa Basin. J. China Coal Soc. 2007, 494–498. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Feng, Z. Evolution charactics of pore structure during lignite seam spontaneous combustion developing. J. China Coal Soc. 2010, 35, 1490–1495. [Google Scholar] [CrossRef]
- Zhou, F. Study on the coexistence of gas and coal spontaneous combustion (I):disaster mechanism. J. China Coal Soc. 2012, 37, 843–849. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. Experimental Investigation and Mathematical Modeling of Gas Diffusivity by Carbon Dioxide and Methane Kinetic Adsorption. Ind. Eng. Chem. Res. 2019, 58, 12392–12400. [Google Scholar] [CrossRef]
- Wang, D. Thermodynamic disaster in coal mine and its characteristics. J. China Coal Soc. 2018, 43, 137–142. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wang, J.; Zhang, X.; Wang, J.; Zhou, C. Study on the Effect of Extraneous Moisture on the Spontaneous Combustion of Coal and Its Mechanism of Action. Energies 2020, 13, 1969. [Google Scholar] [CrossRef]
- Küçük, A.; Kadıoğlu, Y.; Gülaboğlu, M. A study of spontaneous combustion characteristics of a turkish lignite: Particle size, moisture of coal, humidity of air. Combust. Flame 2003, 133, 255–261. [Google Scholar] [CrossRef]
- Daryayehsalameh, B.; Nabavi, M.; Vaferi, B. Modeling of CO2 capture ability of [Bmim][BF4] ionic liquid using connectionist smart paradigms. Environ. Technol. Innov. 2021, 22, 101484. [Google Scholar] [CrossRef]
- Mazarei, M.; Davarpanah, A.; Ebadati, A.; Mirshekari, B. The feasibility analysis of underground gas storage during an integration of improved condensate recovery processes. J. Pet. Explor. Prod. Technol. 2018, 9, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Wang, G.; Zhang, J. Study on Spontaneous Combustion Tendency of Coals with Different Metamorphic Grade at Low Moisture Content Based on TPO-DSC. Energies 2019, 12, 3890. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Xin, H.; Wang, D.; Qi, G. A rapid method for determining the R70 self-heating rate of coal. Thermochim. Acta 2013, 571, 21–27. [Google Scholar] [CrossRef]
- Wang, H.; Dlugogorski, B.Z.; Kennedy, E.M. Coal oxidation at low temperatures: Oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog. Energy Combust. Sci. 2003, 29, 487–513. [Google Scholar] [CrossRef]
- Xin, H.H.; Wang, D.-M.; Qi, X.-Y.; Zhong, X.-X.; Ma, L.-Y.; Dou, G.-L.; Wang, H.-T. Oxygen consumption and chemisorption in low-temperature oxidation of sub-bituminous pulverized coal. Spectrosc. Lett. 2018, 51, 104–111. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, F.; Li, H.; Xu, H. Experimental Study on Coalfield Fire Control Technology under Complex Conditions. Energy Technol. Manag. 2020, 193, 118–119. [Google Scholar]
- Sun, W.; Shi, J.; Su, W. Risk assessment and application of coalfield fire area based on AHP-fuzzy comprehensive evaluation. China Energy Environ. Prot. 2020, 42, 12–15. [Google Scholar] [CrossRef]
- Zheng, X.; Jia, Y.; Guo, J.; Wen, H.; Wang, B. Research status and prospect of coalfield fire monitoring technologies. Ind. Mine Autom. 2019, 45, 9–13, 64. [Google Scholar] [CrossRef]
- Zheng, K. Research on the Characteristics of Coal Smoldering Combustion and Fire Extinguishment for Coalfield Fires; China University of Mining and Technology: Xuzhou, China, 2017. [Google Scholar]
- Hu, W.; Jun, G.; Yongfei, J.; Wang, D.; Kai, W. Development of testing device for characteristic parameters of high temperature oxidation combustion. Ind. Mine Autom. 2015, 41, 14–18. [Google Scholar] [CrossRef]
- Qu, Z.; Zhou, X.; Li, T. Volatilization combustion mechanism analysis and experiment of coalfield fire. J. Chongqing Univ. 2011, 34, 122–127. [Google Scholar] [CrossRef]
- Wedler, C.; Span, R.; Richter, M. Comparison of micro- and macropore evolution of coal char during pyrolysis. Fuel 2020, 275, 117845. [Google Scholar] [CrossRef]
- Lü, H.F.; Deng, J.; Li, D.-J.; Xu, F.; Xiao, Y.; Shu, C.-M. Effect of oxidation temperature and oxygen concentration on macro characteristics of pre-oxidised coal spontaneous combustion process. Energy 2021, 227, 120431. [Google Scholar] [CrossRef]
- Valdés, C.F.; Chejne, F. Effect of reaction atmosphere on the products of slow pyrolysis of coals. J. Anal. Appl. Pyrolysis 2017, 126, 105–117. [Google Scholar] [CrossRef]
- Zhang, G.; Su, A.; Zhang, Y.; Wang, P. Shrinkage Character in the Process of Semi-coke Formation. Chiang Mai J. Sci. 2015, 42, 401–406. [Google Scholar]
- Yörük, C.R.; Meriste, T.; Sener, S.; Kuusik, R.; Trikkel, A. Thermogravimetric analysis and process simulation of oxy-fuel combustion of blended fuels including oil shale, semicoke, and biomass. Int. J. Energy Res. 2018, 42, 2213–2224. [Google Scholar] [CrossRef]
- Tran, Q.A.; Stanger, R.; Xie, W.; Smith, N.; Lucas, J.; Wall, T. Linking Thermoplastic Development and Swelling with Molecular Weight Changes of a Coking Coal and Its Pyrolysis Products. Energy Fuels 2016, 30, 3906–3916. [Google Scholar] [CrossRef]
- Sun, M.; Ning, X.; Zhang, J.; Li, K.; Tang, Q.; Liu, Z.; Wang, G.; Wang, H. Combustion kinetics and structural features of bituminous coal before and after modification process. J. Therm. Anal. Calorim. 2017, 131, 983–992. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Ono, Y.; Tsubouchi, N. Evolution profile of gases during coal carbonization and relationship between their amounts and the fluidity or coke strength. Fuel 2019, 237, 735–744. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhang, J.-L.; Shao, J.-G.; Sun, H.; Zuo, H.-B. Thermogravimetric Analysis of Coal Char Combustion Kinetics. J. Iron Steel Res. Int. 2014, 21, 897–904. [Google Scholar] [CrossRef]
- Yong, Z.; Jian, Y.; Ai, C.; Hong, Z. Study on coal combustion features with thermal balance. Coal Sci. Technol. 2004, 8–11. [Google Scholar] [CrossRef]
- Wei, L. Study on Combustion Mechanism of Micro-pulverised Coal. Proc. Chin. Soc. Electr. Eng. 2007, 20, 71–74. [Google Scholar]
- Liu, H.; Wang, F.; Ren, T. Research on the Characteristics of the Coal–Oxygen Reaction in a Lean-Oxygen Environment Caused by Methane. Energy Fuels 2019, 33, 9215–9223. [Google Scholar] [CrossRef]
- Arenillas, A.; Rubiera, F.; Pis, J.; Cuesta, M.; Iglesias, M.; Jiménez, A.; Suárez-Ruiz, I. Thermal behaviour during the pyrolysis of low rank perhydrous coals. J. Anal. Appl. Pyrolysis 2003, 68–69, 371–385. [Google Scholar] [CrossRef]
- Chen, Y.; Mori, S.; Pan, W. Estimating the combustibility of various coals by TG-DTA. Fuel Energy Abstr. 1995, 36, 171. [Google Scholar] [CrossRef]
- Chen, Y.; Mori, S.; Pan, W.-P. Studying the mechanisms of ignition of coal particles by TG-DTA. Thermochim. Acta 1996, 275, 149–158. [Google Scholar] [CrossRef]
- Hu, X.; Xie, J.; Cai, W.; Wang, R.; Davarpanah, A. Thermodynamic effects of cycling carbon dioxide injectivity in shale reservoirs. J. Pet. Sci. Eng. 2020, 195, 107717. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Che, D. Ignition and Kinetics Analysis of Coal Combustion in Low Oxygen Concentration. Energy Sources Part A Recover. Util. Environ. Eff. 2012, 34, 810–819. [Google Scholar] [CrossRef]
- Ma, L.; Wang, D.; Xin, H.; Qi, X.; Dou, G. The competitive reaction mechanism between oxidation and pyrolysis consumption during low-rank coal combustion at lean-oxygen conditions: A quantitative calculation based on thermogravimetric analyses. Can. J. Chem. Eng. 2018, 96, 2575–2585. [Google Scholar] [CrossRef]
- Pinchuk, V.; Sharabura, T.; Kuzmin, A. The peculiar influence of the mineral impurities content in coal-water fuel on the regularities of fuel drop ignition and combustion. J. Energy Inst. 2020, 93, 911–921. [Google Scholar] [CrossRef]
- Xin, H.; Wang, H.; Kang, W.; Di, C.; Qi, X.; Zhong, X.; Wang, D.; Liu, F. The reburning thermal characteristics of residual structure of lignite pyrolysis. Fuel 2020, 259, 116226. [Google Scholar] [CrossRef]
- Chen, L.; Qi, X.; Yang, J.; Xin, H. Thermogravimetric and infrared spectral analysis of candle coal pyrolysis under low-oxygen concentration. Thermochim. Acta 2021, 696, 178840. [Google Scholar] [CrossRef]
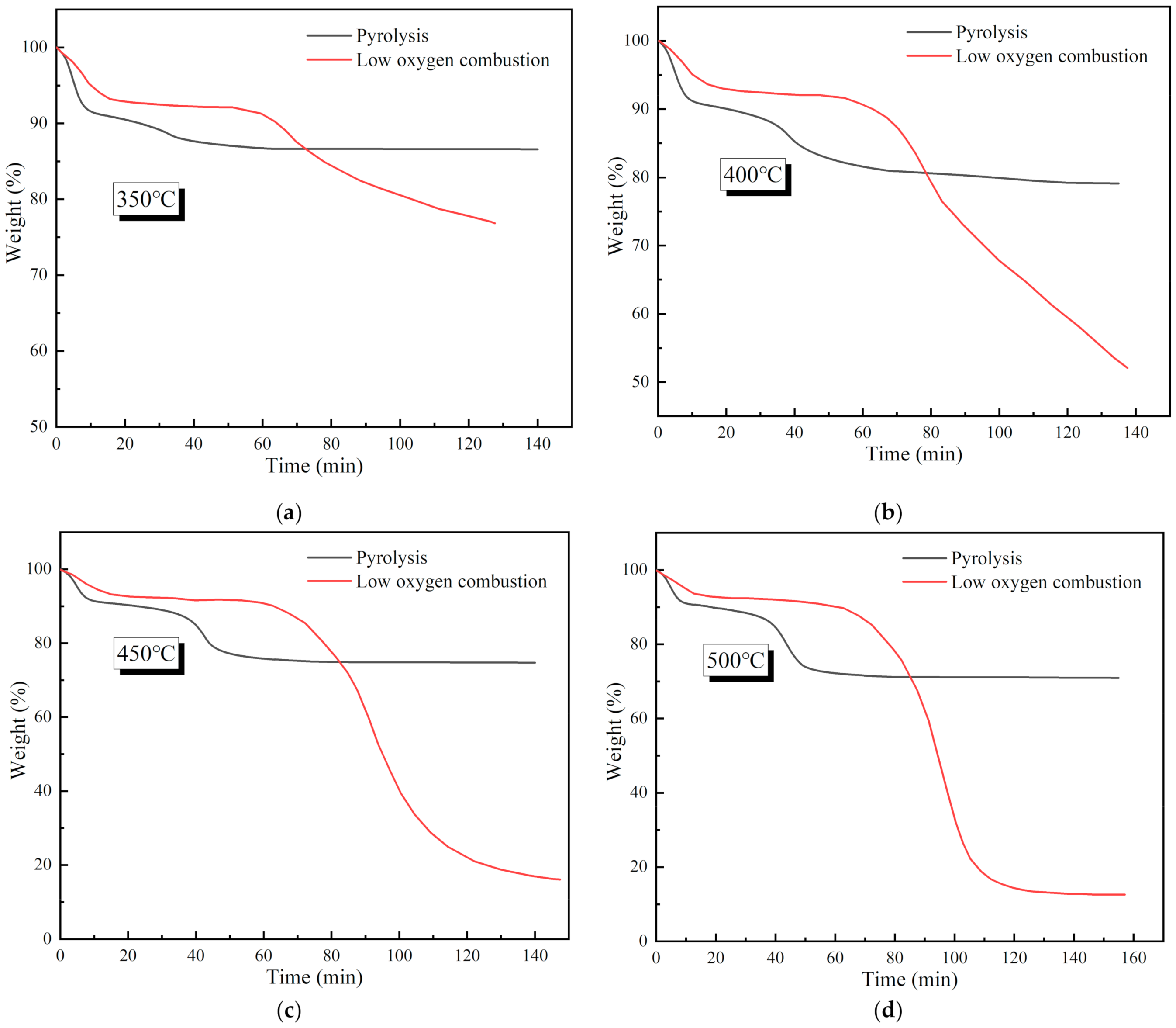
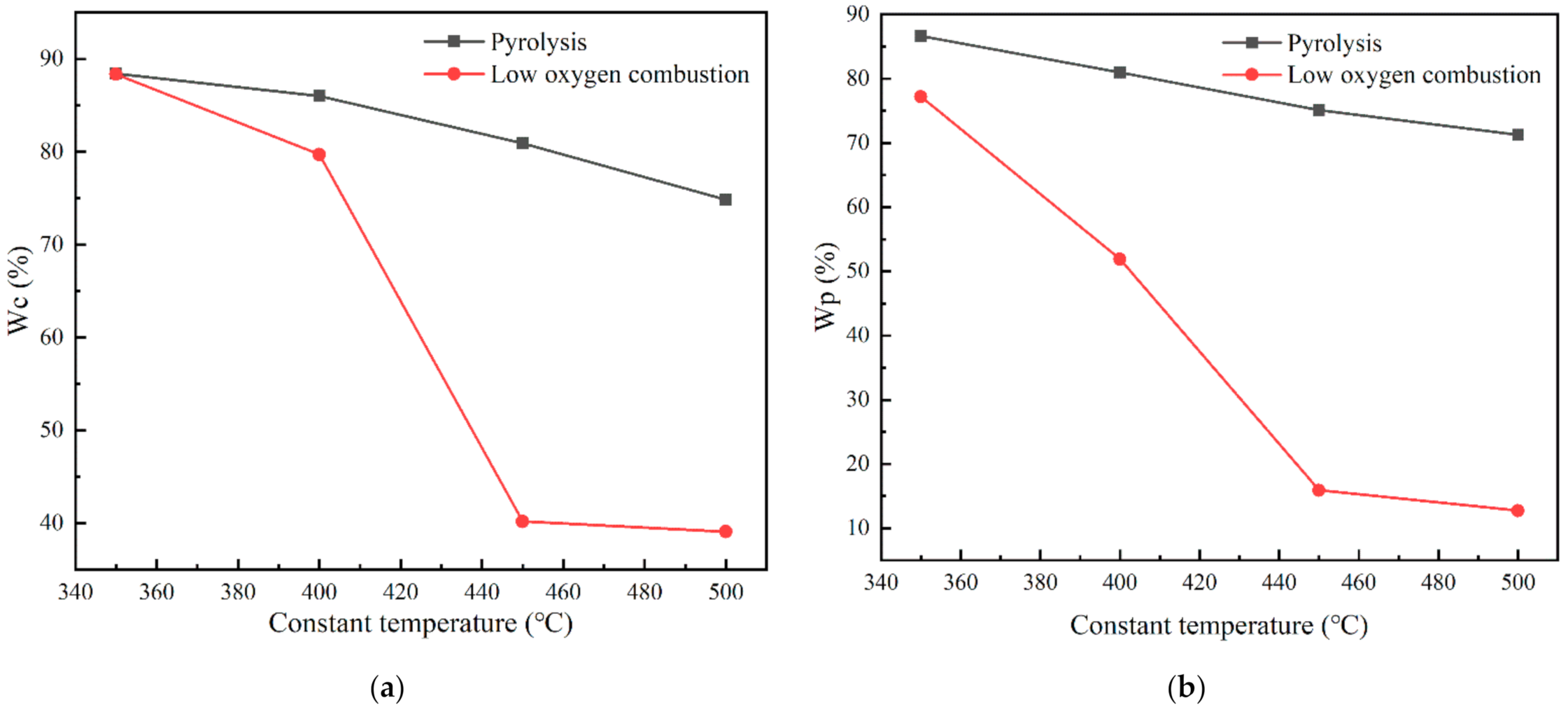
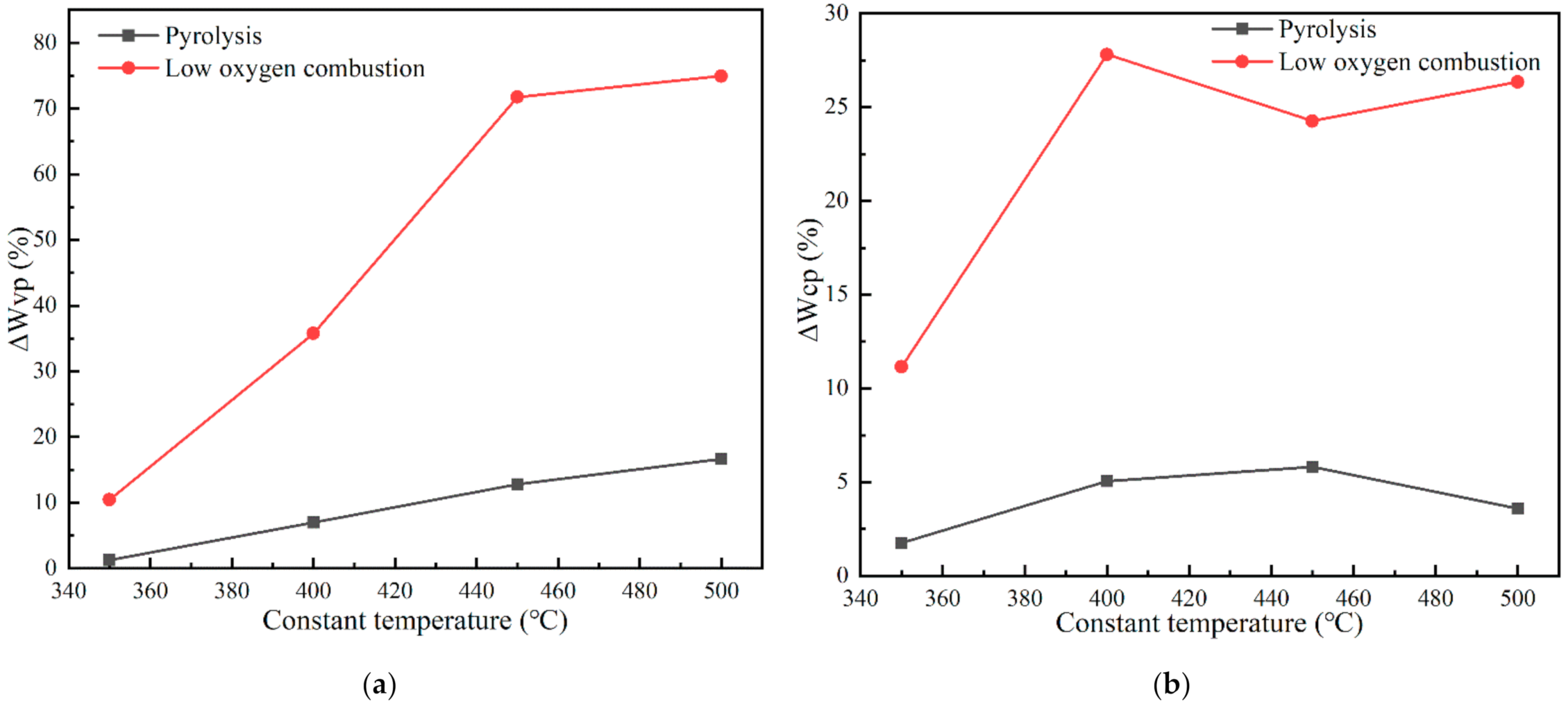
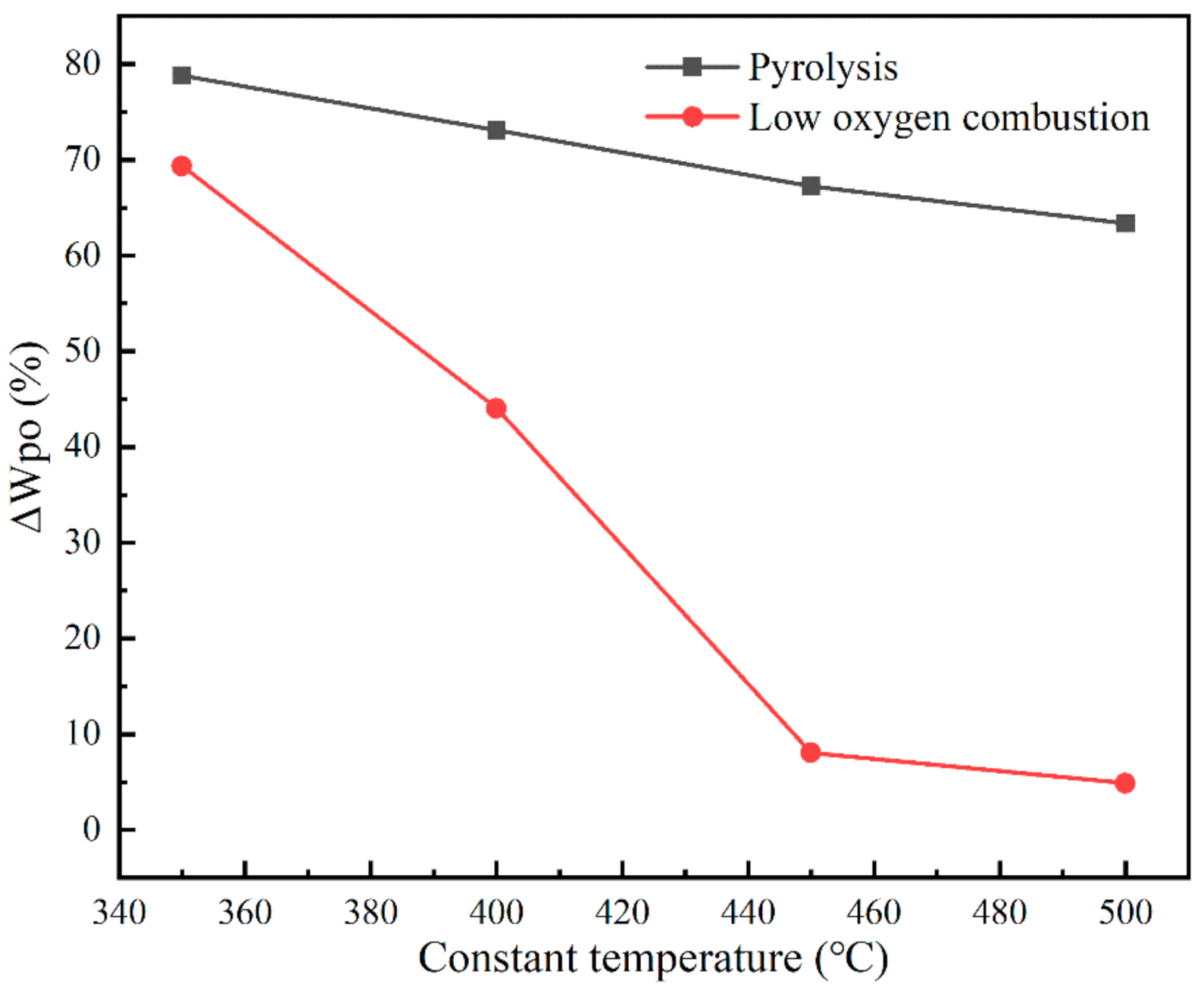

| Proximate Analysis % | Ultimate Analysis % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Equipment | SDTGA5000 | Vario MICRO | |||||||
| Content (%) | Moisture | Ash | Volatile matter | Fixed carbon | Carbon | Hydrogen | Oxygen | Nitrogen | Sulfur |
| LHG long flame coal | 8.99 | 6.74 | 29.64 | 54.63 | 65.50 | 2.947 | 30.97 | 0.162 | 0.418 |
| Experiment Types | Coal Samples | Reaction Gas | Initial Temperature (°C) | Termination Temperature (°C) | Heating Rate (°C/min) | Constant Temperature Time (min) |
|---|---|---|---|---|---|---|
| Constant temperature experiment | Raw coals in pyrolysis | N2 | 25 | 350 | 10 | 150 |
| 400 | ||||||
| 450 | ||||||
| 500 | ||||||
| Raw coals in low oxygen combustion | 5% Q2, 95% N2 | 350 | 10 | 150 | ||
| 400 | ||||||
| 450 | ||||||
| 500 | ||||||
| Temperature programmed experiment | Residues in pyrolysis | Dry air (21% Q2, 79% N2) | 25 | 800 | 5 | 0 |
| Residues in low oxygen combustion | ||||||
| Raw coal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, W.; Xin, H.; Wang, D.; Di, C.; Liu, L. Characteristics of Pyrolysis and Low Oxygen Combustion of Long Flame Coal and Reburning of Residues. Energies 2021, 14, 2944. https://doi.org/10.3390/en14102944
Wang H, Zhang W, Xin H, Wang D, Di C, Liu L. Characteristics of Pyrolysis and Low Oxygen Combustion of Long Flame Coal and Reburning of Residues. Energies. 2021; 14(10):2944. https://doi.org/10.3390/en14102944
Chicago/Turabian StyleWang, Hua, Wei Zhang, Haihui Xin, Deming Wang, Cuicui Di, and Lu Liu. 2021. "Characteristics of Pyrolysis and Low Oxygen Combustion of Long Flame Coal and Reburning of Residues" Energies 14, no. 10: 2944. https://doi.org/10.3390/en14102944





