The Effect of RME-1-Butanol Blends on Combustion, Performance and Emission of a Direct Injection Diesel Engine
Abstract
:1. Introduction
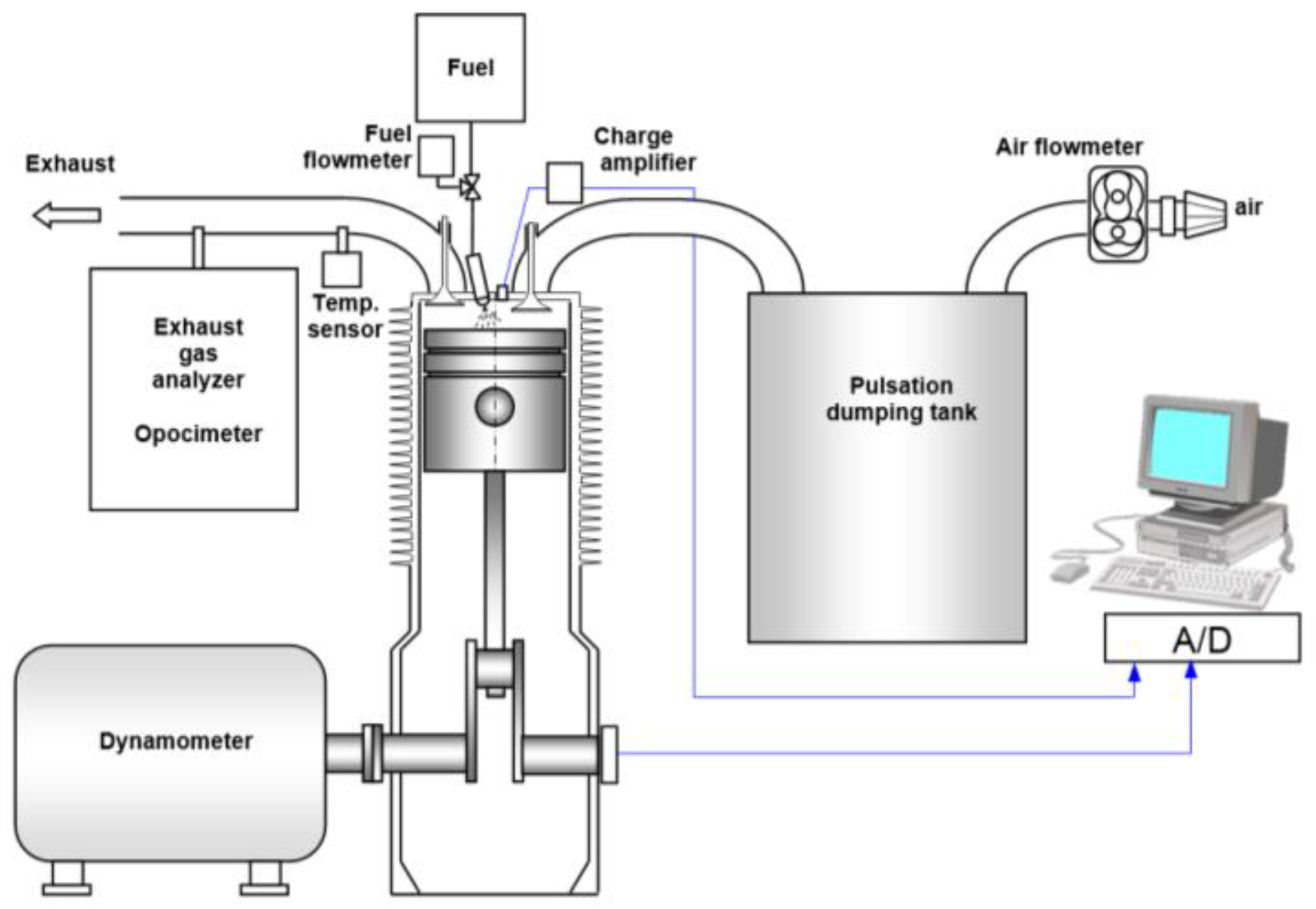
2. Materials and Methods
2.1. Fuel Characteristics
2.2. Calculation Methodology
- chamber instabilities—due to the occurrence of combustion inside a chamber (shock instabilities, fluid-dynamic instabilities associated with the chamber);
- intrinsic instabilities—chemical-kinetic instabilities, diffusive-thermal instabilities, hydrodynamics instabilities;
- system instabilities—caused by feed-system interactions, exhaust/intake-system interactions.
3. Results
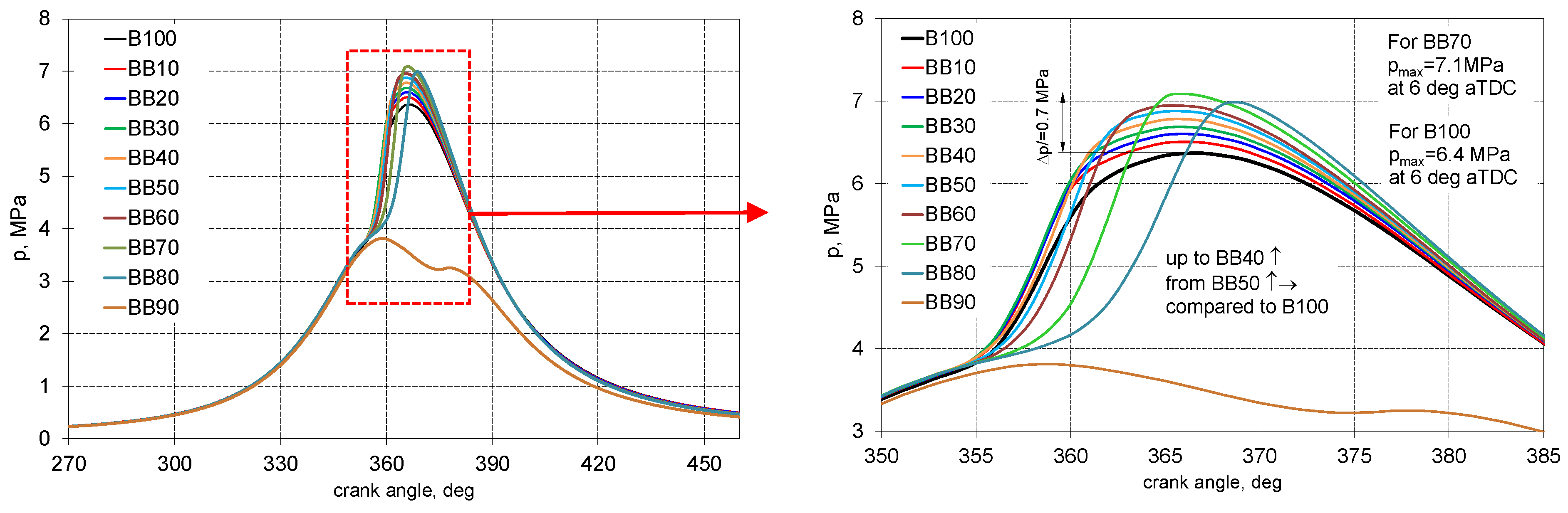
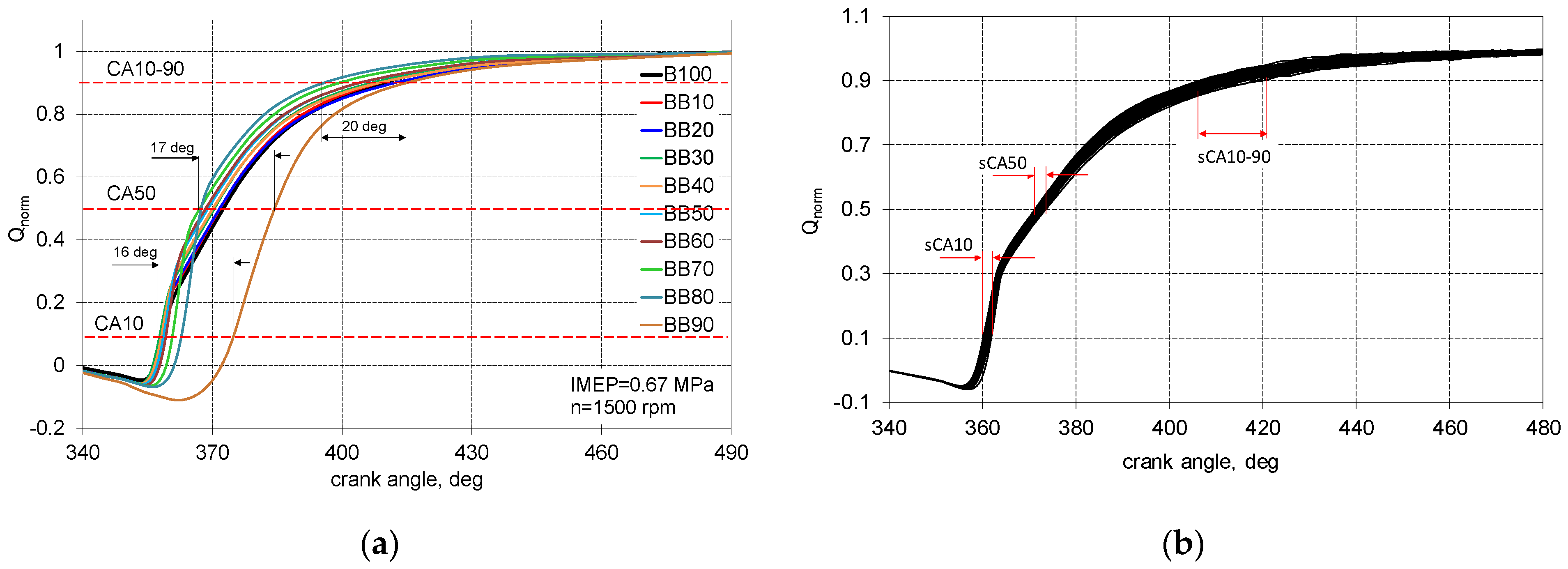
3.1. Characteristics of the Combustion Process
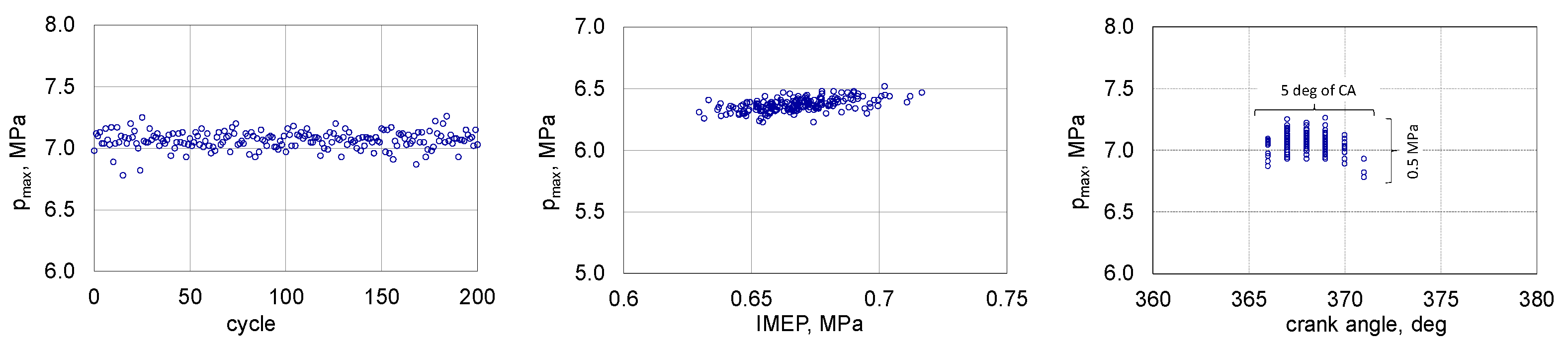
3.2. Stability of Combustion Process
3.3. Emission Characteristics
4. Conclusions
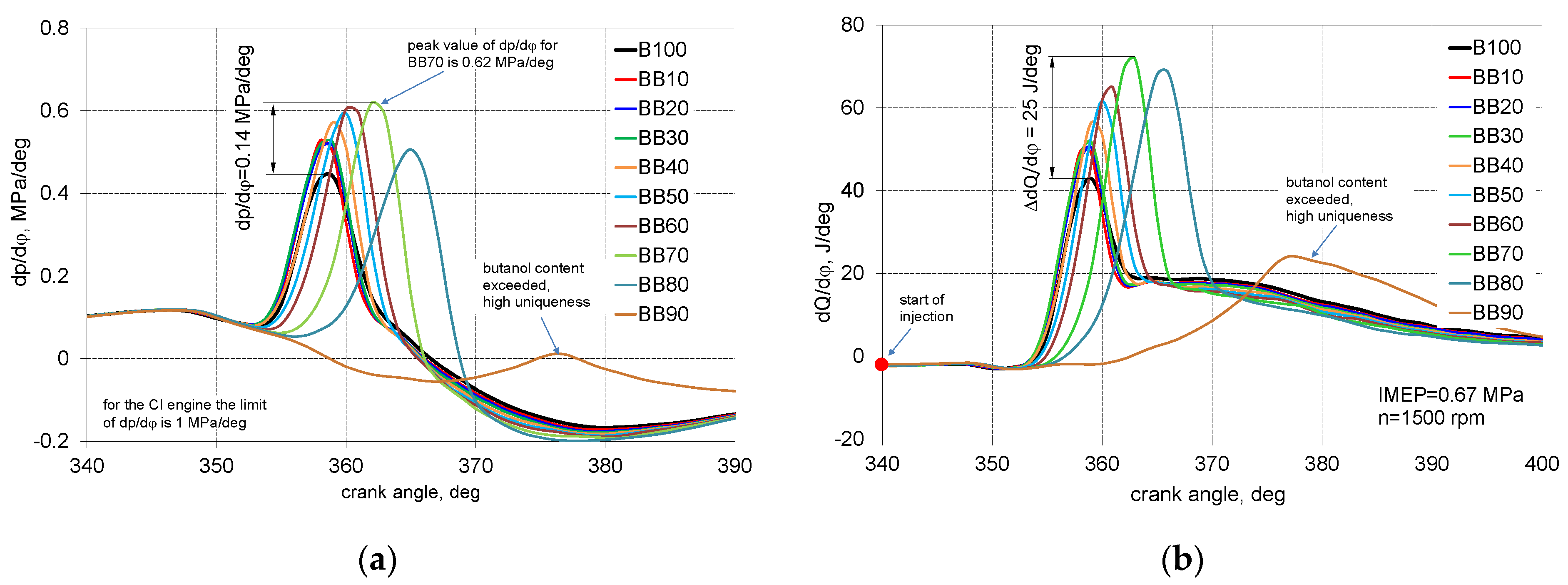
- Up to 40% of 1-Butanol energetic fraction there was only increase in peak pressure, for larger shares of 1-butanol, it was an increase in the value of peak pressure and was moved further from TDC;
- The highest value of dp/dφ and dQ/dφ was obtained for BB70 and was equal, respectively, 0.62 MPa/degree and 71.7 J/degree;
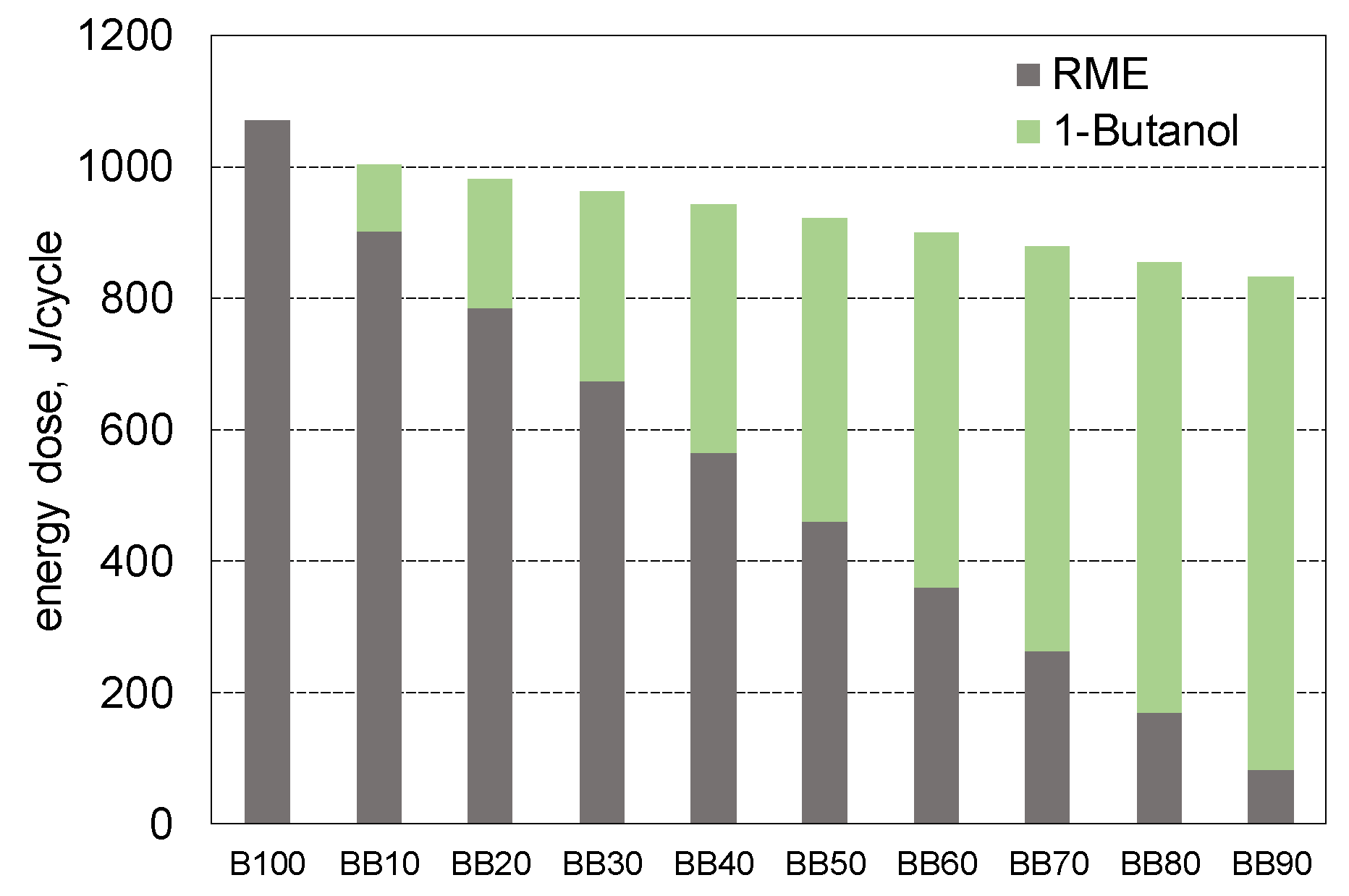
- With the increase in 1-butanol fraction in blend the energy demand of the engine decrease, up to BB80, what result in decrease in BSEC by near to 9% compared to reference case;
- With the increase in 1-butanol fraction (up to 80%) the thermal efficiency of the engine increased, for engine powered by BB80 it reaches value of 39.2% and it was higher by 3.6% compared to engine powered by reference fuel;
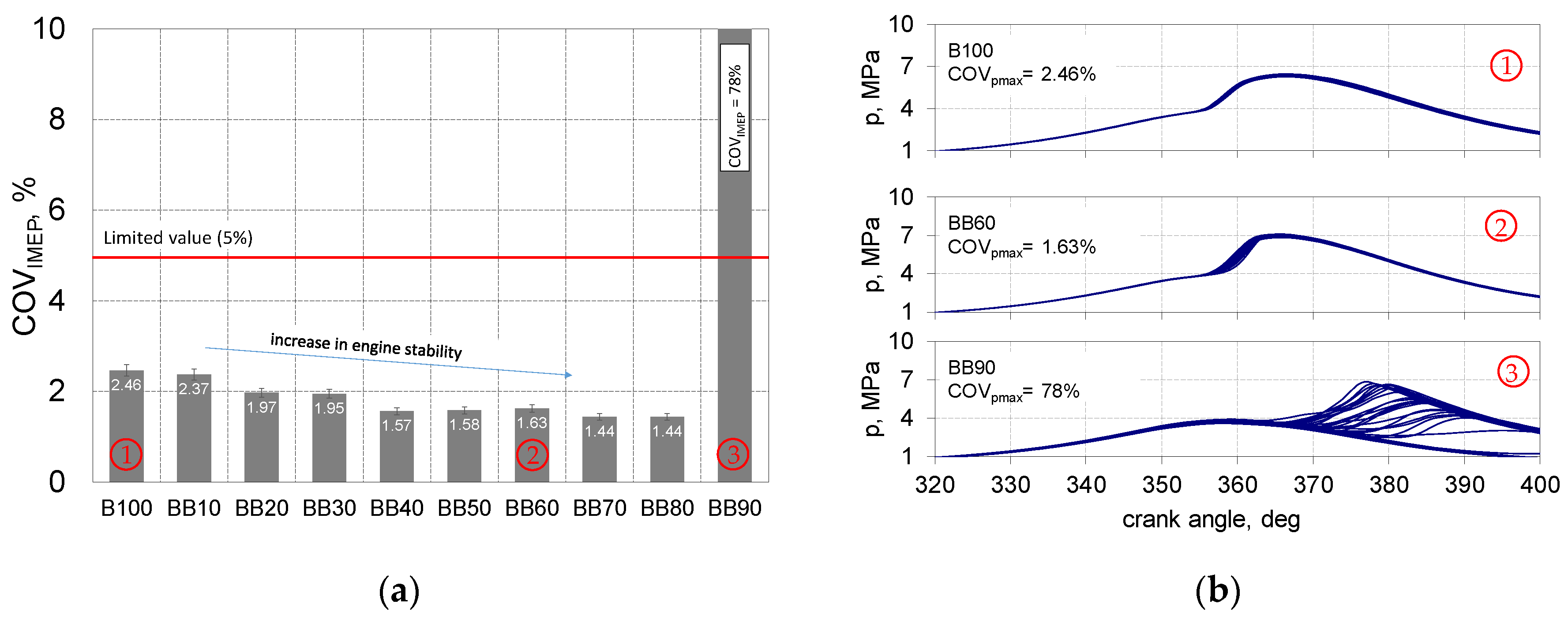
- With the increase in the share of 1-butanol in the mixture with RME, the stability of the engine’s operation increased up to 80% of energetic share 1-butanol;
- The share of 1-butanol is conducive to increasing the stability of the engine operation, for BB80 there was an almost double increase in the value of the function f (IMEP) in relation to B100;
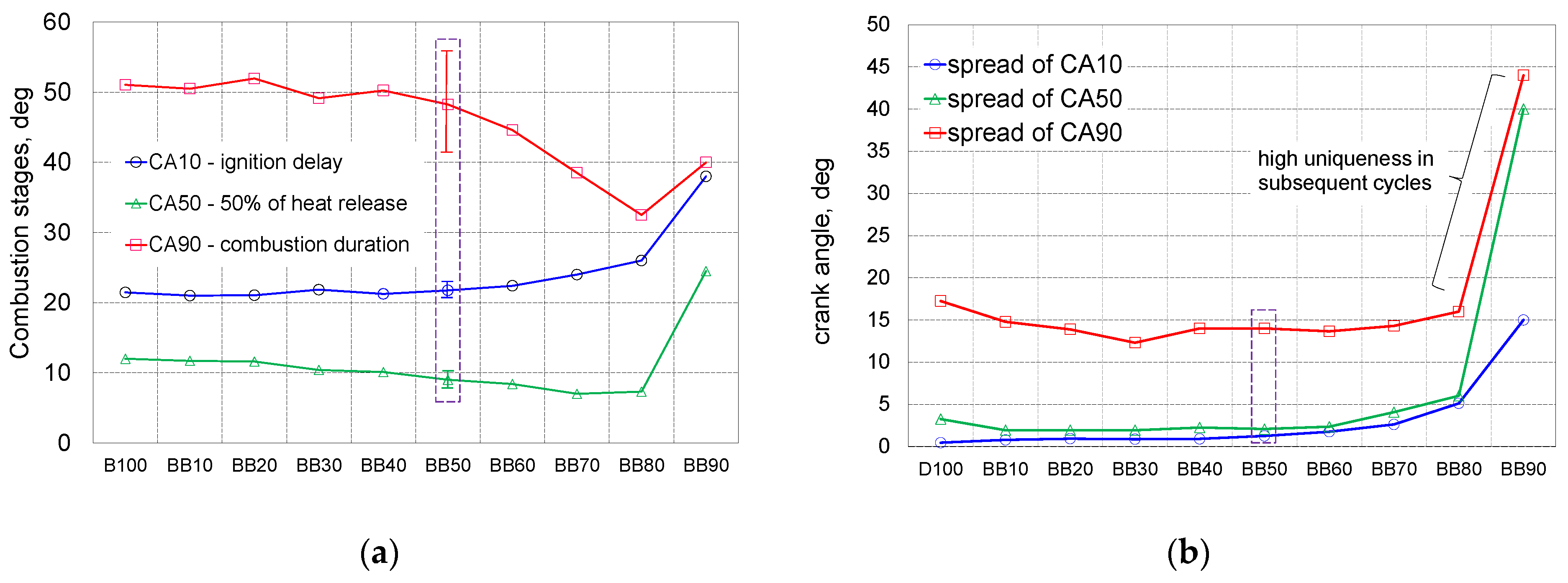
- Up to 50% of 1-butanol energetic fraction ID is at the same level equal to 21.5 degrees, exceeding this share of 1-butanol, ID increases, for the BB80, there was an increase in ID by 5 degrees compared to the reference fuel;
- Up to 40% of 1-butanol fraction CD is stable and equal to 50 degrees of CA, exceeding the 40% share of 1-Butanol, the combustion time is decreasing;
- In case of ID spread that up to 50% of 1-butanol fraction the repeatability of the combustion start was very stable and within the limits 1.5 degrees, for larger shares of alternative fuel, the uniqueness of the distribution reaches the scope of 5 degrees for BB80;
- The end of combustion is determined with great uncertainty, for BB80, the combustion duration is set equal to 33 degrees but the spread of this parameter is 8 degrees of CA.
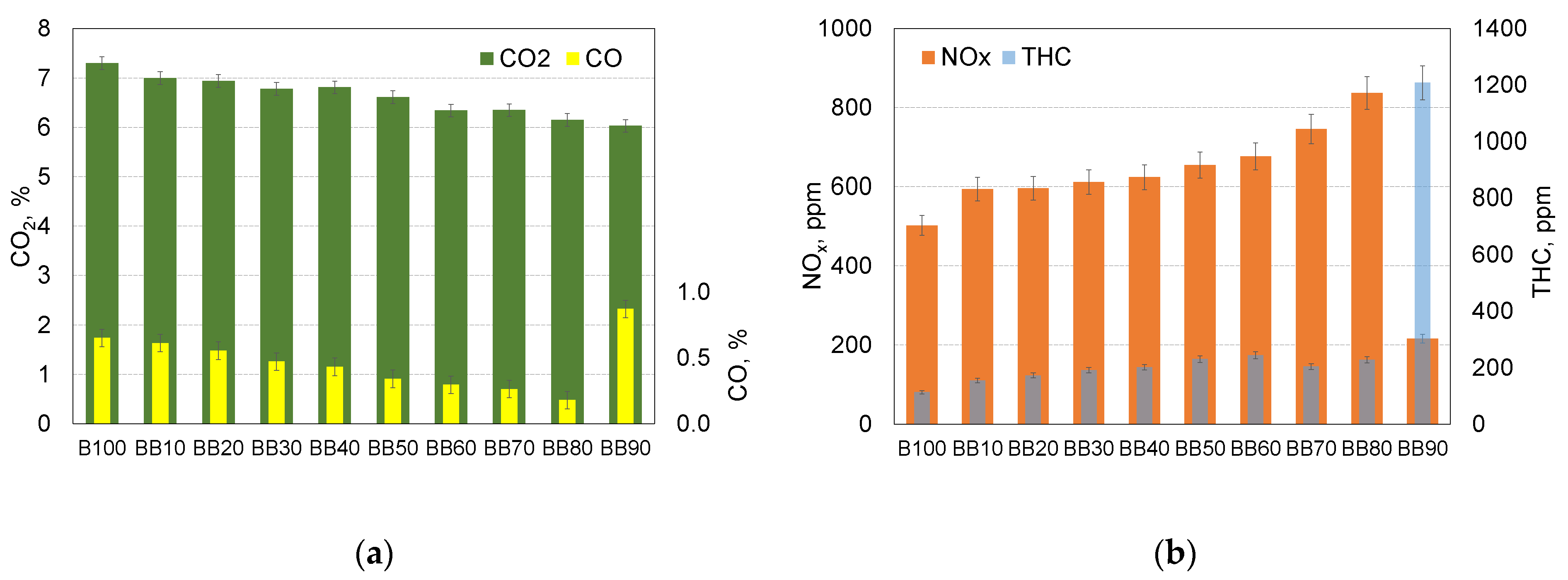
- In terms of the assessment of the emission, it was found:
- In case of BB80 the decrease in CO emission reduced to 0.178% and it was over 3.7-times lower compared to reference fuel;
- The highest THC emission was noticed for the combustion of BB60 blend and it was equal to 244 ppm. It was 100% more compared to the reference fuel;
- The highest value of NOx emission was obtained for BB80 and it was 837ppm, it was 67% more compared to the combustion of the reference fuel;
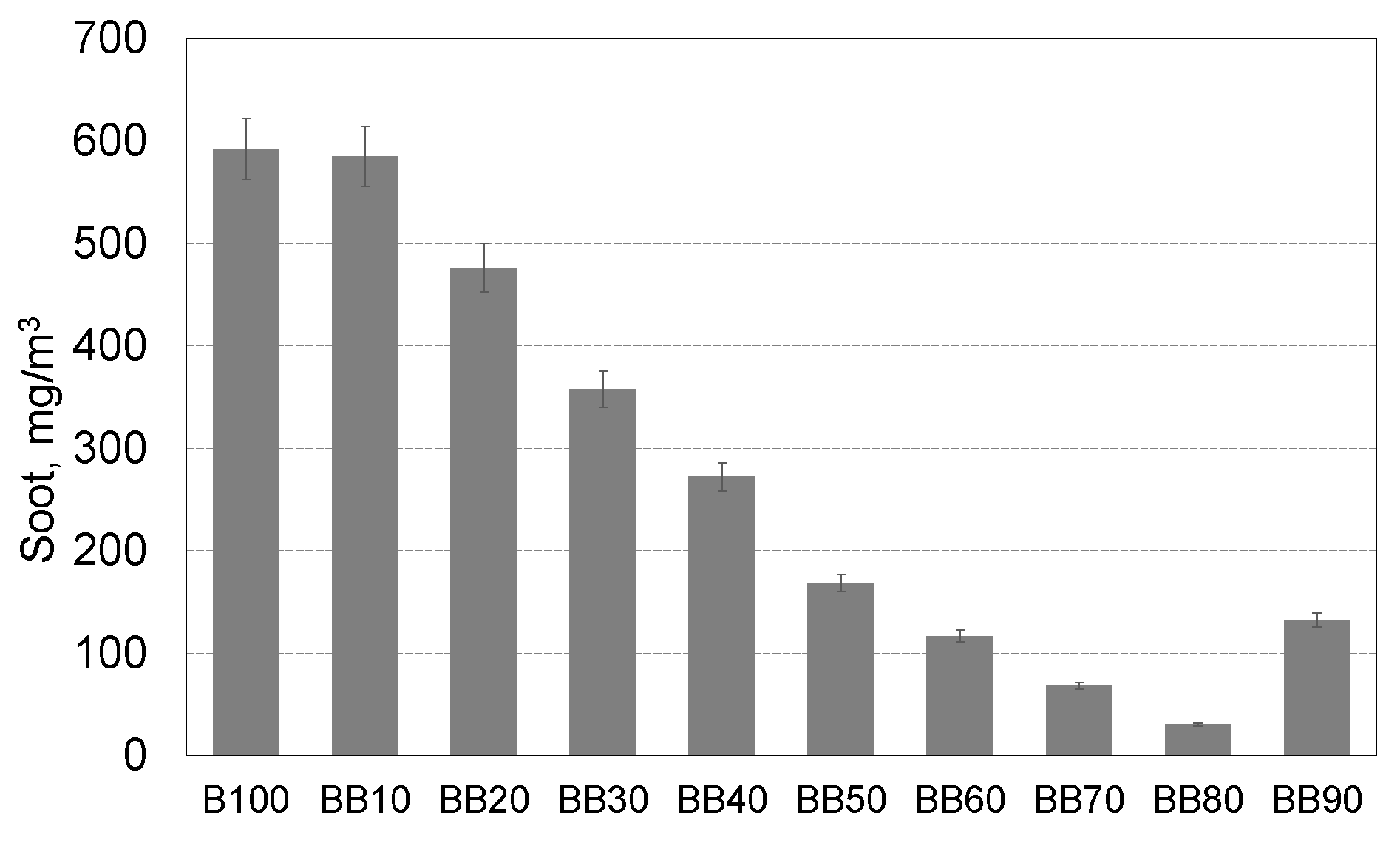
- The participation of 1-Butanol in the co-combustion process with RME has a positive effect on the soot emission, already for the 50% energetic share of 1-butanol, the soot emission was over 3.5 times reduced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeevahan, J.; Sriramanjaneyulu, G.; Durairaj, R.; Mageshwaran, G. Experimental investigation of the suitability of 1-butanol blended with biodiesel as an alternative biofuel in diesel engines. Biocatal. Agric. Biotechnol. 2018, 15, 72–77. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Pyrc, M.; Gruca, M.; Kočiško, M. Study on co-combustion of diesel fuel with oxygenated alcohols in a compression ignition dual-fuel engine. Fuel 2018, 221, 329–345. [Google Scholar] [CrossRef]
- Tutak, W.; Lukács, K.; Szwaja, S.; Bereczky, Á. Alcohol–diesel fuel combustion in the compression ignition engine. Fuel 2015, 154, 196–206. [Google Scholar] [CrossRef]
- Rimkus, A.; Žaglinskis, J.; Stravinskas, S.; Rapalis, P.; Matijošius, J.; Bereczky, Á. Research on the Combustion, Energy and Emission Parameters of Various Concentration Blends of Hydrotreated Vegetable Oil Biofuel and Diesel Fuel in a Compression-Ignition Engine. Energies 2019, 12, 2978. [Google Scholar] [CrossRef] [Green Version]
- Jamrozik, A.; Tutak, W.; Pyrc, M.; Sobiepański, M. Effect of diesel/biodiesel/ethanol blend on combustion, performance and emissions characteristics on a direct injection diesel engine. Therm. Sci. 2017, 21, 591–604. [Google Scholar] [CrossRef] [Green Version]
- Rajak, U.; Nashine, P.; Verma, T.N. Characteristics of microalgae spirulina biodiesel with the impact of n-butanol addition on a CI engine. Energy 2019, 189, 116311. [Google Scholar] [CrossRef]
- Siwale, L.; Lukacs, K.; Torok, A.; Bereczky, Á.; Mbarawa, M.; Penninger, A.; Kolesnikov, A. N-Butanol-Diesel (D2) Blend Fired in a Turbo-Charged Compression Ignition Engine: Performance and Combustion Characteristics. Improv. Trends Intern. Combust. Engines 2018, 3, 21. [Google Scholar]
- Goga, G.; Chauhan, B.S.; Mahla, S.K.; Cho, H.M. Performance and emission characteristics of diesel engine fueled with rice bran biodiesel and n-butanol. Energy Rep. 2019, 5, 78–83. [Google Scholar] [CrossRef]
- Killol, A.; Reddy, N.; Paruvada, S.; Murugan, S. Experimental studies of a diesel engine run on biodiesel n-butanol blends. Renew. Energy 2019, 135, 687–700. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Kumar, M. Impact of n-butanol as an additive with eucalyptus biodiesel-diesel blends on the performance and emission parameters of the diesel engine. Fuel 2020, 277, 118178. [Google Scholar] [CrossRef]
- Atmanli, A.; Yilmaz, N. A comparative analysis of n-butanol/diesel and 1-pentanol/diesel blends in a compression ignition engine. Fuel 2018, 234, 161–169. [Google Scholar] [CrossRef]
- Raman, L.A.; Deepanraj, B.; Rajakumar, S.; Sivasubramanian, V. Experimental investigation on performance, combustion and emission analysis of a direct injection diesel engine fuelled with rapeseed oil biodiesel. Fuel 2019, 246, 69–74. [Google Scholar] [CrossRef]
- Qi, D.; Lee, C.; Jia, C.; Wang, P.; Wu, S. Experimental investigations of combustion and emission characteristics of rapeseed oil–diesel blends in a two cylinder agricultural diesel engine. Energy Convers. Manag. 2014, 77, 227–232. [Google Scholar] [CrossRef]
- Nuortila, C.; Help, R.; Sirviö, K.; Suopanki, H.; Heikkilä, S.; Niemi, S. Selected Fuel Properties of Alcohol and Rapeseed Oil Blends. Energies 2020, 13, 3821. [Google Scholar] [CrossRef]
- Xiao, H.; Guo, F.; Li, S.; Wang, R.; Yang, X. Combustion performance and emission characteristics of a diesel engine burning biodiesel blended with n-butanol. Fuel 2019, 258, 115887. [Google Scholar] [CrossRef]
- Xiao, H.; Guo, F.; Wang, R.; Yang, X.; Li, S.; Ruan, J. Combustion performance and emission characteristics of diesel engine fueled with iso-butanol/biodiesel blends. Fuel 2020, 268, 117387. [Google Scholar] [CrossRef]
- Zhen, X.; Wang, Y.; Liu, D. Bio-butanol as a new generation of clean alternative fuel for SI (spark ignition) and CI (compression ignition) engines. Renew. Energy 2020, 147, 2494–2521. [Google Scholar] [CrossRef]
- Qi, D.; Bae, C.; Feng, Y.; Jia, C.; Bian, Y. Preparation, characterization, engine combustion and emission characteristics of rapeseed oil based hybrid fuels. Renew. Energy 2013, 60, 98–106. [Google Scholar] [CrossRef]
- Çelikten, I.; Koca, A.; Arslan, M.A. Comparison of performance and emissions of diesel fuel, rapeseed and soybean oil methyl esters injected at different pressures. Renew. Energy 2010, 35, 814–820. [Google Scholar] [CrossRef]
- Lešnik, L.; Biluš, I. The effect of rapeseed oil biodiesel fuel on combustion, performance, and the emission formation process within a heavy-duty DI diesel engine. Energy Convers. Manag. 2016, 109, 140–152. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Zhao, W.; Li, Z.; Huang, G.; Zhang, Y.; Qian, Y.; Lu, X. Experimental investigation of direct injection dual fuel of n-butanol and biodiesel on Intelligent Charge Compression Ignition (ICCI) Combustion mode. Appl. Energy 2020, 266, 114884. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Eryilmaz, T.; Arslan, M. A comparative analysis of the engine performance, exhaust emissions and combustion behaviors of a compression ignition engine fuelled with biodiesel/diesel/1-butanol (C4 alcohol) and biodiesel/diesel/n-pentanol (C5 alcohol) fuel blends. Energy 2018, 165, 1332–1351. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Gnatowska, R. Combustion of different reactivity fuel mixture in a dual fuel engine. Therm. Sci. 2018, 22, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Tutak, W.; Jamrozik, A. Comparative Analysis of Combustion Stability of Diesel/Ethanol Utilization by Blend and Dual Fuel. Processes 2019, 7, 946. [Google Scholar] [CrossRef] [Green Version]
- Jamrozik, A.; Tutak, W.; Gnatowska, R.; Nowak, Ł. Comparative analysis of the combustion stability of diesel-methanol and die-sel-ethanol in a dual fuel engine. Energies 2019, 12, 971. [Google Scholar] [CrossRef] [Green Version]
- Satsangi, D.P.; Tiwari, N. Experimental investigation on combustion, noise, vibrations, performance and emissions characteristics of diesel/n-butanol blends driven genset engine. Fuel 2018, 221, 44–60. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jakubowski, M.; Jaworski, A.; Lubas, J.; Balawender, K. Effect of temperature on tribological properties of 1-butanol–diesel fuel blends—Preliminary experimental study using the HFRR method. Fuel 2021, 296, 120700. [Google Scholar] [CrossRef]
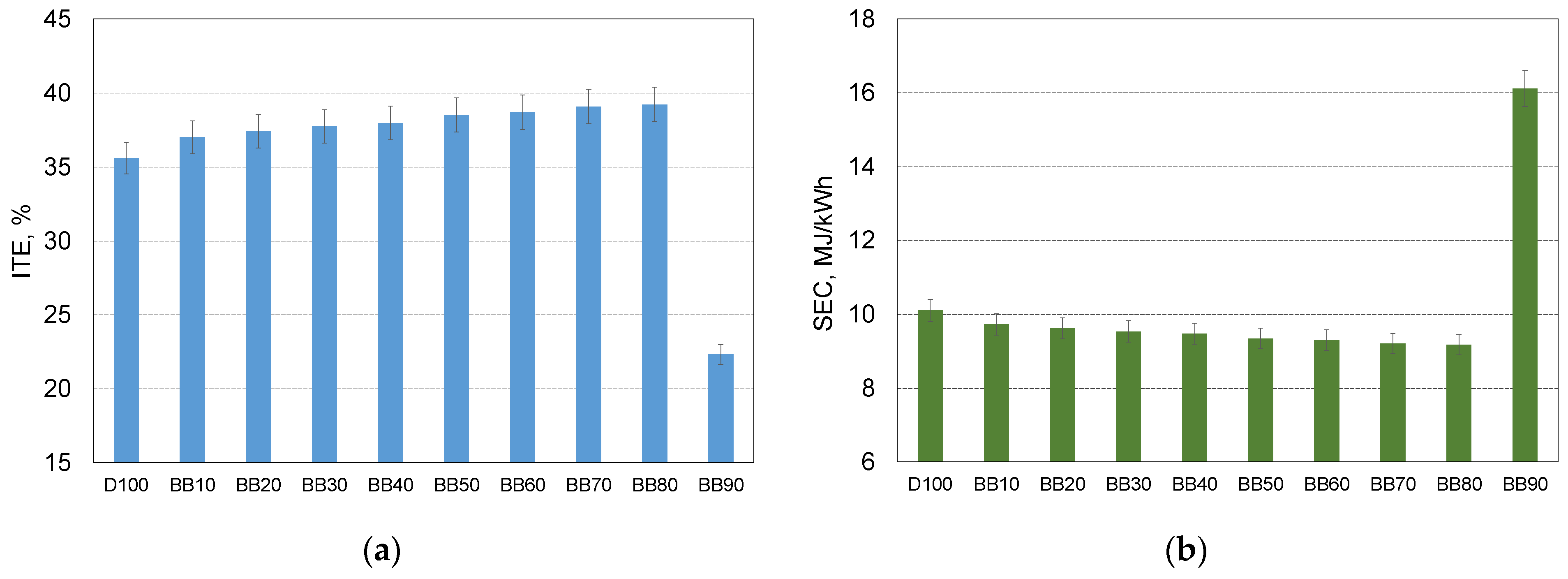
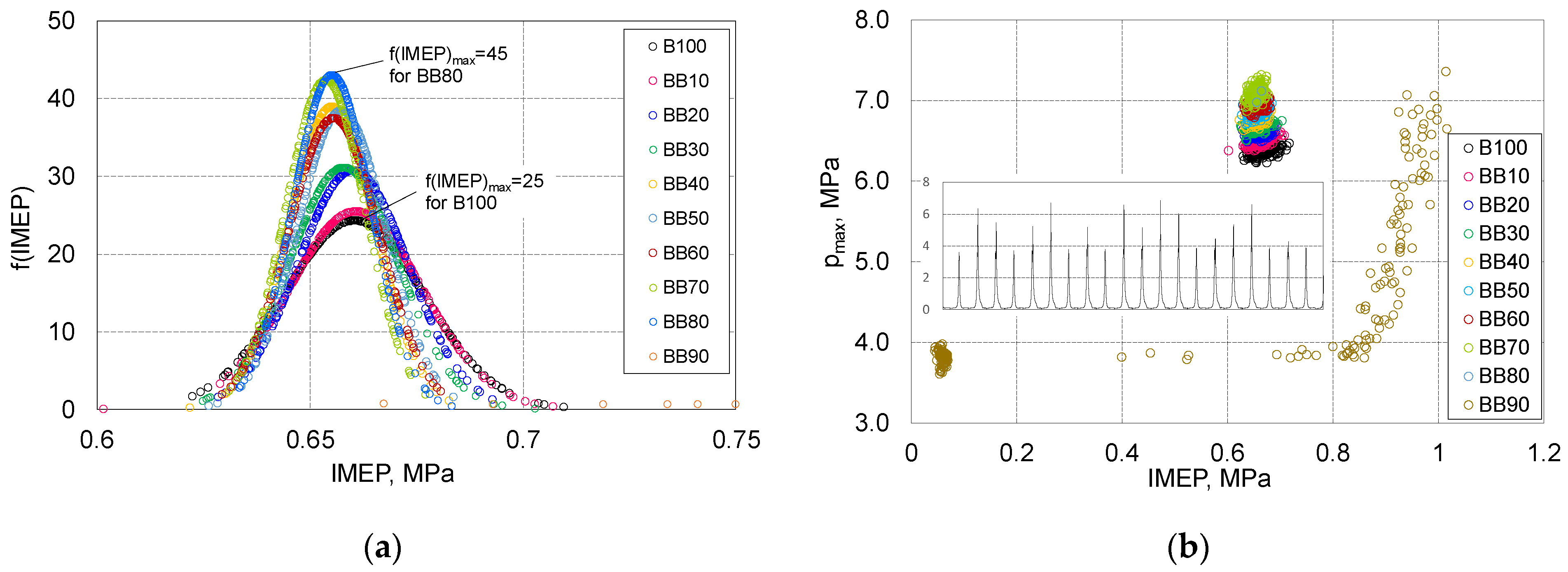
| Title 1 | Title 2 |
|---|---|
| Type of engine | 4-stroke compression ignition |
| Number of cylinders | 1 |
| Bore | 90 mm |
| Stroke | 90 mm |
| Displacement volume | 573 cm3 |
| Number of valves | 2 |
| Compression ratio | 17 |
| Engine speed | 1500 rpm |
| Fuel injection | mechanical direct injection |
| Fuel injection timing | 20 degress bTDC |
| Apparatus | Measuring Range | Resolution | Accuracy from Measured Value | Absolute Accuracy |
|---|---|---|---|---|
| CO | 0.000–10.000% vol. | 0.001% vol. | … | … |
| 0.000–5.000% vol. | 0.001% vol | ±5% | ±0.06% vol. | |
| HC | 0–9999 ppm vol. | 1 ppm vol. | … | … |
| 0–2000 ppm vol. | 1 ppm vol. | ±5% | ±12 ppm vol. | |
| CO2 | 0.00–18.00% vol. | 0.01% vol. | … | … |
| 0.00–16.00% vol. | 0.01% vol. | ±5% | ±0.5% vol. | |
| O2 | 0.00–22.00% vol. | 0.01% vol. | … | … |
| 0.00–21.00% vol. | 0.01% vol. | ±4% | ±0.1% vol. | |
| NO | 0–5000 ppm vol. | 1 ppm vol. | ±4% | ±25 ppm vol. |
| 0–4000 ppm vol. | 1 ppm vol. | ±8% | ±50 ppm vol. | |
| λ | 0.500–9.999 | 0.001 | … | … |
| 0.700–1.300 | 0.001 | ±4% | … |
| Parameter | RME | 1-Butanol |
|---|---|---|
| Chemical formula | CH3(CH2)nCOOH3 | C4H10O |
| Cetane number | 56 | 17–25 |
| Density at 1 atm and 15 °C (kg/m3) | 855 | 810 |
| Lower heating value (MJ/kg) | 37.1 | 33.2 |
| Heat of evaporation (kJ/kg) | 250 | 585 |
| Auto-ignition temperature (°C) | >101 | 343 |
| Flash point (°C) | 91–135 | 35 |
| Stoichiometric air-fuel ratio | 12.5 | 11.2 |
| Kinematic viscosity at 40 °C (mm2/s) | 4.51 | 2.63 |
| Oxygen content (wt%) | 10.8 | 21.6 |
| Properties | B100 | BB10 | BB20 | BB30 | BB40 | BB50 | BB60 | BB70 | BB80 | BB90 |
|---|---|---|---|---|---|---|---|---|---|---|
| RME, (% energy) | 100 | 90 | 80 | 70 | 60 | 50 | 40 | 30 | 20 | 10 |
| 1-Butanol, (% energy) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| LHV, (MJ/kg) | 37.1 | 36.7 | 36.3 | 35.9 | 35.5 | 35.1 | 34.7 | 34.4 | 34.0 | 33.6 |
| Heat of evap., (kJ/kg) | 250 | 283.5 | 317 | 350.5 | 384 | 417.5 | 451 | 484.5 | 518 | 551.5 |
| Oxygen content, (%) | 10.8 | 11.88 | 12.96 | 14.04 | 15.12 | 16.2 | 17.28 | 18.36 | 19.44 | 20.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutak, W.; Jamrozik, A.; Grab-Rogaliński, K. The Effect of RME-1-Butanol Blends on Combustion, Performance and Emission of a Direct Injection Diesel Engine. Energies 2021, 14, 2941. https://doi.org/10.3390/en14102941
Tutak W, Jamrozik A, Grab-Rogaliński K. The Effect of RME-1-Butanol Blends on Combustion, Performance and Emission of a Direct Injection Diesel Engine. Energies. 2021; 14(10):2941. https://doi.org/10.3390/en14102941
Chicago/Turabian StyleTutak, Wojciech, Arkadiusz Jamrozik, and Karol Grab-Rogaliński. 2021. "The Effect of RME-1-Butanol Blends on Combustion, Performance and Emission of a Direct Injection Diesel Engine" Energies 14, no. 10: 2941. https://doi.org/10.3390/en14102941






