Physicochemical Properties of Activated Carbons Produced from Coffee Waste and Empty Fruit Bunch by Chemical Activation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Preparation
2.2. Compositional Analysis of Coffee Waste and Empty Fruit Bunch
2.3. Preparation for Chemical Activation from Coffee Waste and Empty Fruit Bunch
2.4. Thermal Stability and Van Krevelen Diagram of Produced Activated Carbons
2.5. Surface Features of Produced Activated Carbons
3. Results and Discussion
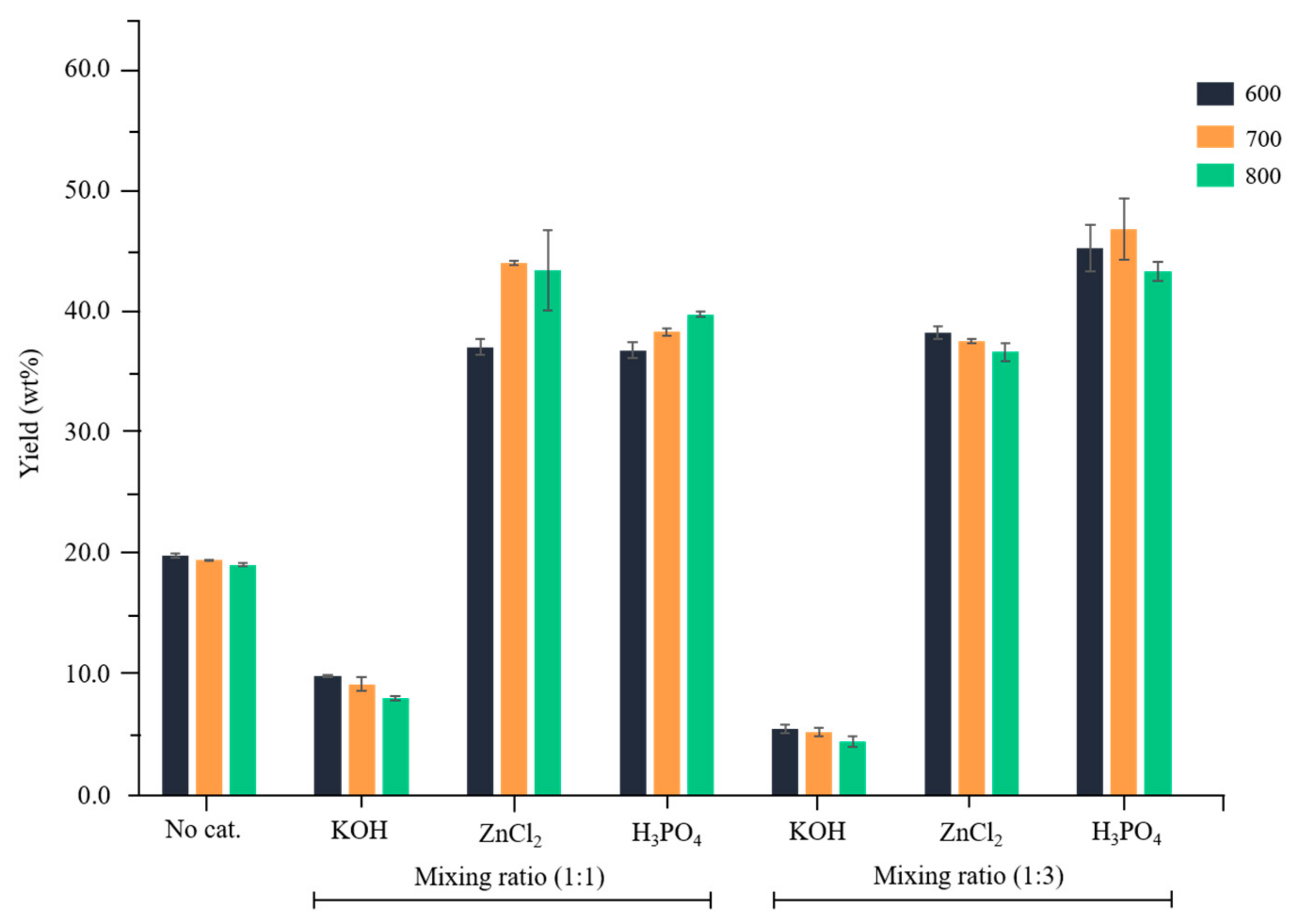
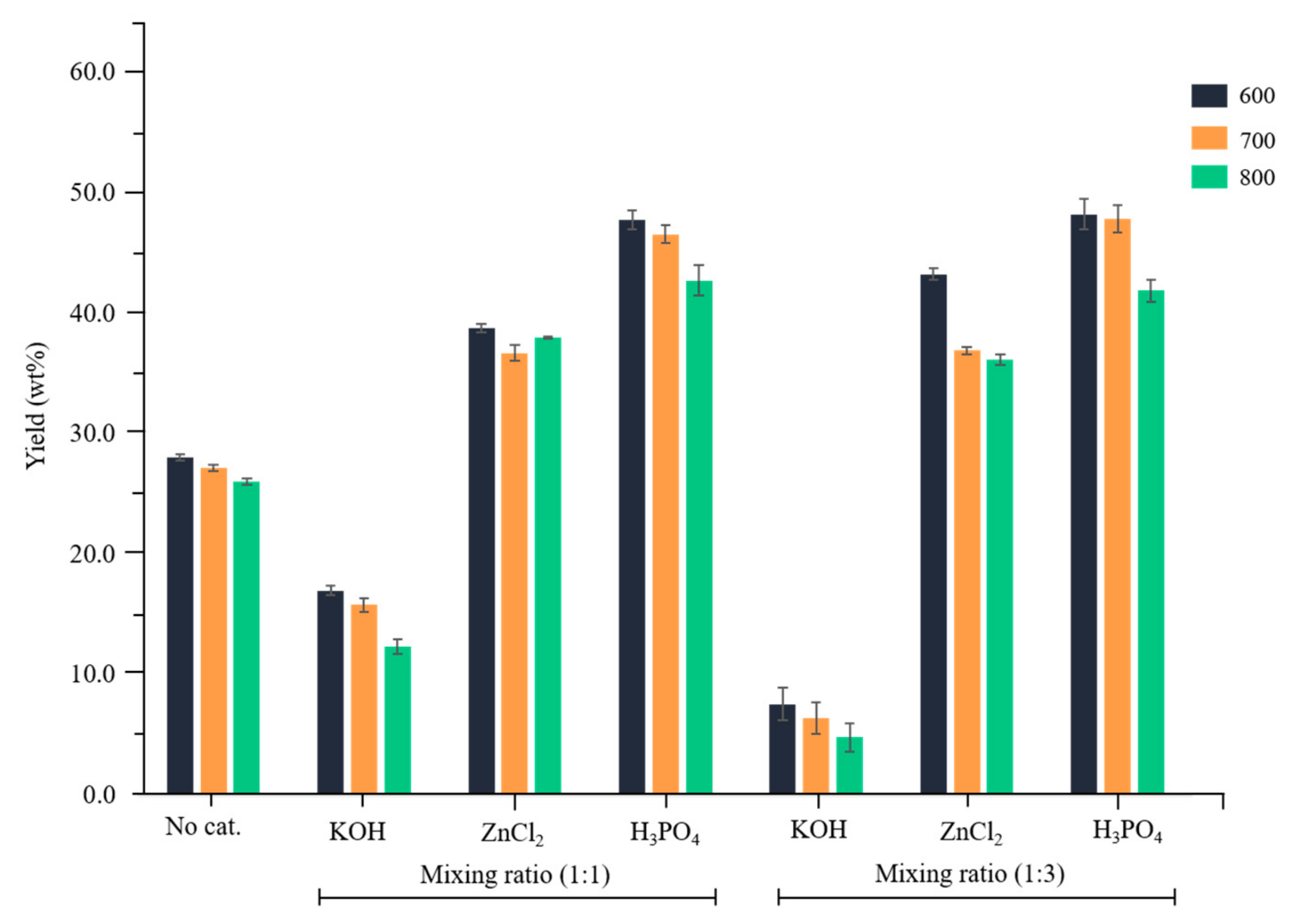
3.1. Yields of Activated Carbon Prepared by Chemical Activation
3.2. Elemental Analysis of Produced Activated Carbons
3.3. Van Krevelen Diagram
3.4. Thermal Degradation Behavior of Activated Carbons
3.5. Surface Morphology of Produced Activated Carbons
3.6. Determination of Surface Area and Pore Volume of Activated Carbons
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernandes, A.S.; Mello, F.V.C.; Thode Filho, S.; Carpes, R.M.; Honório, J.G.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E.R.A. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Blinová, L.; Sirotiak, M.; Bartošová, A.; Soldán, M. Faculty of Materials Science and Technology in Trnava Review: Utilization of Waste From Coffee Production. Res. Pap. 2017, 25, 91–102. [Google Scholar]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Palamae, S.; Dechatiwongse, P.; Choorit, W.; Chisti, Y.; Prasertsan, P. Cellulose and hemicellulose recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carbohydr. Polym. 2017, 155, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Supian, M.A.F.; Amin, K.N.M.; Jamari, S.S.; Mohamad, S. Production of cellulose nanofiber (CNF) from empty fruit bunch (EFB) via mechanical method. J. Environ. Chem. Eng. 2020, 8, 103024. [Google Scholar] [CrossRef]
- Sumathi, S.; Chai, S.P.; Mohamed, A.R. Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2008, 12, 2404–2421. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, X.; Zeng, J.; Loh, S.K.; Choo, Y.M.; Liu, D. Robust enzymatic hydrolysis of Formiline-pretreated oil palm empty fruit bunches (EFB) for efficient conversion of polysaccharide to sugars and ethanol. Bioresour. Technol. 2014, 166, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Cao, Q.; Xie, K.C.; Lv, Y.K.; Bao, W.R. Process effects on activated carbon with large specific surface area from corn cob. Bioresour. Technol. 2006, 97, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Yuliusman; Al Farouq, F.; Sipangkar, S.P.; Fatkhurrahman, M.; Putri, S.A. Preparation and characterization of activated carbon from corn stalks by chemical activation with KOH and NaOH. AIP Conf. Proc. 2020, 2255. [Google Scholar] [CrossRef]
- Oh, G.H.; Park, C.R. Preparation and characteristics of rice-straw-based porous carbons with high adsorption capacity. Fuel 2002, 81, 327–336. [Google Scholar] [CrossRef]
- Mak, S.M.; Tey, B.T.; Cheah, K.Y.; Siew, W.L.; Tan, K.K. Porosity characteristics and pore developments of various particle sizes palm kernel shells activated carbon (PKSAC) and its potential applications. Adsorption 2009, 15, 507–519. [Google Scholar] [CrossRef]
- Gonçalves, M.; Guerreiro, M.C.; Oliveira, L.C.A.; Solar, C.; Nazarro, M.; Sapag, K. Micro mesoporous activated carbon from coffee husk as biomass waste for environmental applications. Waste Biomass Valorization 2013, 4, 395–400. [Google Scholar] [CrossRef]
- Rajeshwarisivaraj; Sivakumar, S.; Senthilkumar, P.; Subburam, V. Carbon from Cassava peel, an agricultural waste, as an adsorbent in the removal of dyes and metal ions from aqueous solution. Bioresour. Technol. 2001, 80, 233–235. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crops Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Chandra, T.C.; Mirna, M.M.; Sunarso, J.; Sudaryanto, Y.; Ismadji, S. Activated carbon from durian shell: Preparation and characterization. J. Taiwan Inst. Chem. Eng. 2009, 40, 457–462. [Google Scholar] [CrossRef]
- Uçar, S.; Erdem, M.; Tay, T.; Karagöz, S. Preparation and characterization of activated carbon produced from pomegranate seeds by ZnCl 2 activation. Appl. Surf. Sci. 2009, 255, 8890–8896. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Klein, M.; Kardaś, D.; Łuczak, J. Activated carbon produced by pyrolysis of waste wood and straw for potential wastewater adsorption. Materials 2020, 13, 2047. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Pirajan, J.C. Synthesis of activated carbon mesoporous from coffee waste and its application in adsorption zinc and mercury ions from aqueous solution. E J. Chem. 2012, 9, 938–948. [Google Scholar] [CrossRef]
- Hwang, H.; Sahin, O.; Choi, J.W. Manufacturing a super-active carbon using fast pyrolysis char from biomass and correlation study on structural features and phenol adsorption. RSC Adv. 2017, 7, 42192–42202. [Google Scholar] [CrossRef]
- Örkün, Y.; Karatepe, N.; Yavuz, R. Influence of temperature and impregnation ratio of H3PO 4 on the production of activated carbon from hazelnut shell. Acta Phys. Pol. A 2012, 121, 277–280. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Castello, D.; Fiori, L. Granular activated carbon from grape seeds hydrothermal char. Appl. Sci. 2018, 8, 331. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, H.; Choi, J.W. Effects of transition metals on hydrothermal liquefaction of empty fruit bunches (EFB) for conversion to biofuel and valuable chemicals. Energy 2018, 162, 1–9. [Google Scholar] [CrossRef]
- Hwang, H.; Oh, S.; Choi, I.G.; Choi, J.W. Catalytic effects of magnesium on the characteristics of fast pyrolysis products-Bio-oil, bio-char, and non-condensed pyrolytic gas fractions. J. Anal. Appl. Pyrolysis 2015, 113, 27–34. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis. Lab. Anal. Proced. 2008, 1617, 65–71. [Google Scholar]
- Thithai, V.; Choi, J.W. Physicochemical Properties of Activated Carbon Produced From Corn Stover by Chemical Activation Under Various Catalysts and Temperatures. For. Bioenergy 2020, 30, 8–16. [Google Scholar] [CrossRef]
- Caturla, F.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Preparation of activated carbon by chemical activation with ZnCl2. Carbon 1991, 29, 999–1007. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chem. Eng. J. 2013, 217, 345–353. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.Y.; Cho, T.S.; Choi, J.W. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.P.; Choi, H.S.; Choi, J.W.; Choi, Y.S. Fast pyrolysis of Miscanthus sinensis in fluidized bed reactors: Characteristics of product yields and biocrude oil quality. Energy 2013, 60, 44–52. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. High surface area microporous activated carbons prepared from Fox nut (Euryale ferox) shell by zinc chloride activation. Appl. Surf. Sci. 2015, 356, 753–761. [Google Scholar] [CrossRef]
- Okman, I.; Karagöz, S.; Tay, T.; Erdem, M. Activated carbons from grape seeds by chemical activation with potassium carbonate and potassium hydroxide. Appl. Surf. Sci. 2014, 293, 138–142. [Google Scholar] [CrossRef]
- Mohanty, K.; Das, D.; Biswas, M.N. Preparation and characterization of activated carbons from Sterculia alata nutshell by chemical activation with zinc chloride to remove phenol from wastewater. Adsorption 2006, 12, 119–132. [Google Scholar] [CrossRef]
- Varil, T.; Bergna, D.; Lahti, R.; Romar, H.; Hu, T.; Lassi, U. Activated carbon production from peat using ZnCl2: Characterization and applications. BioResources 2017, 12, 8078–8092. [Google Scholar] [CrossRef]
- Sych, N.V.; Trofymenko, S.I.; Poddubnaya, O.I.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Porous structure and surface chemistry of phosphoric acid activated carbon from corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Guo, Y.; Rockstraw, D.A. Activated carbons prepared from rice hull by one-step phosphoric acid activation. Microporous Mesoporous Mater. 2007, 100, 12–19. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H 3 PO 4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D.D. The preparation of activated carbon from macadamia nutshell by chemical activation. Carbon 1997, 35, 1723–1732. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Sathishkumar, K. Removal of methylene blue dye from aqueous solution by activated carbon prepared from cashew nut shell as a new low-cost adsorbent. Korean J. Chem. Eng. 2011, 28, 149–155. [Google Scholar] [CrossRef]




| Elemental Analysis (wt%) | Coffee Waste | Empty Fruit Bunch |
|---|---|---|
| Carbon | 51.5 | 45.8 |
| Hydrogen | 8.1 | 6.6 |
| Nitrogen | 2.0 | 0.6 |
| Sulfur | 0.5 | 0.5 |
| Oxygen a | 38.0 | 46.6 |
| Component analysis (wt%) b | ||
| Holocellulose | 44.5 | 73.1 |
| Lignin | 19.5 | 22.1 |
| Extractive | 18.9 | 3.0 |
| Ash | 1.3 | 5.4 |
| Proximate analysis (wt%) | ||
| Volatile matter | 83.6 | 74.2 |
| Fixed carbon | 16.3 | 25.7 |
| Inorganic Compound Analysis (mg L−1) | Coffee Waste | Empty Fruit Bunch |
|---|---|---|
| Aluminum | 0.387 | 10.364 |
| Calcium | 13.405 | 21.710 |
| Iron | 0.682 | 18.266 |
| Magnesium | 16.661 | 8.006 |
| Potassium | 4.859 | 7.744 |
| Copper | 5.927 | 4.690 |
| Silicon | 1.940 | 23.483 |
| Sample | Properties (wt%) | Temp (°C) | Activating Agents and Mixing Ratios | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No Act a | KOH | ZnCl2 | H3PO4 | ||||||
| 1:0 | 1:1 | 1:3 | 1:1 | 1:3 | 1:1 | 1:3 | |||
| 600 | 78.4 | 72.0 | 78.1 | 79.1 | 85.9 | 70.4 | 55.7 | ||
| Carbon | 700 | 79.1 | 79.4 | 88.3 | 73.2 | 85.3 | 63.3 | 49.1 | |
| 800 | 79.8 | 85.6 | 92.7 | 70.7 | 82.1 | 59.8 | 46.3 | ||
| 600 | 1.8 | 0.9 | 1.1 | 1.5 | 1.4 | 2.0 | 1.9 | ||
| CWAC | Hydrogen | 700 | 1.1 | 0.4 | 0.4 | 0.9 | 1.1 | 1.7 | 1.5 |
| 800 | 0.7 | 0.3 | 0.2 | 0.7 | 0.8 | 1.5 | 1.3 | ||
| 600 | 3.5 | 2.1 | 0.3 | 3.1 | 2.8 | 2.5 | 2.3 | ||
| Nitrogen | 700 | 3.6 | 0.8 | 0.2 | 3.1 | 3.4 | 2.8 | 2.7 | |
| 800 | 3.5 | 0.4 | 0.1 | 2.8 | 3.4 | 2.7 | 2.5 | ||
| 600 | 16.3 | 25.0 | 20.5 | 16.4 | 9.8 | 25.1 | 40.1 | ||
| Oxygen b | 700 | 16.3 | 19.5 | 11.0 | 22.8 | 10.2 | 32.2 | 46.7 | |
| 800 | 15.9 | 13.7 | 7.0 | 25.8 | 13.7 | 36.0 | 50.0 | ||
| 600 | 68.5 | 67.0 | 66.3 | 78.1 | 77.6 | 62.3 | 63.0 | ||
| Carbon | 700 | 74.1 | 72.8 | 73.0 | 75.9 | 85.8 | 55.5 | 51.7 | |
| 800 | 73.3 | 74.0 | 79.1 | 76.3 | 84.3 | 55.1 | 54.7 | ||
| 600 | 0.8 | 0.9 | 1.2 | 1.2 | 1.4 | 1.4 | |||
| EFBAC | Hydrogen | 700 | 0.9 | 0.4 | 0.5 | 0.8 | 0.9 | 1.1 | 1.1 |
| 800 | 0.5 | 0.3 | 0.3 | 0.6 | 0.6 | 1.0 | 0.8 | ||
| 600 | 0.8 | 0.7 | 0.4 | 1.1 | 0.9 | 0.8 | 0.5 | ||
| Nitrogen | 700 | 0.7 | 0.5 | 0.3 | 1.0 | 1.2 | 0.8 | 0.7 | |
| 800 | 0.8 | 0.5 | 0.3 | 1.1 | 1.2 | 0.8 | 0.9 | ||
| 600 | 29.3 | 31.5 | 32.4 | 19.7 | 20.4 | 35.5 | 35.1 | ||
| Oxygen b | 700 | 24.2 | 26.3 | 26.1 | 22.2 | 12.1 | 42.6 | 46.5 | |
| 800 | 25.3 | 25.2 | 20.2 | 22.1 | 14.0 | 43.1 | 43.6 | ||
| Sample | Properties (wt%) | Temp (°C) | Activating Agents and Mixing Ratios | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No Act * | KOH | ZnCl2 | H3PO4 | ||||||
| 1:0 | 1:1 | 1:3 | 1:1 | 1:3 | 1:1 | 1:3 | |||
| CWAC | 600 | 16.9 | 29.3 | 26.6 | 19.6 | 20.1 | 25.0 | 29.9 | |
| Volatile matter | 700 | 12.1 | 23.7 | 42.5 | 27.2 | 20.3 | 19.5 | 22.2 | |
| 800 | 14.4 | 16.4 | 30.7 | 27.3 | 20.0 | 20.2 | 22.4 | ||
| 600 | 83.1 | 70.7 | 73.4 | 80.4 | 79.9 | 75.0 | 70.1 | ||
| Fixed carbon | 700 | 87.9 | 76.2 | 57.5 | 72.8 | 79.8 | 80.5 | 77.8 | |
| 800 | 85.6 | 83.5 | 63.3 | 72.7 | 79.9 | 79.8 | 77.6 | ||
| EFBAC | 600 | 16.6 | 25.0 | 30.6 | 14.4 | 17.4 | 16.0 | 16.3 | |
| Volatile matter | 700 | 13.0 | 16.9 | 24.7 | 13.5 | 9.0 | 14.0 | 13.7 | |
| 800 | 11.2 | 17.7 | 23.0 | 13.9 | 9.4 | 13.0 | 11.0 | ||
| 600 | 83.4 | 75.0 | 69.4 | 85.6 | 82.6 | 84.0 | 83.7 | ||
| Fixed carbon | 700 | 87.0 | 83.1 | 75.2 | 86.5 | 91.0 | 86.0 | 86.3 | |
| 800 | 88.8 | 82.2 | 77.0 | 86.1 | 90.6 | 87.0 | 89.0 | ||
| Sample | Temperature (°C) | Ratio (w/w) | SBET a (m2/g) | Vtotal b (cm3/g) | Vmicro c (cm3/g) | Vmeso d (cm3/g) | Davg e (nm) |
|---|---|---|---|---|---|---|---|
| CWAC | 600 | 1:0 | 0.00 | 0.00 | 0.00 | 0.00 | 1.33 |
| 700 | 1:0 | 0.40 | 0.00 | 0.00 | 0.00 | 1.98 | |
| 800 | 1:0 | 1.07 | 0.00 | 0.00 | 0.00 | 10.98 | |
| CWACK | 600 | 1:1 | 1162 | 0.47 | 0.45 | 0.04 | 1.62 |
| 700 | 1:1 | 1642 | 0.67 | 0.63 | 0.06 | 1.63 | |
| 800 | 1:1 | 1912 | 0.82 | 0.77 | 0.12 | 1.72 | |
| CWACZ | 600 | 1:1 | 1060 | 0.66 | 0.44 | 0.32 | 2.49 |
| 700 | 1:1 | 990 | 0.61 | 0.41 | 0.29 | 2.47 | |
| 800 | 1:1 | 851 | 0.53 | 0.35 | 0.26 | 2.50 | |
| CWACP | 600 | 1:1 | 554 | 0.34 | 0.24 | 0.16 | 2.44 |
| 700 | 1:1 | 320 | 0.22 | 0.13 | 0.12 | 2.70 | |
| 800 | 1:1 | 381 | 0.23 | 0.13 | 0.10 | 2.37 | |
| CWACK | 600 | 1:3 | 2218 | 1.19 | 1.04 | 0.52 | 2.15 |
| 700 | 1:3 | 2934 | 1.94 | 1.64 | 1.31 | 2.65 | |
| 800 | 1:3 | 3068 | 2.86 | 1.33 | 2.46 | 3.73 | |
| CWACZ | 600 | 1:3 | 1074 | 1.55 | 0.25 | 1.31 | 5.77 |
| 700 | 1:3 | 986 | 1.32 | 0.24 | 1.10 | 5.36 | |
| 800 | 1:3 | 939 | 1.23 | 0.24 | 1.00 | 5.22 | |
| CWACP | 600 | 1:3 | 524 | 0.52 | 0.17 | 0.38 | 3.97 |
| 700 | 1:3 | 346 | 0.37 | 0.11 | 0.29 | 4.32 | |
| 800 | 1:3 | 372 | 0.35 | 0.13 | 0.24 | 3.74 |
| Sample | Temperature (°C) | Ratio (w/w) | SBET a (m2/g) | Vtotal b (cm3/g) | Vmicro c (cm3/g) | Vmeso d (cm3/g) | Davg e (nm) |
|---|---|---|---|---|---|---|---|
| EFBAC | 600 | 1:0 | 59.69 | 0.04 | 0.00 | 0.00 | 2.72 |
| 700 | 1:0 | 71.59 | 0.02 | 0.00 | 0.00 | 1.37 | |
| 800 | 1:0 | 38.37 | 0.01 | 0.00 | 0.00 | 1.20 | |
| EFBACK | 600 | 1:1 | 720 | 0.34 | 0.29 | 0.05 | 1.88 |
| 700 | 1:1 | 948 | 0.43 | 0.36 | 0.07 | 1.80 | |
| 800 | 1:1 | 1214 | 0.63 | 0.48 | 0.19 | 2.08 | |
| EFBACZ | 600 | 1:1 | 1083 | 0.66 | 0.55 | 0.32 | 2.42 |
| 700 | 1:1 | 947 | 0.57 | 0.44 | 0.28 | 2.41 | |
| 800 | 1:1 | 945 | 0.55 | 0.45 | 0.25 | 2.32 | |
| EFBACP | 600 | 1:1 | 651 | 0.48 | 0.31 | 0.30 | 2.94 |
| 700 | 1:1 | 531 | 0.40 | 0.24 | 0.25 | 3.01 | |
| 800 | 1:1 | 646 | 0.44 | 0.31 | 0.24 | 2.70 | |
| EFBACK | 600 | 1:3 | 1229 | 0.57 | 0.52 | 0.12 | 1.85 |
| 700 | 1:3 | 1610 | 0.80 | 0.75 | 0.23 | 1.99 | |
| 800 | 1:3 | 2147 | 1.31 | 1.17 | 0.70 | 2.44 | |
| EFBACZ | 600 | 1:3 | 1278 | 1.27 | 0.60 | 0.99 | 3.98 |
| 700 | 1:3 | 1261 | 1.07 | 0.65 | 0.75 | 3.40 | |
| 800 | 1:3 | 1219 | 1.05 | 0.57 | 0.76 | 3.45 | |
| EFBACP | 600 | 1:3 | 932 | 1.20 | 0.11 | 1.05 | 5.14 |
| 700 | 1:3 | 626 | 0.63 | 0.20 | 0.50 | 4.02 | |
| 800 | 1:3 | 753 | 0.63 | 0.27 | 0.45 | 3.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thithai, V.; Jin, X.; Ajaz Ahmed, M.; Choi, J.-W. Physicochemical Properties of Activated Carbons Produced from Coffee Waste and Empty Fruit Bunch by Chemical Activation Method. Energies 2021, 14, 3002. https://doi.org/10.3390/en14113002
Thithai V, Jin X, Ajaz Ahmed M, Choi J-W. Physicochemical Properties of Activated Carbons Produced from Coffee Waste and Empty Fruit Bunch by Chemical Activation Method. Energies. 2021; 14(11):3002. https://doi.org/10.3390/en14113002
Chicago/Turabian StyleThithai, Vilaysit, Xuanjun Jin, Muhammed Ajaz Ahmed, and Joon-Weon Choi. 2021. "Physicochemical Properties of Activated Carbons Produced from Coffee Waste and Empty Fruit Bunch by Chemical Activation Method" Energies 14, no. 11: 3002. https://doi.org/10.3390/en14113002
APA StyleThithai, V., Jin, X., Ajaz Ahmed, M., & Choi, J.-W. (2021). Physicochemical Properties of Activated Carbons Produced from Coffee Waste and Empty Fruit Bunch by Chemical Activation Method. Energies, 14(11), 3002. https://doi.org/10.3390/en14113002






