Review on the Safe Use of Ammonia Fuel Cells in the Maritime Industry
Abstract
:1. Introduction
1.1. Emissions
1.2. Regulations
1.3. Fuel Cells
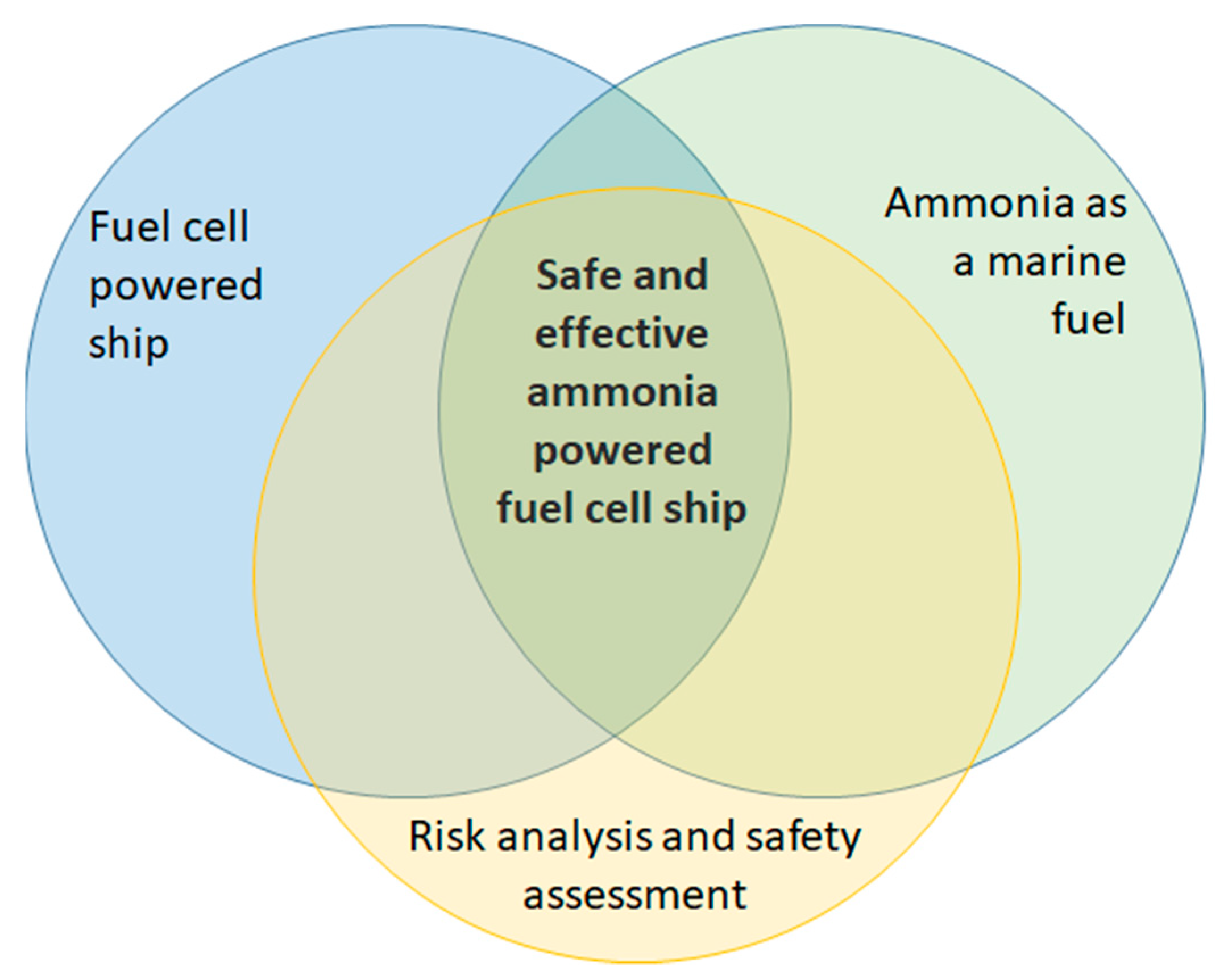
1.4. Scope
2. Literature Review
2.1. Alternative Fuels
2.2. Fuel Cells
2.2.1. Ammonia Fuel Cells
| Source | Fuels | Power Plant | Technology | |
|---|---|---|---|---|
| [67,69] | Ammonia | Land-based | SOFC | |
| [70] | Ammonia | Vehicle | Direct FC | |
| [71] | Ammonia | Railway | Molten alkaline fuel cell | |
| [36,74] | Ammonia | Hydrogen | Land-based | SOFC |
| [77] | Ammonia | Biomethane | Land-based | SOFC |
| [75] | Ammonia | Hydrogen | Alkaline fuel cells | |
| [72] | Ammonia | Biogas | Land-based | SOFC |
| [76] | Ammonia | vehicle | SOFC | |
| [32] | Ammonia | HFO | Container ship | SOFC |
| PEMFC | ||||
| Diesel engine | ||||
| Diesel electric | ||||
| [73] | Ammonia | hydrogen | Land-based | SOFC |
| [48] | Ammonia | hydrogen | Land-based | SOFC |
2.2.2. Future Perspectives and Challenges of Ammonia Fuel Cells
2.3. Safety and Reliability
2.3.1. Risk Assessment Methods
2.3.2. Ammonia Fuel Cells Safety in Shipping
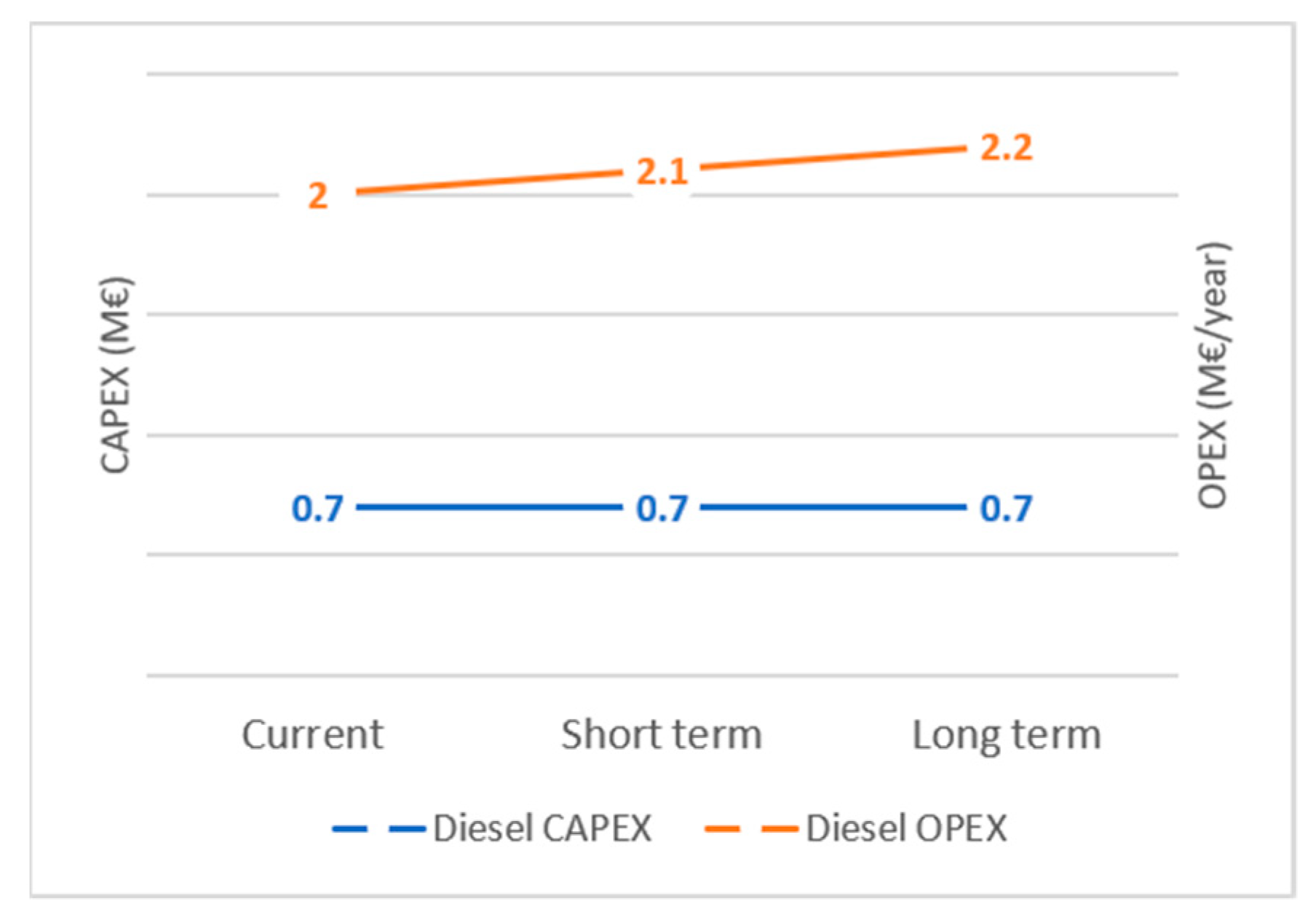
3. Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbasi, R.; Setzler, B.P.; Wang, J.; Zhao, Y.; Wang, T.; Gottesfeld, S.; Yan, Y. Low-temperature direct ammonia fuel cells: Recent developments and remaining challenges. Curr. Opin. Electrochem. 2020, 21, 335–344. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- ABS. Setting the Course to Low Carbon Shipping. 2019. Available online: https://absinfo.eagle.org/acton/attachment/16130/f-982b1623-4d26-4b04-91c5-25453a6e2fba/1/-/-/-/-/low-carbon-shipping-outlook.pdf?utm_term=DOWNLOAD&utm_campaign=2019LowCarbonShippingOutlook&utm_content=email&utm_source=Act-On+Software&utm_medium=email (accessed on 15 April 2021).
- Ahn, J.; Park, S.H.; Noh, Y.; Choi, B.I.; Ryu, J.; Chang, D.; Brendstrup, K.L.M. Performance and availability of a marine generator-solid oxide fuel cell-gas turbine hybrid system in a very large ethane carrier. J. Power Sources 2018, 399, 199–206. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.M.; Dincer, I. A novel ammonia molten alkaline fuel cell based integrated powering system for clean rail transportation. Energy 2020, 201, 117620. [Google Scholar] [CrossRef]
- Alkaner, S.; Zhou, P. A comparative study on life cycle analysis of molten carbon fuel cells and diesel engines for marine application. J. Power Sources 2006, 158, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.; Bows, A. Executing a Scharnow turn: Reconciling shipping emissions with international commitments on climate change. Carbon Manag. 2012, 3, 615–628. [Google Scholar] [CrossRef]
- Anderson, M.K. Ammonia safety, a global perspective. In Proceedings of the Technion Ammonia Conference, 29 October–3 November 2017; Available online: http://chemeng.technion.ac.il/wp-content/uploads/2017/11/M.-Kent-Anderson.pdf (accessed on 15 April 2021).
- Anjana, N.S.; Amarnath, A.; Harindranathan Nair, M.V. Toxic hazards of ammonia release and population vulnerability assessment using geographical information system. J. Environ. Manag. 2018, 210, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, D.; Xydis, G. A literature review on hydrogen refuelling stations and infrastructure. Current status and future prospects. Renew. Sustain. Energy Rev. 2019, 113, 109292. [Google Scholar] [CrossRef]
- Aziz, M.; TriWijayanta, A.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Baldi, F.; Azzi, A.; Maréchal, F. From renewable energy to ship fuel: Ammonia as an energy vector and mean for energy storage. Comput. Aided Chem. Eng. 2019, 46, 1747–1752. [Google Scholar] [CrossRef]
- Baldi, F.; Moret, S.; Tammi, K.; Maréchal, F. The role of solid oxide fuel cells in future ship energy systems. Energy 2020, 194, 116811. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I. Energy and exergy analyses of a combined ammonia-fed solid oxide fuel cell system for vehicular applications. Int. J. Hydrog. Energy 2011, 36, 11128–11136. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Cinti, G. Operation of a solid oxide fuel cell based power system with ammonia as a fuel: Experimental test and system design. Energies 2020, 13, 6173. [Google Scholar] [CrossRef]
- Ben-Daya, M.; Knezevic, J.; Raouf, A.; Ait-Kadi, D. Handbook of Maintenance Management and Engineering, 1st ed.; Ben-Daya, M., Knezevic, J., Raouf, A., Ait-Kadi, D., Eds.; Springer: Heidelberg, Germany, 2009; Volume 1. [Google Scholar] [CrossRef]
- Bertsche, B. Reliability in Automotive and Mechanical Engineering, 1st ed.; Springer: Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Environmental impact categories of hydrogen and ammonia driven transoceanic maritime vehicles: A comparative evaluation. Int. J. Hydrog. Energy 2018, 43, 4583–4596. [Google Scholar] [CrossRef]
- Van Biert, L. Solid Oxide Fuel Cells for Ships: System Integration Concepts with Reforming Thermal Cycles. Ph.D. Thesis, TU Delft University, Delft, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Borunda, M.; Jaramillo, O.A.; Reyes, A.; Ibargüengoytia, P.H. Bayesian networks in renewable energy systems: A bibliographical survey. Renew. Sustain. Energy Rev. 2016, 62, 32–45. [Google Scholar] [CrossRef]
- Bourne, C.; Nietsch, T.; Griffiths, D.; Morley, J. Applications of Fuel Cells in Surface Ships; DTI Sustainable Energy Programmes. 2001. Available online: https://www.osti.gov/etdeweb/biblio/20249928 (accessed on 12 April 2021).
- Brinks, H. Ammonia as a marine fuel. In Proceedings of the Alternative Fuels Online Conference, Online, 15 October 2020. [Google Scholar]
- Budi Santosa, H.; Kasim, F.; Yudha Prasetyo, E.; Yahya Ayash, M. Mitigating accidental ammonia (NH3) release from storage tank based on risk distribution using ALOHA and fault tree analysis in PT. AIP Conf. Proc. 2020, 2223, 40006. [Google Scholar] [CrossRef]
- Cai, B.; Kong, X.; Liu, Y.; Lin, J.; Yuan, X.; Xu, H.; Ji, R. Application of Bayesian Networks in Reliability Evaluation. IEEE Trans. Ind. Inform. 2019, 15, 2146–2157. [Google Scholar] [CrossRef] [Green Version]
- Camilo Gomez Trillos, J.; Wilken, D.; Brand, U.; Vogt, T.; GTrillos, J.C.; Brand, U.; Vogt, T.; Wilken, D. Life cycle assessment of a hydrogen and fuel cell ropax ferry prototype. In Progress in Life Cycle Assessment 2019; Springer: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- CDNSWC. Handbook of Reliability Prediction Procedures for Mechanical Equipment; Carderockdiv, NSWC-92/L01; CDNSWC: Carderock, MD, USA, 2010. [Google Scholar]
- Chisalita, D.-A.; Petrescu, L.; Cormos, C.-C. Environmental evaluation of european ammonia production considering various hydrogen supply chains. Renew. Sustain. Energy Rev. 2020, 130, 109964. [Google Scholar] [CrossRef]
- Cinti, G.; Barelli, L.; Bidini, G. The use of ammonia as a fuel for transport: Integration with solid oxide fuel cells. AIP Conf. Proc. 2019, 2191. [Google Scholar] [CrossRef]
- Cluster, O.H. Hydrogen and Ammonia Infrastructure Safety and Risk Information and Guidance; Lloyd’s Register: London, UK, 2020. [Google Scholar]
- Storwold, D.P. Detailed Test Plan for the Jack Rabbit Test Program; Analysis Center, Science and Technology Directorate: Washington, DC, USA, 2010. [Google Scholar]
- Dammers, E.; McLinden, C.A.; Griffin, D.; Shephard, M.W.; Van Der Graaf, S.; Lutsch, E.; Schaap, M.; Gainairu-Matz, Y.; Fioletov, V.; Van Damme, M.; et al. NH3 emissions from large point sources derived from CrIS and IASI satellite observations. Atmos. Chem. Phys. Discuss. 2019, 19, 12261–12293. [Google Scholar] [CrossRef] [Green Version]
- Damo, U.; Ferrari, M.; Turan, A.; Massardo, A. Solid oxide fuel cell hybrid system: A detailed review of an environmentally clean and efficient source of energy. Energy 2019, 168, 235–246. [Google Scholar] [CrossRef] [Green Version]
- De-Troya, J.J.; Álvarez, C.; Fernández-Garrido, C.; Carral, L. Analysing the possibilities of using fuel cells in ships. Int. J. Hydrog. Energy 2016, 41, 2853–2866. [Google Scholar] [CrossRef]
- Deniz, C.; Zincir, B. Environmental and economical assessment of alternative marine fuels. J. Clean. Prod. 2016, 113, 438–449. [Google Scholar] [CrossRef]
- Det Norske Veritas (DNV). Technology Qualification; July 2013; Volume 81. Available online: http://www2.dnvgl.com/DNV-RP-A203 (accessed on 12 April 2021).
- Dimopoulos, G.G.; Stefanatos, I.C.; Kakalis, N.M. Exergy analysis and optimisation of a marine molten carbonate fuel cell system in simple and combined cycle configuration. Energy Convers. Manag. 2016, 107, 10–21. [Google Scholar] [CrossRef]
- DNV-GL. Use of Methanol as Fuel Methanol as Marine Fuel: Environmental Benefits, Technology Readiness, and Economic Feasibility, IMO; DNV-GL: Belum, Norway, 2016. [Google Scholar]
- DNV-GL. Rules for Classifaction Part 6 Chapter 2 Propulsion, Power Generation and Auxiliary Systems. 2019. Available online: https://rules.dnv.com/docs/pdf/DNV/RU-SHIP/2019-10/DNVGL-RU-SHIP-Pt6Ch2.pdf (accessed on 12 April 2021).
- DNV GL. Assessment of Selected Ternative Fuels and technologies. Imo 2018, 391, 1–48. Available online: http://www.imo.org/en/KnowledgeCentre/IndexofIMOResolutions/Maritime-Safety-Committee-(MSC)/Documents/MSC.391(95).pdf (accessed on 12 April 2021).
- DNV GL. Energy Transition Outlook 2020. A Global and Regional Forecast to 2050; DNV GL: Belum, Norway, 2020. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Horri, B.A. Progress in Material Development for Low-Temperature Solid Oxide Fuel Cells: A Review. Energies 2021, 14, 1280. [Google Scholar] [CrossRef]
- Fuerte, A.; Valenzuela, R.; Escudero, M.; Daza, L. Ammonia as efficient fuel for SOFC. J. Power Sources 2009, 192, 170–174. [Google Scholar] [CrossRef]
- Giap, V.-T.; Lee, Y.D.; Kim, Y.S.; Ahn, K.Y.; Kim, D.H.; Lee, J.I. System simulation and exergetic evaluation of hybrid propulsion system for crude oil tanker: A hybrid of solid-oxide fuel cell and gas engine. Energy Convers. Manag. 2020, 223, 113265. [Google Scholar] [CrossRef]
- Goldwire, H.C.J.; McRae, T.G.; Johnson, C.W.; Hipple, D.L.; Koopman, R.P.; McClure, J.W.; Morris, L.K.; Cederwall, R.T. Desert Tortoise Series Data Report; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1983. [Google Scholar]
- Gottesfeld, S. The Direct Ammonia Fuel Cell and a Common Pattern of Electrocatalytic Processes. J. Electrochem. Soc. 2018, 165, J3405–J3412. [Google Scholar] [CrossRef]
- Grasham, O.; Dupont, V.; Camargo-Valero, M.A.; García-Gutiérrez, P.; Cockerill, T. Combined ammonia recovery and solid oxide fuel cell use at wastewater treatment plants for energy and greenhouse gas emission improvements. Appl. Energy 2019, 240, 698–708. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, Z.; An, L. Carbon-free sustainable energy technology: Direct ammonia fuel cells. J. Power Sources 2020, 476, 228454. [Google Scholar] [CrossRef]
- Hagen, A.; Langnickel, H.; Sun, X. Operation of solid oxide fuel cells with alternative hydrogen carriers. Int. J. Hydrog. Energy 2019, 44, 18382–18392. [Google Scholar] [CrossRef]
- Han, J.; Charpentier, J.-F.; Tang, T. An Energy Management System of a Fuel Cell/Battery Hybrid Boat. Energies 2014, 7, 2799–2820. [Google Scholar] [CrossRef] [Green Version]
- Hansson, J.; Brynolf, S.; Fridell, E.; Lehtveer, M. The Potential Role of Ammonia as Marine Fuel—Based on Energy Systems Modeling and Multi-Criteria Decision Analysis. Sustainability 2020, 12, 3265. [Google Scholar] [CrossRef] [Green Version]
- De Gusmão, A.P.H.; Silva, M.M.; Poleto, T.; e Silva, L.C.; Costa, A.P.C.S. Cybersecurity risk analysis model using fault tree analysis and fuzzy decision theory. Int. J. Inf. Manag. 2018, 43, 248–260. [Google Scholar] [CrossRef]
- Huijsmans, J.; Kraaij, G.; Makkus, R.; Rietveld, G.; Sitters, E.; Reijers, H. An analysis of endurance issues for MCFC. J. Power Sources 2000, 86, 117–121. [Google Scholar] [CrossRef]
- ICS. Reducing CO2 Emissions to Zero: The “Paris Agreement for Shipping”; International Chamber of Shipping: London, UK, 2018; Volume 16. [Google Scholar]
- Igder, M.A.; Rafiei, M.; Boudjadar, J.; Khooban, M.-H. Reliability and Safety Improvement of Emission-Free Ships: Systemic Reliability-Centered Maintenance. IEEE Trans. Transp. Electrif. 2021, 7, 256–266. [Google Scholar] [CrossRef]
- IMO. Annex 9, Resolution MEPC.213(63), 2012 Guidelines for the Development of a Ship Energy Efficiency Management Plan (SEEMP); IMO: London, UK, 2012. [Google Scholar]
- IMO. Annex 5, Resolution MEPC.245(66), Guidelines on the Method of Calculation of the Attained Energy Efficiency Design Index (EEDI) for New Ships; IMO: London, UK, 2014. [Google Scholar]
- IMO. Reducing Greenhouse Gas Emissions from Ships; IMO: London, UK, 2020; Available online: http://www.imo.org/en/MediaCentre/HotTopics/Pages/Reducing-greenhouse-gas-emissions-from-ships.aspx (accessed on 4 May 2021).
- Inal, O.B.; Deniz, C. Assessment of fuel cell types for ships: Based on multi-criteria decision analysis. J. Clean. Prod. 2020, 265, 121734. [Google Scholar] [CrossRef]
- Isermann, R. Fault-Diagnosis Systems. An Introduction from Fault Detection to Fault Tolerance; Isermann, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-3-540-30368-8. Available online: https://link.springer.com/content/pdf/10.1007%2F3-540-30368-5.pdf (accessed on 12 April 2021).
- Jeerh, G.; Zhang, M.; Tao, S. Recent progress in ammonia fuel cells and their potential applications. J. Mater. Chem. A 2021, 9, 727–752. [Google Scholar] [CrossRef]
- Jordan, J. Shipping Industry Needs to Look beyond IMO 2020 to Lower-Carbon Fuels. S&P Glob. Platts 2019. Available online: https://blogs.platts.com/2019/07/24/shipping-beyond-imo-2020-lower-carbon/ (accessed on 1 April 2021).
- Kang, J.; Sun, L.; Soares, C.G. Fault Tree Analysis of floating offshore wind turbines. Renew. Energy 2019, 133, 1455–1467. [Google Scholar] [CrossRef]
- Kim, K.; Roh, G.; Kim, W.; Chun, K. A Preliminary Study on an Alternative Ship Propulsion System Fueled by Ammonia: Environmental and Economic Assessments. J. Mar. Sci. Eng. 2020, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, M.; Muroyama, H.; Suzuki, S.; Saito, M.; Koide, T.; Takahashi, Y.; Horiuchi, T.; Yamasaki, H.; Matsumoto, S.; Kubo, H.; et al. Development of 1 kW-class Ammonia-fueled Solid Oxide Fuel Cell Stack. Fuel Cells 2020, 20, 80–88. [Google Scholar] [CrossRef]
- Kobbacy, K.A. Complex System Maintenance Handbook, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Lan, R.; Tao, S. Direct Ammonia Alkaline Anion-Exchange Membrane Fuel Cells. Electrochem. Solid-State Lett. 2010, 13, B83–B86. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Ammonia as a Suitable Fuel for Fuel Cells. Front. Energy Res. 2014, 2, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Langseth, H.; Portinale, L. Bayesian networks in reliability. Reliab. Eng. Syst. Saf. 2007, 92, 92–108. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.J.; Shin, D.; Lee, J.P.; Yoo, D.W.; Choi, H.K.; Kim, H.J. Hybrid photovoltaic/diesel green ship operating in standalone and grid-connected mode in South Korea—Experimental investigation. Energy 2012, 49, 580–583. [Google Scholar] [CrossRef]
- Lewis, R.J.S. Hawley’s Condensed Chemical Dictionary, 12th ed.; Van Nostrand Reinhold: New York, NY, USA, 1993. [Google Scholar]
- Lindstad, H. GHG Emission Reduction Potential of EU-Related Maritime Transport and on its Impacts; TNO: The Hague, The Netherlands, 2015. [Google Scholar]
- Lloyd’s Register; UMAS. Techno-Economic Assessment of Zero-Carbon Fuels; Lloyds Register: London, UK, 2020. [Google Scholar]
- Ma, Q.; Ma, J.; Zhou, S.; Yan, R.; Gao, J.; Meng, G. A high-performance ammonia-fueled SOFC based on a YSZ thin-film electrolyte. J. Power Sources 2007, 164, 86–89. [Google Scholar] [CrossRef]
- MAN Energy Solutions. Engineering the Future Two-Stroke Green-Ammonia Engine. 2019. Available online: https://marine.man-es.com/docs/librariesprovider6/test/engineering-the-future-two-stroke-green-ammonia-engine.pdf?sfvrsn=7f4dca2_4 (accessed on 1 April 2021).
- Mardones, C.; Flores, B. Effectiveness of a CO2 tax on industrial emissions. Energy Econ. 2018, 71, 370–382. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Mckinlay, C.J.; Turnock, S.R.; Hudson, D.A. A Comparison of hydrogen and ammonia for future long distance shipping fuels. In Proceedings of the LNG/LPG and Alternative Fuels Ships Conference, London, UK, 29–30 January 2020. [Google Scholar]
- McMahon, J. Get Ready for 1.5¢ Renewable Electricity, Steven Chu Says, Which Could Unleash Hydrogen Economy. 2019. Available online: https://www.forbes.com/sites/jeffmcmahon/2019/04/02/get-ready-for-1-5¢-renewable-electricity-steven-chu-says-which-could-unleash-hydrogen-economy/?sh=342c3a601c01 (accessed on 1 April 2021).
- MEPC. Reduction of GHG emissions from ships. Fourth IMO GHG Study 2020. IMO 2020, 53. [Google Scholar] [CrossRef]
- Mobley, K.; Higgins, L.; Wikoff, D. Maintenance Engineering Handbook, 7th ed.; Mobley, R.K., Ed.; McGraw Hill: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Mohanty, A.R. Machinery Condtion Monitoring, 1st ed.; Taylor & Francis: Abingdon, UK, 2015. [Google Scholar]
- Mokashi, A.; Wang, J.; Vermar, A. A study of reliability-centred maintenance in maritime operations. Mar. Policy 2002, 26, 325–335. [Google Scholar] [CrossRef]
- Namboothiri, N.V.; Soman, A. Consequence assessment of anhydrous ammonia release using CFD-probit analysis. Process. Saf. Prog. 2018, 37, 525–534. [Google Scholar] [CrossRef]
- National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 6; The National Academics Press: New York, NY, USA, 2007. [Google Scholar]
- National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 6; National Academies Press (US): New York, NY, USA, 2008. [Google Scholar]
- NCE. Norwegian Future Value Chains for Liquid Hydrogen; NCE Maritime CleanTech: Stord, Norway, 2019; Volume 84, Available online: https://maritimecleantech.no/wp-content/uploads/2016/11/Report-liquid-hydrogen.pdf?fbclid=IwAR3uqivsh0dF3_VBQd8UB_0cgVtnf3XIM1of2xG7Y2WAS07e3OHzoTT-_9Q (accessed on 1 April 2021).
- Nielsen, M.; Bengtsson, R.; Jones, C.; Nyrén, K.; Ott, S.; Ride, D. Design of the Fladis Field Experiments with Dispersion of Liquefied Ammonia; US Department of Energy: Washington, DC, USA, 1994.
- Nielsen, M.; Ott, S. Fladis Field Experiments, Final Report; Riso National Laboratory: Roskilde, Denmark, 1996. [Google Scholar]
- OECD. Decarbonising Maritime Transport. Pathways to Zero-Carbon Shipping by 2035; International Transport Forum, 2018; Volume 86. Available online: https://www.itf-oecd.org/sites/default/files/docs/decarbonising-maritime-transport.pdf (accessed on 5 May 2021).
- Okanishi, T.; Okura, K.; Srifa, A.; Muroyama, H.; Matsui, T.; Kishimoto, M.; Saito, M.; Iwai, H.; Yoshida, H.; Koide, T.; et al. Comparative Study of Ammonia-fueled Solid Oxide Fuel Cell Systems. Fuel Cells 2017, 17, 383–390. [Google Scholar] [CrossRef]
- Pearl, J. Bayesian Networks: A Model of Self-Activated Memory for Evidential Reasoning; UCLA: Los Angeles, CA, USA, 1985. [Google Scholar]
- Pearl, J. Prpbabilistic reasoning in intelligent systems. In Probabilistic Reasoning in Intelligent Systems, 2nd ed.; Morgan, M.B., Ed.; Morgan Kaufmann: San Francisco, CA, USA, 1988. [Google Scholar] [CrossRef]
- Rausan, M.; Hoyland, A. System Reliability Theory: Models, Statistical Methods, and Applications, 2nd ed.; Shewhart, W., Wilks, S., Eds.; Wikey & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Rosli, R.; Sulong, A.; Daud, W.; Zulkifley, M.; Husaini, T.; Rosli, M.; Majlan, E.H.; Haque, M. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrog. Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Ruijters, E.; Stoelinga, M. Fault tree analysis: A survey of the state-of-the-art in modeling, analysis and tools. Comput. Sci. Rev. 2015, 15–16, 29–62. [Google Scholar] [CrossRef] [Green Version]
- RVIM. Guidelines for Quantitative Risk Assessment (Purple Book) CPR 18E; Publicatiereeks Gevaarlijky Stoffen: Rotterdam, The Netherlands, 2005. [Google Scholar]
- ShipFC. Grant Agreement Number: 875156—ShipFC, 2019.
- Siddiqui, O.; Ishaq, H.; Dincer, I. Experimental investigation of improvement capability of ammonia fuel cell performance with addition of hydrogen. Energy Convers. Manag. 2020, 205, 112372. [Google Scholar] [CrossRef]
- Silva, J.C.M.; Da Silva, S.G.; De Souza, R.F.; Buzzo, G.S.; Spinacé, E.V.; Neto, A.O.; Assumpção, M.H. PtAu/C electrocatalysts as anodes for direct ammonia fuel cell. Appl. Catal. A Gen. 2015, 490, 133–138. [Google Scholar] [CrossRef]
- Singhal, S. Solid oxide fuel cells for stationary, mobile, and military applications. Solid State Ion. 2002, 152–153, 405–410. [Google Scholar] [CrossRef]
- Smith, R.; Mobley, K. Rules of Thumb for Maintenance and Reliability Engineers, 1st ed.; Butterworth-Heinemann: Woburn, MA, USA, 2008. [Google Scholar] [CrossRef]
- Spreafico, C.; Russo, D.; Rizzi, C. A state-of-the-art review of FMEA/FMECA including patents. Comput. Sci. Rev. 2017, 25, 19–28. [Google Scholar] [CrossRef]
- Stoeckl, B.; Preininger, M.; Subotić, V.; Megel, S.; Folgner, C.; Hochenauer, C. Towards a wastewater energy recovery system: The utilization of humidified ammonia by a solid oxide fuel cell stack. J. Power Sources 2020, 450, 227608. [Google Scholar] [CrossRef]
- Stopford, M. Three Maritime Scenarios 2020–2050; Seatrade Maritime: Colchester, UK, 2020. [Google Scholar]
- The Royal Society. Ammonia: Zero-Carbon Fertiliser, Fuel and Energy Store. Policy Briefing; Issue Accessed 25 August 2020; 2020. Available online: https://royalsociety.org/-/media/policy/projects/green-ammonia/green-ammonia-policy-briefing.pdf (accessed on 1 March 2021).
- Thiem, C.; Gentner, C.; Ackermann, G. Methanol powered fuel cell systems for marine applications. In Proceedings of the International Conference on Shipping in Changing Climates Technologies, Operations, Logistics and Policies towards Meeting 2050 Emission Targets, Glasgow, UK, 7 September–8 September 2015. [Google Scholar]
- Tixier, J.; Dusserre, G.; Salvi, O.; Gaston, D. Review of 62 risk analysis methodologies of industrial plants. J. Loss Prev. Process. Ind. 2002, 15, 291–303. [Google Scholar] [CrossRef] [Green Version]
- TNO. Methods for the Determination of Possible Damage to People and Objects Resulting from Releases of Hazardous Materials (Green Book); Instituut voor Milieu- en Energietechnologie TNO Prins Maurits Laboratorium TNO, Ed.; Committee for the Prevention of Disasters: Den Haag, The Netherlands, 1992. [Google Scholar]
- TNO. Methods for the Calculation of Physical Effects due to Releases of Hazardous Materials (Yellow Book) CPR 14E, 3rd ed.; Publicatiereeks Gevaarlijke Stoffen 2: Rotterdam, The Netherlands, 2005. [Google Scholar]
- Tronstad, T.; Åstrand, H.H.; Haugom, G.P.; Langfeldt, L. Study on the Use of Fuel Cells in Shipping; EMSA, EU: Lisbon, Portugal, 2017. [Google Scholar]
- UNCTAD. Review of Maritime Transport. J. Chem. Inf. Modeling 2020, 53. Available online: https://unctad.org/system/files/official-document/rmt2020_en.pdf (accessed on 1 March 2021).
- US Department of Energy. Comparison of Fuel Cell Technologies; Fuel Cell Technology Offices: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Utah Valley University. Jack Rabbit Project. 2010. Available online: https://www.uvu.edu/es/jack-rabbit/ (accessed on 1 March 2021).
- Uwemedimo, E.; Doymus, M.; Eylul, D. Understanding regulations, sustainable shipping, alternative marine fuels and LNG. In Proceedings of the 8th Global LNG Bunkering Summit 2020, Amsterdam, The Netherlands, 28–30 January 2020. [Google Scholar]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Weast, R.C. CRC Handbook of Chemistry and Physics, 65th ed.; Astle, M., Beyer, W.H., Eds.; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Wojcik, A.; Middleton, H.; Damopoulos, I.; Van Herle, J. Ammonia as a fuel in solid oxide fuel cells. J. Power Sources 2003, 118, 342–348. [Google Scholar] [CrossRef]
- Xing, H.; Spence, S.; Chen, H. A comprehensive review on countermeasures for CO2 emissions from ships. Renew. Sustain. Energy Rev. 2020, 134, 110222. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC. J. Power Sources 2007, 172, 133–144. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Zhao, Y.; Setzler, B.P.; Wang, J.; Nash, J.; Wang, T.; Xu, B.; Yan, Y. An Efficient Direct Ammonia Fuel Cell for Affordable Carbon-Neutral Transportation. Joule 2019, 3, 2472–2484. [Google Scholar] [CrossRef]


| Fuel | Energy Density LHV (MJ/kg) | Volumetric Energy Density (GJ/m3) | Renewable Synthetic Production Cost (MJ/MJ) | Storage Pressure (Bar) | Liquified Storage Temperature (°C) |
|---|---|---|---|---|---|
| Compressed hydrogen | 120 | 4.7 | 1.7 | 700 | 20 |
| Liquid hydrogen | 120 | 8.5 | 1.8 | 1 | −253 |
| Ethanol | 26.7 | 21.1 | 3.6 | 1 | 20 |
| Methanol | 19.9 | 15.8 | 2.6 | 1 | 20 |
| Liquid methane | 50 | 23.4 | 2.3 | 1 | −162 |
| Liquid ammonia | 18.6 | 12.7 | 1.8 | 1 or 10 | −34 or 20 |
| LT-PEMFC | HT-PEMFC | PAFC | MCFC | SOFC | |
|---|---|---|---|---|---|
| Operating Temperature (°C) | 65–85 | 120–180 | 150–200 | 700–800 | 700–1000 |
| Durability | High | Medium | Medium | Low | Low |
| Start-up Time | Low | Medium | Medium | High | Very High |
| CAPEX | High | Medium | High | Low | Medium |
| Complexity | High | Medium | Medium | Medium | Low |
| Power Density | Medium | Medium | Low | High | Very High |
| Electrical Efficiency (%) | 60 | 60 | 40 | 50 | 60 |
| Level | Exposure Time | Repercussion | ||
|---|---|---|---|---|
| 10 min | 30 min | 60 min | ||
| AEGL-1 | 30 ppm | 30 ppm | 30 ppm | ‘discomfort, irritation, or asymptomatic nonsensory effects’ |
| AEGL-2 | 220 ppm | 220 ppm | 160 ppm | ‘irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape’ |
| AEGL-3 | 2700 ppm | 1600 ppm | 1100 ppm | ‘life-threatening health effects or death’ |
| SHIP Type | Event | Accident | Consequences | |
|---|---|---|---|---|
| Personnel Safety | Environment | |||
| Container | Hull/Machinery Damage | Leak of ammonia cargo | None | None |
| Fishing vessel | Hull/Machinery Damage | Ammonia leak in refrigeration | Fatalities | None |
| Fishing vessel | Hull/Machinery Damage | Explosion in engine room following rapture of ammonia storage | Fatalities | None |
| Fishing vessel | Hull/Machinery Damage | Ammonia leak after burst of refrigeration pipe | Fatalities | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheliotis, M.; Boulougouris, E.; Trivyza, N.L.; Theotokatos, G.; Livanos, G.; Mantalos, G.; Stubos, A.; Stamatakis, E.; Venetsanos, A. Review on the Safe Use of Ammonia Fuel Cells in the Maritime Industry. Energies 2021, 14, 3023. https://doi.org/10.3390/en14113023
Cheliotis M, Boulougouris E, Trivyza NL, Theotokatos G, Livanos G, Mantalos G, Stubos A, Stamatakis E, Venetsanos A. Review on the Safe Use of Ammonia Fuel Cells in the Maritime Industry. Energies. 2021; 14(11):3023. https://doi.org/10.3390/en14113023
Chicago/Turabian StyleCheliotis, Michail, Evangelos Boulougouris, Nikoletta L Trivyza, Gerasimos Theotokatos, George Livanos, George Mantalos, Athanasios Stubos, Emmanuel Stamatakis, and Alexandros Venetsanos. 2021. "Review on the Safe Use of Ammonia Fuel Cells in the Maritime Industry" Energies 14, no. 11: 3023. https://doi.org/10.3390/en14113023









