Experimental Characterization of Phase Change Materials for Refrigeration Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Determination of Phase Change Temperature and Phase Change Enthalpy
2.2.2. Determination of Density
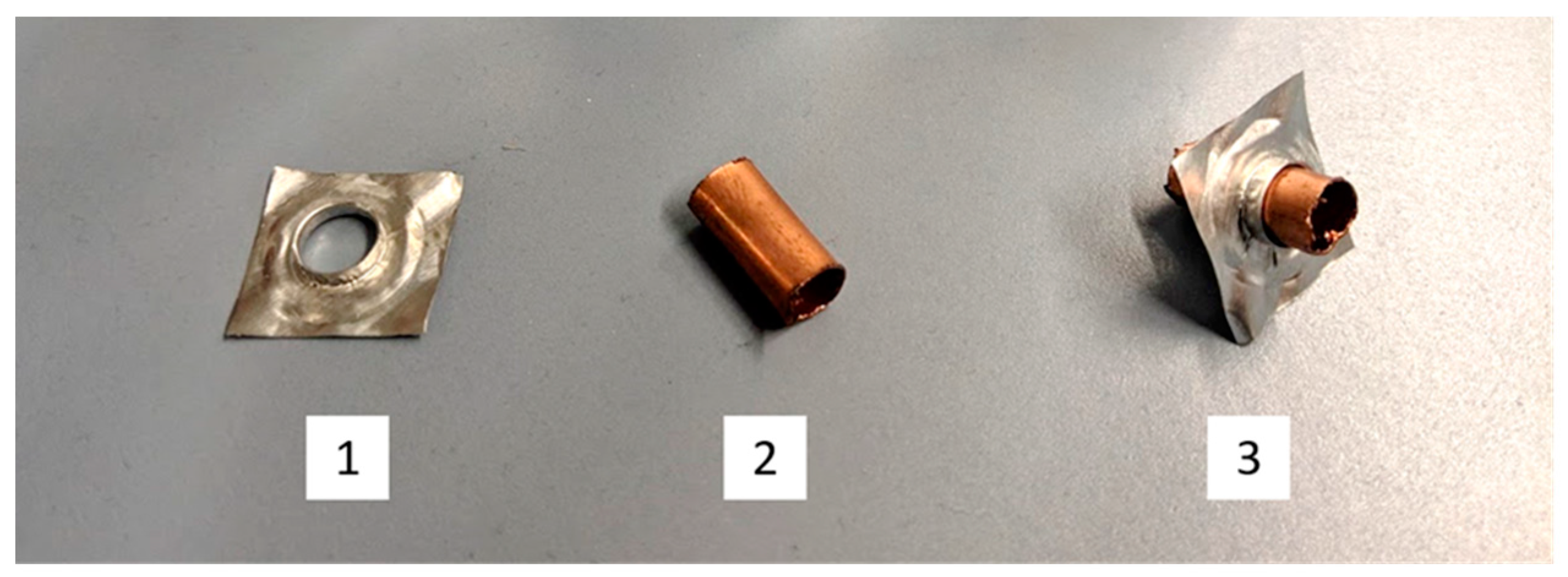
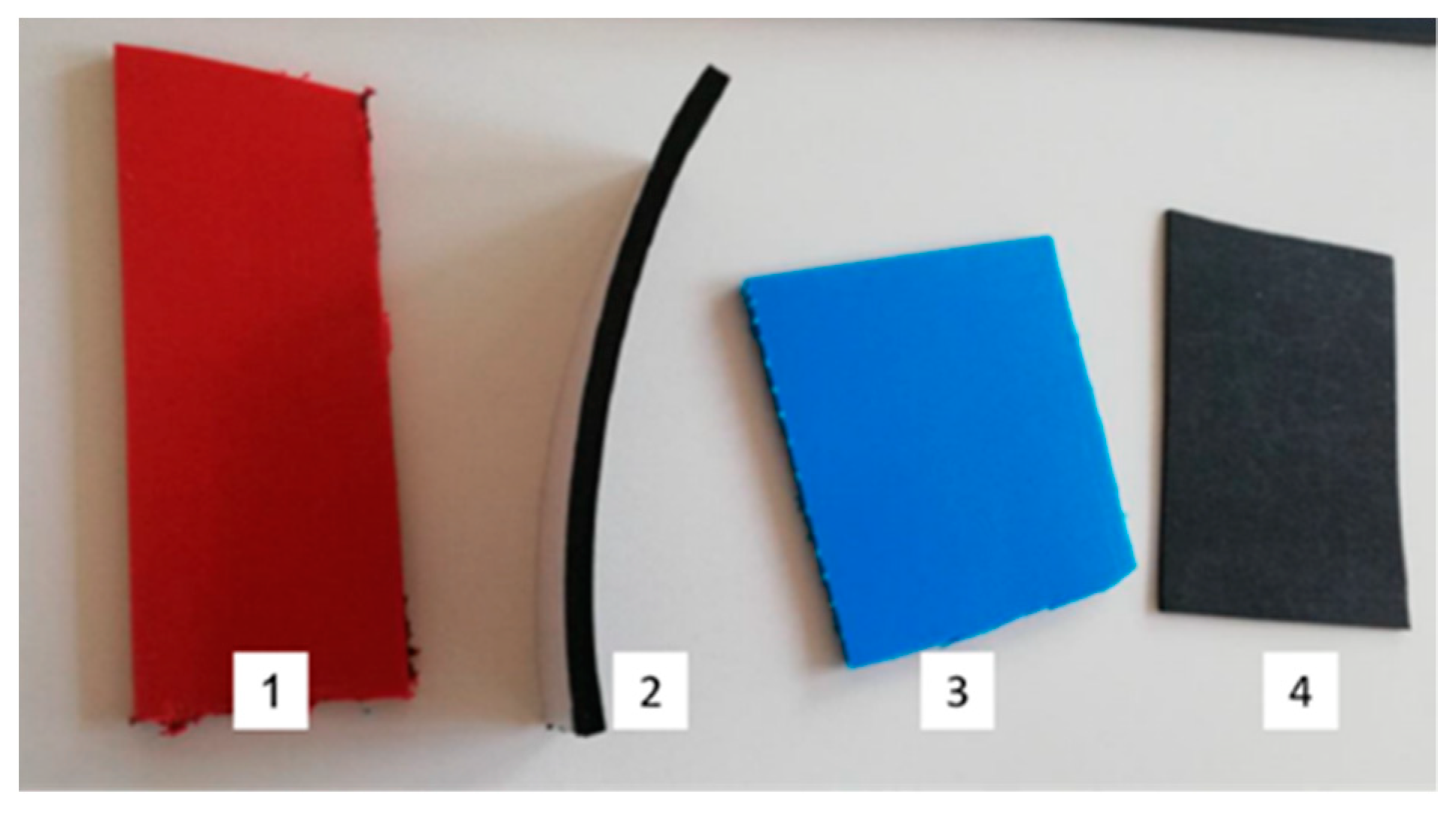
2.2.3. Determination of Material Compatibility
3. Results
3.1. Measurement of the Thermo-Physical Properties
3.2. Material Compatibility Tests
3.2.1. Metallic Samples
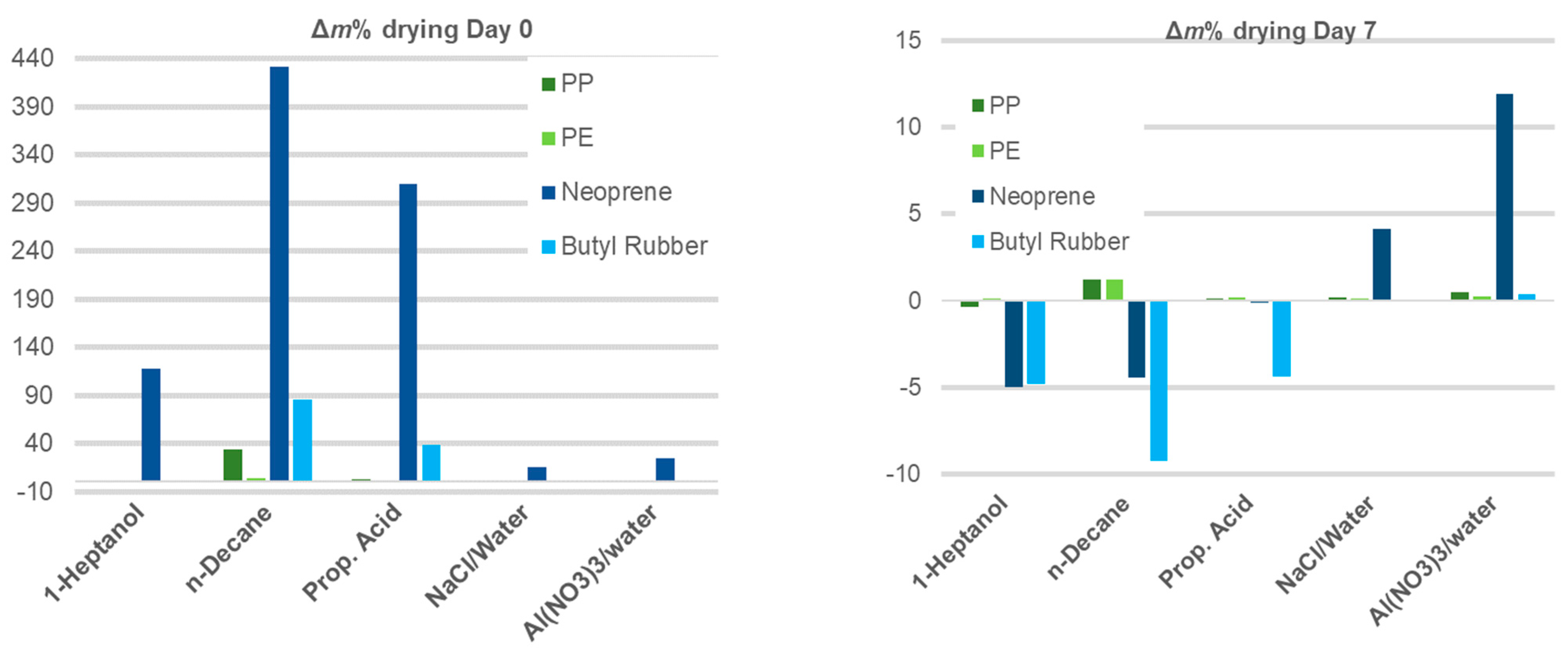
3.2.2. Polymeric Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleiter, T.; Steinbach, J.; Ragwitz, M. Mapping and Analyses of the Current and Future (2020–2030) Heating/Cooling Fuel Deployment (Fossil/Renewables)—Work Package 1: Final Energy Consumption for the Year 2012. 2016. Available online: https://ec.europa.eu/energy/studies/mapping-and-analyses-current-and-future-2020-2030-heatingcooling-fuel-deployment_en (accessed on 15 April 2020).
- Fleiter, T.; Steinbach, J.; Ragwitz, M. Mapping and Analyses of the Current and Future (2020–2030) Heating/Cooling Fuel Deployment (Fossil/Renewables)—Work Package 2: Assessment of the Technologies for the Year 2012. 2016. Available online: https://ec.europa.eu/energy/studies/mapping-and-analyses-current-and-future-2020-2030-heatingcooling-fuel-deployment_en (accessed on 15 April 2020).
- Vakiloroaya, V.; Samali, B.; Fakhar, A.; Pishghadam, K. A review of different strategies for HVAC energy saving. Energy Convers. Manag. 2014, 77, 738–754. [Google Scholar] [CrossRef]
- Zhu, K.; Li, X.; Campana, P.E.; Li, H.; Yan, J. Techno-economic feasibility of integrating energy storage systems in refrigerated warehouses. Appl. Energy 2018, 216, 348–357. [Google Scholar] [CrossRef]
- Arteconi, A.; Ciarrocchi, E.; Pan, Q.; Carducci, F.; Comodi, G.; Polonara, F.; Wang, R. Thermal energy storage coupled with PV panels for demand side management of industrial building cooling loads. Appl. Energy 2017, 185, 1984–1993. [Google Scholar] [CrossRef]
- Bakan, K.; Dincer, I.; Rosen, M.A. Exergoeconomic analysis of glycol cold thermal energy storage systems. Int. J. Energy Res. 2007, 32, 215–225. [Google Scholar] [CrossRef]
- Lavan, Z.; Thompson, J. Experimental study of thermally stratified hot water storage tanks. Sol. Energy 1977, 19, 519–524. [Google Scholar] [CrossRef]
- Oró, E.; Miró, L.; Barreneche, C.; Martorell, I.; Farid, M.M.; Cabeza, L.F. Corrosion of metal and polymer containers for use in PCM cold storage. Appl. Energy 2013, 109, 449–453. [Google Scholar] [CrossRef]
- Oró, E.; De Gracia, A.; Castell, A.; Farid, M.; Cabeza, L. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 2012, 99, 513–533. [Google Scholar] [CrossRef] [Green Version]
- Joybari, M.M.; Haghighat, F.; Moffat, J.; Sra, P. Heat and cold storage using phase change materials in domestic refrigeration systems: The state-of-the-art review. Energy Build. 2015, 106, 111–124. [Google Scholar] [CrossRef]
- Li, G.; Hwang, Y.; Radermacher, R.; Chun, H.-H. Review of cold storage materials for subzero applications. Energy 2013, 51, 1–17. [Google Scholar] [CrossRef]
- Suamir, I.N.; Rasta, I.M.; Tsamos, K.M. Development of Corn-Oil Ester and Water Mixture Phase Change Materials for Food Refrigeration Applications. Energy Procedia 2019, 161, 198–206. [Google Scholar] [CrossRef]
- Konuklu, Y.; Şahan, N.; Paksoy, H. 2.14 Latent Heat Storage Systems. Compr. Energy Syst. 2018, 2, 396–434. [Google Scholar]
- Schroder, J.; Gawron, K. Latent heat storage. Int. J. Energy Res. 1981, 5, 103–109. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Müller, L.; Rubio-Pérez, G.; Bach, A.; Muñoz-Rujas, N.; Aguilar, F.; Worlitschek, J. Consistent DSC and TGA Methodology as Basis for the Measurement and Comparison of Thermo-Physical Properties of Phase Change Materials. Materials 2020, 13, 4486. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, L.F.; Illa, J.; Roca, J.; Badia, F.; Mehling, H.; Hiebler, S.; Ziegler, F. Immersion corrosion tests on metal-salt hydrate pairs used for latent heat storage in the 32 to 36 °C temperature range. Mater. Corros. 2001, 52, 140–146. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.; Hippeli, S.; Hiebler, S. PCM-module to improve hot water heat stores with stratification. Renew. Energy 2003, 28, 699–711. [Google Scholar] [CrossRef]
- García-Romero, A.; Delgado, A.; Urresti, A.; Martín, K.; Sala, J. Corrosion behaviour of several aluminium alloys in contact with a thermal storage phase change material based on Glauber’s salt. Corros. Sci. 2009, 51, 1263–1272. [Google Scholar] [CrossRef]
- ASTM. ASTM G 31-72—Standard Practise for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- International Organization for Standardization. Korrosion von Metallen und Legierungen—Entfernen von Korrosionsprodukten von Korrosionsprobekörpern (ISO 8407: 2009); SNV Schweizerische Normen: Winterthur, Switzerland, 2014. [Google Scholar]
- ASTM D543. Standard Practices for Evaluating the Resistance of Plastics to Chemical Reagents; No. Reapproved 2013; ASTM International: West Conshohocken, PA, USA, 2015; Volume i, pp. 3–9. [Google Scholar]
- Al-Jimaz, A.S.; Al-Kandary, J.A.; Abdul-Latif, A.-H.M. Densities and viscosities for binary mixtures of phenetole with 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol, 1-nonanol, and 1-decanol at different temperatures. Fluid Phase Equilibria 2004, 218, 247–260. [Google Scholar] [CrossRef]
- Singh, S.; Bahadur, I.; Redhi, G.G.; Ebenso, E.E.; Ramjugernath, D. Density and speed of sound of 1-ethyl-3-methylimidazolium ethyl sulphate with acetic or propionic acid at different temperatures. J. Mol. Liq. 2014, 199, 518–523. [Google Scholar] [CrossRef]
- Simion, A.I.; Grigoraş, C.G.; Roşu, A.M.; Gavrilă, L. Mathematical modelling of density and viscosity of nacl aqueous solutions. J. Agrolimentary Process. Technol. 2015, 21, 41–52. [Google Scholar]
- Rathgeber, C.; Miró, L.; Cabeza, L.F.; Hiebler, S. Measurement of enthalpy curves of phase change materials via DSC and T-History: When are both methods needed to estimate the behaviour of the bulk material in applications? Thermochim. Acta 2014, 596, 79–88. [Google Scholar] [CrossRef]
- Kabelac, S.; Kind, M.; Martin, H.; Mewes, D.; Schaber, K.; Stephan, P. VDI-Wärmeatlas, 11th ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Acree, W.E. Thermodynamic properties of organic compounds: Enthalpy of fusion and melting point temperature compilation. Thermochim. Acta 1991, 189, 37–56. [Google Scholar] [CrossRef]
- Martin, J.; Andon, R. Thermodynamic properties of organic oxygen compounds Part LII. Molar heat capacity of ethanoic, propanoic, and butanoic acids. J. Chem. Thermodyn. 1982, 14, 679–688. [Google Scholar] [CrossRef]
- Browne, M.C.; Boyd, E.; McCormack, S.J. Investigation of the corrosive properties of phase change materials in contact with metals and plastic. Renew. Energy 2017, 108, 555–568. [Google Scholar] [CrossRef]
- Verein Deutscher Ingenieure. VDI-Wärmeatlas; Verein Deutscher Ingenieure: Düsseldorf, Germany, 1991. [Google Scholar]




| Substance | Formula | Producer | CAS-No° | Amount | Purity |
|---|---|---|---|---|---|
| 1-Heptanol | CH3(CH2)6OH | Sigma Aldrich | 111-70-6 | 250 mL | 98% |
| n-Decane | C10H22 | Roth | 124-18-5 | 500 mL | 99% |
| Propionic acid | C3H6O2 | Sigma Aldrich | 79-09-4 | 500 ml | 99.5% |
| Sodium chloride | NaCl | Sigma Aldrich | 7647-14-5 | 250 g | 99.5% |
| Aluminum nitrate nonahydrate | Al(NO3)3·9H2O | Sigma Aldrich | 7784-27-2 | 100 g | 98% |
| 1-Heptanol | n-Decane | Propionic Acid | NaCl/Water 23.3 wt.% | Al(NO3)3/Water 30.5 wt.% | |
|---|---|---|---|---|---|
| ρl, exp. kg/L | 0.8245 ± 0.0018 | 0.7351 ± 0.0006 | 0.9940 ± 0.0005 | 1.1754 ± 0.0005 | 1.2963 ± 0.0000 |
| ρl, Lit. Kg/L | 0.819 (25 °C) [23] | 0.73 (20 °C) [14] | 0.98 (25 °C) [24] | 1.174 (20 °C) [25] | 1.283 (liquid) [15] |
| Tm, Onset °C | Tm, Peak °C | Tm, Lit. °C | Tc, Onset °C | Tc, Peak °C | Super-Cooling °C | ΔHm kJ/kg | ΔHm, lit. kJ/kg | ΔHm,vol. kJ/L | |
|---|---|---|---|---|---|---|---|---|---|
| 1-Heptanol | −35.2 ± 0.43 | −32.81 ± 0.23 | −34.1 [27] | −45.38 ± 0.37 | −45.83 ± 0.34 | 10.18 ± 0.57 | 116.26 ± 1.07 | 156.4 [27] | 95.86 ± 0.912 |
| n-Decane | −30.38 ± 0.00 | −27.91 ± 0.03 | −29.7 [28] | −35.86 ± 0.30 | −36.03 ± 0.29 | 5.78 ± 0.30 | 202.79 ± 0.45 | 202 [28] | 149.07 ± 0.388 |
| Propionic acid | −23.46 ± 0.03 | −20.88 ± 0.01 | −20.5 [29] | −40.89 ± 0.2 | −40.99 ± 0.2 | 17.43 ± 0.20 | 150.45 ± 0.45 | 143.9 [29] | 149.55 ± 0.447 |
| NaCl/water | −20.94 ± 4.07 | −16.80 ± 0.05 | −21.2 [9] | −38.36 ± 7.00 | −39.20 ± 1.98 | 17.42 ± 8.10 | 256.98 ± 0.18 | 233 [9] | 302.05 ± 0.212 |
| Al(NO3)3/water | −30.38 ± 0.45 | −25.9 ± 0.12 | −30.6 [9] | −53.93 ±0.06 | −55.76 ±0.84 | 23.55 ± 0.45 | 174.22 ± 0.55 | 131.5 [9] | 225.84 ± 0.71 |
| Sample Type | Weight Initial (mg) | Surface Area, Initial (mm2) | Mass Loss (mg), Mechanical | Mass Loss %, Mechanical | Mass Loss (mg), Chemical | Mass Loss %, Chemical | |
|---|---|---|---|---|---|---|---|
| 1-Heptanol | only Al | 95.4 | 714.1 | 0.1 | 0.1 | 0.1280 | 0.134 |
| only Cu | 809.4 | 741.9 | 0.1 | 0.0 | 0.1848 | 0.023 | |
| Al mix | 83.2 | 623.7 | 0.1 | 0.2 | 0.1691 | 0.203 | |
| Cu mix | 804.7 | 737.6 | 0.2 | 0.0 | 0.2897 | 0.036 | |
| n-Decane | only Al | 72.9 | 546.5 | 0.1 | 0.1 | - | - |
| only Cu | 749.4 | 687.7 | 0.1 | 0.0 | 0.2 | 0.023 | |
| Al mix | 72.1 | 540.4 | 0.1 | 0.1 | - | - | |
| Cu mix | 785.9 | 720.7 | 0.1 | 0.0 | 0.2 | 0.023 | |
| Propionic acid | only Al | 81.4 | 610.0 | 0.1 | 0.2 | 0.2 | 0.192 |
| only Cu | 839.6 | 769.1 | 24.8 | 3.0 | - | - | |
| Al mix | 84.6 | 633.7 | 0.2 | 0.2 | - | - | |
| Cu mix | 862.6 | 789.9 | 17.0 | 2.0 | - | - | |
| NaCl/ water | only Al | 84.1 | 630.4 | 0.1 | 0.1 | 0.2 | 0.283 |
| only Cu | 796.9 | 730.6 | 4.6 | 0.6 | 5.7 | 0.721 | |
| Al mix | 66.8 | 501.2 | 1.2 | 1.8 | 2.3 | 3.422 | |
| Cu mix | 740.1 | 679.3 | 0.6 | 0.1 | 0.6 | 0.083 | |
| Al(NO3)3/ water | only Al | 82.9 | 620.9 | 82.9 * | 100.0 * | - | - |
| only Cu | 856.9 | 784.8 | 95.7 | 11.2 | - | - | |
| Al mix | 69.4 | 520.3 | 69.4 * | 100.0 * | - | - | |
| Cu mix | 818.5 | 750.1 | 99.7 | 12.2 | - | - |
| Corrosion Rate mg/cm2/yr | Recommendation |
|---|---|
| >1000 | Completely destroyed within days |
| 100–999 | Not recommended for service greater than one month |
| 50–99 | Not recommended for service greater than one year |
| 10–49 | Caution recommended |
| <9.9 | Recommended for long term service |
| Mass Gain% | ||||||
|---|---|---|---|---|---|---|
| Polymer | Days of Drying | 1-Heptanol | n-Decane | Prop. Acid | NaCl/Water | Al(NO3)3/Water |
| PP | 0 | 1.62 | 34.31 | 3.32 | 0.48 | 1.14 |
| 1 | 0.03 | 10.69 | 1.66 | 0.50 | 1.08 | |
| 7 | −0.37 | 1.24 | 0.12 | 0.17 | 0.46 | |
| PE | 0 | 0.66 | 3.46 | 0.63 | 0.37 | 0.36 |
| 1 | 0.16 | 2.78 | 1.04 | 0.59 | 0.61 | |
| 7 | 0.11 | 1.20 | 0.20 | 0.11 | 0.27 | |
| Neopre-ne | 0 | 117.74 | 430.95 | 309.24 | 15.24 | 24.04 |
| 1 | 21.22 | −1.41 | 5.16 | 8.03 | 19.94 | |
| 7 | −4.96 | −4.42 | −0.10 | 4.16 | 11.89 | |
| Butylrubber | 0 | −0.48 | 85.48 | 38.29 | −0.12 | 0.46 |
| 1 | −2.12 | −2.18 | 17.56 | 0.92 | 0.94 | |
| 7 | −4.81 | −9.24 | −4.38 | −0.02 | 0.38 | |
| Thermophysical Properties | Corrosion Rate | Compatibility with Polymers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/cm2 yr) | |||||||||||
| Δh (kWh/m3) | Tm (°C) | ΔT (°C) | Al | Cu | Al (mix) | Cu (mix) | PP | PE | Neopren | Butyl Rubber | |
| 1-Heptanol | 26.6 | −35.2 | 10.2 | 0.2 | 0.2 | 0.2 | 0.3 | no swel./ low extr. | no swel./ no extr. | medium swel./ high extr. | no swel./ high extr. |
| n-Decane | 41.4 | −30.4 | 5.8 | 0.1 | 0.2 | 0.1 | 0.2 | low swel./ no extr. | no swel./ no extr. | high swel./ high extr. | medium swel./ high extr. |
| Prop. Acid | 41.5 | −23.46 | 17.4 | 0.2 | 24.5 | 0.2 | 16.4 | no swel./ no extr. | no swel./ no extr. | high swel./ no extr. | medium swel./ high extr. |
| NaCl/ H2O | 83.9 | −20.94 | 17.4 | 0.3 * | 6.8 | 4 * | 0.8 | no swel./ no extr. | no swel./ no extr. | low swel./ no extr. | no swel./ no extr. |
| Al(NO3)3/ H2O | 62.73 | −30.38 | 23.55 | 101.5 | 92.7 | 101.4 | 101.1 | no swel./ no extr. | no swel./ no extr. | low swel./ no extr. | low swel./ no extr. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamatiou, A.; Müller, L.; Zimmermann, R.; Hillis, J.; Oliver, D.; Fisher, K.; Zaglio, M.; Worlitschek, J. Experimental Characterization of Phase Change Materials for Refrigeration Processes. Energies 2021, 14, 3033. https://doi.org/10.3390/en14113033
Stamatiou A, Müller L, Zimmermann R, Hillis J, Oliver D, Fisher K, Zaglio M, Worlitschek J. Experimental Characterization of Phase Change Materials for Refrigeration Processes. Energies. 2021; 14(11):3033. https://doi.org/10.3390/en14113033
Chicago/Turabian StyleStamatiou, Anastasia, Lukas Müller, Roger Zimmermann, Jamie Hillis, David Oliver, Kate Fisher, Maurizio Zaglio, and Jörg Worlitschek. 2021. "Experimental Characterization of Phase Change Materials for Refrigeration Processes" Energies 14, no. 11: 3033. https://doi.org/10.3390/en14113033
APA StyleStamatiou, A., Müller, L., Zimmermann, R., Hillis, J., Oliver, D., Fisher, K., Zaglio, M., & Worlitschek, J. (2021). Experimental Characterization of Phase Change Materials for Refrigeration Processes. Energies, 14(11), 3033. https://doi.org/10.3390/en14113033







