Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review
Abstract
:1. Introduction
2. Philosophies and Concept of Sorption Heat Storage
- Adsorption, a surface interaction phenomenon generally between a solid and a gas.
- Absorption, a volume interaction phenomenon in which the adsorbate molecules diffuse into the adsorbent volume (e.g., a gas or a liquid that goes into solution in another liquid).
- Physical adsorption or physisorption: the fixation of adsorbate molecules to the adsorbent surface is done primarily by van der Waals forces (10–100 meV) and the forces due to polarization electrostatic interactions for adsorbents with an ionic structure (e.g., zeolite). It occurs without changing the molecular structure and is generally reversible: the adsorbed molecules can be desorbed by reducing the pressure or increasing the temperature.
- The chemical adsorption or chemisorption: the process is the result of a chemical reaction with the formation of chemical bonds between the adsorbate and adsorbent surface. The binding energy (1–10 eV) is much stronger than that of physical adsorption and the process may be irreversible.
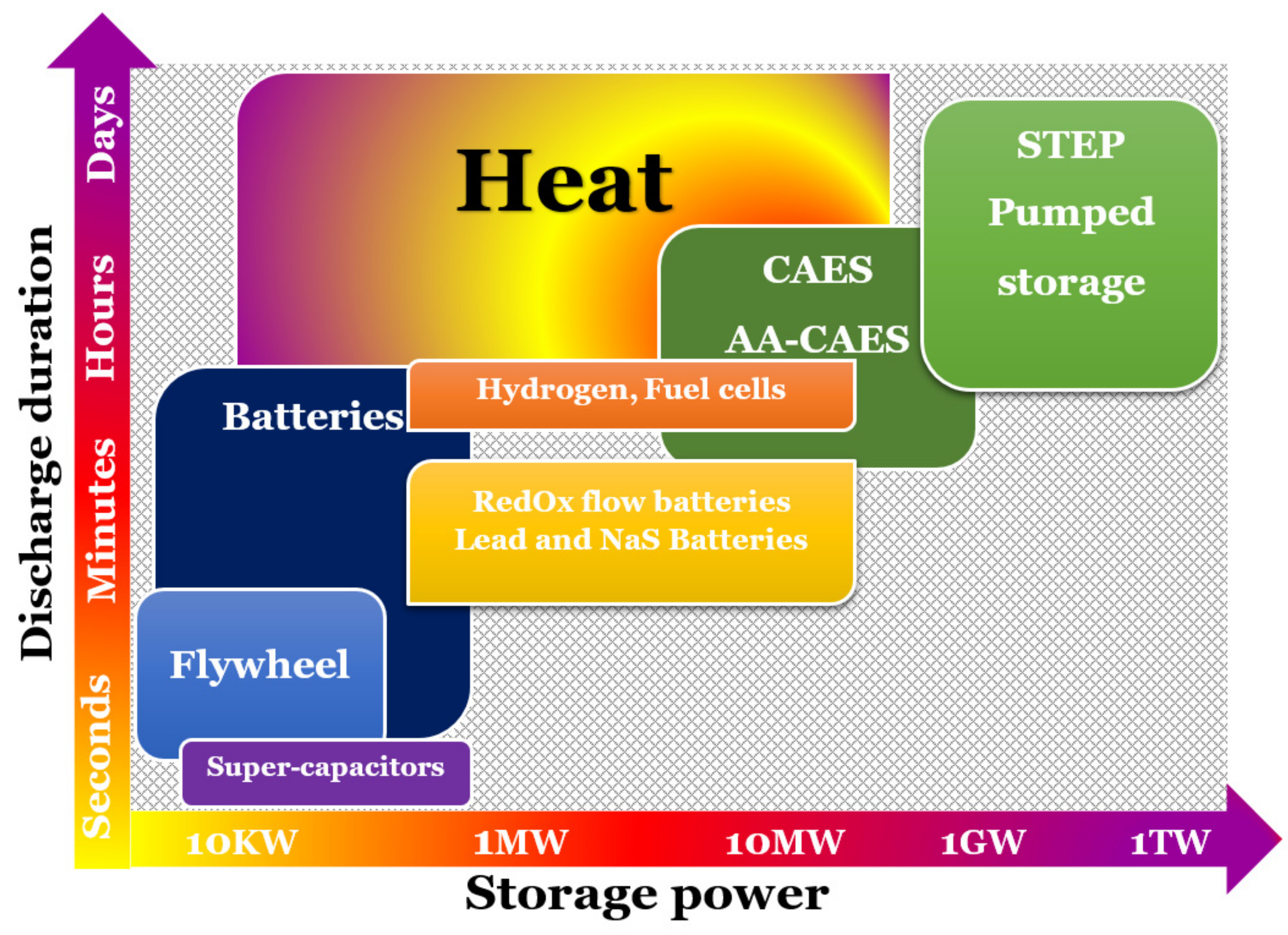
- The storage period (hours, days, or weeks) determines the time-consumption of the energy stored.
- The word “power” refers to how easily energy stored in a device can be charged and discharged. Expressly, power capacity (W) refers to the maximum amount of power that the storage device can produce during discharging. The power density (W/L) is the ratio of the power capacity to the energy storage system’s capacity.
- The amount of energy absorbed in the storage device throughout the charging process under nominal conditions is known as the energy storage capacity or energy capacity. The amount of stored energy in a device after it has been charged is determined by the storage process, storage medium, and system size.
- Energy density, also known as volumetric heat power, is the ratio of stored energy to the volume of the energy storage unit.
- The time required to charge or discharge the device is determined by the charge and discharge time. The cycling capacity, or number of cycles, is defined as the maximum number of charge–discharge cycles possible under the given conditions.
- Self-discharge is the amount of energy initially accumulated and dissipated over a given non-use time.
- The ratio of energy delivered and the energy required to charge the storage device is known as efficiency. It takes into account the energy losses that occur during storage and the charge/discharge cycle.
- The rate at which energy is adsorbed or released is known as the response time [h].
- The time needed for the storage device in order to release energy after each recharge is referred to as the cycle life.
- Cost is a metrics that describes the overall cost in terms of total capacity (€/kWh) or power (€/kW). These are the storage equipment’s capital costs as well as the operating and repair costs over its lifetime.
- The cost per unit of energy divided by the storage efficiency ratio is recognized as cost per output (useful) energy.
- The cost per cycle is calculated by dividing the cost per unit of energy by the cycle life.
3. An Overview about the Use of Salts
4. Recent Research of Salt Hydrate Material in TCES Applications

5. Composites Based Salt Hydrates
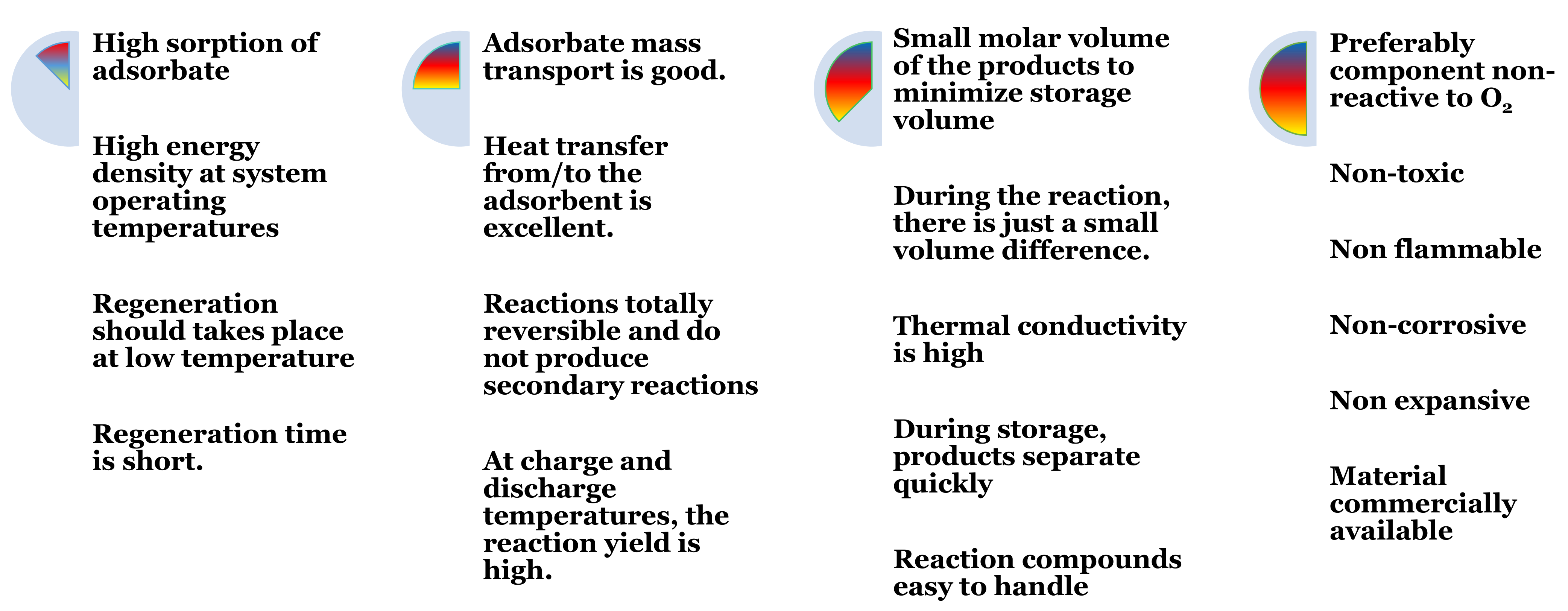
- Due to their high and well-developed porosity, these adsorbents can contain significant quantities of salt hydrate.
- During the hydration process, water vapor can adsorb on the porous matrix by van der Waals forces, and on the anhydrous salt by chemisorption to form salt hydrates.
- The saturated salt solution on the surface of the salt hydrates may be stored in a porous matrix without leakage of the solution, even though excessive water sorption and deliquescence occur. This increases the mechanical strength and overall ability of the products to be sorted.
- The efficiency of heat and mass transfer can be increased by the use of abundant channels.
- The swelling of adsorbents decreases the transfer of heat and severe agglomeration decreases the transfer of mass.
- A porous matrix allows salt hydrates to be confined into pores on a micrometer scale, resulting in a larger specific surface area and more gas diffusion pathways.
- Reasonable drop in pressure to speed up the reaction. In addition, the porous matrix has a great thermal conductivity.
- Composite properties help prevent salt hydrates from swelling and agglomerating, and enhance the heat and mass transfer and the stability of the cycle.
- The mixing parameters can be adjusted to change the properties of the materials.
- The properties of salt hydrates in composite adsorbents and change monomeric materials.
- Changing factors such as material type, pore size, mixing ratio, and preparation conditions can modify the adsorption properties within a certain range.
6. Composites Based Binary Salts
7. Safety, Corrosion Effect, and Recommendations
8. Conclusions and Outlook
- Decrease of unoccupied pores;
- Probable deliquescence;
- Leakage of salt from the composite; and
- Degradation.
- In terms of material, the strategic task is to decrease the prices of the available materials, with the aim to make TES sorption systems more competitive. At this stage, many efforts are devoted to the use of cheaper raw materials and diminishing the hydrophilicity of traditional zeolites, which needs high energy consumption (desorption temperature) that is unattainable, for instance, by traditional solar thermal collectors.
- A deep examination on sorption heat storage is strongly required at diverse scales and several conditions can help to compare experimental studies in a standardized way. Recently, metal organic frameworks (MOFs) and aluminophosphates have shown a remarkable result that needs more focus, thanks to their promising features. Materials for TES sorption systems require more research to discover an appropriate active material with acceptable energy density, hydrothermal stability, and cyclability under the operating conditions of the device.
- Composite materials are being studied in order to diminish the instabilities at salt hydrate material levels. If a high enough desorption temperature is achieved, the host matrices can be made of a porous material that can also act as an adsorbent. Small pore sizes, which are needed for the matrix to participate in the sorption process, result in little salt impregnated in the matrix. Ineffective materials including expanded graphite, sand, silica gel, and vermiculite, on the other hand, have been studied solely for structural support. Numerous studies have been published, but further work is being done to find promising working pairs. Diminished mass transport inside the matrix pores as well as salt deliquescence or overhydration can lead to active material leakage. Finally, the experimental conditions of the examined studies are heterogeneous and some of them are far from the characteristic conditions of low-temperature heat storage. Furthermore, more research is required to fully and deeply comprehend the kinetics and mechanisms that have occurred by using composite materials in TES sorption systems.
- For upcoming studies on sorption heat storage systems, some critical points also should be considered: For instance, the energy density at different stages of the search need to be determined by setting a common reference temperature. In addition, along with the energy density, the required volume used must be defined.
- Only a few studies have concentrated on the economic viability of the systems. This is partly due to the fact that analysis is always at the material and laboratory scale; thus, broad economic surveys will possibly lead to misleading results.
- The economic feasibility of the system has not been well examined because there are many challenges not yet achieved at material and lab-scales. Consequently, broad economic surveys would likely lead to ambiguous outcomes. Nevertheless, the central indicators allied to material cost, system complexity, and auxiliary energy consumption system should be considered in order to have an idea about the estimation of system cost-effectiveness.
- The cost of materials will already give an idea of how profitable and potential the system will be in a given application. For cost estimation, all components and auxiliary systems must be considered when increasing the scale. Supplementary economic considerations that can be highlighted at commercial scale (Prototype) linked to system operations such as lifetime and maintenance costs may perhaps also be involved to evaluate the rentability analysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| TES | Thermal energy storage |
| AEO | Annual energy outlook |
| REST | Renewable energy storage technologies |
| EES | Electrical energy storage |
| ECES | Electrochemical energy storage |
| MES | Mechanical energy storage |
| CES | Chemical energy storage |
| SHS | Sensible heat storage |
| LHS | Latent heat storage |
| TCES | Thermochemical energy storage |
| CAES | Compressed air energy storage |
| AA-CAES | Advanced adiabatic compressed air energy storage system |
| STEP | Pumping energy transfer stations |
| Cp | Heat capacity (J/(kg K)) |
| PCM | Phase change material |
| ∆H | Standard reaction enthalpy (J/mol) |
| Tfusion | Fusion temperature (°C) |
| ΔT | Temperature difference (°C) |
| pvap | Vapor pressure (mbar) |
| ECN | Energy Research Center of the Netherlands |
| RHD | Relative humidity of deliquescence |
| λ | Thermal conductivity (W/m °C) |
| PSD | Pore size distribution (nm) |
| ACF | Activated carbon fiber |
| LD50 | Lethal Dose for 50% of subjects |
| MSDS | Material Safety Data Sheet |
| MOFs | Metal organic frameworks |
References
- Leonzio, G. Solar systems integrated with absorption heat pumps and thermal energy storages: State of art. Renew. Sustain. Energy Rev. 2017, 70, 492–505. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A review on long-term sorption solar energy storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396. [Google Scholar] [CrossRef]
- Matera, F.V.; Gatto, I.; Patti, A.; Passalacqua, E. Fuel cell performance assessment for closed-loop renewable energy systems. J. Energy Chem. 2016, 25, 531–538. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Nagel, T.; Beckert, S.; Lehmann, C.; Gläser, R.; Kolditz, O. Multi-physical continuum models of thermochemical heat storage and transformation in porous media and powder beds—A review. Appl. Energy 2016, 178, 323–345. [Google Scholar] [CrossRef]
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef]
- Peng, X.; Root, T.W.; Maravelias, C.T. Storing solar energy with chemistry: The role of thermochemical storage in concentrating solar power. Green Chem. 2017, 19, 2427–2438. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy storage technologies and real life applications—A state of the art review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef] [Green Version]
- Desai, F.; Seyedhassantehrani, N.; Shagar, M.; Gu, S.; Asmatulu, R. Preparation and characterization of KOH-treated electrospun nanofiber mats as electrodes for iron-based redox-flow batteries. J. Energy Storage 2020, 27, 101053. [Google Scholar] [CrossRef]
- Energy Information Administration (EIA). Energy Information Administration: Annual Energy Outlook 2018 with Projections to 2050; EIA: Washington, DC, USA, 2018.
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- Pereira da Cunha, J.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Desai, F.; Sunku Prasad, J.; Muthukumar, P.; Rahman, M.M. Thermochemical energy storage system for cooling and process heating applications: A review. Energy Convers. Manag. 2021, 229, 113617. [Google Scholar] [CrossRef]
- Berseneff, B.; Perrin, M.; Tran-Quoc, T.; Brault, P.; Mermilliod, N.; Hadjsaid, N.; Delaplagne, T.; Martin, N.; Crouzevialle, B. The significance of energy storage for renewable energy generation and the role of instrumentation and measurement. IEEE Instrum. Meas. Mag. 2014, 17, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Baeyens, J.; Cáceres, G.; Degrève, J.; Lv, Y. Thermal energy storage: Recent developments and practical aspects. Prog. Energy Combust. Sci. 2016, 53, 1–40. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. Closed and open thermochemical energy storage: Energy- and exergy-based comparisons. Energy 2012, 41, 83–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Li, T.; Zhao, Y. Thermochemical Characterizations of Novel Vermiculite-LiCl Composite Sorbents for Low-Temperature Heat Storage. Energies 2016, 9, 854. [Google Scholar] [CrossRef] [Green Version]
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the art on gas–solid thermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A review of chemical heat pump technology and applications. Appl. Therm. Eng. 2001, 21, 1489–1519. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Zhao, Y.J.; Li, T.X.; Riffat, S.B.; Wajid, N.M. Development and thermochemical characterizations of vermiculite/SrBr2 composite sorbents for low-temperature heat storage. Energy 2016, 115, 120–128. [Google Scholar] [CrossRef]
- Hasnain, S.M. Review on sustainable thermal energy storage technologies, Part I: Heat storage materials and techniques. Energy Convers. Manag. 1998, 39, 1127–1138. [Google Scholar] [CrossRef]
- Hasnain, S.M. Review on sustainable thermal energy storage technologies, Part II: Cool thermal storage. Energy Convers. Manag. 1998, 39, 1139–1153. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M. Thermal Energy Storage Systems and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9780470747063. [Google Scholar]
- Shabgard, H.; Bergman, T.L.; Sharifi, N.; Faghri, A. High temperature latent heat thermal energy storage using heat pipes. Int. J. Heat Mass Transf. 2010, 53, 2979–2988. [Google Scholar] [CrossRef]
- Ermis, K.; Erek, A.; Dincer, I. Heat transfer analysis of phase change process in a finned-tube thermal energy storage system using artificial neural network. Int. J. Heat Mass Transf. 2007, 50, 3163–3175. [Google Scholar] [CrossRef]
- Iten, M.; Liu, S. A work procedure of utilising PCMs as thermal storage systems based on air-TES systems. Energy Convers. Manag. 2014, 77, 608–627. [Google Scholar] [CrossRef]
- Caraballo, A.; Galán-Casado, S.; Caballero, Á.; Serena, S. Molten Salts for Sensible Thermal Energy Storage: A Review and an Energy Performance Analysis. Energies 2021, 14, 1197. [Google Scholar] [CrossRef]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Khare, S.; Dell’Amico, M.; Knight, C.; McGarry, S. Selection of materials for high temperature latent heat energy storage. Sol. Energy Mater. Sol. Cells 2012, 107, 20–27. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Sircar, S. Book Review. Principles of Adsorption and Adsorption Processes. Langmuir 1985, 1, 529. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—from theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Thinsurat, K.; Bao, H.; Ma, Z.; Roskilly, A.P. Performance study of solar photovoltaic-thermal collector for domestic hot water use and thermochemical sorption seasonal storage. Energy Convers. Manag. 2019, 180, 1068–1084. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.T.; Xu, Z.Y.; Wang, R.Z. Enhanced sorption heat transportation cycles with large concentration glide. Energy Convers. Manag. 2019, 201, 112145. [Google Scholar] [CrossRef]
- Airò Farulla, G.; Cellura, M.; Guarino, F.; Ferraro, M. A Review of Thermochemical Energy Storage Systems for Power Grid Support. Appl. Sci. 2020, 10, 3142. [Google Scholar] [CrossRef]
- Enescu, D.; Chicco, G.; Porumb, R.; Seritan, G. Thermal Energy Storage for Grid Applications: Current Status and Emerging Trends. Energies 2020, 13, 340. [Google Scholar] [CrossRef] [Green Version]
- Del Pero, C.; Aste, N.; Paksoy, H.; Haghighat, F.; Grillo, S.; Leonforte, F. Energy storage key performance indicators for building application. Sustain. Cities Soc. 2018, 40, 54–65. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, L.F.; Solé, A.; Barreneche, C. Review on sorption materials and technologies for heat pumps and thermal energy storage. Renew. Energy 2017, 110, 3–39. [Google Scholar] [CrossRef] [Green Version]
- Abedin, A.H. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Kohlhepp, P.; Harb, H.; Wolisz, H.; Waczowicz, S.; Müller, D.; Hagenmeyer, V. Large-scale grid integration of residential thermal energy storages as demand-side flexibility resource: A review of international field studies. Renew. Sustain. Energy Rev. 2019, 101, 527–547. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Luo, L. A review of potential materials for thermal energy storage in building applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.Z.; Li, Y. A review of available technologies for seasonal thermal energy storage. Sol. Energy 2014, 103, 610–638. [Google Scholar] [CrossRef]
- Ding, Y.; Riffat, S.B. Thermochemical energy storage technologies for building applications: A state-of-the-art review. Int. J. Low Carbon Technol. 2013, 8, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Wang, R.Z.; Wang, L.W. Sorption thermal storage for solar energy. Prog. Energy Combust. Sci. 2013, 39, 489–514. [Google Scholar] [CrossRef]
- Ervin, G. Solar heat storage using chemical reactions. J. Solid State Chem. 1977, 22, 51–61. [Google Scholar] [CrossRef]
- Fopah-Lele, A.; Tamba, J.G. A review on the use of SrBr2·6H2O as a potential material for low temperature energy storage systems and building applications. Sol. Energy Mater. Sol. Cells 2017, 164, 175–187. [Google Scholar] [CrossRef]
- Krese, G.; Koželj, R.; Butala, V.; Stritih, U. Thermochemical seasonal solar energy storage for heating and cooling of buildings. Energy Build. 2018, 164, 239–253. [Google Scholar] [CrossRef]
- Lizana, J.; Chacartegui, R.; Barrios-Padura, A.; Ortiz, C. Advanced low-carbon energy measures based on thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2018, 82, 3705–3749. [Google Scholar] [CrossRef]
- Kuznik, F.; Johannes, K.; Obrecht, C.; David, D. A review on recent developments in physisorption thermal energy storage for building applications. Renew. Sustain. Energy Rev. 2018, 94, 576–586. [Google Scholar] [CrossRef]
- Sunku Prasad, J.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- Palomba, V.; Frazzica, A. Recent advancements in sorption technology for solar thermal energy storage applications. Sol. Energy 2019, 192, 69–105. [Google Scholar] [CrossRef]
- Fumey, B.; Weber, R.; Baldini, L. Sorption based long-term thermal energy storage—Process classification and analysis of performance limitations: A review. Renew. Sustain. Energy Rev. 2019, 111, 57–74. [Google Scholar] [CrossRef]
- Wu, H.; Salles, F.; Zajac, J. A Critical Review of Solid Materials for Low-Temperature Thermochemical Storage of Solar Energy Based on Solid-Vapour Adsorption in View of Space Heating Uses. Molecules 2019, 24, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Zhao, Q.; Huang, H.; Mao, H.; Liu, Y.; Xiao, Y. Applications of low-temperature thermochemical energy storage systems for salt hydrates based on material classification: A review. Sol. Energy 2021, 214, 149–178. [Google Scholar] [CrossRef]
- van de Voort, I.M. Characterization of a thermochemical storage material. Master’s Thesis, Eindhoven University, Eindhoven, The Netherlands, 2007. [Google Scholar]
- Ferchaud, C.; Zondag, H.; Veldhuis, J.; de Boer, R. Study of the reversible water vapour sorption process of MgSO4. 7H2O and MgCl2. 6H2O under the conditions of seasonal solar heat storage. J. Phys. Conf. Ser. 2012, 395, 012069. [Google Scholar] [CrossRef] [Green Version]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K.L. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Wagman, D.D.; Evans, W.H.; Parker, V.B.; Schumm, R.H.; Halow, I.; Bailey, S.M.; Churney, K.L.; Nuttall, R.L. The NBS Tables of Chemical Thermodynamic Properties: Selected Values for Inorganic and C1 and C2 Organic Substances in SI Units; American Chemical Society and the American Institute of Physics for the National Bureau of Standards: New York, NY, USA, 1982; Volume 11. [Google Scholar]
- Yasuda, K. Method of Recovering Heat of Reaction 1986. U.S. Patent 4,616,692, 14 October 1986. [Google Scholar]
- Resh, H. Physical Constants of Inorganic Compounds. In Hydroponic Food Production; CRC Press: Boca Raton, FL, USA, 2012; pp. 485–486. [Google Scholar]
- Visscher, K.; Veldhuis, J.B.J.; Oonk, H.A.J.; Ekeren, P.J.; Van Blok, J.G. Compacte Chemische Seizoensopslag van Zonnewarmte. 2004. Available online: https://adoc.pub/compacte-chemische-seizoensopslag-van-zonnewarmte-eindrappor.html (accessed on 26 May 2021).
- Iyimen-Schwarz, Z.; Lechner, M.D. Energiespeicherung durch chemische reaktionen. I. DSC-messungen zur quantitativen verfolgung der enthalpieänderungen von speicherstoffen für die hin- und rückreaktion. Thermochim. Acta 1983, 68, 349–361. [Google Scholar] [CrossRef]
- Grevel, K.-D.; Majzlan, J. Internally consistent thermodynamic data for magnesium sulfate hydrates. Geochim. Cosmochim. Acta 2009, 73, 6805–6815. [Google Scholar] [CrossRef]
- Zondag, H.; Kikkert, B.; Smeding, S.; de Boer, R.; Bakker, M. Prototype thermochemical heat storage with open reactor system. Appl. Energy 2013, 109, 360–365. [Google Scholar] [CrossRef]
- Guarini, G.G.T.; Piccini, S. The dehydration of Na2S2O3·5H2O single crystals as studied by thermal analysis and optical microscopy. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 331. [Google Scholar] [CrossRef]
- Trausel, F.; de Jong, A.-J.; Cuypers, R. A Review on the Properties of Salt Hydrates for Thermochemical Storage. Energy Procedia 2014, 48, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Mauran, S.; Lahmidi, H.; Goetz, V. Solar heating and cooling by a thermochemical process. First experiments of a prototype storing 60 kWh by a solid/gas reaction. Sol. Energy 2008, 82, 623–636. [Google Scholar] [CrossRef]
- Michel, B.; Mazet, N.; Mauran, S.; Stitou, D.; Xu, J. Thermochemical process for seasonal storage of solar energy: Characterization and modeling of a high density reactive bed. Energy 2012. [Google Scholar] [CrossRef] [Green Version]
- Lahmidi, H.; Mauran, S.; Goetz, V. Definition, test and simulation of a thermochemical storage process adapted to solar thermal systems. Sol. Energy 2006, 80, 883–893. [Google Scholar] [CrossRef]
- van Essen, V.M.; Cot Gores, J.; Bleijendaal, L.P.J.; Zondag, H.A.; Schuitema, R.; Bakker, M.; van Helden, W.G.J. Characterization of Salt Hydrates for Compact Seasonal Thermochemical Storage. In Proceedings of the ASME 2009 3rd International Conference on Energy Sustainability; ASMEDC, 2009; Amer Society of Mechanical: New York, NY, USA, 2009; Volume 2, pp. 825–830. [Google Scholar]
- Deshpande, D.A.; Ghormare, K.R.; Deshpande, N.D.; Tankhiwale, A.V. Dehydration of crystalline K2CO3·1.5 H2O. Thermochim. Acta 1983. [Google Scholar] [CrossRef]
- Galwey, A.K.; Brown, M.E. Thermal Decomposition of Ionic Solids: Chemical Properties and Reactivities of Ionic Crystalline Phases; Elsevier: Amsterdam, The Netherlands, 1999; ISBN 0444824375. [Google Scholar]
- Mirzaev, S.M.; Yakubov, Y.N.; Akhmedov, A.A.; Boltaev, S.A.; Shodiev, O.K. Experimental study of the temperature dependence of the CaCl2, SrCl2, CaCl2·6H2O, and SrCl2·6H2O absorbent heat conductivity. Appl. Sol. Energy (English Transl. Geliotekhnika) 1996, 32, 65–70. [Google Scholar]
- Michel, B.; Neveu, P.; Mazet, N. Comparison of closed and open thermochemical processes, for long-term thermal energy storage applications. Energy 2014, 72, 702–716. [Google Scholar] [CrossRef] [Green Version]
- Fopah Lele, A.; Kuznik, F.; Rammelberg, H.U.; Schmidt, T.; Ruck, W.K.L. Thermal decomposition kinetic of salt hydrates for heat storage systems. Appl. Energy 2015, 154, 447–458. [Google Scholar] [CrossRef]
- Balasubramanian, G.; Ghommem, M.; Hajj, M.R.; Wong, W.P.; Tomlin, J.A.; Puri, I.K. Modeling of thermochemical energy storage by salt hydrates. Int. J. Heat Mass Transf. 2010, 53, 5700–5706. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Glaznev, I.S.; Freni, A.; Restuccia, G. Kinetics of water sorption on SWS-1L (calcium chloride confined to mesoporous silica gel): Influence of grain size and temperature. Chem. Eng. Sci. 2006, 61, 1453–1458. [Google Scholar] [CrossRef]
- Prieto, C.; Cooper, P.; Fernández, A.I.; Cabeza, L.F. Review of technology: Thermochemical energy storage for concentrated solar power plants. Renew. Sustain. Energy Rev. 2016, 60, 909–929. [Google Scholar] [CrossRef] [Green Version]
- Visscher, K.; Veldhuis, J.B.J. Comparison of candidate materials for seasonal storage of solar heat through dynamic simulation of building and renewable energy system. In Proceedings of the IBPSA 2005—International Building Performance Simulation Association 2005, Montreal, QC, Canada, 15–18 August 2005. [Google Scholar]
- Bertsch, F.; Mette, B.; Asenbeck, S.; Kerskes, H.; Müller-Steinhagen, H. Low temperature chemical heat storage—An investigation of hydration reactions. In Proceedings of the Effstock Conference 2009, Stockholm, Sweden, 14–17 July 2009. [Google Scholar]
- van Essen, M.; Bleijendaal, L.P.J.; Kikkert, B.W.J.; Zondag, H.A.; Bakker, M.; Bach, P.W. Development of a Compact Heat Storage System Based on Salt Hydrates. In Proceedings of the EuroSun 2010 Conference, Freiburg, Germany, 28 September–1 October 2010; pp. 1–8. [Google Scholar]
- Fopah Lele, A.; Kuznik, F.; Opel, O.; Ruck, W.K.L. Performance analysis of a thermochemical based heat storage as an addition to cogeneration systems. Energy Convers. Manag. 2015, 106, 1327–1344. [Google Scholar] [CrossRef]
- Ferchaud, C.J.; Zondag, H.A.; Rubino, A.; De Boer, R. Seasonal sorption heat storage—Research on thermochemical materials and storage performance. In Proceedings of the Heat Power Cycle 2012, Alkmaar, The Netherlands, 10–12 September 2012; pp. 1–7. [Google Scholar]
- Zondag, H.A.; Van Essen, V.M.; Bleijendaal, L.P.J.; Kikkert, B.W.J.; Bakker, M. Application of MgCl2.6H2O for Thermochemical Seasonal Solar Heat Storage; U.S. Department of Energy: Washington, DC, USA, 2010.
- van Essen, V.M.; Zondag, H.A.; Gores, J.C.; Bleijendaal, L.P.J.; Bakker, M.; Schuitema, R.; van Helden, W.G.J.; He, Z.; Rindt, C.C.M. Characterization of MgSO4 Hydrate for Thermochemical Seasonal Heat Storage. J. Sol. Energy Eng. 2009, 131. [Google Scholar] [CrossRef]
- Ferchaud, C.; Zondag, H.; De Boer, R.; Rindt, C. Characterization of the sorption process in thermochemical materials for seasonal solar heat storage application. In Proceedings of the 12th International Conference on Energy Storage, Lleida, Spain, 16–18 March 2012; pp. 1–10. [Google Scholar]
- Donkers, P.A.J.; Pel, L.; Adan, O.C.G. Experimental studies for the cyclability of salt hydrates for thermochemical heat storage. J. Energy Storage 2016, 5, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, F.; Jaehnig, D.; Asenbeck, S.; Kerskes, H.; Drueck, H.; Wagner, W.; Weiss, W. Comparison of the Thermal Performance of a Solar Heating System with Open and Closed Solid Sorption Storage. Energy Procedia 2014, 48, 280–289. [Google Scholar] [CrossRef] [Green Version]
- de Jong, A.-J.; Trausel, F.; Finck, C.; van Vliet, L.; Cuypers, R. Thermochemical Heat Storage—System Design Issues. Energy Procedia 2014, 48, 309–319. [Google Scholar] [CrossRef] [Green Version]
- de Boer, R.; Haije, W.G.; Veldhuis, J.B.J.; Smeding Petten, S.F. Solid-Sorption Cooling with Integrated Thermal Storage. The SWEAT Prototype; U.S. Department of Energy location: Washington, DC, USA, 2004.
- Donkers, P.A.J.; Sögütoglu, L.C.; Huinink, H.P.; Fischer, H.R.; Adan, O.C.G. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Beckert, S.; Pel, L.; Stallmach, F.; Steiger, M.; Adan, O.C.G. Water Transport in MgSO4·7H2O during Dehydration in View of Thermal Storage. J. Phys. Chem. C 2015, 119, 28711–28720. [Google Scholar] [CrossRef]
- Posern, K.; Kaps, C. Humidity controlled calorimetric investigation of the hydration of MgSO4 hydrates. J. Therm. Anal. Calorim. 2008, 92, 905–909. [Google Scholar] [CrossRef]
- Rammelberg, H.U.; Schmidt, T.; Ruck, W. Hydration and dehydration of salt hydrates and hydroxides for thermal energy storage—Kinetics and energy release. Energy Procedia 2012, 30, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Barreneche, C.; Fernández, A.I.; Cabeza, L.F.; Cuypers, R. Thermophysical characterization and thermal cycling stability of two TCM: CaCl2 and zeolite. Appl. Energy 2015, 137, 726–730. [Google Scholar] [CrossRef] [Green Version]
- Molenda, M.; Stengler, J.; Linder, M.; Wörner, A. Reversible hydration behavior of CaCl2 at high H2O partial pressures for thermochemical energy storage. Thermochim. Acta 2013, 560, 76–81. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Rammelberg, H.U.; Lele, A.F.; Korhammer, K.; Watts, B.A.; Schmidt, T.; Ruck, W.K.L. A review on the use of calcium chloride in applied thermal engineering. Appl. Therm. Eng. 2015, 75, 513–531. [Google Scholar] [CrossRef]
- Posern, K.; Kaps, C. Calorimetric studies of thermochemical heat storage materials based on mixtures of MgSO4 and MgCl2. Thermochim. Acta 2010, 502, 73–76. [Google Scholar] [CrossRef]
- Conde, M.R. Properties of aqueous solutions of lithium and calcium chlorides: Formulations for use in air conditioning equipment design. Int. J. Therm. Sci. 2004, 43, 367–382. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, K.-H.; Kim, H. Effective Thermal Conductivity of Graphite—Metallic Salt Complex for Chemical Heat Pumps. J. Thermophys. Heat Transf. 1999, 13, 481–488. [Google Scholar] [CrossRef]
- Mauran, S.; Prades, P.; L’Haridon, F. Heat and mass transfer in consolidated reacting beds for thermochemical systems. Heat Recover. Syst. CHP 1993, 13, 315–319. [Google Scholar] [CrossRef]
- Wang, K.; Wu, J.Y.; Wang, R.Z.; Wang, L.W. Effective thermal conductivity of expanded graphite–CaCl2 composite adsorbent for chemical adsorption chillers. Energy Convers. Manag. 2006, 47, 1902–1912. [Google Scholar] [CrossRef]
- Abhat, A.; Huy, T.Q. Heat and mass transfer considerations in a thermochemicalenergy storage system based on solid-gas reactions. Sol. Energy 1983, 30, 93–98. [Google Scholar] [CrossRef]
- Dellero, T.; Sarmeo, D.; Touzain, P. A chemical heat pump using carbon fibers as additive. Part I: Enhancement of thermal conduction. Appl. Therm. Eng. 1999, 19, 991–1000. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, K.-H. Gas permeability of expanded graphite–metallic salt composite. Appl. Therm. Eng. 2001, 21, 453–463. [Google Scholar] [CrossRef]
- Druske, M.-M.; Fopah-Lele, A.; Korhammer, K.; Rammelberg, H.U.; Wegscheider, N.; Ruck, W.; Schmidt, T. Developed Materials for Thermal Energy Storage: Synthesis and Characterization. Energy Procedia 2014, 61, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Kerskes, H.; Mette, B.; Bertsch, F.; Asenbeck, S.; Drück, H. Development of a Thermo-Chemical Energy Storage for Solar Thermal Applications. In Proceedings of the ISES Solar World Congress 2011, Freiburg, Germany, 28 August–2 September 2011; pp. 1–12. [Google Scholar]
- Yu, N.; Wang, R.Z.; Lu, Z.S.; Wang, L.W. Study on consolidated composite sorbents impregnated with LiCl for thermal energy storage. Int. J. Heat Mass Transf. 2015, 84, 660–670. [Google Scholar] [CrossRef]
- Kerskes, H.; Asenbeck, S.; Mette, B.; Bertsch, F.; Müller-Steinhagen, H. Experimental and Numerical Investigations on Thermo Chemical Heat Storage. In Proceedings of the EuroSun 2010 Conference, International Solar Energy Society, Freiburg, Germany, 28 September–1 October 2010; pp. 1–10. [Google Scholar]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. Water sorption heats on silica-alumina-based composites for interseasonal heat storage. J. Therm. Anal. Calorim. 2014. [Google Scholar] [CrossRef]
- Hongois, S.; Kuznik, F.; Stevens, P.; Roux, J.-J. Development and characterisation of a new MgSO4-zeolite composite for long-term thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1831–1837. [Google Scholar] [CrossRef]
- Tokarev, M.; Gordeeva, L.; Romannikov, V.; Glaznev, I.; Aristov, Y. New composite sorbent CaCl2 in mesopores for sorption cooling/heating. Int. J. Therm. Sci. 2002, 41, 470–474. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Wang, R.; Xu, Y.; Wang, S.; Li, X. Study of the performance of activated carbon–methanol adsorption systems concerning heat and mass transfer. Appl. Therm. Eng. 2003, 23, 1605–1617. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Grekova, A.D.; Krieger, T.A.; Aristov, Y.I. Adsorption properties of composite materials (LiCl+LiBr)/silica. Microporous Mesoporous Mater. 2009, 126, 262–267. [Google Scholar] [CrossRef]
- Ristić, A.; Henninger, S.K. Sorption Composite Materials for Solar Thermal Energy Storage. Energy Procedia 2014, 48, 977–981. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Glaznev, I.S.; Savchenko, E.V.; Malakhov, V.V.; Aristov, Y.I. Impact of phase composition on water adsorption on inorganic hybrids “salt/silica. J. Colloid Interface Sci. 2006, 301, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Veselovskaya, J.V.; Critoph, R.E.; Thorpe, R.N.; Metcalf, S.; Tokarev, M.M.; Aristov, Y.I. Novel ammonia sorbents “porous matrix modified by active salt” for adsorptive heat transformation: 3. Testing of “BaCl2/vermiculite” composite in a lab-scale adsorption chiller. Appl. Therm. Eng. 2010, 30, 1188–1192. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Cacciola, G.; Restuccia, G. Selective water sorbents for multiple applications, 1. CaCl2 confined in mesopores of silica gel: Sorption properties. React. Kinet. Catal. Lett. 1996, 59, 325–333. [Google Scholar] [CrossRef]
- Aristov, Y.I. New family of solid sorbents for adsorptive cooling: Material scientist approach. J. Eng. Thermophys. 2007, 16, 63–72. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Mette, B.; Kerskes, H.; Drück, H.; Müller-Steinhagen, H. Experimental and numerical investigations on the water vapor adsorption isotherms and kinetics of binderless zeolite 13X. Int. J. Heat Mass Transf. 2014, 71, 555–561. [Google Scholar] [CrossRef]
- Shigeishi, R.A.; Langford, C.H.; Hollebone, B.R. Solar energy storage using chemical potential changes associated with drying of zeolites. Sol. Energy 1979, 23, 489–495. [Google Scholar] [CrossRef]
- Aristov, Y.I. Challenging offers of material science for adsorption heat transformation: A review. Appl. Therm. Eng. 2013, 50, 1610–1618. [Google Scholar] [CrossRef]
- Erlund, R.; Zevenhoven, R. Thermal energy storage (TES) capacity of a lab scale magnesium hydro carbonates/silica gel system. J. Energy Storage 2019, 25, 100907. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Wang, R. Composite Reactive Block for Heat Transformer System and Improvement of System Performance. J. Chem. Eng. JAPAN 2007, 40, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.G.; Wang, R.Z. A consolidated calcium chloride-expanded graphite compound for use in sorption refrigeration systems. Carbon N. Y. 2007, 45, 390–396. [Google Scholar] [CrossRef]
- Wang, K.; Wu, J.Y.; Wang, R.Z.; Wang, L.W. Composite adsorbent of CaCl2 and expanded graphite for adsorption ice maker on fishing boats. Int. J. Refrig. 2006, 29, 199–210. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, R.Z.; Li, T.X.; Nomura, Y. Investigation of a 10 kWh sorption heat storage device for effective utilization of low-grade thermal energy. Energy 2016, 113, 739–747. [Google Scholar] [CrossRef]
- Askalany, A.A.; Salem, M.; Ismail, I.M.; Ali, A.H.H.; Morsy, M.G. A review on adsorption cooling systems with adsorbent carbon. Renew. Sustain. Energy Rev. 2012, 16, 493–500. [Google Scholar] [CrossRef]
- Suzuki, M. Activated carbon fiber: Fundamentals and applications. Carbon N. Y. 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Tso, C.Y.; Chao, C.Y.H.; Fu, S.C. Performance analysis of a waste heat driven activated carbon based composite adsorbent—Water adsorption chiller using simulation model. Int. J. Heat Mass Transf. 2012, 55, 7596–7610. [Google Scholar] [CrossRef]
- Tso, C.Y.; Chao, C.Y.H. Activated carbon, silica-gel and calcium chloride composite adsorbents for energy efficient solar adsorption cooling and dehumidification systems. Int. J. Refrig. 2012, 35, 1626–1638. [Google Scholar] [CrossRef]
- Entezari, A.; Ejeian, M.; Wang, R.Z. Extraordinary air water harvesting performance with three phase sorption. Mater. Today Energy 2019, 13, 362–373. [Google Scholar] [CrossRef]
- Liu, H.; Nagano, K.; Sugiyama, D.; Togawa, J.; Nakamura, M. Honeycomb filters made from mesoporous composite material for an open sorption thermal energy storage system to store low-temperature industrial waste heat. Int. J. Heat Mass Transf. 2013, 65, 471–480. [Google Scholar] [CrossRef]
- Casey, S.P.; Elvins, J.; Riffat, S.; Robinson, A. Salt impregnated desiccant matrices for ‘open’ thermochemical energy storage—Selection, synthesis and characterisation of candidate materials. Energy Build. 2014, 84, 412–425. [Google Scholar] [CrossRef]
- Guan, W.; Li, J.; Qian, T.; Wang, X.; Deng, Y. Preparation of paraffin/expanded vermiculite with enhanced thermal conductivity by implanting network carbon in vermiculite layers. Chem. Eng. J. 2015, 277, 56–63. [Google Scholar] [CrossRef]
- Willers, E.; Wanner, M.; Groll, M. A multi-hydride thermal wave device for simultaneous heating and cooling. J. Alloys Compd. 1999, 293–295, 915–918. [Google Scholar] [CrossRef]
- Haije, W.G.; Veldhuis, J.B.J.; Smeding, S.F.; Grisel, R.J.H. Solid/vapour sorption heat transformer: Design and performance. Appl. Therm. Eng. 2007, 27, 1371–1376. [Google Scholar] [CrossRef] [Green Version]
- Aristov, Y.; Restuccia, G.; Cacciola, G.; Parmon, V. A family of new working materials for solid sorption air conditioning systems. Appl. Therm. Eng. 2002, 22, 191–204. [Google Scholar] [CrossRef]
- Dawoud, B.; Aristov, Y. Experimental study on the kinetics of water vapor sorption on selective water sorbents, silica gel and alumina under typical operating conditions of sorption heat pumps. Int. J. Heat Mass Transf. 2003, 46, 273–281. [Google Scholar] [CrossRef]
- Ferchaud, C.; Zondag, H.A.; De Boer, R. Material research on salt hydrates for seasonal heat storage application in a residential environment. In Proceedings of the International Symposium on Innovative Materials for Processes in Energy Systems 2013, Fukuoka, Japan, 4–6 September 2013. [Google Scholar]
- Tanashev, Y.Y.; Krainov, A.V.; Aristov, Y.I. Thermal conductivity of composite sorbents “salt in porous matrix” for heat storage and transformation. Appl. Therm. Eng. 2013, 61, 401–407. [Google Scholar] [CrossRef]
- Ponomarenko, I.V.; Glaznev, I.S.; Gubar, A.V.; Aristov, Y.I.; Kirik, S.D. Synthesis and water sorption properties of a new composite “CaCl2 confined into SBA-15 pores. Microporous Mesoporous Mater. 2010, 129, 243–250. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites ‘salt inside porous matrix’ for adsorption heat transformation: A current state-of-the-art and new trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef] [Green Version]
- Aristov, Y.I. Selective water sorbents, an new family of materials for adsorption cooling/heat: State-of-the art. In Proceedings of the Vminsk International Seminar “Heat Pipe, Heat Pumps, Refrigerators, Minsk, Belarus, 8–11 September 2003. [Google Scholar]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. Enhancing the heat storage density of silica–alumina by addition of hygroscopic salts (CaCl2, Ba(OH)2, and LiNO3). Sol. Energy Mater. Sol. Cells 2015, 140, 351–360. [Google Scholar] [CrossRef]
- Ristić, A.; Maučec, D.; Henninger, S.K.; Kaučič, V. New two-component water sorbent CaCl2-FeKIL2 for solar thermal energy storage. Microporous Mesoporous Mater. 2012, 164, 266–272. [Google Scholar] [CrossRef]
- Jänchen, J.; Ackermann, D.; Stach, H.; Brösicke, W. Studies of the water adsorption on Zeolites and modified mesoporous materials for seasonal storage of solar heat. Sol. Energy 2004, 76, 339–344. [Google Scholar] [CrossRef]
- The IEA Solar Heating and Cooling Task 32. Thermal Energy Storage for Solar and Low Energy Buildings: State of the Art; International Energy Agency (IEA): Lleida, Spain, 2005; ISBN 9788484098775. [Google Scholar]
- Xue, B.; Ye, S.; Zhang, L.; Wei, X.; Nakaso, K.; Fukai, J. High-temperature steam generation from low-grade waste heat from an adsorptive heat transformer with composite zeolite-13X/CaCl2. Energy Convers. Manag. 2019, 186, 93–102. [Google Scholar] [CrossRef]
- Casey, S.P.; Aydin, D.; Riffat, S.; Elvins, J. Salt impregnated desiccant matrices for ‘open’ thermochemical energy storage—Hygrothermal cyclic behaviour and energetic analysis by physical experimentation. Energy Build. 2015, 92, 128–139. [Google Scholar] [CrossRef]
- Tso, C.Y.; Chan, K.C.; Chao, C.Y.H.; Wu, C.L. Experimental performance analysis on an adsorption cooling system using zeolite 13X/CaCl2 adsorbent with various operation sequences. Int. J. Heat Mass Transf. 2015, 85, 343–355. [Google Scholar] [CrossRef]
- Nonnen, T.; Beckert, S.; Gleichmann, K.; Brandt, A.; Unger, B.; Kerskes, H.; Mette, B.; Bonk, S.; Badenhop, T.; Salg, F.; et al. A Thermochemical Long-Term Heat Storage System Based on a Salt/Zeolite Composite. Chem. Eng. Technol. 2016, 39, 2427–2434. [Google Scholar] [CrossRef]
- Whiting, G.; Grondin, D.; Bennici, S.; Auroux, A. Heats of water sorption studies on zeolite–MgSO4 composites as potential thermochemical heat storage materials. Sol. Energy Mater. Sol. Cells 2013, 112, 112–119. [Google Scholar] [CrossRef]
- Chan, K.C.; Tso, C.Y.; Wu, C.; Chao, C.Y.H. Enhancing the performance of a zeolite 13X/CaCl2–water adsorption cooling system by improving adsorber design and operation sequence. Energy Build. 2018, 158, 1368–1378. [Google Scholar] [CrossRef]
- Mahon, D.; Claudio, G.; Eames, P.C. An experimental investigation to assess the potential of using MgSO4 impregnation and Mg2+ ion exchange to enhance the performance of 13X molecular sieves for interseasonal domestic thermochemical energy storage. Energy Convers. Manag. 2017, 150, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.Q.; Tso, C.Y.; Chan, K.C.; Wu, C.L.; Chao, C.Y.H.; Chen, J.; He, W.; Luo, S.W. Experimental investigation on composite adsorbent—Water pair for a solar-powered adsorption cooling system. Appl. Therm. Eng. 2018, 131, 649–659. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Z.; Xie, Y.; Ren, Y.; Ye, F.; Ju, X. Study of the hydration behavior of zeolite-MgSO4 composites for long-term heat storage. Appl. Therm. Eng. 2018, 129, 250–259. [Google Scholar] [CrossRef]
- Xu, S.Z.; Lemington; Wang, R.Z.; Wang, L.W.; Zhu, J. A zeolite 13X/magnesium sulfate–water sorption thermal energy storage device for domestic heating. Energy Convers. Manag. 2018, 171, 98–109. [Google Scholar] [CrossRef]
- Xu, S.Z.; Wang, R.Z.; Wang, L.W.; Zhu, J. Performance characterizations and thermodynamic analysis of magnesium sulfate-impregnated zeolite 13X and activated alumina composite sorbents for thermal energy storage. Energy 2019, 167, 889–901. [Google Scholar] [CrossRef]
- Chan, K.C.; Chao, C.Y.H.; Sze-To, G.N.; Hui, K.S. Performance predictions for a new zeolite 13X/CaCl2 composite adsorbent for adsorption cooling systems. Int. J. Heat Mass Transf. 2012, 55, 3214–3224. [Google Scholar] [CrossRef]
- Cortés, F.B.; Chejne, F.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Moreno-Castilla, C. Water sorption on silica- and zeolite-supported hygroscopic salts for cooling system applications. Energy Convers. Manag. 2012, 53, 219–223. [Google Scholar] [CrossRef]
- Whiting, G.T.; Grondin, D.; Stosic, D.; Bennici, S.; Auroux, A. Zeolite–MgCl2 composites as potential long-term heat storage materials: Influence of zeolite properties on heats of water sorption. Sol. Energy Mater. Sol. Cells 2014, 128, 289–295. [Google Scholar] [CrossRef]
- Jänchen, J.; Ackermann, D.; Weiler, E.; Stach, H.; Brösicke, W. Calorimetric investigation on zeolites, AlPO4′s and CaCl2 impregnated attapulgite for thermochemical storage of heat. Thermochim. Acta 2005, 434, 37–41. [Google Scholar] [CrossRef]
- Gantenbein, P.; Rindt, C. Collection of Experimental Data on the Behavior of TCM/PCM-Materials to Benchmark Numerical Codes; Report A3.2 IEA SHC Task 42; International Energy Agency: Paris, France, 2012. [Google Scholar]
- Alefeld, G.; Maier-Laxhuber, P.; Rothmeyer, M. Thermochemical Heat Storage and Heat Transformation with Zeolites as Absorbents. In New Energy Conservation Technologies and Their Commercialization; Springer: Berlin/Heidelberg, Germany, 1981; pp. 796–820. [Google Scholar]
- Petrova, N.; Mizota, T.; Fujiwara, K. Hydration Heats of Zeolites for Evaluation of Heat Exchangers. J. Therm. Anal. Calorim. 2001, 64, 157–166. [Google Scholar] [CrossRef]
- Mizota, T.; Matsui, K.; Kasai, T.; Nakayama, N. Hydration enthalpies of synthetic Na-A, cation-exchanged-A and some natural zeolites for evaluating as heat exchange absorbents. Thermochim. Acta 1995, 266, 331–341. [Google Scholar] [CrossRef]
- Korhammer, K.; Druske, M.-M.; Fopah-Lele, A.; Rammelberg, H.U.; Wegscheider, N.; Opel, O.; Osterland, T.; Ruck, W. Sorption and thermal characterization of composite materials based on chlorides for thermal energy storage. Appl. Energy 2016, 162, 1462–1472. [Google Scholar] [CrossRef]
- Stach, H.; Mugele, J.; Jänchen, J.; Weiler, E. Influence of Cycle Temperatures on the Thermochemical Heat Storage Densities in the Systems Water/Microporous and Water/Mesoporous Adsorbents. Adsorption 2005, 11, 393–404. [Google Scholar] [CrossRef]
- Ng, E.-P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Aristov, Y. Concept of adsorbent optimal for adsorptive cooling/heating. Appl. Therm. Eng. 2014, 72, 166–175. [Google Scholar] [CrossRef]
- Krajnc, A.; Varlec, J.; Mazaj, M.; Ristić, A.; Logar, N.Z.; Mali, G. Superior Performance of Microporous Aluminophosphate with LTA Topology in Solar-Energy Storage and Heat Reallocation. Adv. Energy Mater. 2017, 7, 1601815. [Google Scholar] [CrossRef]
- Courbon, E.; D’Ans, P.; Permyakova, A.; Skrylnyk, O.; Steunou, N.; Degrez, M.; Frère, M. Further improvement of the synthesis of silica gel and CaCl2 composites: Enhancement of energy storage density and stability over cycles for solar heat storage coupled with space heating applications. Sol. Energy 2017, 157, 532–541. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, Y.; Shen, C.; Shi, J.; Xu, G.; Xiao, X.; Cao, R. Water sorption properties of functionalized MIL-101(Cr)-X (X = –NH2, –SO3H, H, –CH3, –F) based composites as thermochemical heat storage materials. Microporous Mesoporous Mater. 2019, 285, 129–136. [Google Scholar] [CrossRef]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. CaCl2-containing composites as thermochemical heat storage materials. Sol. Energy Mater. Sol. Cells 2017, 172, 177–185. [Google Scholar] [CrossRef]
- Zhu, D.; Wu, H.; Wang, S. Experimental study on composite silica gel supported CaCl2 sorbent for low grade heat storage. Int. J. Therm. Sci. 2006, 45, 804–813. [Google Scholar] [CrossRef]
- Xu, J.X.; Li, T.X.; Chao, J.W.; Yan, T.S.; Wang, R.Z. High energy-density multi-form thermochemical energy storage based on multi-step sorption processes. Energy 2019, 185, 1131–1142. [Google Scholar] [CrossRef]
- Liu, H.; Nagano, K.; Togawa, J. A composite material made of mesoporous siliceous shale impregnated with lithium chloride for an open sorption thermal energy storage system. Sol. Energy 2015, 111, 186–200. [Google Scholar] [CrossRef]
- Opel, O.; Rammelberg, H.U.; Gérard, M.; Ruck, W. Thermochemical storage materials research-TGA/DSC-hydration studies. In Proceedings of the 1st International Conference for Sustainable Energy Storage, Ann Arbor, MI, USA, 18–22 June 2006. [Google Scholar]
- D’Ans, P.; Courbon, E.; Permyakova, A.; Nouar, F.; Simonnet-Jégat, C.; Bourdreux, F.; Malet, L.; Serre, C.; Frère, M.; Steunou, N. A new strontium bromide MOF composite with improved performance for solar energy storage application. J. Energy Storage 2019, 25, 100881. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Houben, J.; Fischer, H.; Huinink, H.P. Stabilization of K2CO3 in vermiculite for thermochemical energy storage. Renew. Energy 2020, 150, 990–1000. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Li, T.X. Thermochemical characterizations of high-stable activated alumina/LiCl composites with multistage sorption process for thermal storage. Energy 2018, 156, 240–249. [Google Scholar] [CrossRef]
- Mehrabadi, A.; Farid, M. New salt hydrate composite for low-grade thermal energy storage. Energy 2018, 164, 194–203. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Li, J.; Kobayashi, N.; Osaka, Y.; He, Z.; Yuan, H. The effect of 3D carbon nanoadditives on lithium hydroxide monohydrate based composite materials for highly efficient low temperature thermochemical heat storage. RSC Adv. 2018, 8, 8199–8208. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, H.; Yang, X.; Bai, Y.; Li, J.; Kobayashi, N.; Kubota, M. Hydrophilic substance assisted low temperature LiOH·H2O based composite thermochemical materials for thermal energy storage. Appl. Therm. Eng. 2018, 128, 706–711. [Google Scholar] [CrossRef]
- Ristić, A.; Zabukovec Logar, N. New Composite Water Sorbents CaCl2-PHTS for Low-Temperature Sorption Heat Storage: Determination of Structural Properties. Nanomaterials 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. Effect of aluminum sulfate addition on the thermal storage performance of mesoporous SBA-15 and MCM-41 materials. Sol. Energy Mater. Sol. Cells 2016, 149, 232–241. [Google Scholar] [CrossRef]
- Rammelberg, H.U.; Myrau, M.; Schmidt, T.; Ruck, W. An Optimization of Salt Hydrates for Thermochemical Heat Storage. In Innovative Materials for Processes in Energy Systems; Impres: Santa Fe Springs, CA, USA, 2013; pp. 550–555. [Google Scholar]
- Rammelberg, H.U.; Osterland, T.; Priehs, B.; Opel, O.; Ruck, W.K.L. Thermochemical heat storage materials—Performance of mixed salt hydrates. Sol. Energy 2016, 136, 571–589. [Google Scholar] [CrossRef]
- Ejeian, M.; Entezari, A.; Wang, R.Z. Solar powered atmospheric water harvesting with enhanced LiCl/MgSO4/ACF composite. Appl. Therm. Eng. 2020, 176, 115396. [Google Scholar] [CrossRef]
- Entezari, A.; Ge, T.S.; Wang, R.Z. Water adsorption on the coated aluminum sheets by composite materials (LiCl + LiBr)/silica gel. Energy 2018, 160, 64–71. [Google Scholar] [CrossRef]
- Bissell, A.J.; Pulham, O.; Oliver, D. Strontium bromide phase change material 2015. W.O. Patent 2,015,025,175, 24 February 2015. [Google Scholar]
- Solé, A.; Miró, L.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Corrosion Test of Salt Hydrates and Vessel Metals for Thermochemical Energy Storage. Energy Procedia 2014, 48, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Power, M.; Lanno, R.P. Risk assessment of chemicals: An introduction. Aquat. Toxicol. 1997, 38, 199–201. [Google Scholar] [CrossRef]







| SHS System | LHS System | TCES System | |
|---|---|---|---|
| Principle description | SHS storage involves increasing the temperature of an element and recovering this energy by dropping its temperature during the discharge phase. Then, we use the heat capacity, Cp, of the element, which is the amount of energy needed, per unit mass, to increase (or decrease) its temperature by 1 °C. The discharge temperature cannot be higher than the charge temperature. For applications where the operating temperature is between 0 and 100 °C, the most widely-used material is water, for example, in the domestic hot water tank of a home. Indeed, it is a non-toxic and inexpensive product. There is also the use of certain rocks or concrete. Beyond 100 °C, it is possible to use solid elements such as concrete at high temperatures or refractory ceramics, but the necessary volumes are important. Generally, we can find liquid storage systems for molten salts, pressurized water, or organic oils. For high temperature applications, molten salts are the most widely used material. This is due to their high volumetric heat capacity, a high boiling point, high temperature stability, and their vapor pressure being close to zero. Additionally, they are relatively cheap, readily available, neither toxic nor flammable, and can act as a heat transfer fluid as well as a TES material. However, they have certain disadvantages: they have a relatively high melting point (generally >200 °C), which results in them solidifying in pipes in the absence of a heat source and thus necessitates the installation of antifreeze systems; they also have high viscosity and low thermal conductivity compared to other fluids [27]. | LHS is the amount of energy required to change the state of a solid, liquid or gas, called phase change material, PCM. In LHS systems, common transformations are from solid to liquid or from liquid to gas. The temperature range corresponding to the phase change of a PCM should be relatively small (20 to 80 °C). The materials used in LHS are numerous and allow working over a wide temperature range (example: 0 °C for water; 318 °C for sodium hydroxide). Latent heat storage in ‘low’ temperature range below 220 °C, ‘medium’ range up to 420 °C, and ‘high’ range greater than 420 °C is associated with a solar power tower as the point focus system. Materials for potential use as PCMs are mostly organic compounds, inorganic salts, and their eutectics. Inorganic materials such as salt hydrates, metals, and eutectics as well as organic compounds such as paraffin waxes, esters, acids, and alcohols have been studied [28]. According to the literature, molten salts have received more attention for heat storage applications than molten metals and alloys [29]. | TCES involves reversible reactions, endothermic in one direction and exothermic in the other direction. These may be physical or chemical phenomena, described as follows: A + ∆H ⇔ B + C During the storage process, the charging phase corresponds to an endothermic decomposition reaction of a chemical element A into two products B and C stored separately at room temperature. The phase of discharge corresponds to the exothermic synthesis of the chemical element A by association of the two components B and C. It can correspond to physisorption or chemisorption phenomena, but in all cases, the heat released comes from the breaking of the bonds between the various components. TCES can be applied to energy storage at less than 100 °C and between 100–400 °C, the physisorption being characterized by a low enthalpy (<50 kJ/mol of material) and chemisorption, by a high enthalpy (>100 kJ/mol of material). TCES is defined according to two criteria: is the process open or closed, and is the reactor integrated or separate from the storage system. In a closed circuit, the storage of the sorbate is an internal element of the process. In an open circuit, in contrast, there is a transfer of material with the outside of the process in order to supply the sorbate to the reactor. In both cases, sorbent storage is a component of the system. |
| Volumetric energy density storage | Small (15–50 kWh/m3) | Medium (50–100 kWh/m3) | High (100–700 kWh/m3) |
| Gravimetric energy density storage | Small (0.02–0.03 kWh/kg) | Medium (0.05–0.1 kWh/kg) | High (0.5–1.0 kWh/kg) |
| Capacity | 10–50 (kWh/t) | 50–100 (kWh/t) | 120–250 (kWh/t) |
| Power | 0.001–10.0 (MW) | 0.001–1.0 (MW) | 0.01–1.0 (MW) |
| Efficiency | 50–90% | 75–90% | 75–100% |
| Cost | 0.1–10 (€/kWh) | 10–50 (€/kWh) | 8–100 (€/kWh) |
| Storage temperature | Charging step temperature | Charging step temperature | Ambient temperature |
| Storage period | Limited due to thermal losses to surroundings | Limited due to thermal losses to surroundings | Theoretically unlimited |
| Energy transport | Shorter distance | Shorter distance | Theoretically long distance |
| Maturity | Industrial scale | Pilot-scale | Laboratory and pilot-scale |
| Technology | Simple | Simple | Complex |
| Author(s) | Highlights | Ref. |
|---|---|---|
| Fopah-Lele and Tamba (2017) | SrBr2·6H2O was chosen for its thermal properties and sorption characteristics in heating and cooling applications. Several studies on SrBr2·6H2O were conducted at the laboratory and prototype scales. | [49] |
| Krese et al. (2018) | The use of solar energy in TCES technologies and systems for building applications was highlighted. The numerical modeling of device design for open and closed sorption storage systems was the focus of this project. | [50] |
| Lizana et al. (2018) | An analysis of recent developments in thermal energy storage technology for zero-energy building applications was presented. Sensible, latent, and thermochemical thermal energy storage systems were used to investigate different types of materials and mechanisms for heat storage. | [51] |
| Kuznik et al. (2018) | The idea of a building-based physisorption heat storage system was introduced. Concentrated on physisorption materials (activated carbon, silica gel, zeolites, and composite salts), reactors (fixed bed and fluidized bed), and experimental prototypes. | [52] |
| Sunku Prasad et al. (2019) | Reviewed TCES systems operating over 300 °C. Under the category of solid–gas reactions, authors discussed carbonates, hydroxides, metal hydrides, and redox reactions; Under the category of gas–gas reactions, authors discussed ammonia synthesis/dissociation, methane reforming, and the SO3/O2/SO2 TCES system. A variety of reactor designs for solid–gas and gas–gas reactions were described. Cyclic experiments on solid–gas reaction materials were described. | [53] |
| Palomba et al. (2019) | A review of the most recent developments in the field of sorption TES. The results of activity analysis at the materials and device levels are presented. In addition, the most recent events and sponsored programs in the field of sorption TES are highlighted. | [54] |
| Fumey et al. (2019) | Open fixed, open transported, closed fixed, and closed transported are the four basic sorption thermal energy storage processes. Temperature effectiveness is a universal metric for comparing the efficiency of sorption heat storage systems. In terms of temperature effectiveness, closed transported sorption thermal energy storage systems perform much better. | [55] |
| Wu et al. (2019) | In light of space heating applications, a critical review of solid materials for low-temperature thermochemical storage of solar energy based on solid-vapor adsorption. The current report is about low-temperature thermochemical storage for space heating, which is dependent on vapor adsorption into solid adsorbents. | [56] |
| Desai et al. (2021) | For cooling and process heating applications, a thermochemical energy storage device is used. Metal hydride-based cooling and heating systems are defined in detail. For medium-temperature applications, salt hydrates are discussed. Solar-powered cooling and heating systems conceptual designs. | [13] |
| Lin et al. (2021) | Based on material classification, applications of low-temperature thermochemical energy storage systems for salt hydrates. This review established a fair classification of salt hydrates for TCES systems, summarizing material properties, appropriate reactor types, applications, and device prototype optimization based on material properties. | [57] |
| Present review | The materials, mechanisms, and storage principles of sorption and reaction-based TCES systems are discussed in this study. TCES materials and applications at low and medium temperatures are the focus. A comprehensive review of recent research on the use of mono and binary salts imbedded in host matrixes. The safety of various materials is discussed. |
| Hydrated Salt | Dehydrated Salt | Tfusion (°C) | Density (kg/m3) | Reaction Enthalpy (kJ/molmat) | Energy Storage Density (kWh/m3mat) |
|---|---|---|---|---|---|
| Al2(SO4)3.18H2O | Al2(SO4)3.8H2O | 88 | 1690 | 554.5 [61] | 391 [60] |
| CaBr2.6H2O | CaBr2.0.3H2O | 38.2 | 2295 | 353.9 [61,62]; 372.6 [61,63] | 732 [60] |
| CaCl2.2H2O | CaCl2.H2O | 176 | 1850 | 47 [58] | 167 [64] |
| CaCl2.6H2O | CaCl2.H2O | 29 | 1710 | 277 [65] | 601 [60] |
| Ce(SO4)2.4H2O | Ce(SO4)2.2H2O | 180 | 3910 | 58 [60] | 156 [60] |
| K2CO3.1.5H2O | K2CO3 | 891 | 2155 | 95.5 [61]; 96.7 [61] | 346 [60] |
| LaCl3.7H2O | LaCl3.H2O | 91 | 2223 | 355.5 [61] | 591 [60] |
| La(NO3)3.6H2O | La(NO3)3.1.5H2O | 40 | 2347 | 260.4 [61] | 392 [60] |
| LiCl.H2O | LiCl | 99 | 1700 | 62.2 [61] | 486 [60]; 253 [2] |
| LiNO3.3H2O | LiNO3 | 29.9 | 1550 | 165.8 [61] | 580 [60] |
| MgBr2.6H2O | MgBr2.4H2O | 152–165 | 2000 | 144.9 [61] | - |
| MgBr2.6H2O | MgBr2 | 152–165 | 2000 | 439.7 [61] | 276 [60] |
| MgCl2.6H2O | MgCl2.2H2O | 117 | 1569 | 255 [65]; 220.02 (183.83 (i)) [59] | - |
| MgSO4.7H2O (ii) | MgSO4.H2O | 49.2 | 1680 | 335.7–336 [58,66] | 547 [60]; 556 [60,67] |
| MgSO4.7H2O (ii) | MgSO4 | 49.2 | 1680 | 411 [58] | 632 [60]; 639 [60,64] |
| MgSO4.H2O | MgSO4 | 200 | 2570 | 75 [58] | 780 [2]; 760 [59] |
| Na2S2O3.5H2O | Na2S2O3 | 48.3 | 1690–1580 | 279.9 [61,68]; 312.2 [61,69] | 361 [64] |
| SrBr2.6H2O | SrBr2.H2O | 88.6 | 2386 | 337 [70,71,72] | 529 [60] |
| SrCl2.6H2O | SrCl2 | 61 | 1960 | 342 [65]; 348,962 [61,66] | 628 [60]; 400 [72] |
| Zn(NO3)2.6H2O | Zn(NO3)2 | 36.4 | 2067 | 372 [61]; 376.9 [61] | 698 [60] |
| CaSO4.2H2O | CaSO4 | 128 | 2320 | 105 [61] | 817 [60] |
| Remarks on Hydrated Salts for Heat Storage Applications | |||||
| Hydrated salt | Dehydrated salt | Remarks | |||
| Al2 (SO4)3.18H2O | Al2 (SO4)3.8H2O | Low level of corrosiveness; very small temperature difference during hydration of Al2(SO4)3.5H2O (∆T = from 1 to 10 °C) [67,73] | |||
| CaCl2.2H2O | CaCl2.H2O | Hydration of the anhydrous to dihydrate results in the formation of a gel, which reduces the porosity of the bed and the rehydration capacity of the material [73]. Cost = 0.11 €/kg [69] | |||
| CaCl2.6H2O | CaCl2.H2O | ||||
| K2CO3.1.5H2O | K2CO3 | The rate of dehydration was strongly affected by the presence of water vapor [74] | |||
| LiCl.H2O | LiCl | Lithium resources tend to be depleted which could result in additional material costs in the future. Cost = 3600 €/m3 [2] | |||
| LiNO3.3H2O | LiNO3 | Lithium resources tend to be depleted which could result in additional material costs in the future. Cost = 3600 €/m3 [2] | |||
| MgCl2.6H2O | MgCl2.2H2O | Formation of HCl above 115 °C [59,60] and decomposition of MgCl2.2H2O into MgOHCl above 130 °C [59] ≥ material deterioration and corrosively. Over-hydration below 40 °C ≥ liquefaction consequently loss of performance [59]. Cost = 0.154 €/kg [69] | |||
| MgSO4.7H2O | MgSO4.H2O | Very low reaction kinetics. Recrystallization of MgSO4.7H2O possible only at a water vapor pressure > 60 mbar [59]. Cost = 4870 €/m3 [2]/3.8 €/kg [69] | |||
| MgSO4.7H2O | MgSO4 | Non-corrosive, non-toxic; Most of the energy stored at less than 90 °C. Cost = 4870 €/m3 [2],/3.8 €/kg [69] | |||
| MgSO4.H2O | MgSO4 | ||||
| Na2S2O3.5H2O | Na2S2O3 | Formation of a thin surface layer that interferes with the release of water [75]. Poor reversibility in real conditions [60]. | |||
| SrBr2.6H2O | SrBr2.H2O | Total dehydration from 80 °C [69] | |||
| Zn(NO3)2.6H2O | Zn(NO3)2 | Observation of melting during tests but the reaction remains reversible. There are inconsistencies in the literature [60]. | |||
| CaSO4.2H2O | CaSO4 | Poor reversibility in real conditions [60] | |||
| Matrix | Salt | Salt Content/wt.% | Energy Storage Capacity | Adsorption T (°C) | Desorption T (°C) | Relative Humidity RH% | Ref. | Years |
|---|---|---|---|---|---|---|---|---|
| Silica gel | CaCl2 | 43 | 1080 J/g | 30 | 80 | 32 | [177] | 2017 |
| MIL-101(Cr)–NH2 | CaCl2 | 45 | 1205 J/g | 30 | 120 | 30 | [178] | 2019 |
| Bentonite | CaCl2 | 40 | 700 J/g | 25 | 150 | 65 | [112] | 2010 |
| Silica gel | CaCl2 | 15 | 746 J/g | 20 | 150 | 30 | [179] | 2017 |
| Bentonite | CaCl2 | 15 | 719 J/g | 20 | 150 | 30 | [179] | 2017 |
| Aluminum oxide | CaCl2 | 15 | 576 J/g | 20 | 150 | 30 | [179] | 2017 |
| Silica gel | CaCl2 | 35 | 1000 J/g | 30 | 90 | 80 | [180] | 2006 |
| FeKIL2 | CaCl2 | 7 | 560 J/g | 40 | 150 | 75 | [150] | 2012 |
| Expanded natural graphite | CaCl2 | 63 | 1268 J/g | 25 | 200 | 63 | [109] | 2014 |
| Activated carbon foam | CaCl2 | 90 | 701 J/g | 25 | 200 | 63 | [109] | 2014 |
| Expanded natural Graphite; Activated carbon foam | KCl; CaCl2 | 31–90 | 1451–1310 J/g | 25 | 200 | 31–63 | [172] | 2016 |
| Zeolite | MgCl2 | 24.5 | 1368 J/g | 30 | 300 | 68 | [181] | 2019 |
| Zeolites Na–Y | MgCl2 | 15 | 1173 J/g | 20 | 150 | 55 | [166] | 2014 |
| Zeolites H–Y | MgCl2 | 15 | 970 J/g | 20 | 150 | 55 | [166] | 2014 |
| Zeolites Na–Y | MgSO4 | 15 | 1090 J/g | 20 | 150 | 55 | [157] | 2013 |
| Zeolites H–Y | MgSO4 | 15 | 867 J/g | 20 | 150 | 55 | [157] | 2013 |
| Wakkanai siliceous shale (WSS) | LiCl; CaCl2 | 9.6 | 0.2 GJ/m3 | 25 | 150 | 69 | [182] | 2015 |
| Silica gel, zeolite 13X; vermiculite | CaCl2, MgSO4, Ca(NO3)2 LiNO3 LiBr | 2–65 | 0.18 GJ/m3 | 30 | 140 | - | [138] | 2014 |
| Graphite, copper, zeolite A, sand | MgCl2 | 46–69 | 0.56 GJ/m3 | 35 | 200 | 21–39 | [183] | 2006 |
| Activated carbon, silica solution, expanded graphite | LiCl | 32–45 | 0.72–1.43 GJ/m3 | 30 | 90 | 60 | [111] | 2015 |
| Zeolite 13X; silica gel | MgSO4 | 15 | 0.6 GJ/m3 | 25 | 150 | 68 | [114] | 2011 |
| Attapulgite | CaCl2 | 30 | 1.08 (40 °C) 0.41 GJ/m3 (60 °C) | 40 | 400 | - | [167] | 2005 |
| MIL-101(Cr) | SrBr2 | 63 | 375 Wh/Kg | 30 | 80 | - | [184] | 2019 |
| vermiculite | K2CO3 | 69 | 0.9 GJ/m3 | 30–50 | 75–95 | - | [185] | 2020 |
| activated alumina | LiCl | 14.68 | 1041.5 J/g | 20 | 120 | 80 | [186] | 2018 |
| Zeolithe 13X | MgSO4 | 15 | 550.8 J/g | 25 | 250 | - | [162] | 2018 |
| Expanded clay | SrCl2 | 40 | 29 kW/m3 | 20 | 110 | 71 | [187] | 2018 |
| Pumice | SrCl2 | 14 | 7.3 kW/m3 | 20 | 110 | 71 | [187] | 2018 |
| 3D-nickel-carbon nanotubes | LiOH | 14 | 3935 J/g | 30 | 150 | 64 | [188] | 2018 |
| Zeolite 13X | LiOH | 80 | 1949 J/g | 30 | 150 | 70 | [189] | 2018 |
| Plugged hexagonal templated silicate (PHTS) | CaCl2 | 20 | 1199 J/g | 30 | 120 | - | [190] | 2019 |
| SBA-15 | Al2(SO4)3 | 7 | 334 J/g | 22 | 150 | 30 | [191] | 2016 |
| MCM-41 | CaCl2 | 37.7 | 2.1 kJ/g | - | 150 | - | [115] | 2002 |
| MCM-41 | Al2(SO4)3 | 7 | 612 J/g | 22 | 150 | 30 | [191] | 2016 |
| Salt | LD50 (mg/kg) | Chemical Stability | Point of Concern |
|---|---|---|---|
| GdCl3 | 102 | Rare earth | |
| EuCl3 | 3527 | Rare earth | |
| CrCl2 | 1870 | Instable Cr2+ | Instable |
| LiCl | 1629 | Price | |
| LiBr | 1800 | Price | |
| FeCl2 | 895 | Instable Fe2+ | Instable |
| CsF | N/A | Price | |
| Ca(ClO4)2 | 4500 | Explosive | Safety |
| CuCl2 | 584 | Price | |
| Na2S | 208 | H2S formation | Safety/Instable |
| RbF | N/A | Price | |
| CrCl2 | 1870 | Instable Cr2+ | Instable |
| CaCl2 | 1940 | Deliquescence and higher hydrates | |
| Mg(NO3)2 | 5440 | Loss of N2 | Instable |
| LiNO2 | N/A | Loss of N2 | Price |
| Mg(NO3)2 | 5440 | Loss of N2 | Instable |
| LiI | 6500 | Price | |
| LaCl3 | 2370 | Rare earth | |
| KAl(SO4)2 | 6986 | Kinetics | |
| MnI2 | N/A | Safety | |
| VOSO4 | N/A | Price/Safety | |
| K2CO3 | 1870 | ||
| MgCl2 | 3800 | HCl formation | Instable |
| Na2S | 208 | H2S formation | Safety/Instable |
| Na2S | 208 | H2S formation | Safety/Instable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbair, M.; Bennici, S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies 2021, 14, 3105. https://doi.org/10.3390/en14113105
Zbair M, Bennici S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies. 2021; 14(11):3105. https://doi.org/10.3390/en14113105
Chicago/Turabian StyleZbair, Mohamed, and Simona Bennici. 2021. "Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review" Energies 14, no. 11: 3105. https://doi.org/10.3390/en14113105
APA StyleZbair, M., & Bennici, S. (2021). Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies, 14(11), 3105. https://doi.org/10.3390/en14113105







