Inhomogeneities and Cell-to-Cell Variations in Lithium-Ion Batteries, a Review
Abstract
1. Introduction
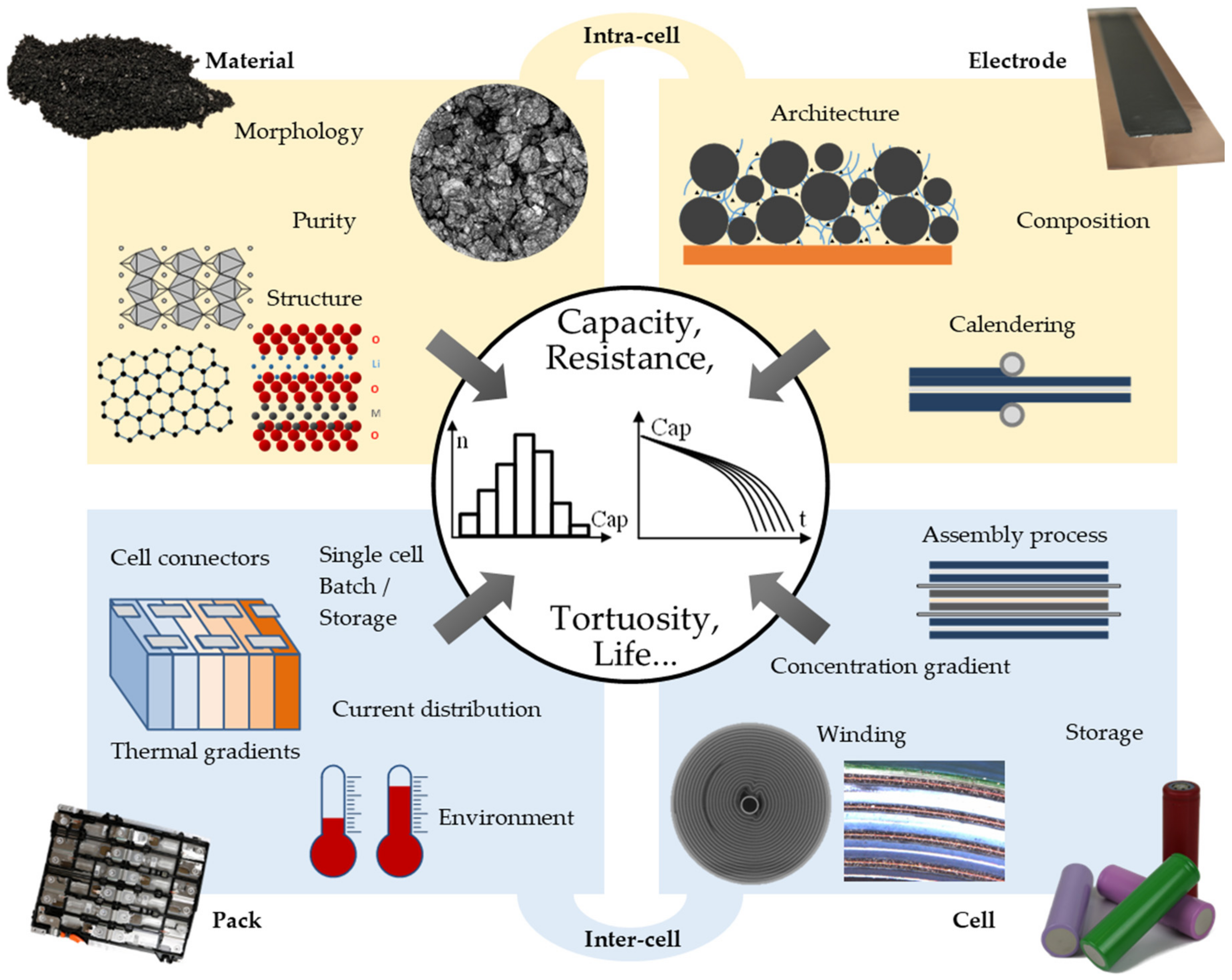
2. Origins of Cell-to-Cell Variations (ctcV) and Inhomogeneities
2.1. Material and Electrode Levels
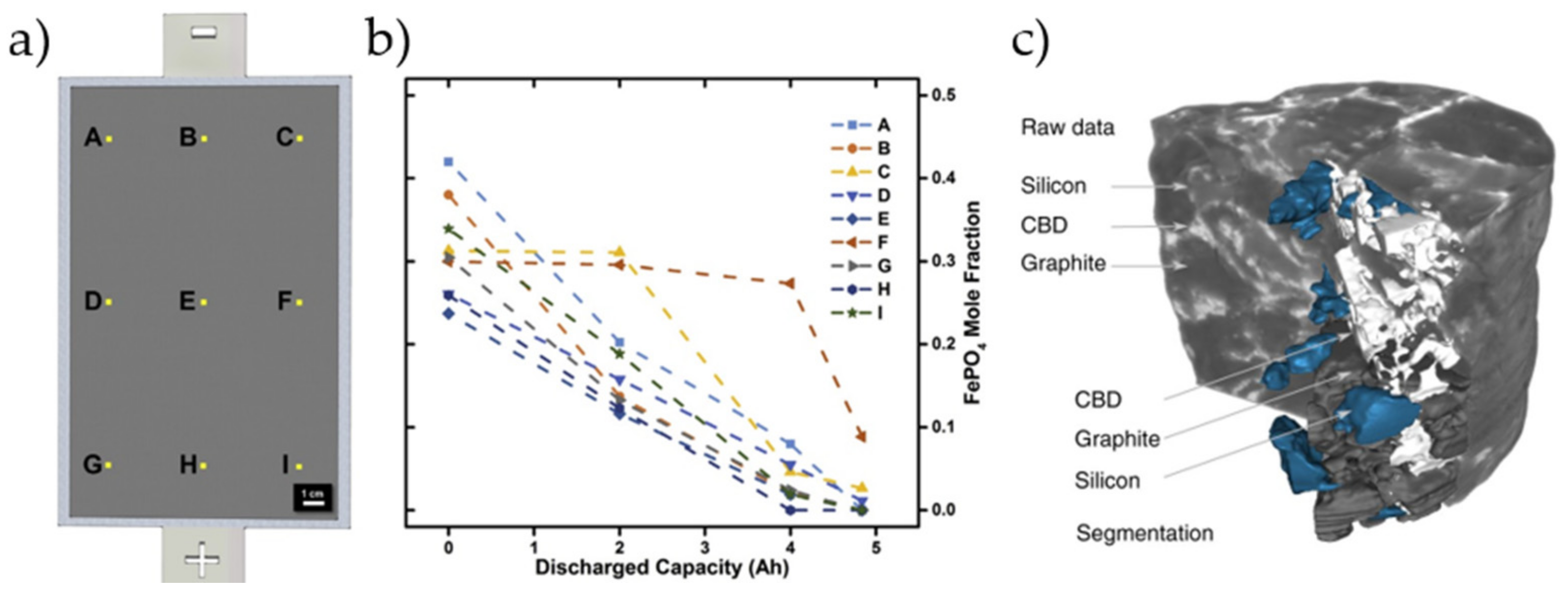
2.1.1. Microstructure and Composition
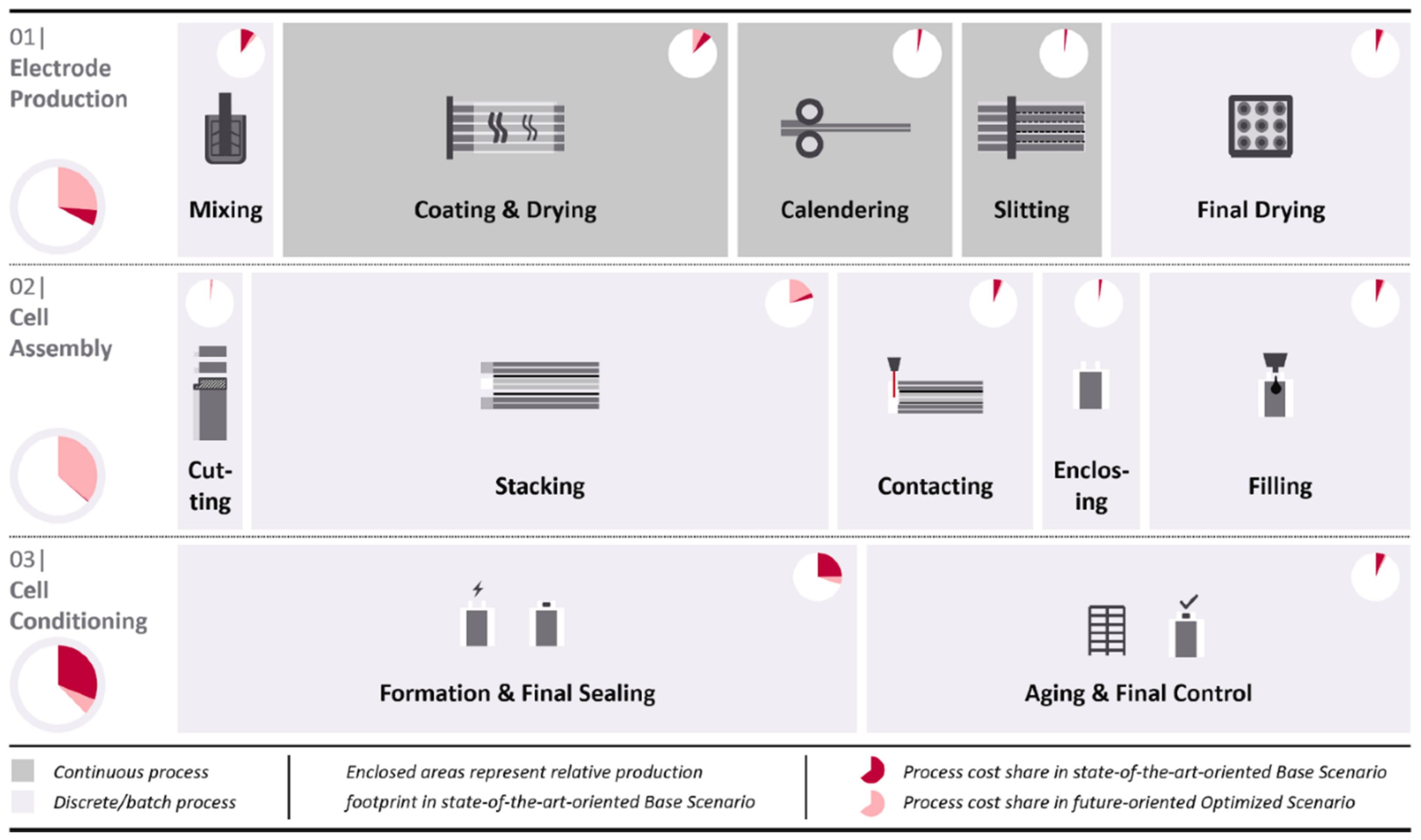
2.1.2. Electrode Fabrication
2.2. Cell Level
2.2.1. Intrinsic
2.2.2. Cycling
2.3. Pack Level
3. Methods to Track Inhomogeneities
3.1. Large-Scale Research Facilities
3.1.1. High-Energy X-ray
3.1.2. X-ray Tomography
3.1.3. Neutron Diffraction
3.2. Laboratory Scale Tracking
3.2.1. Cell Modifications
3.2.2. Spatially Resolved Bulk Measurements
3.2.3. Visual Inspection of Post-Mortem Cells
3.2.4. Electron Microscopy
3.2.5. Computer Tomography
3.2.6. Magnetic Resonance
3.2.7. Acoustics
3.2.8. Electrochemical Characterization
3.2.9. Characterization of Inhomogeneous Cell Degradation
3.3. Field/Usage Data
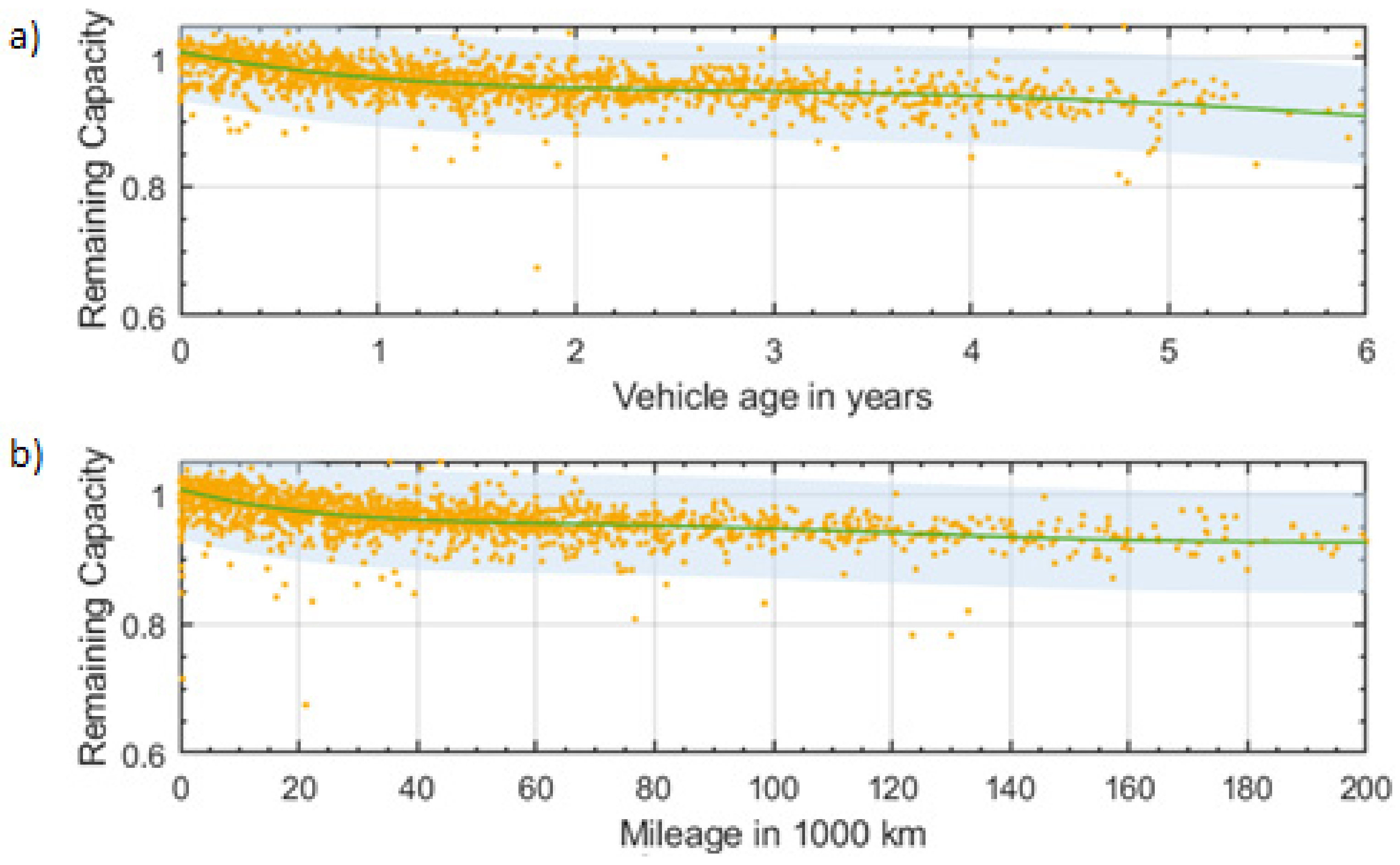
3.3.1. Usage Data
3.3.2. Consumer Reports
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumpf, K.; Rheinfeld, A.; Schindler, M.; Keil, J.; Schua, T.; Jossen, A. Influence of Cell-to-Cell Variations on the Inhomogeneity of Lithium-Ion Battery Modules. J. Electrochem. Soc. 2018, 165, A2587–A2607. [Google Scholar] [CrossRef]
- Palacin, M.R. Battery Materials Design Essentials. Acc. Mater. Res. 2021. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.; Chong, Y.; Yuan, H.; Huang, J.-Q.; Zhang, Q. Advanced Electrode Processing of Lithium Ion Batteries: A Review of Powder Technology in Battery Fabrication. Particuology 2021, 57, 56–71. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-Ion Battery Aging Mechanisms and Diagnosis Method for Automotive Applications: Recent Advances and Perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Yu, T.F.; Widanage, W.D.; Marco, J. Critical Review of Non-Invasive Diagnosis Techniques for Quantification of Degradation Modes in Lithium-Ion Batteries. Renew. Sustain. Energy Rev. 2019, 109, 138–159. [Google Scholar] [CrossRef]
- Boebinger, M.G.; Lewis, J.A.; Sandoval, S.E.; McDowell, M.T. Understanding Transformations in Battery Materials Using in Situ and Operando Experiments: Progress and Outlook. ACS Energy Lett. 2020, 5, 335–345. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.; Amine, K. State-of-the-Art Characterization Techniques for Advanced Lithium-Ion Batteries. Nat. Energy 2017, 2, 17011. [Google Scholar] [CrossRef]
- Harks, P.P.R.M.L.; Mulder, F.M.; Notten, P.H.L. In Situ Methods for Li-Ion Battery Research: A Review of Recent Developments. J. Power Sources 2015, 288, 92–105. [Google Scholar] [CrossRef]
- Schindler, M.; Sturm, J.; Ludwig, S.; Schmitt, J.; Jossen, A. Evolution of Initial Cell-to-Cell Variations During a Three-Year Production Cycle. eTransportation 2021, 100102. [Google Scholar] [CrossRef]
- Harris, S.J.; Lu, P. Effects of Inhomogeneities—Nanoscale to Mesoscale—On the Durability of Li-Ion Batteries. J. Phys. Chem. C 2013, 117, 6481–6492. [Google Scholar] [CrossRef]
- Bach, T.C.; Schuster, S.F.; Fleder, E.; Müller, J.; Brand, M.J.; Lorrmann, H.; Jossen, A.; Sextl, G. Nonlinear Aging of Cylindrical Lithium-Ion Cells Linked to Heterogeneous Compression. J. Energy Storage 2016, 5, 212–223. [Google Scholar] [CrossRef]
- Grün, T.; Stella, K.; Wollersheim, O. Influence of Circuit Design on Load Distribution and Performance of Parallel-Connected Lithium Ion Cells for Photovoltaic Home Storage Systems. J. Energy Storage 2018, 17, 367–382. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature Effect and Thermal Impact in Lithium-Ion Batteries: A Review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Demortière, A.; Fleutot, B.; Delobel, B.; Delacourt, C.; Cooper, S.J. The Electrode Tortuosity Factor: Why the Conventional Tortuosity Factor Is Not Well Suited for Quantifying Transport in Porous Li-Ion Battery Electrodes and What to Use Instead. NPJ Comput. Mater. 2020, 6, 123. [Google Scholar] [CrossRef]
- Forouzan, M.M.; Chao, C.-W.; Bustamante, D.; Mazzeo, B.A.; Wheeler, D.R. Experiment and Simulation of the Fabrication Process of Lithium-Ion Battery Cathodes for Determining Microstructure and Mechanical Properties. J. Power Sources 2016, 312, 172–183. [Google Scholar] [CrossRef]
- Thorat, I.V.; Stephenson, D.E.; Zacharias, N.A.; Zaghib, K.; Harb, J.N.; Wheeler, D.R. Quantifying Tortuosity in Porous Li-Ion Battery Materials. J. Power Sources 2009, 188, 592–600. [Google Scholar] [CrossRef]
- Antartis, D.; Dillon, S.; Chasiotis, I. Effect of Porosity on Electrochemical and Mechanical Properties of Composite Li-Ion Anodes. J. Compos. Mater. 2015, 49, 1849–1862. [Google Scholar] [CrossRef]
- Elango, R.; Nadeina, A.; Cadiou, F.; De Andrade, V.; Demortière, A.; Morcrette, M.; Seznec, V. Impact of Electrode Porosity Architecture on Electrochemical Performances of 1 Mm-Thick LiFePO4 Binder-Free Li-Ion Electrodes Fabricated by Spark Plasma Sintering. J. Power Sources 2021, 488, 229402. [Google Scholar] [CrossRef]
- Sieg, J.; Storch, M.; Fath, J.; Nuhic, A.; Bandlow, J.; Spier, B.; Sauer, D.U. Local Degradation and Differential Voltage Analysis of Aged Lithium-Ion Pouch Cells. J. Energy Storage 2020, 30, 101582. [Google Scholar] [CrossRef]
- Ecker, M.; Shafiei Sabet, P.; Sauer, D.U. Influence of Operational Condition on Lithium Plating for Commercial Lithium-Ion Batteries—Electrochemical Experiments and Post-Mortem-Analysis. Appl. Energy 2017, 206, 934–946. [Google Scholar] [CrossRef]
- Suthar, B.; Northrop, P.W.C.; Rife, D.; Subramanian, V.R. Effect of Porosity, Thickness and Tortuosity on Capacity Fade of Anode. J. Electrochem. Soc. 2015, 162, A1708. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Effect of Porosity on the Thick Electrodes for High Energy Density Lithium Ion Batteries for Stationary Applications. Batteries 2016, 2, 35. [Google Scholar] [CrossRef]
- Müller, S.; Eller, J.; Ebner, M.; Burns, C.; Dahn, J.; Wood, V. Quantifying Inhomogeneity of Lithium Ion Battery Electrodes and Its Influence on Electrochemical Performance. J. Electrochem. Soc. 2018, 165, A339–A344. [Google Scholar] [CrossRef]
- Dubarry, M.; Gaubicher, J.; Guyomard, D.; Steunou, N.; Livage, J.; Dupré, N.; Grey, C.P. Synthesis of Li1+αV3O8 via a Gel Precursor: Part II, from Xerogel to the Anhydrous Material. Chem. Mater. 2006, 18, 629–636. [Google Scholar] [CrossRef]
- Pavoni, F.H.; Sita, L.E.; dos Santos, C.S.; da Silva, S.P.; da Silva, P.R.C.; Scarminio, J. LiCoO2 Particle Size Distribution as a Function of the State of Health of Discarded Cell Phone Batteries. Powder Technol. 2018, 326, 78–83. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.-S.; Song, W.-L.; Fang, D. Effect of Defects on Diffusion Behaviors of Lithium-Ion Battery Electrodes: In Situ Optical Observation and Simulation. ACS Appl. Mater. Interfaces 2018, 10, 43623–43630. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Fang, A.; Haataja, M.P.; Arnold, C.B. Size Dependence of Transport Non-Uniformities on Localized Plating in Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1147–A1155. [Google Scholar] [CrossRef]
- Pouraghajan, F.; Thompson, A.I.; Hunter, E.E.; Mazzeo, B.; Christensen, J.; Subbaraman, R.; Wray, M.; Wheeler, D. The Effects of Cycling on Ionic and Electronic Conductivities of Li–Ion Battery Electrodes. J. Power Sources 2021, 492, 229636. [Google Scholar] [CrossRef]
- Forouzan, M.M.; Mazzeo, B.A.; Wheeler, D.R. Modeling the Effects of Electrode Microstructural Heterogeneities on Li-Ion Battery Performance and Lifetime. J. Electrochem. Soc. 2018, 165, A2127–A2144. [Google Scholar] [CrossRef]
- Vogel, J.E.; Forouzan, M.M.; Hardy, E.E.; Crawford, S.T.; Wheeler, D.R.; Mazzeo, B.A. Electrode Microstructure Controls Localized Electronic Impedance in Li-Ion Batteries. Electrochim. Acta 2019, 297, 820–825. [Google Scholar] [CrossRef]
- Zhou, W. Effects of External Mechanical Loading on Stress Generation during Lithiation in Li-Ion Battery Electrodes. Electrochim. Acta 2015, 185, 28–33. [Google Scholar] [CrossRef]
- Christensen, J. Modeling Diffusion-Induced Stress in Li-Ion Cells with Porous Electrodes. J. Electrochem. Soc. 2010, 157, A366. [Google Scholar] [CrossRef]
- Lin, N.; Jia, Z.; Wang, Z.; Zhao, H.; Ai, G.; Song, X.; Bai, Y.; Battaglia, V.; Sun, C.; Qiao, J.; et al. Understanding the Crack Formation of Graphite Particles in Cycled Commercial Lithium-Ion Batteries by Focused Ion Beam—Scanning Electron Microscopy. J. Power Sources 2017, 365, 235–239. [Google Scholar] [CrossRef]
- Dai, K.; Wang, Z.; Ai, G.; Zhao, H.; Yuan, W.; Song, X.; Battaglia, V.; Sun, C.; Wu, K.; Liu, G. The Transformation of Graphite Electrode Materials in Lithium-Ion Batteries after Cycling. J. Power Sources 2015, 298, 349–354. [Google Scholar] [CrossRef]
- Sun, F.; Markötter, H.; Dong, K.; Manke, I.; Hilger, A.; Kardjilov, N.; Banhart, J. Investigation of Failure Mechanisms in Silicon Based Half Cells during the First Cycle by Micro X-Ray Tomography and Radiography. J. Power Sources 2016, 321, 174–184. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing Nanostructured Si Anodes for High Energy Lithium Ion Batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, G.; Liu, N.; Carney, T.J.; Yang, Y.; Cui, Y. Engineering Empty Space between Si Nanoparticles for Lithium-Ion Battery Anodes. Nano Lett. 2012, 12, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, X.; Wang, Z.; Chen, L. Cracking Causing Cyclic Instability of LiFePO4 Cathode Material. J. Power Sources 2005, 140, 125–128. [Google Scholar] [CrossRef]
- Gabrisch, H.; Wilcox, J.; Doeff, M.M. TEM Study of Fracturing in Spherical and Plate-like LiFePO4 Particles. Electrochem. Solid State Lett. 2008, 11, A25. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation Diagnostics for Lithium Ion Cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Liaw, B.Y. Cell Degradation in Commercial LiFePO4 Cells with High-Power and High-Energy Designs. J. Power Sources 2014, 258, 408–419. [Google Scholar] [CrossRef]
- Schmidt, O.; Thomitzek, M.; Röder, F.; Thiede, S.; Herrmann, C.; Krewer, U. Modeling the Impact of Manufacturing Uncertainties on Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 060501. [Google Scholar] [CrossRef]
- Higa, K.; Zhao, H.; Parkinson, D.Y.; Barnard, H.; Ling, M.; Liu, G.; Srinivasan, V. Electrode Slurry Particle Density Mapping Using X-ray Radiography. J. Electrochem. Soc. 2017, 164, A380. [Google Scholar] [CrossRef]
- Lenze, G.; Bockholt, H.; Schilcher, C.; Froböse, L.; Jansen, D.; Krewer, U.; Kwade, A. Impacts of Variations in Manufacturing Parameters on Performance of Lithium-Ion-Batteries. J. Electrochem. Soc. 2018, 165, A314–A322. [Google Scholar] [CrossRef]
- Dreger, H.; Haselrieder, W.; Kwade, A. Influence of Dispersing by Extrusion and Calendering on the Performance of Lithium-Ion Battery Electrodes. J. Energy Storage 2019, 21, 231–240. [Google Scholar] [CrossRef]
- Kenney, B.; Darcovich, K.; MacNeil, D.D.; Davidson, I.J. Modelling the Impact of Variations in Electrode Manufacturing on Lithium-Ion Battery Modules. J. Power Sources 2012, 213, 391–401. [Google Scholar] [CrossRef]
- Rynne, O.; Dubarry, M.; Molson, C.; Nicolas, E.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Rochefort, D.; Dollé, M. Exploiting Materials to Their Full Potential, a Li-Ion Battery Electrode Formulation Optimization Study. ACS Appl. Energy Mater. 2020, 3, 2935–2948. [Google Scholar] [CrossRef]
- Mauler, L.; Duffner, F.; Leker, J. Economies of Scale in Battery Cell Manufacturing: The Impact of Material and Process Innovations. Appl. Energy 2021, 286, 116499. [Google Scholar] [CrossRef]
- Haselrieder, W.; Ivanov, S.; Christen, D.K.; Bockholt, H.; Kwade, A. Impact of the Calendering Process on the Interfacial Structure and the Related Electrochemical Performance of Secondary Lithium-Ion Batteries. ECS Trans. 2013, 50, 59–70. [Google Scholar] [CrossRef]
- Ngandjong, A.C.; Lombardo, T.; Primo, E.N.; Chouchane, M.; Shodiev, A.; Arcelus, O.; Franco, A.A. Investigating Electrode Calendering and Its Impact on Electrochemical Performance by Means of a New Discrete Element Method Model: Towards a Digital Twin of Li-Ion Battery Manufacturing. J. Power Sources 2021, 485, 229320. [Google Scholar] [CrossRef]
- Kang, H.; Lim, C.; Li, T.; Fu, Y.; Yan, B.; Houston, N.; De Andrade, V.; De Carlo, F.; Zhu, L. Geometric and Electrochemical Characteristics of LiNi1/3Mn1/3Co1/3O2 Electrode with Different Calendering Conditions. Electrochim. Acta 2017, 232, 431–438. [Google Scholar] [CrossRef]
- Schmidt, D.; Kamlah, M.; Knoblauch, V. Highly Densified NCM-Cathodes for High Energy Li-Ion Batteries: Microstructural Evolution during Densification and Its Influence on the Performance of the Electrodes. J. Energy Storage 2018, 17, 213–223. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; White, R.E. Quantifying Cell-to-Cell Variations in Lithium Ion Batteries. Int. J. Electrochem. 2012, 2012, e395838. [Google Scholar] [CrossRef]
- Shin, D.; Poncino, M.; Macii, E.; Chang, N. A Statistical Model-Based Cell-to-Cell Variability Management of Li-Ion Battery Pack. IEEE Trans. Comput. Aided Des. Integr. Circuits Syst. 2015, 34, 252–265. [Google Scholar] [CrossRef]
- Rucci, A.; Ngandjong, A.C.; Primo, E.N.; Maiza, M.; Franco, A.A. Tracking Variabilities in the Simulation of Lithium Ion Battery Electrode Fabrication and Its Impact on Electrochemical Performance. Electrochim. Acta 2019, 312, 168–178. [Google Scholar] [CrossRef]
- Duquesnoy, M.; Lombardo, T.; Chouchane, M.; Primo, E.N.; Franco, A.A. Data-Driven Assessment of Electrode Calendering Process by Combining Experimental Results, in Silico Mesostructures Generation and Machine Learning. J. Power Sources 2020, 480, 229103. [Google Scholar] [CrossRef]
- Leithoff, R.; Fröhlich, A.; Dröder, K. Investigation of the Influence of Deposition Accuracy of Electrodes on the Electrochemical Properties of Lithium-Ion Batteries. Energy Technol. 2020, 8, 1900129. [Google Scholar] [CrossRef]
- Paxton, W.A.; Zhong, Z.; Tsakalakos, T. Tracking Inhomogeneity in High-Capacity Lithium Iron Phosphate Batteries. J. Power Sources 2015, 275, 429–434. [Google Scholar] [CrossRef]
- Ziesche, R.F.; Arlt, T.; Finegan, D.P.; Heenan, T.M.M.; Tengattini, A.; Baum, D.; Kardjilov, N.; Markötter, H.; Manke, I.; Kockelmann, W.; et al. 4D Imaging of Lithium-Batteries Using Correlative Neutron and X-Ray Tomography with a Virtual Unrolling Technique. Nat. Commun. 2020, 11, 777. [Google Scholar] [CrossRef]
- Baumhöfer, T.; Brühl, M.; Rothgang, S.; Sauer, D.U. Production Caused Variation in Capacity Aging Trend and Correlation to Initial Cell Performance. J. Power Sources 2014, 247, 332–338. [Google Scholar] [CrossRef]
- Miyatake, S.; Susuki, Y.; Hikihara, T.; Itoh, S.; Tanaka, K. Discharge Characteristics of Multicell Lithium-Ion Battery with Nonuniform Cells. J. Power Sources 2013, 241, 736–743. [Google Scholar] [CrossRef]
- Gogoana, R.; Pinson, M.B.; Bazant, M.Z.; Sarma, S.E. Internal Resistance Matching for Parallel-Connected Lithium-Ion Cells and Impacts on Battery Pack Cycle Life. J. Power Sources 2014, 252, 8–13. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Cugnet, M.; Liaw, B.Y.; Gering, K.; Sazhin, S.; Jamison, D.; Michelbacher, C. Evaluation of Commercial Lithium-Ion Cells Based on Composite Positive Electrode for Plug-in Hybrid Electric Vehicle Applications. Part I: Initial Characterizations. J. Power Sources 2011, 196, 10328–10335. [Google Scholar] [CrossRef]
- Paul, S.; Diegelmann, C.; Kabza, H.; Tillmetz, W. Analysis of Ageing Inhomogeneities in Lithium-Ion Battery Systems. J. Power Sources 2013, 239, 642–650. [Google Scholar] [CrossRef]
- Rumpf, K.; Naumann, M.; Jossen, A. Experimental Investigation of Parametric Cell-to-Cell Variation and Correlation Based on 1100 Commercial Lithium-Ion Cells. J. Energy Storage 2017, 14, 224–243. [Google Scholar] [CrossRef]
- An, F.; Chen, L.; Huang, J.; Zhang, J.; Li, P. Rate Dependence of Cell-to-Cell Variations of Lithium-Ion Cells. Sci. Rep. 2016, 6, 35051. [Google Scholar] [CrossRef]
- Schuster, S.F.; Brand, M.J.; Berg, P.; Gleissenberger, M.; Jossen, A. Lithium-Ion Cell-to-Cell Variation during Battery Electric Vehicle Operation. J. Power Sources 2015, 297, 242–251. [Google Scholar] [CrossRef]
- Dubarry, M.; Vuillaume, N.; Liaw, B.Y. Origins and Accommodation of Cell Variations in Li-Ion Battery Pack Modeling. Int. J. Energy Res. 2010, 34, 216–231. [Google Scholar] [CrossRef]
- Devie, A.; Dubarry, M. Durability and Reliability of Electric Vehicle Batteries under Electric Utility Grid Operations. Part 1: Cell-to-Cell Variations and Preliminary Testing. Batteries 2016, 2, 28. [Google Scholar] [CrossRef]
- Xie, L.; Ren, D.; Wang, L.; Chen, Z.; Tian, G.; Amine, K.; He, X. A Facile Approach to High Precision Detection of Cell-to-Cell Variation for Li-Ion Batteries. Sci. Rep. 2020, 10, 7182. [Google Scholar] [CrossRef]
- Carter, R.; Klein, E.J.; Atkinson, R.W.; Love, C.T. Mechanical Collapse as Primary Degradation Mode in Mandrel-Free 18650 Li-Ion Cells Operated at 0 °C. J. Power Sources 2019, 437, 226820. [Google Scholar] [CrossRef]
- Pfrang, A.; Kersys, A.; Kriston, A.; Sauer, D.U.; Rahe, C.; Käbitz, S.; Figgemeier, E. Long-Term Cycling Induced Jelly Roll Deformation in Commercial 18650 Cells. J. Power Sources 2018, 392, 168–175. [Google Scholar] [CrossRef]
- Willenberg, L.K.; Dechent, P.; Fuchs, G.; Teuber, M.; Eckert, M.; Graff, M.; Kürten, N.; Sauer, D.U.; Figgemeier, E. The Development of Jelly Roll Deformation in 18650 Lithium-Ion Batteries at Low State of Charge. J. Electrochem. Soc. 2020. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. The Effects of Defects on Localized Plating in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A1365. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Ion Transport Restriction in Mechanically Strained Separator Membranes. J. Power Sources 2013, 226, 149–155. [Google Scholar] [CrossRef]
- Mühlbauer, M.J.; Petz, D.; Baran, V.; Dolotko, O.; Hofmann, M.; Kostecki, R.; Senyshyn, A. Inhomogeneous Distribution of Lithium and Electrolyte in Aged Li-Ion Cylindrical Cells. J. Power Sources 2020, 475, 228690. [Google Scholar] [CrossRef]
- Petz, D.; Mühlbauer, M.J.; Schökel, A.; Achterhold, K.; Pfeiffer, F.; Pirling, T.; Hofmann, M.; Senyshyn, A. Heterogeneity of Graphite Lithiation in State-of-the-Art Cylinder-Type Li-Ion Cells. Batter. Supercaps 2021, 4, 327–335. [Google Scholar] [CrossRef]
- Mühlbauer, M.J.; Dolotko, O.; Hofmann, M.; Ehrenberg, H.; Senyshyn, A. Effect of Fatigue/Ageing on the Lithium Distribution in Cylinder-Type Li-Ion Batteries. J. Power Sources 2017, 348, 145–149. [Google Scholar] [CrossRef]
- [Infographic] Galaxy Note7: What We Discovered. Available online: https://news.samsung.com/global/infographic-galaxy-note7-what-we-discovered (accessed on 25 February 2021).
- Loveridge, M.J.; Remy, G.; Kourra, N.; Genieser, R.; Barai, A.; Lain, M.J.; Guo, Y.; Amor-Segan, M.; Williams, M.A.; Amietszajew, T.; et al. Looking Deeper into the Galaxy (Note 7). Batteries 2018, 4, 3. [Google Scholar] [CrossRef]
- Werner, D.; Paarmann, S.; Wiebelt, A.; Wetzel, T. Inhomogeneous Temperature Distribution Affecting the Cyclic Aging of Li-Ion Cells. Part II: Analysis and Correlation. Batteries 2020, 6, 12. [Google Scholar] [CrossRef]
- Osswald, P.J.; Erhard, S.V.; Rheinfeld, A.; Rieger, B.; Hoster, H.E.; Jossen, A. Temperature Dependency of State of Charge Inhomogeneities and Their Equalization in Cylindrical Lithium-Ion Cells. J. Power Sources 2016, 329, 546–552. [Google Scholar] [CrossRef]
- Grandjean, T.; Barai, A.; Hosseinzadeh, E.; Guo, Y.; McGordon, A.; Marco, J. Large Format Lithium Ion Pouch Cell Full Thermal Characterisation for Improved Electric Vehicle Thermal Management. J. Power Sources 2017, 359, 215–225. [Google Scholar] [CrossRef]
- Carter, R.; Kingston, T.A.; Atkinson, R.W.; Parmananda, M.; Dubarry, M.; Fear, C.; Mukherjee, P.P.; Love, C.T. Directionality of Thermal Gradients in Lithium-Ion Batteries Dictates Diverging Degradation Modes. Cell Rep. Phys. Sci. 2021, 100351. [Google Scholar] [CrossRef]
- Paarmann, S.; Cloos, L.; Technau, J.; Wetzel, T. Measurement of the Temperature Influence on the Current Distribution in Li-Ion Batteries. Energy Technol. 2020, ente.202000862. [Google Scholar] [CrossRef]
- Moretti, A.; Carvalho, D.V.; Ehteshami, N.; Paillard, E.; Porcher, W.; Brun-Buisson, D.; Ducros, J.-B.; de Meatza, I.; Eguia-Barrio, A.; Trad, K.; et al. A Post-Mortem Study of Stacked 16 Ah Graphite//LiFePO4 Pouch Cells Cycled at 5 °C. Batteries 2019, 5, 45. [Google Scholar] [CrossRef]
- Robinson, J.B.; Maier, M.; Alster, G.; Compton, T.; Brett, D.J.L.; Shearing, P.R. Spatially Resolved Ultrasound Diagnostics of Li-Ion Battery Electrodes. Phys. Chem. Chem. Phys. 2019, 21, 6354–6361. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A Review of Gas Evolution in Lithium Ion Batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Michalowski, P.; Gräfenstein, A.; Knipper, M.; Plaggenborg, T.; Schwenzel, J.; Parisi, J. Examining Inhomogeneous Degradation of Graphite/Carbon Black Composite Electrodes in Li-Ion Batteries by Lock-In Thermography. J. Electrochem. Soc. 2017, 164, A2251. [Google Scholar] [CrossRef]
- Devie, A.; Dubarry, M.; Liaw, B.Y. Overcharge Study in Li4Ti5O12 Based Lithium-Ion Pouch Cell: I. Quantitative Diagnosis of Degradation Modes. J. Electrochem. Soc. 2015, 162, A1033. [Google Scholar] [CrossRef]
- Devie, A.; Dubarry, M.; Wu, H.-P.; Wu, T.-H.; Liaw, B.Y. Overcharge Study in Li4Ti5O12 Based Lithium-Ion Pouch Cell. J. Electrochem. Soc. 2016, 163, A2611. [Google Scholar] [CrossRef]
- Devie, A.; Baure, G.; Dubarry, M. Intrinsic Variability in the Degradation of a Batch of Commercial 18650 Lithium-Ion Cells. Energies 2018, 11, 1031. [Google Scholar] [CrossRef]
- Harris, S.J.; Harris, D.J.; Li, C. Failure Statistics for Commercial Lithium Ion Batteries: A Study of 24 Pouch Cells. J. Power Sources 2017, 342, 589–597. [Google Scholar] [CrossRef]
- Li, W.; Sengupta, N.; Dechent, P.; Howey, D.; Annaswamy, A.; Sauer, D.U. Online Capacity Estimation of Lithium-Ion Batteries with Deep Long Short-Term Memory Networks. J. Power Sources 2021, 482, 228863. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Bruen, T.; Widanage, W.D.; Gama-Valdez, M.A.; Marco, J. A Study of Cell-to-Cell Interactions and Degradation in Parallel Strings: Implications for the Battery Management System. J. Power Sources 2016, 329, 574–585. [Google Scholar] [CrossRef]
- Brand, M.J.; Schmidt, P.A.; Zaeh, M.F.; Jossen, A. Welding Techniques for Battery Cells and Resulting Electrical Contact Resistances. J. Energy Storage 2015, 1, 7–14. [Google Scholar] [CrossRef]
- Taylor, J.; Barai, A.; Ashwin, T.R.; Guo, Y.; Amor-Segan, M.; Marco, J. An Insight into the Errors and Uncertainty of the Lithium-Ion Battery Characterisation Experiments. J. Energy Storage 2019, 24, 100761. [Google Scholar] [CrossRef]
- Bruen, T.; Marco, J. Modelling and Experimental Evaluation of Parallel Connected Lithium Ion Cells for an Electric Vehicle Battery System. J. Power Sources 2016, 310, 91–101. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Y.; Zhao, X. Influence of Connecting Plate Resistance upon LiFePO4 Battery Performance. Appl. Energy 2015, 147, 353–360. [Google Scholar] [CrossRef]
- Offer, G.J.; Yufit, V.; Howey, D.A.; Wu, B.; Brandon, N.P. Module Design and Fault Diagnosis in Electric Vehicle Batteries. J. Power Sources 2012, 206, 383–392. [Google Scholar] [CrossRef]
- Naguib, M.; Kollmeyer, P.; Emadi, A. Lithium-Ion Battery Pack Robust State of Charge Estimation, Cell Inconsistency, and Balancing: Review. IEEE Access 2021, 9, 50570–50582. [Google Scholar] [CrossRef]
- Dubarry, M.; Vuillaume, N.; Liaw, B.Y. From Single Cell Model to Battery Pack Simulation for Li-Ion Batteries. J. Power Sources 2009, 186, 500–507. [Google Scholar] [CrossRef]
- Dubarry, M.; Pastor-Fernández, C.; Baure, G.; Yu, T.F.; Widanage, W.D.; Marco, J. Battery Energy Storage System Modeling: Investigation of Intrinsic Cell-to-Cell Variations. J. Energy Storage 2019, 23, 19–28. [Google Scholar] [CrossRef]
- Feng, F.; Hu, X.; Hu, L.; Hu, F.; Li, Y.; Zhang, L. Propagation Mechanisms and Diagnosis of Parameter Inconsistency within Li-Ion Battery Packs. Renew. Sustain. Energy Rev. 2019, 112, 102–113. [Google Scholar] [CrossRef]
- Liu, X.; Ai, W.; Naylor Marlow, M.; Patel, Y.; Wu, B. The Effect of Cell-to-Cell Variations and Thermal Gradients on the Performance and Degradation of Lithium-Ion Battery Packs. Appl. Energy 2019, 248, 489–499. [Google Scholar] [CrossRef]
- Neupert, S.; Kowal, J. Inhomogeneities in Battery Packs. World Electr. Veh. J. 2018, 9, 20. [Google Scholar] [CrossRef]
- Wu, B.; Yufit, V.; Marinescu, M.; Offer, G.J.; Martinez-Botas, R.F.; Brandon, N.P. Coupled Thermal–Electrochemical Modelling of Uneven Heat Generation in Lithium-Ion Battery Packs. J. Power Sources 2013, 243, 544–554. [Google Scholar] [CrossRef]
- Dubarry, M.; Devie, A.; Stein, K.; Tun, M.; Matsuura, M.; Rocheleau, R. Battery Energy Storage System Battery Durability and Reliability under Electric Utility Grid Operations: Analysis of 3 Years of Real Usage. J. Power Sources 2017, 338, 65–73. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, X.; Shang, B.; Li, G. Unbalanced Discharging and Aging Due to Temperature Differences among the Cells in a Lithium-Ion Battery Pack with Parallel Combination. J. Power Sources 2016, 306, 733–741. [Google Scholar] [CrossRef]
- Chiu, K.-C.; Lin, C.-H.; Yeh, S.-F.; Lin, Y.-H.; Huang, C.-S.; Chen, K.-C. Cycle Life Analysis of Series Connected Lithium-Ion Batteries with Temperature Difference. J. Power Sources 2014, 263, 75–84. [Google Scholar] [CrossRef]
- An, F.; Huang, J.; Wang, C.; Li, Z.; Zhang, J.; Wang, S.; Li, P. Cell Sorting for Parallel Lithium-Ion Battery Systems: Evaluation Based on an Electric Circuit Model. J. Energy Storage 2016, 6, 195–203. [Google Scholar] [CrossRef]
- Lyu, C.; Song, Y.; Wang, L.; Li, J.; Zhang, B.; Liu, E. A New Method for Lithium-Ion Battery Uniformity Sorting Based on Internal Criteria. J. Energy Storage 2019, 25, 100885. [Google Scholar] [CrossRef]
- Wang, T.; Tseng, K.J.; Zhao, J.; Wei, Z. Thermal Investigation of Lithium-Ion Battery Module with Different Cell Arrangement Structures and Forced Air-Cooling Strategies. Appl. Energy 2014, 134, 229–238. [Google Scholar] [CrossRef]
- Gering, K.L.; Sazhin, S.V.; Jamison, D.K.; Michelbacher, C.J.; Liaw, B.Y.; Dubarry, M.; Cugnet, M. Investigation of Path Dependence in Commercial Lithium-Ion Cells Chosen for Plug-in Hybrid Vehicle Duty Cycle Protocols. J. Power Sources 2011, 196, 3395–3403. [Google Scholar] [CrossRef]
- Keil, J.; Paul, N.; Baran, V.; Keil, P.; Gilles, R.; Jossen, A. Linear and Nonlinear Aging of Lithium-Ion Cells Investigated by Electrochemical Analysis and In-Situ Neutron Diffraction. J. Electrochem. Soc. 2019, 166, A3908. [Google Scholar] [CrossRef]
- Raj, T.; Wang, A.A.; Monroe, C.W.; Howey, D.A. Investigation of Path-Dependent Degradation in Lithium-Ion Batteries**. Batter. Supercaps 2020, 3, 1377–1385. [Google Scholar] [CrossRef]
- Simolka, M.; Heger, J.-F.; Kaess, H.; Biswas, I.; Friedrich, K.A. Influence of Cycling Profile, Depth of Discharge and Temperature on Commercial LFP/C Cell Ageing: Post-Mortem Material Analysis of Structure, Morphology and Chemical Composition. J. Appl. Electrochem. 2020, 50, 1101–1117. [Google Scholar] [CrossRef]
- Anseán, D.; Dubarry, M.; Devie, A.; Liaw, B.Y.; García, V.M.; Viera, J.C.; González, M. Operando Lithium Plating Quantification and Early Detection of a Commercial LiFePO4 Cell Cycled under Dynamic Driving Schedule. J. Power Sources 2017, 356, 36–46. [Google Scholar] [CrossRef]
- Liaw, B.Y.; Dubarry, M. From Driving Cycle Analysis to Understanding Battery Performance in Real-Life Electric Hybrid Vehicle Operation. J. Power Sources 2007, 174, 76–88. [Google Scholar] [CrossRef]
- Martinez-Laserna, E.; Sarasketa-Zabala, E.; Stroe, D.-I.; Swierczynski, M.; Warnecke, A.; Timmermans, J.M.; Goutam, S.; Rodriguez, P. Evaluation of Lithium-Ion Battery Second Life Performance and Degradation. In Proceedings of the 2016 IEEE Energy Conversion Congress and Exposition (ECCE), Milwaukee, WI, USA, 18–22 September 2016; pp. 1–7. [Google Scholar]
- Hossain, E.; Murtaugh, D.; Mody, J.; Faruque, H.M.R.; Sunny, M.S.H.; Mohammad, N. A Comprehensive Review on Second-Life Batteries: Current State, Manufacturing Considerations, Applications, Impacts, Barriers Potential Solutions, Business Strategies, and Policies. IEEE Access 2019, 7, 73215–73252. [Google Scholar] [CrossRef]
- Glazer, M.P.B.; Okasinski, J.S.; Almer, J.D.; Ren, Y. High-Energy X-ray Scattering Studies of Battery Materials. MRS Bull. 2016, 41, 460–465. [Google Scholar] [CrossRef]
- Müller, S.; Pietsch, P.; Brandt, B.-E.; Baade, P.; Andrade, V.D.; Carlo, F.D.; Wood, V. Quantification and Modeling of Mechanical Degradation in Lithium-Ion Batteries Based on Nanoscale Imaging. Nat. Commun. 2018, 9, 2340. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; An, K.; Feng, Z.; Liang, C.; Harris, S.J. In-Situ Observation of Inhomogeneous Degradation in Large Format Li-Ion Cells by Neutron Diffraction. J. Power Sources 2013, 236, 163–168. [Google Scholar] [CrossRef]
- Senyshyn, A.; Mühlbauer, M.J.; Dolotko, O.; Hofmann, M.; Ehrenberg, H. Homogeneity of Lithium Distribution in Cylinder-Type Li-Ion Batteries. Sci. Rep. 2015, 5, 18380. [Google Scholar] [CrossRef]
- Paul, N.; Wandt, J.; Seidlmayer, S.; Schebesta, S.; Mühlbauer, M.J.; Dolotko, O.; Gasteiger, H.A.; Gilles, R. Aging Behavior of Lithium Iron Phosphate Based 18650-Type Cells Studied by in Situ Neutron Diffraction. J. Power Sources 2017, 345, 85–96. [Google Scholar] [CrossRef]
- Paul, N.; Keil, J.; Kindermann, F.M.; Schebesta, S.; Dolotko, O.; Mühlbauer, M.J.; Kraft, L.; Erhard, S.V.; Jossen, A.; Gilles, R. Aging in 18650-Type Li-Ion Cells Examined with Neutron Diffraction, Electrochemical Analysis and Physico-Chemical Modeling. J. Energy Storage 2018, 17, 383–394. [Google Scholar] [CrossRef]
- Zinth, V.; von Lüders, C.; Wilhelm, J.; Erhard, S.V.; Hofmann, M.; Seidlmayer, S.; Rebelo-Kornmeier, J.; Gan, W.; Jossen, A.; Gilles, R. Inhomogeneity and Relaxation Phenomena in the Graphite Anode of a Lithium-Ion Battery Probed by in Situ Neutron Diffraction. J. Power Sources 2017, 361, 54–60. [Google Scholar] [CrossRef]
- Petz, D.; Mühlbauer, M.J.; Baran, V.; Frost, M.; Schökel, A.; Paulmann, C.; Chen, Y.; Garcés, D.; Senyshyn, A. Lithium Heterogeneities in Cylinder-Type Li-Ion Batteries—Fatigue Induced by Cycling. J. Power Sources 2020, 448, 227466. [Google Scholar] [CrossRef]
- Fleming, J.; Amietszajew, T.; Charmet, J.; Roberts, A.J.; Greenwood, D.; Bhagat, R. The Design and Impact of In-Situ and Operando Thermal Sensing for Smart Energy Storage. J. Energy Storage 2019, 22, 36–43. [Google Scholar] [CrossRef]
- McTurk, E.; Birkl, C.R.; Roberts, M.R.; Howey, D.A.; Bruce, P.G. Minimally Invasive Insertion of Reference Electrodes into Commercial Lithium-Ion Pouch Cells. ECS Electrochem. Lett. 2015, 4, A145. [Google Scholar] [CrossRef]
- Osswald, P.J.; Erhard, S.V.; Noel, A.; Keil, P.; Kindermann, F.M.; Hoster, H.; Jossen, A. Current Density Distribution in Cylindrical Li-Ion Cells during Impedance Measurements. J. Power Sources 2016, 314, 93–101. [Google Scholar] [CrossRef]
- Wang, H.; Whitacre, J.F. Inhomogeneous Aging of Cathode Materials in Commercial 18650 Lithium Ion Battery Cells. J. Energy Storage 2021, 35, 102244. [Google Scholar] [CrossRef]
- Warnecke, A.J. Degradation Mechanisms in NMC-Based Lithium-Ion Batteries. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2017. [Google Scholar]
- Gyenes, B.; Stevens, D.A.; Chevrier, V.L.; Dahn, J.R. Understanding Anomalous Behavior in Coulombic Efficiency Measurements on Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A278–A283. [Google Scholar] [CrossRef]
- Juarez-Robles, D.; Jeevarajan, J.A.; Mukherjee, P.P. Degradation-Safety Analytics in Lithium-Ion Cells: Part I. Aging under Charge/Discharge Cycling. J. Electrochem. Soc. 2020, 167, 160510. [Google Scholar] [CrossRef]
- Spingler, F.B.; Naumann, M.; Jossen, A. Capacity Recovery Effect in Commercial LiFePO4 / Graphite Cells. J. Electrochem. Soc. 2020, 167, 040526. [Google Scholar] [CrossRef]
- Burns, J.C.; Stevens, D.A.; Dahn, J.R. In-Situ Detection of Lithium Plating Using High Precision Coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Lewerenz, M.; Warnecke, A.; Sauer, D.U. Post-Mortem Analysis on LiFePO4|Graphite Cells Describing the Evolution & Composition of Covering Layer on Anode and Their Impact on Cell Performance. J. Power Sources 2017, 369, 122–132. [Google Scholar] [CrossRef]
- Fleury, X.; Noh, M.H.; Geniès, S.; Thivel, P.X.; Lefrou, C.; Bultel, Y. Fast-Charging of Lithium Iron Phosphate Battery with Ohmic-Drop Compensation Method: Ageing Study. J. Energy Storage 2018, 16, 21–36. [Google Scholar] [CrossRef]
- Käbitz, S. Untersuchung der Alterung von Lithium-Ionen-Batterien Mittels Elektroanalytik und Elektrochemischer Impedanzspektroskopie. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2016. [Google Scholar]
- Pfrang, A.; Kersys, A.; Kriston, A.; Sauer, D.U.; Rahe, C.; Käbitz, S.; Figgemeier, E. Geometrical Inhomogeneities as Cause of Mechanical Failure in Commercial 18650 Lithium Ion Cells. J. Electrochem. Soc. 2019, 166, A3745–A3752. [Google Scholar] [CrossRef]
- Burow, D.; Sergeeva, K.; Calles, S.; Schorb, K.; Börger, A.; Roth, C.; Heitjans, P. Inhomogeneous Degradation of Graphite Anodes in Automotive Lithium Ion Batteries under Low-Temperature Pulse Cycling Conditions. J. Power Sources 2016, 307, 806–814. [Google Scholar] [CrossRef]
- Rahe, C.; Kelly, S.T.; Rad, M.N.; Sauer, D.U.; Mayer, J.; Figgemeier, E. Nanoscale X-Ray Imaging of Ageing in Automotive Lithium Ion Battery Cells. J. Power Sources 2019, 433, 126631. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, R.; Zhang, K.; Lee, S.-J.; Mu, L.; Liu, P.; Waters, C.K.; Spence, S.; Xu, Z.; Wei, C.; et al. Quantification of Heterogeneous Degradation in Li-Ion Batteries. Adv. Energy Mater. 2019, 9, 1900674. [Google Scholar] [CrossRef]
- Li, L.; Hou, J. Capacity Detection of Electric Vehicle Lithium-Ion Batteries Based on X-Ray Computed Tomography. RSC Adv. 2018, 8, 25325–25333. [Google Scholar] [CrossRef]
- Ilott, A.J.; Mohammadi, M.; Schauerman, C.M.; Ganter, M.J.; Jerschow, A. Rechargeable Lithium-Ion Cell State of Charge and Defect Detection by in-Situ inside-out Magnetic Resonance Imaging. Nat. Commun. 2018, 9, 1776. [Google Scholar] [CrossRef]
- Krachkovskiy, S.A.; Foster, J.M.; Bazak, J.D.; Balcom, B.J.; Goward, G.R. Operando Mapping of Li Concentration Profiles and Phase Transformations in Graphite Electrodes by Magnetic Resonance Imaging and Nuclear Magnetic Resonance Spectroscopy. J. Phys. Chem. C 2018, 122, 21784–21791. [Google Scholar] [CrossRef]
- Wiemers-Meyer, S.; Winter, M.; Nowak, S. Mechanistic Insights into Lithium Ion Battery Electrolyte Degradation—A Quantitative NMR Study. Phys. Chem. Chem. Phys. 2016, 18, 26595–26601. [Google Scholar] [CrossRef] [PubMed]
- Bauermann, L.P.; Mesquita, L.V.; Bischoff, C.; Drews, M.; Fitz, O.; Heuer, A.; Biro, D. Scanning Acoustic Microscopy as a Non-Destructive Imaging Tool to Localize Defects inside Battery Cells. J. Power Sources Adv. 2020, 6, 100035. [Google Scholar] [CrossRef]
- Dubarry, M.; Baure, G. Perspective on Commercial Li-Ion Battery Testing, Best Practices for Simple and Effective Protocols. Electronics 2020, 9, 152. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.-S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberšček, M.; et al. Application of Electrochemical Impedance Spectroscopy to Commercial Li-Ion Cells: A Review. J. Power Sources 2020, 480, 228742. [Google Scholar] [CrossRef]
- Bloom, I.; Jansen, A.N.; Abraham, D.P.; Knuth, J.; Jones, S.A.; Battaglia, V.S.; Henriksen, G.L. Differential Voltage Analyses of High-Power, Lithium-Ion Cells 1. Technique and Application. J. Power Sources 2005, 139, 295–303. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y. Identify Capacity Fading Mechanism in a Commercial LiFePO4 Cell. J. Power Sources 2009, 194, 541–549. [Google Scholar] [CrossRef]
- Lewerenz, M.; Marongiu, A.; Warnecke, A.; Sauer, D.U. Differential Voltage Analysis as a Tool for Analyzing Inhomogeneous Aging: A Case Study for LiFePO4|Graphite Cylindrical Cells. J. Power Sources 2017, 368, 57–67. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Gao, Y.; Guo, Q. Recognition of Battery Aging Variations for LiFePO4 Batteries in 2nd Use Applications Combining Incremental Capacity Analysis and Statistical Approaches. J. Power Sources 2017, 360, 180–188. [Google Scholar] [CrossRef]
- Tanim, T.R.; Shirk, M.G.; Bewley, R.L.; Dufek, E.J.; Liaw, B.Y. Fast Charge Implications: Pack and Cell Analysis and Comparison. J. Power Sources 2018, 381, 56–65. [Google Scholar] [CrossRef]
- Chang, L.; Wang, C.; Zhang, C.; Xiao, L.; Cui, N.; Li, H.; Qiu, J. A Novel Fast Capacity Estimation Method Based on Current Curves of Parallel-Connected Cells for Retired Lithium-Ion Batteries in Second-Use Applications. J. Power Sources 2020, 459, 227901. [Google Scholar] [CrossRef]
- Krupp, A.; Ferg, E.; Schuldt, F.; Derendorf, K.; Agert, C. Incremental Capacity Analysis as a State of Health Estimation Method for Lithium-Ion Battery Modules with Series-Connected Cells. Batteries 2021, 7, 2. [Google Scholar] [CrossRef]
- Lewerenz, M.; Warnecke, A.; Sauer, D.U. Introduction of Capacity Difference Analysis (CDA) for Analyzing Lateral Lithium-Ion Flow to Determine the State of Covering Layer Evolution. J. Power Sources 2017, 354, 157–166. [Google Scholar] [CrossRef]
- Prosser, R.; Offer, G.; Patel, Y. Lithium-Ion Diagnostics: The First Quantitative In-Operando Technique for Diagnosing Lithium Ion Battery Degradation Modes under Load with Realistic Thermal Boundary Conditions. J. Electrochem. Soc. 2021, 168, 030532. [Google Scholar] [CrossRef]
- Delobel, B. Lessons Learned from Field Data Analysis, and Future Challenges—Renault EV 2019. Available online: http://cii-resource.com/cet/AABE-03-17/Presentations/BMGT/Delobel_Bruno.pdf (accessed on 1 June 2021).
- Salinas, F.; Krüger, L.; Neupert, S.; Kowal, J. A Second Life for Li-Ion Cells Rescued from Notebook Batteries. J. Energy Storage 2019, 24, 100747. [Google Scholar] [CrossRef]
- Myall, D. 30 KWh Nissan Leaf Firmware Update to Correct Capacity Reporting. FlipTheFleet 2018. Available online: https://flipthefleet.org/2018/30-kwh-nissan-leaf-firmware-update-to-correct-capacity-reporting/ (accessed on 1 June 2021).
- MaxRange Tesla Battery Survey. Available online: https://teslamotorsclub.com/tmc/threads/maxrange.35978/ (accessed on 7 March 2021).








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, D.; Dechent, P.; Junker, M.; Sauer, D.U.; Dubarry, M. Inhomogeneities and Cell-to-Cell Variations in Lithium-Ion Batteries, a Review. Energies 2021, 14, 3276. https://doi.org/10.3390/en14113276
Beck D, Dechent P, Junker M, Sauer DU, Dubarry M. Inhomogeneities and Cell-to-Cell Variations in Lithium-Ion Batteries, a Review. Energies. 2021; 14(11):3276. https://doi.org/10.3390/en14113276
Chicago/Turabian StyleBeck, David, Philipp Dechent, Mark Junker, Dirk Uwe Sauer, and Matthieu Dubarry. 2021. "Inhomogeneities and Cell-to-Cell Variations in Lithium-Ion Batteries, a Review" Energies 14, no. 11: 3276. https://doi.org/10.3390/en14113276
APA StyleBeck, D., Dechent, P., Junker, M., Sauer, D. U., & Dubarry, M. (2021). Inhomogeneities and Cell-to-Cell Variations in Lithium-Ion Batteries, a Review. Energies, 14(11), 3276. https://doi.org/10.3390/en14113276







