1. Introduction
The criteria used for pricing diamonds in the jewelry trade are the 4 C’s [
1] (Carat, Color, Cut, and Clarity). The color grade and the size are the most significant properties for evaluating a diamond’s price. Gemological laboratories determine a diamond’s color grading using human eyes and by comparing the diamonds with a set of predefined color grade master diamonds [
2]. Furthermore, in the last 50 years, some optical equipment in the infrared range and in the UV–visible spectrum have been frequently used for ranking a diamond’s color [
3,
4,
5,
6,
7,
8,
9,
10].
The yellow to brownish color which occurs in a diamond is the main indicator of color degradation. Nitrogen (N) atom contamination is responsible (in at least 95% cases) for a diamond’s color degradation, inducing a yellow-brown tint [
6,
11,
12,
13]. The nitrogen atom is smaller, at ~56 pm, compared to the carbon atoms, 67 pm [
14]; thus, it can contaminate the diamond crystal when carbon atoms are replaced. A brown color occurs (~1–3%) due to plastic deformation: slight deviations from an ideal structure can exist in the crystal as a result of the carbon atoms’ position [
15,
16]. In addition, rarely, boron (B) atoms (which are small compared to carbon atoms) may replace carbon in the diamond, producing a bluish-gray color (e.g., the well-known Hope diamond [
4,
7,
17,
18]). The nitrogen content in the diamonds is due to an impurity, which is spread homogenously in the diamond crystal during the formation of the natural diamond in the Earth’s crust magma.
The letters D-M represent the color grading system [
3]; an earlier letter alphabetically indicates a better quality, and a higher price. Diamonds that have no nitrogen concentration at all (defined as type 2a) and no plastic deformation are denoted as D type in the gemological certificate of the diamond.
As previously mentioned, to evaluate the color grade for rough stones, two main methods are used: UV–visible or infrared spectroscopy [
3,
19].
For the UV–visible range, a commercial unit is used, but the accuracy is poor. For the infrared spectral range, the nitrogen content can be identified by analyzing the absorption peaks and distinguishing them from the absorption peaks of the diamond’s crystal structure. Errors occur in determining the color grading in rough stones because the scattered light is proportional to 1/λ
4. Explicitly, shorter wavelengths will cause the scattered light to increase, causing perturbations and imprecise measurements. Thus, infrared spectroscopy in the micrometer wavelength range (larger λ, 4–25 μm) is superior to the shorter wavelength UV–visible range (larger λ, 250–700 nm). For uncut and rough diamonds, the effect of the surface shape can also be detrimental, causing inaccuracy in determining the color grade. MW spectroscopy has advantages because its range, used here, has a much longer λ and thus more potential to measure a diamond’s electromagnetic/optical properties [
20].
Another advantage is the price of a MW color grading system. A MW detector, if developed, is expected to be much less expensive in a commercial unit than in a FTIR unit (with an estimated price of 25,000 USD per unit). The electromagnetic wave source and the detectors in the MW spectral region are also much cheaper.
To the best of our knowledge, the only reports that have investigated and analyzed nitrogen concentration (or color grading) using MW techniques is [
21] which handled frequencies in the range of 100–110 GHz. However, some work in CVD diamonds [
22] and in nanodiamonds [
23,
24] determined their permittivity in the MW range.
, which is the dielectric permittivity, is a frequency dependent quantity, affected by the type of atoms in the diamond crystal, i.e., nitrogen and carbon. Each atom has a different number of electrons. Thus, different binding forces take place between the nucleus and the surrounding electrons, resulting in a corresponding electronic susceptibility [
25].
The electronic susceptibility of the carbon atoms is given by:
while the contribution of the nitrogen atoms is:
where f is the frequency,
and
represent the electron mass and charge, respectively,
is the number of electrons per each atom (
for carbon, and
for nitrogen) with respective binding frequency
and damping constant
, and
and
are the nitrogen and carbon molecules per unit volume, respectively.
In the last equation, we identify as the nitrogen concentration in the diamond. The dielectric permittivity is revealed to be dependent both on frequency and on the concentration of nitrogen atoms.
In this paper, spectroscopic characterization of the diamond is done using a microwave cavity (waveguide or resonator) described in
Section 2.2.2 and
Section 2.2.3. A comparison is made with the color grading done with infrared spectroscopy (
Section 2.2.1). The characterization is done via transmission or reflection S parameters measurements as described in
Section 2.2.2. The frequency range for both methods is 3.95–26.5 GHz.
3. Results and Discussion
The 4 master diamonds were measured 20 times using the transmission/reflection method. The frequency used in all measurements was the waveguide’s full range.
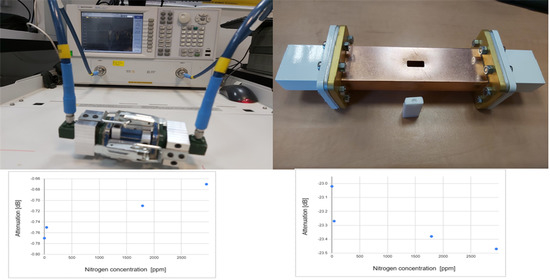
Examining the results of transition S12 magnitude revealed a correlation between the measured signal level in dB (attenuation from the original signal) and the nitrogen concentration.
Figure 10 presents the value of S12 when the master diamonds are inserted into the WR62 waveguide. Each diamond’s average S12 value is presented vs. the frequency. This implies that reducing the nitrogen concentration in a diamond also reduces the S12 transition parameter.
Inspection of
Figure 10 revealed that the nitrogen concentration (and therefore the resultant color grade) can be determined via the transmission S12 measurements. The level in dB of the measurements decreases for lower nitrogen concentrations. The trend of this correlation is within the frequency range of 17.6 GHz. To achieve better discrimination of color grading, we present in
Figure 11 a correlation graph of S12 parameters in dB vs. the nitrogen concentration in ppm (calculated from the absorption values of the 1095 cm
−1 peaks in the infrared spectrum). A linear regression line is also illustrated to show the correlation of the measured S12 with the nitrogen concentration.
The trend of the correlation between S12 and nitrogen concentrations can be seen again in
Figure 11. Noting the linearity of the trend, a direct a mapping from S12 measurements to nitrogen concentration can be employed.
In order to verify a reliable S12 value, we carried out additional measurements. Each diamond type was inserted into the cavity and the parameter S12 was measured by the VNA.
Figure 12 shows the resulting normal statistical distributions of the measured S12 for each diamond type. Each distribution center represents the average value of the S12 measurements for each of the master diamonds, revealing some overlaps.
Figure 12 demonstrates the statistical distribution of the S12 measurements. There is a clear difference between master diamond types 2a and L1, consisting of the lowest and highest nitrogen concentrations, respectively, with a high level of reliability.
Table 2 summarizes the average value and the standard deviation (STD) of S12 at a frequency of 17.72 GHz for the characterized master diamonds. The S12 parameter increases with the nitrogen concentration (
Figure 3,
Figure 4,
Figure 5 and
Figure 6). The same correlation is revealed for the color grade.
Experiments were also carried out at 26 GHz using the waveguide WR42.
Figure 13 displays the S12 parameter (in dB) vs. the nitrogen concentration (in ppm).
The regression line drawn in the
Figure 13, denoted by a dashed line, implies that a decrease in S12 corresponds to an increase in the nitrogen concentration. The strict trend demonstrates that spectroscopic diamond characterization at a frequency near 26.025 GHz can estimate the diamond nitrogen concentration and color. As in
Figure 11, in
Figure 13 a linear trend is demonstrated.
The measurement statistics are displayed for each diamond type as normal distribution graphs in
Figure 14.
As demonstrated, at low nitrogen concentrations almost no overlapping exists between the master diamond 2a (zero nitrogen) and the L master diamond (with the highest concentration level of nitrogen). At this frequency, the evaluation is shown to be robust. The overlapping indicates the probability of incorrect color evaluation.
Table 3 summarizes the mean value and the STD of S12 at a frequency of 26.025 GHz for the master diamonds examined. The S12 parameter decreases as the nitrogen concentration increases (see
Figure 3,
Figure 4,
Figure 5 and
Figure 6). The same trend is observed for the color grade.
With WR90, no correlations in the frequencies were found; however, when measurement of the diamonds using this waveguide at lower frequencies towards its cutoff was attempted, an interesting result was found at around 7 GHz.
Figure 15 and
Figure 16 display normal distributions graphs which present the average and the STD of some frequencies near the cutoff of the WR90 waveguide. Since the cutoff frequency was 6.557 GHz, it was measured from 6–8 GHz.
As before, a correlation is successfully found between the nitrogen concentration and the transmission S12 parameter near 7.0375 GHz and 7.175 GHz. The average values of S12 measurements presented in
Figure 15 and
Figure 16 increase as the nitrogen concentration falls. A higher level of S12 implies a better color grading. Despite the overlap between the distributions in the master diamonds D and H, the average value can be a good indicator for the color grade, since 2a and L1 master diamonds present almost no overlapping between their corresponding transmission and only a small overlap to master diamonds D and H.
Table 4 summarizes the mean values and the STD of S12 measured at a frequency of 7.175 GHz for the master diamonds. The S12 parameter decreases as the nitrogen concentration increases (
Figure 3,
Figure 4,
Figure 5 and
Figure 6).
Table 5 summarizes the mean value and the STD of S12 at a frequency of 7.0375 GHz for the master diamonds. The S12 parameter decreases when the nitrogen increases (
Figure 3,
Figure 4,
Figure 5 and
Figure 6).
As in the WR42 waveguide experiments, almost no overlaps existed between the highest concentration (L1) and the lowest concentration (2a). A small overlap was found between the 2a and L1 master diamonds and the other two D and H diamonds, which have nitrogen concentrations.
In the resonator method, a nitrogen concentration correlated frequency was found using the WR187 waveguide. As expected in such a multi-reflection cavity, the unique periodic spectral signature of such a structure is significant.
Figure 17 displays the resulting S12 for the full frequency range available at this waveguide.
Closer inspection reveals that the S12 peak locations are correlated with the nitrogen concentration, and therefore with the color grade as well.
Figure 18,
Figure 19,
Figure 20 and
Figure 21 display peak shifts at 3.27 GHz, 3.7 GHz, 4.29 GHz, and 5.01 GHz, respectively, obtained for the different diamond types.
Figure 22 displays the statistics of the location of the peaks, revealing that no overlap exists between the 2a and L1 master diamonds.
Figure 18,
Figure 19,
Figure 20 and
Figure 21 show that there is a clear correlation between the nitrogen concentration and the location of the peak which resulted due to resonances. Higher peak frequency is correlated with lower nitrogen concentration. The correlation is similar for all the peaks in the frequency range of 3.15–5.85 GHz. As such, it is reliable indicator for the estimation of the color grade.
Figure 22 implies that the overlapping between the 2a master diamond and the L1 master diamond is negligible, so no false color grade identification is expected using resonator-based spectroscopy for the above color gradings. It indicates that this approach presents more reliable characterizations compared to transmission/reflection measurements. A clear discrimination between H and D master diamonds is revealed.
Table 6 summarizes the average and STD of the spectral peak locations for different master diamonds. The peak frequency decreases as the nitrogen concentration increases.
The results indicate that a strong correlation exists between the nitrogen concentration and the electromagnetic parameters using several methods and a wide range of frequencies. Since the wavelength of the above frequencies is longer, the results show promise for finding a technique for evaluating the color grade of rough and polished diamonds. Further research will elaborate whether and how the size of the diamonds affects the measurements. In addition, a master series of rough diamonds should also be tested using the above methods.