Burnout Investigation of Small Diameter Tubes Immersed in Nanofluids †
Abstract
:1. Introduction
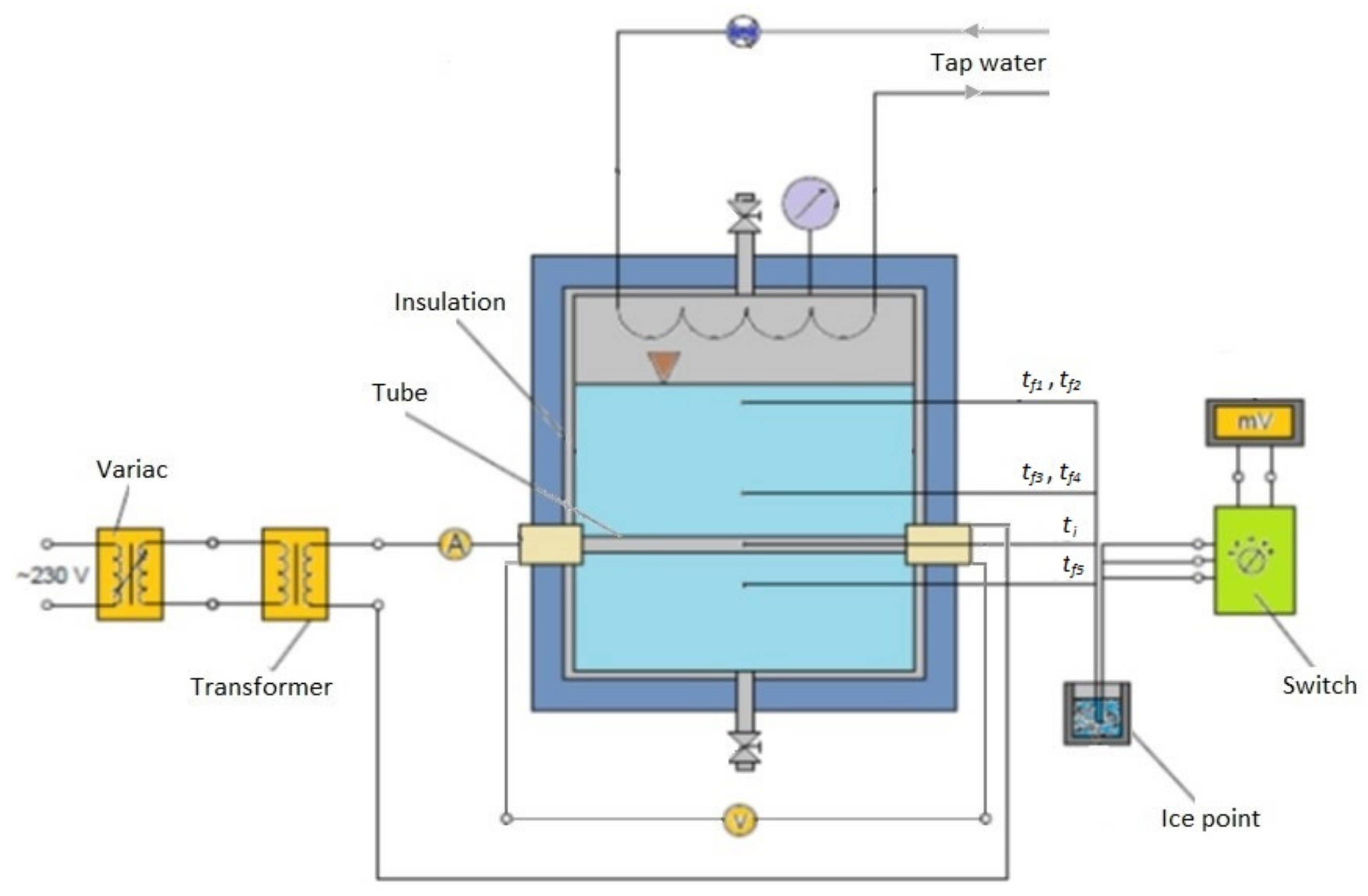
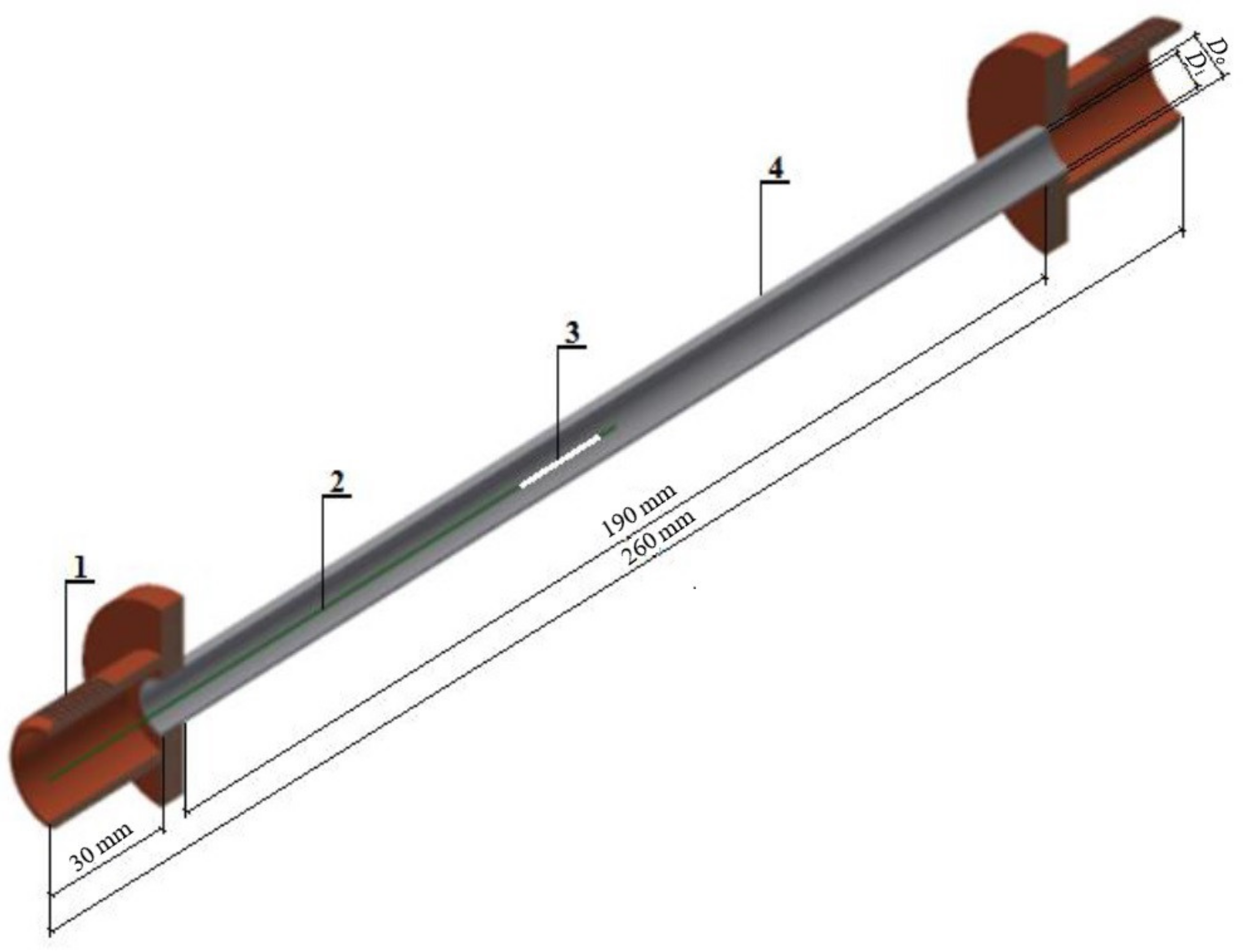
2. Experimental Apparatus and Procedure
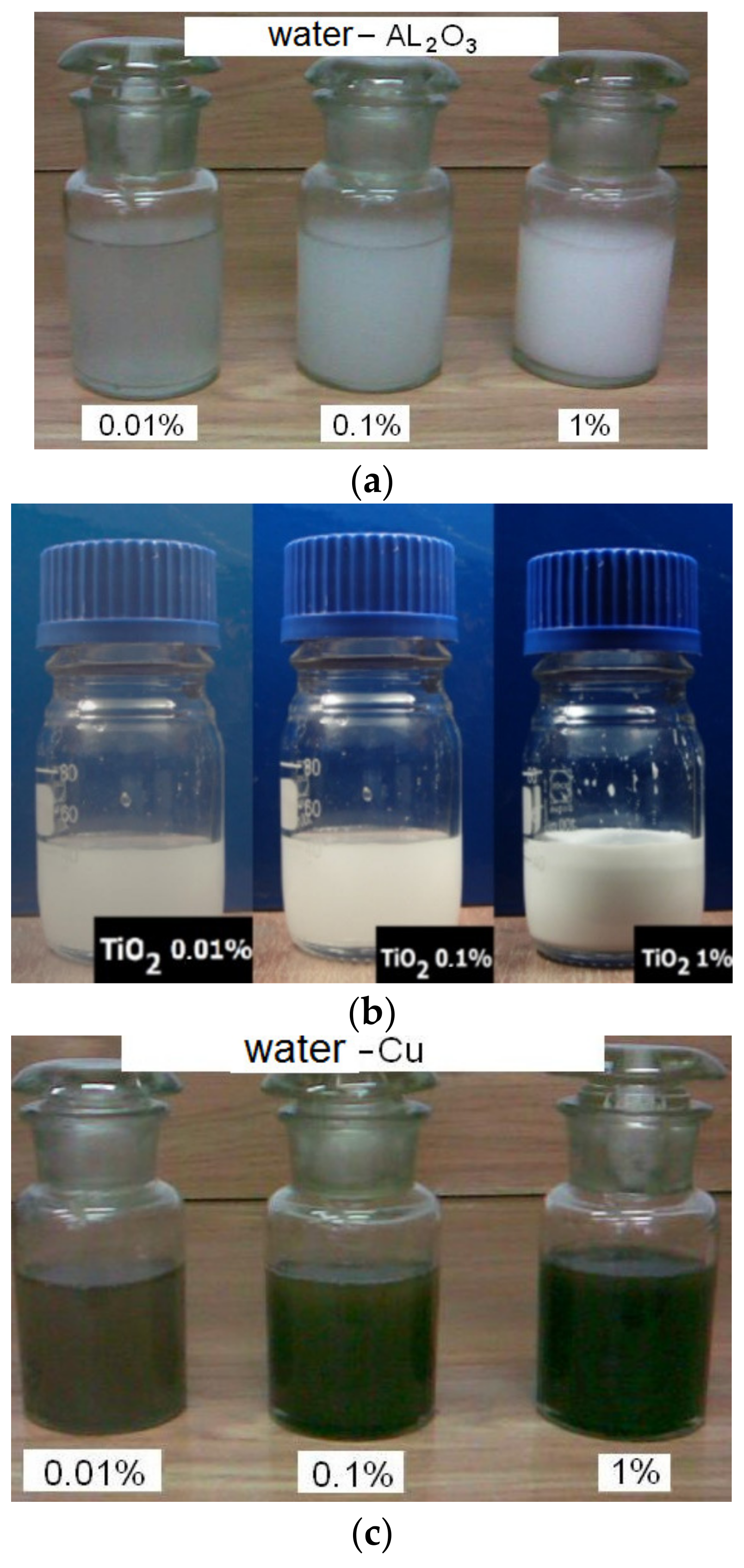
3. Preparation and Characterization of Nanofluids
4. Data Reduction and Uncertainty Estimation
5. Results
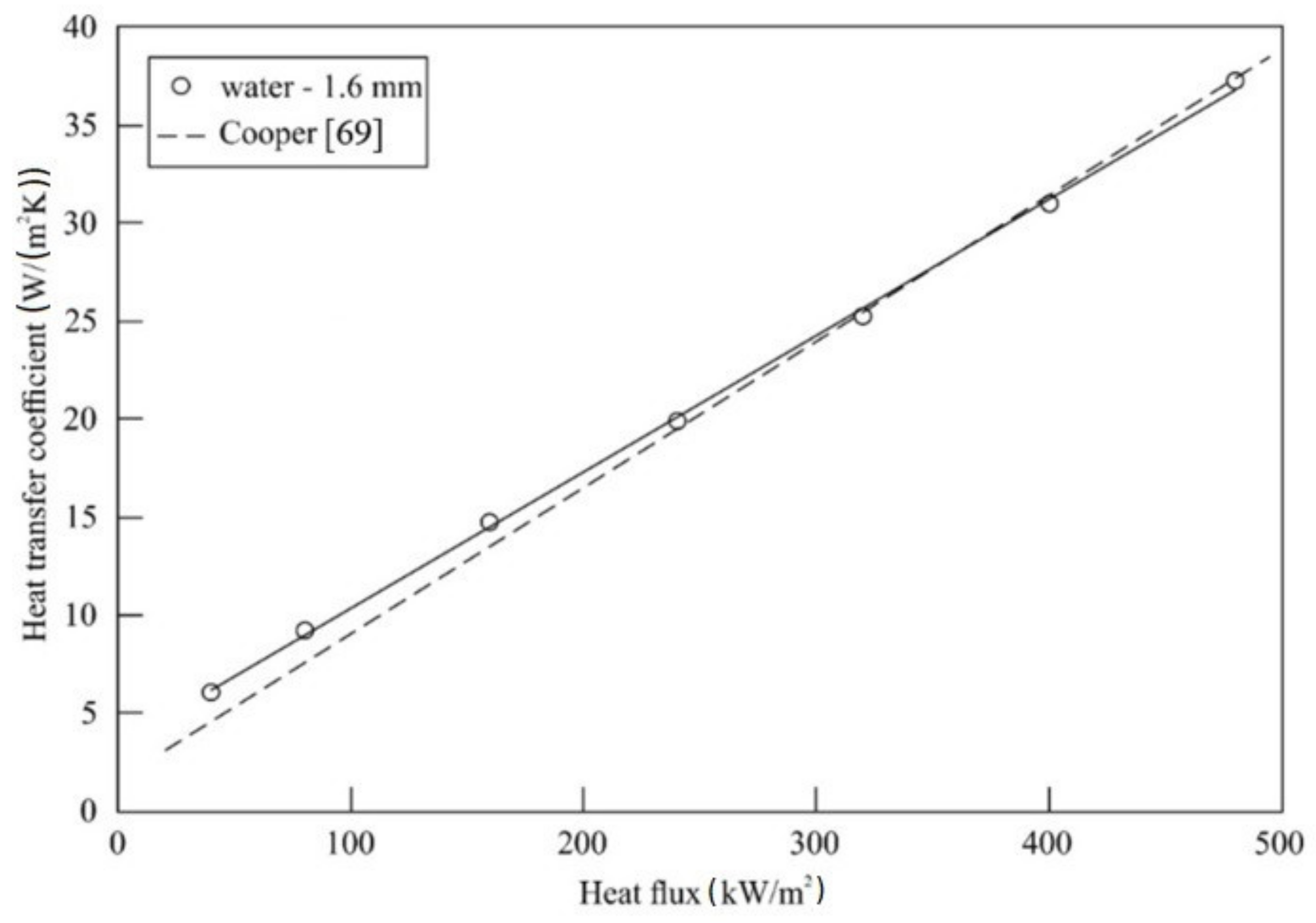
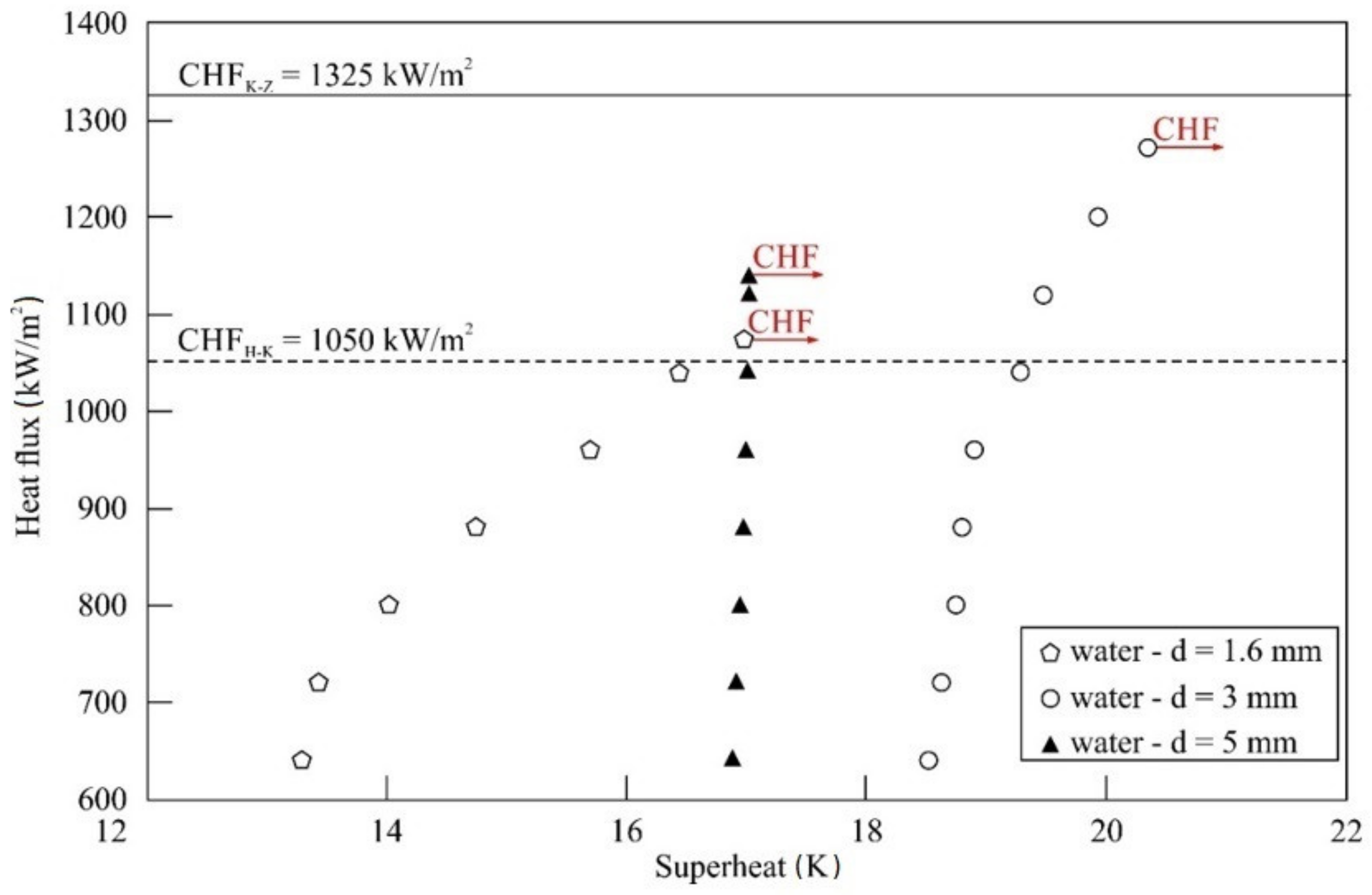
5.1. Impact of Tube Diameter
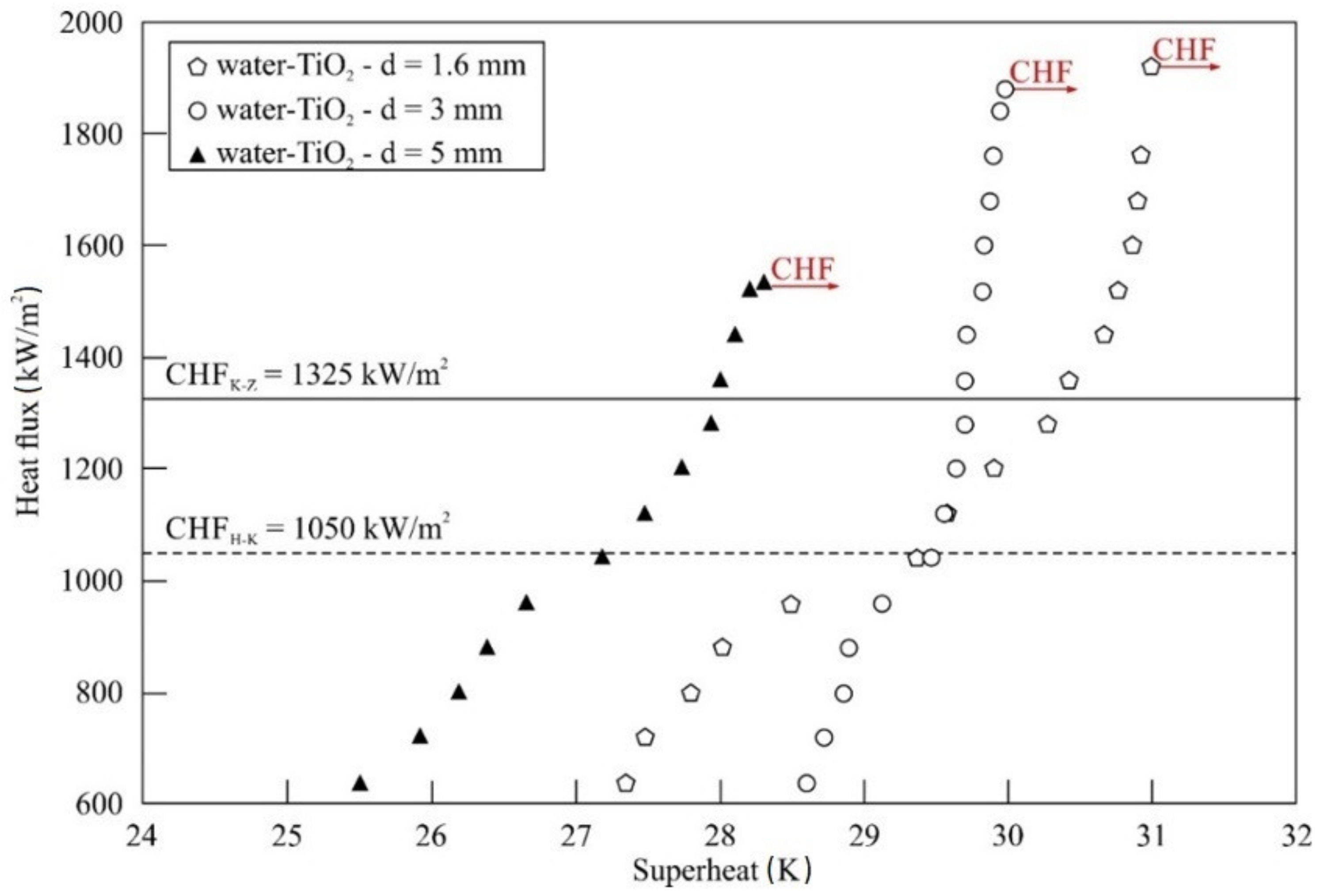
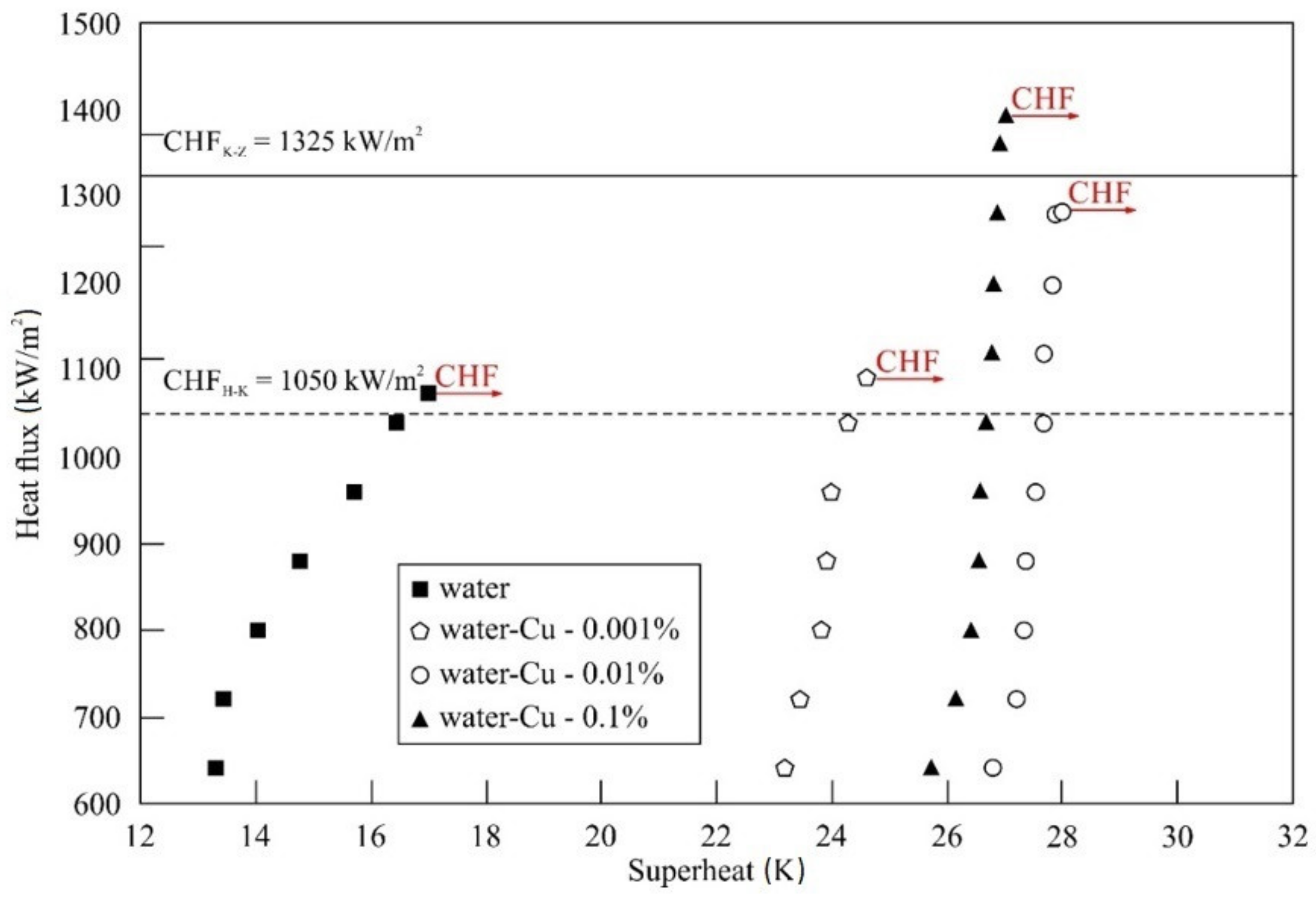
5.2. Impact of Nanoparticle Concentration and Material
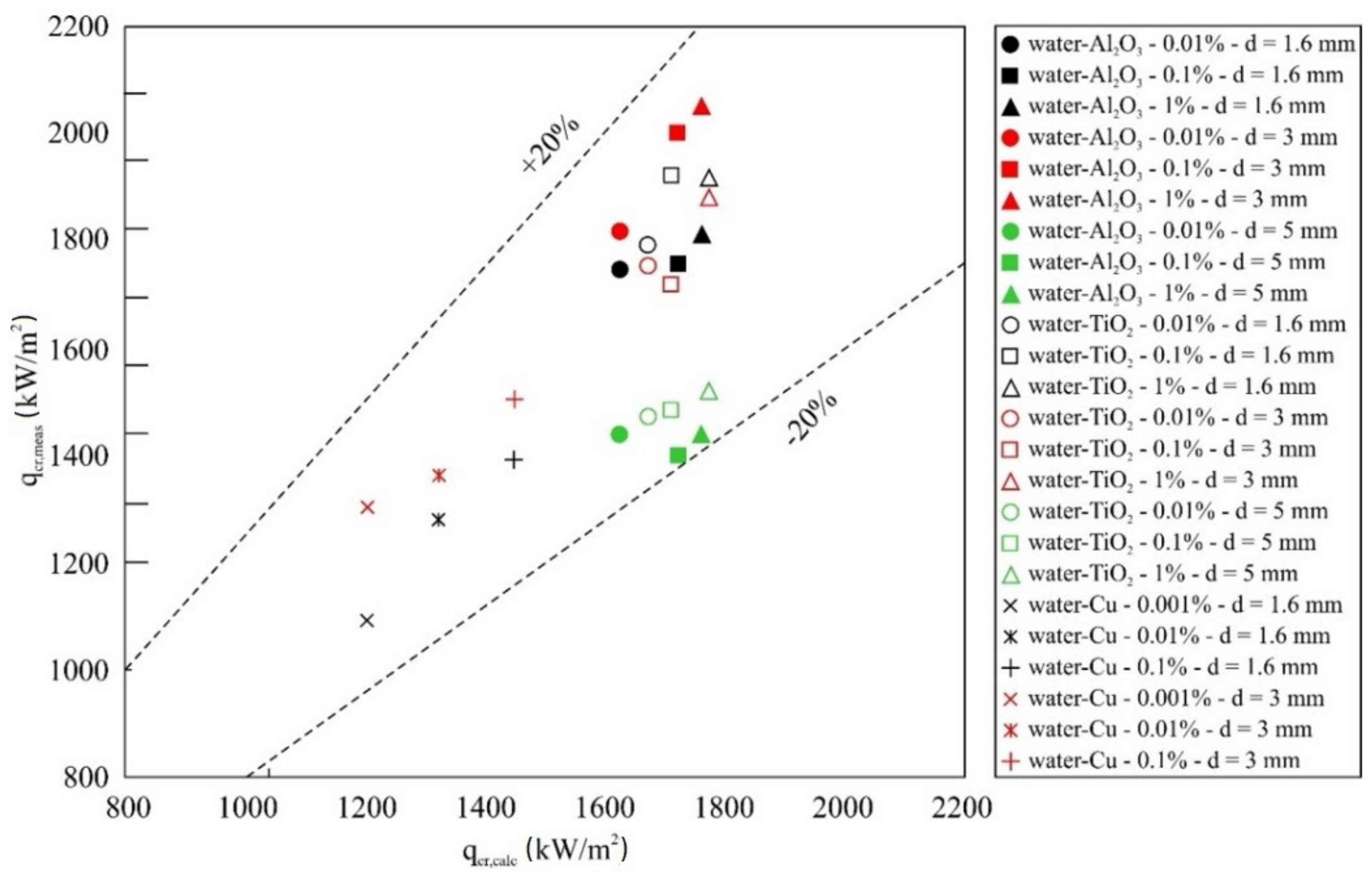
5.3. Correlation Equation
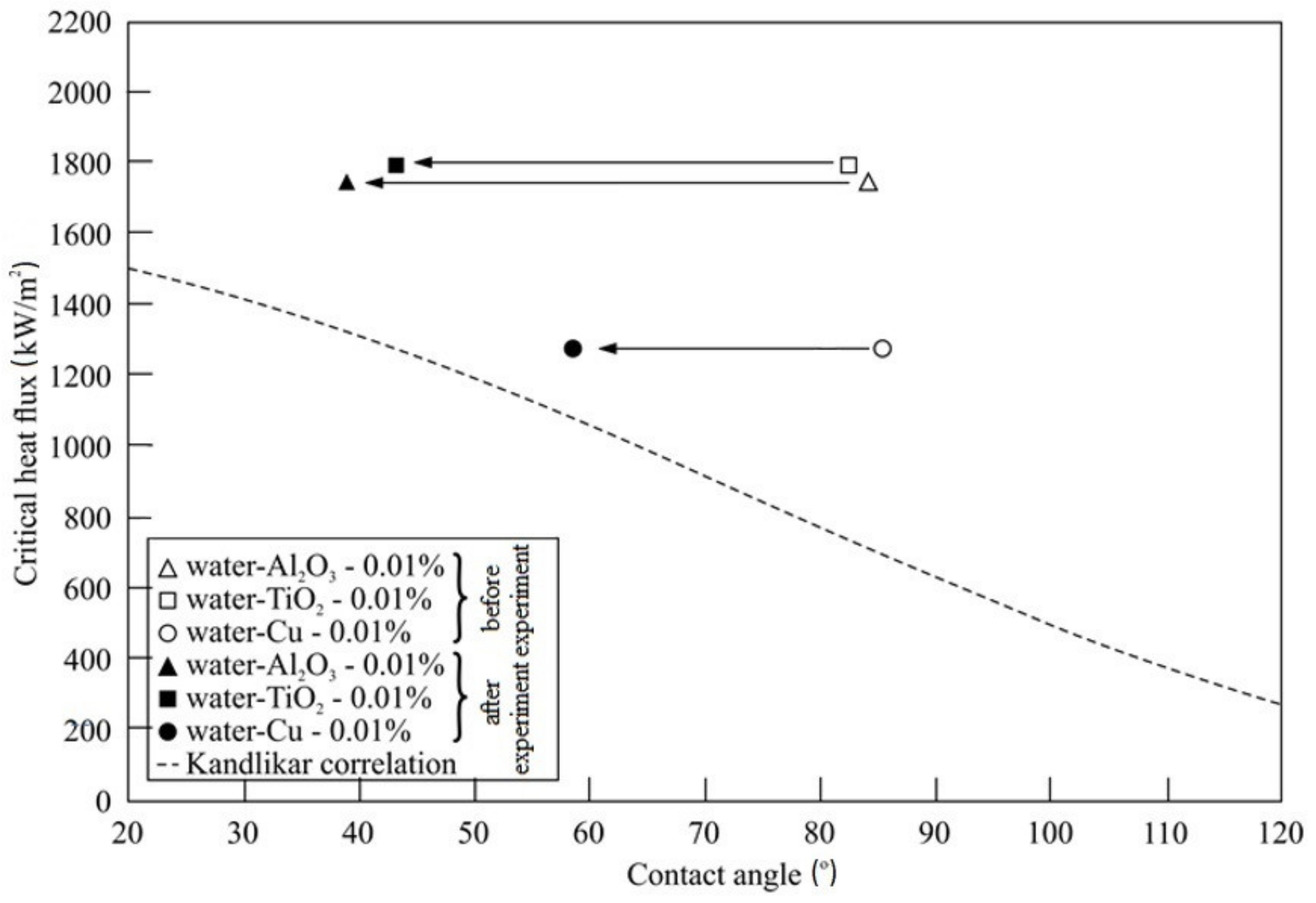
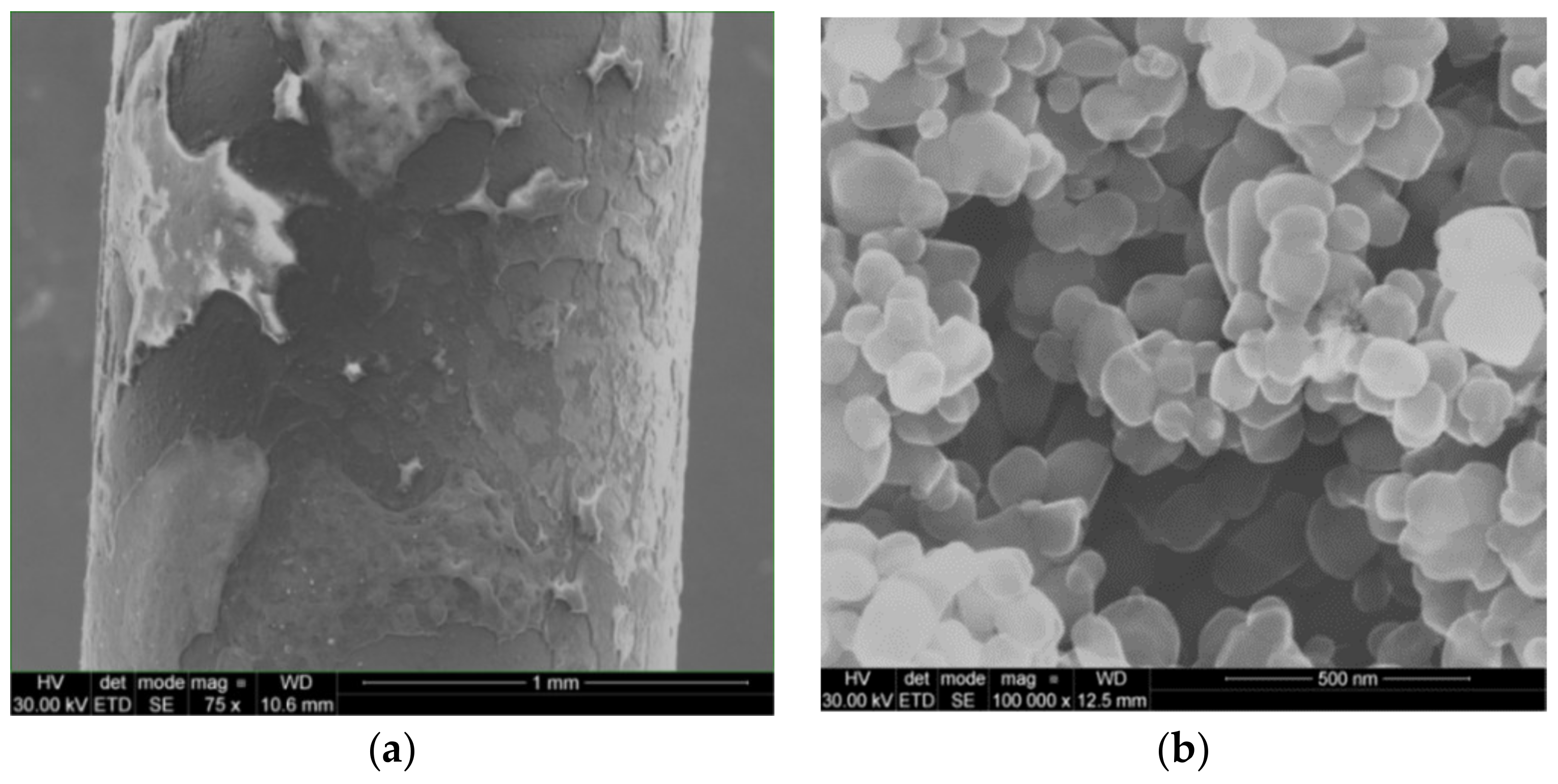
5.4. Nanocoating on Heating Surface
6. Discussion
7. Conclusions
- For all nanofluids tested and stainless steel tubes used, an improvement of CHF compared to distilled water was observed.
- The maximum enhancement of CHF was obtained for water–TiO2 nanofluid and a stainless steel tube of the smallest diameter, Do = 1.6 mm. Compared to distilled water, the improvement was about 178%.
- It was established that self-deposited nanocoatings result in substantial improvement in the wettability of the boiling surface, which is what leads to CHF enhancement.
- Formation of the nanocoatings results in heat transfer degradation—boiling curves are shifted left, towards higher wall superheats.
- An empirical correlation equation for predicting the CHF of water-based nanofluids was developed and verified for Al2O3, TiO2 and Cu nanoparticles and mass concentrations of 0.001%, 0.01%, 0.1% and 1%.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Di | Inner diameter of heated tube | (m) |
| Do | Outer diameter of heated tube | (m) |
| g | Gravitational acceleration | (m/s2) |
| h | Heat transfer coefficient | (W/( m2 K)) |
| hfg | Latent heat of vaporization | (J/kg) |
| It | Current | (A) |
| k | Thermal conductivity | (W/(m K)) |
| L | Tube length | (m) |
| M | Molecular weight | (kg/kmol) |
| Heat flux | (W/m2) | |
| Pr | Reduced pressure | (-) |
| Ra | Mean roughness | (μm) |
| Rp | Mean roughness | (μm) |
| t | Temperature | (°C) |
| Ut | Voltage drop | (V) |
| ΔT | Temperature difference | (K) |
| Greek symbols | ||
| Contact angle | (°) | |
| λ | Thermal conductivity | (W/(mK)) |
| Nanoparticle mass concentration | (-) | |
| Density | (kg/m3) | |
| σ | Surface tension | (J/m2) |
| Subscripts | ||
| f | Fluid | |
| l | Liquid | |
| m | Mass | |
| nf | Nanofluid | |
| t | Tube | |
| v | Vapor | |
| vol | Volume | |
| wt | Mass | |
| Abbreviations | ||
| CHF | Critical Heat Flux | |
| CNT | Carbon Nanotubes | |
| DW | Deionized Water | |
| EEWL | Electrical Explosion of Wire in Liquids | |
| EG | Ethylene Glycol | |
| FCNT | Functionalized Carbon Nanotube | |
| G | Graphene | |
| GO | Graphene Oxide | |
| GON | Graphene Oxide Nanosheets | |
| HTC | Heat Transfer Coefficient | |
| NPB | Nucleate Pool Boiling | |
| OD | Outside Diameter | |
| PG | Polyethylene Glycol | |
| PVP | Polyvinylpyrrolidone | |
| RGO | Reduced Graphene Oxide | |
| SDBS | Sodium Dodecyl Benzene Sulfonate | |
| SDS | Sodium Dodecyl Sulfate | |
| SEM | Scanning Electron Microscope | |
| SS | Stainless Steel | |
| xGnPs | Exfoliated Graphite Nanoplatelets | |
References
- Xie, S.; Beni, M.S.; Cai, J.; Zhao, J. Review of critical-heat-flux enhancement methods. Int. J. Heat Mass Transf. 2018, 122, 275–289. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of pool boiling enhancement with additives and nanofluids. Int. J. Heat Mass Transf. 2018, 124, 423–453. [Google Scholar] [CrossRef]
- Kim, H. Enhancement of critical heat flux in nucleate boiling of nanofluids: A state-of-art review. Nanoscale Res. Lett. 2011, 6, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, H.S.; Kim, M.H. A Review on Critical Heat Flux Enhancement with Nanofluids and Surface Modification. J. Heat Transf. 2012, 134, 024001. [Google Scholar]
- Kamatchi, R.; Venkatachalapathy, S. Parametric study of pool boiling heat transfer with nanofluids for the enhancement of critical heat flux: A review. Int. J. Therm. Sci. 2015, 87, 228–240. [Google Scholar] [CrossRef]
- Bolton, J.; Liu, L.; Hinks, J.; Chai, J. Critical Heat Flux Enhancement Using Nanofluids and Hybrid Nanofluids: A Review. Int. J. Nanosci. Nanotechnol. 2018, 4, 35–56. [Google Scholar]
- Yao, S.; Teng, Z. Effect of Nanofluids on Boiling Heat Transfer Performance. Appl. Sci. 2019, 9, 2818. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Mudawar, I. Review of pool boiling enhancement by surface modification. Int. J. Heat Mass Transf. 2019, 128, 892–933. [Google Scholar] [CrossRef]
- Vafaei, S.; Borca-Tasciuc, T. Role of nanoparticles on nanofluid boiling phenomenon: Nanoparticle deposition. Chem. Eng. Res. Des. 2014, 92, 842–856. [Google Scholar] [CrossRef]
- Shojaeian, M.; Koşar, A. Pool boiling and flow boiling on micro- and nanostructured surfaces. Exp. Therm. Fluid Sci. 2015, 63, 45–73. [Google Scholar] [CrossRef]
- Li, X.; Cole, I.; Tu, J. A review of nucleate boiling on nanoengineered surfaces—The nanostructures, phenomena and mechanisms. Int. J. Heat Mass Transf. 2019, 141, 20–33. [Google Scholar] [CrossRef]
- Sezer, N.; Khan, S.A.; Koç, M. Amelioration of the pool boiling heat transfer performance by colloidal dispersions of carbon black. Int. J. Heat Mass Transf. 2019, 137, 599–608. [Google Scholar] [CrossRef]
- Moghadasi, H.; Malekian, N.; Saffari, H.; Gheitaghy, A.M.; Zhang, G.Q. Recent Advances in the Critical Heat Flux Amelioration of Pool Boiling Surfaces Using Metal Oxide Nanoparticle Deposition. Energies 2020, 13, 4026. [Google Scholar] [CrossRef]
- Kim, D.E.; Yu, D.I.; Jerng, D.W.; Kim, M.H.; Ahn, H.S. Review of boiling heat transfer enhancement on micro/nanostructured surfaces. Exp. Therm. Fluid Sci. 2015, 66, 173–196. [Google Scholar] [CrossRef]
- Lee, S.; Choi, U.S.; Li, S.; Eastman, J.A. Measuring thermal conductivity of fluids containing oxide nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Cieśliński, J.T.; Kaczmarczyk, T.Z. Pool boiling of water-Al2O3 and water-Cu nanofluids on horizontal smooth tubes. Nanoscale Res. Lett. 2011. [Google Scholar] [CrossRef] [Green Version]
- Cieśliński, J.T.; Kaczmarczyk, T.Z. Pool boiling of water-Al2O3 and water-Cu nanofluids on porous coated tubes. Heat Transf. Eng. 2015, 36, 553–563. [Google Scholar] [CrossRef]
- You, S.M.; Kim, J.H.; Kim, K.H. Effect of nanoparticles on critical heat flux of water in pool boiling heat transfer. Appl. Phys. Lett. 2003, 83, 3374–3376. [Google Scholar] [CrossRef]
- Vassallo, P.; Kumar, R.; D’Amico, S. Pool boiling heat transfer experiments in silica-water nanofluids. Int. J. Heat Mass Transf. 2004, 47, 407–411. [Google Scholar] [CrossRef]
- Dinh, N.; Tu, J.; Theofanous, T. Hydrodynamic and physico-chemical nature of burnout in pool boiling. In Proceedings of the 5th International Conference on Multiphase Flow, ICMF’04, Yokohama, Japan, 30 May–4 June 2004. paper No. 296 (CD-ROM). [Google Scholar]
- Moreno, G.; Oldenburg, S.J.; You, S.M.; Kim, J.H. Pool boiling heat transfer of alumina-water, zinc oxide-water and alumina-water–ethylene glycol nanofluids. In Proceedings of the HT2005 ASME Summer Heat Transfer Conference, San Francisco, CA, USA, 17–22 July 2005; pp. 625–632. [Google Scholar]
- Bang, I.C.; Chang, S.H. Boiling heat transfer performance and phenomena of Al2O3-water nanofluids from a plain surface in a pool. Int. J. Heat Mass Transf. 2005, 48, 2407–2419. [Google Scholar] [CrossRef]
- Milanova, D.; Kumar, R. Role of ions in pool boiling heat transfer of pure and silica nanofluids. Appl. Phys. Lett. 2005, 87, 233107. [Google Scholar] [CrossRef]
- Jackson, J.E.; Borgmeyer, B.V.; Wilson, C.A.; Cheng, P.; Bryan, J.E. Characteristics of Nucleate Boiling with Gold Nanoparticles in water. In Proceedings of the IMECE2006, Chicago, IL, USA, 5–10 November 2006. [Google Scholar]
- Kim, H.; Kim, J.; Kim, M.H. Effect of nanoparticles on CHF enhancement in pool boiling of nano-fluids. Int. J. Heat Mass Transf. 2006, 49, 5070–5074. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Kim, M. CHF enhancement in pool boiling of water-TiO2 nanofluids: Effect of nanoparticle-coating on heating surface. In Proceedings of the 13th International Heat Transfer Conference, Sydney, Australia, 13–18 August 2006. paper NAN-22 (CD-ROM). [Google Scholar]
- Kim, S.J.; Bang, I.C.; Buongiorno, J.; Hu, L.W. Effects of nanoparticle deposition on surface wettability influencing boiling heat transfer in nanofluids. Appl. Phys. Lett. 2006, 89, 153107. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Bang, I.; Buongiorno, J.; Hu, L. Surface wettability change during pool boiling of nanofluids and its effect on critical heat flux. Int. J. Heat Mass Transf. 2007, 50, 4105–4116. [Google Scholar] [CrossRef]
- Kashinath, M.R. Parameters Affecting Critical Heat Flux on Nanofluids: Heater size, Pressure Orientation and Anti-Freeze Addition. Master’s Thesis, University of Texas, Arlington, TX, USA, 2006. [Google Scholar]
- Milanova, D.; Kumar, R.; Kuchibhatla, S.; Seal, S. Heat transfer behavior of oxide nanoparticles in pool boiling experiment. In Proceedings of the International Fourth Conference on Nanochannels, Microchannels and Minichannels (ICNMM2006), Limerick, Ireland, 19–21 June 2006. [Google Scholar]
- Kim, H.; Kim, J.; Kim, M. Experimental study on CHF characteristics of water-TiO2 nano-fluids. Nucl. Eng. Technol. 2006, 38, 61–68. [Google Scholar]
- Kim, H.D.; Kim, M.H. Effect of nanoparticle deposition on capillary wicking that influences the critical heat flux in nanofluids. Appl. Phys. Lett. 2007, 91, 014104. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.; Kim, M. Experimental studies on CHF characteristics of nano-fluids at pool boiling. Int. J. Multiph. Flow 2007, 33, 691–706. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, J.; Bao, R. Boiling heat transfer characteristics of nanofluids in a flat heat pipe evaporator with micro-grooved heating surface. Int. J. Multiph. Flow 2007, 33, 1284–1295. [Google Scholar] [CrossRef]
- Coursey, J.; Kim, J. Nanofluid boiling: The effect of surface wettability. Int. J. Heat Fluid Flow 2008, 29, 1577–1585. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, L. Sorption and agglutination phenomenon of nanofluids on a plain heating surface during pool boiling. Int. J. Heat Mass Transf. 2008, 51, 2593–2602. [Google Scholar] [CrossRef]
- Milanova, D.; Kumar, R. Heat transfer behavior of silica nanoparticles in pool boiling experiment. J. Heat Transf. 2008, 130, 042401. [Google Scholar] [CrossRef]
- Golubovic, M.N.; Madhawa Hettiarachchi, H.D.; Worek, W.M.; Minkowycz, W.J. Nanofluids and critical heat flux, experimental and analytical study. Appl. Therm. Eng. 2009, 29, 1281–1288. [Google Scholar] [CrossRef]
- Jo, B.; Jeon, P.S.; Yoo, J.; Kim, H.J. Wide range parametric study for the pool boiling of nanofluids with a circular plate heater. J. Vis. 2009, 12, 37–46. [Google Scholar] [CrossRef]
- Kumar, R.; Milanova, D. Effect of surface tension on nanotube nanofluids. Appl. Phys. Lett. 2009, 94, 073107. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, M. Experimental study of the characteristics and mechanism of pool boiling CHF enhancement using nanofluids. Int. J. Heat Mass Transf. 2009, 45, 991–998. [Google Scholar] [CrossRef]
- Park, K.J.; Jung, D.; Shim, S.E. Nucleate boiling heat transfer in aqueous solutions with carbon nanotubes up to critical heat fluxes. Int. J. Multiph. Flow 2009, 35, 525–532. [Google Scholar] [CrossRef]
- Kim, H.; DeWitt, G.; McKrell, T.; Buongiorno, J.; Hu, L.W. On the quenching of steel and zircaloy spheres in water-based nanofluids with alumina, silica and diamond nanoparticles. Int. J. Multiph. Flow 2009, 35, 427–438. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Preparation and pool boiling characteristics of copper nanofluids over a flat plate heater. Int. J. Heat Mass Transf. 2010, 53, 1673–1681. [Google Scholar] [CrossRef]
- Kwark, S.M.; Kumar, R.; Moreno, G.; Yoo, J.; You, S.M. Pool boiling characteristics of low concentration nanofluids. Int. J. Heat Mass Transf. 2010, 53, 972–981. [Google Scholar] [CrossRef]
- Kwark, S.M.; Moreno, G.; Kumar, R.; Moon, H.; You, S.M. Nanocoating characterization in pool boiling heat transfer of pure water. Int. J. Heat Mass Transf. 2010, 53, 4579–4587. [Google Scholar] [CrossRef]
- Kwark, S.M.; Amaya, M.; Kumar, R.; Moreno, G.; You, S.M. Effects of pressure, orientation, and heater size on pool boiling of water with nanocoated heaters. Int. J. Heat Mass Transf. 2010, 53, 5199–5208. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yang, X.F.; Xiong, J.G. Boiling characteristics of carbon nanotube suspensions under sub-atmospheric pressures. Int. J. Therm. Sci. 2010, 49, 1156–1164. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, H.S.; Kim, M.H. On the mechanism of pool boiling critical heat flux enhancement in nanofluids. J. Heat Transf. 2010, 132, 061501. [Google Scholar] [CrossRef]
- Park, S.D.; Lee, S.W.; Kang, S.; Bang, I.C.; Kim, J.H.; Shin, H.S.; Lee, D.W.; Lee, D.W. Effects of nanofluids containing graphene/graphene-oxide nanosheets on critical heat flux. Appl. Phys. Lett. 2010, 97, 023103. [Google Scholar] [CrossRef] [Green Version]
- Truong, B.; Hu, L.W.; Buongiorno, J.; McKrell, T. Modification of sandblasted plate heaters using nanofluids to enhance pool boiling critical heat flux. Int. J. Heat Mass Transf. 2010, 53, 85–94. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Preparation and pool boiling characteristics of copper silver nanofluids over a flat plate heater. Heat Transf. Eng. 2012, 33, 69–78. [Google Scholar] [CrossRef]
- Park, E.J.; Park, S.D.; Bang, I.C.; Park, Y.-B.; Park, H.W. Critical heat flux characteristics of nanofluids based on exfoliated graphite nanoplatelets (xGnPs). Mater. Lett. 2012, 81, 193–197. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, T.; Jeong, Y.H. Experimental investigation on the CHF enhancement of pool boiling using water-based nanofluid at higher pressure. In Proceedings of the ICAPP 2013, Jeju Island, Korea, 14–18 April 2013. Paper No. 131. [Google Scholar]
- Hiswankar, S.C.; Kshirsagar, J.M. Determination Of Critical Heat Flux In Pool Boiling Using ZnO Nanofluids. Int. J. Eng. Res. Technol. 2013, 2, 2091–2095. [Google Scholar]
- Park, E.; Bang, I.; Park, H. Critical heat flux (CHF) enhancement of the nanofluids by the electrical explosion of a wire in liquids (EEWL) process. Nanotech 2012, 2, 353–356. [Google Scholar]
- Kole, M.; Dey, T.K. Pool Boiling Heat Transfer and Critical Heat Flux Enhancement of Copper Nanoparticles Dispersed in Distilled Water. J. Nanofluids 2014, 3, 1–12. [Google Scholar] [CrossRef]
- Song, S.L.; Lee, J.H.; Chang, S.H. CHF enhancement of SiC nanofluid in pool boiling experiment. Exp. Therm. Fluid Sci. 2014, 52, 12–18. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Hormozi, F.; Silakhori, M.; Peyghambarzadeh, S.M. On the fouling formation of functionalized and non-functionalized carbon nanotube nano-fluids under pool boiling condition. Appl. Therm. Eng. 2016, 95, 433–444. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Hu, Y.; Wang, X.; Zhu, J. Boiling heat transfer characteristics of ethylene glycol and water mixture based ZnO nanofluids in a cylindrical vessel. Int. J. Heat Mass Transf. 2016, 98, 611–615. [Google Scholar] [CrossRef]
- Sulaiman, M.Z.; Matsuo, D.; Enoki, K.; Okawa, T. Systematic measurements of heat transfer characteristics in saturated pool boiling of water-based nanofluids. Int. J. Heat Mass Transf. 2016, 102, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Ham, J.; Kim, H.; Shin, Y.; Cho, H. Experimental investigation of pool boiling characteristics in Al2O3 nanofluid according to surface roughness and concentration. Int. J. Therm. Sci. 2017, 114, 86–97. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.M.; Jerng, D.W.; Kim, E.Y.; Ahn, H.S. Effect of aluminum oxide and reduced graphene oxide mixtures on critical heat flux enhancement. Int. J. Heat Mass Transf. 2018, 116, 858–870. [Google Scholar] [CrossRef]
- Hwang, W.-K.; Choy, S.; Song, S.L.; Lee, J.; Hwang, D.S.; Lee, K.-Y. Enhancement of nanofluid stability and critical heat flux in pool boiling with nanocellulose. Carbohydr. Polym. 2019, 213, 393–402. [Google Scholar] [CrossRef]
- Lienhard, J.H.; Dhir, V.K. Hydrodynamic prediction of peak pool-boiling heat fluxes from finite bodies. J. Heat Transf. 1973, 95, 152–158. [Google Scholar] [CrossRef]
- Bakhru, N.; Lienhard, J.H. Boiling from small cylinders. Int. J. Heat Mass Transf. 1972, 15, 2011–2025. [Google Scholar] [CrossRef]
- Zuber, N. On the stability of boiling heat transfer. Trans. Am. Soc. Mech. Eng. 1958, 80, 711–720. [Google Scholar]
- Zuber, N. The dynamics of vapor bubbles in nonuniform temperature fields. Int. J. Heat Mass Transf. 1961, 2, 83–98. [Google Scholar] [CrossRef]
- Cooper, M.G. Heat Flow in Saturated Nucleate pool Boiling—A Wide-Ranging Examination Using Reduced Properties. Adv. Heat Transf. 1984, 16, 157–239. [Google Scholar]
- Haramura, Y.; Katto, Y. A new hydrodynamic model of critical heat flux, applicable widely to both pool and forced convection boiling on submerged bodies in saturated liquids. Int. J. Heat Mass Transf. 1983, 26, 389–399. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Pool boiling critical heat flux (CHF)—Part 1: Review of mechanisms, models, and correlations. Int. J. Heat Mass Transf. 2018, 117, 1352–1367. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Pool boiling critical heat flux (CHF)—Part 2: Assessment of models and correlations. Int. J. Heat Mass Transf. 2018, 117, 1368–1383. [Google Scholar] [CrossRef]
- Kandlikar, S.G. A theoretical model to predict pool boiling CHF incorporating effects of contact angle and orientation. J. Heat Transf. 2001, 123, 1071–1079. [Google Scholar] [CrossRef]
- Cieśliński, J.T.; Krygier, K.A. Sessile droplet contact angle of water-Al2O3, water-TiO2 and water-Cu nanofluids. Exp. Therm. Fluid Sci. 2014, 59, 258–263. [Google Scholar] [CrossRef]
- Cieśliński, J.T. Nucleate pool boiling on porous metallic coatings. Exp. Therm. Fluid Sci. 2002, 25, 557–564. [Google Scholar] [CrossRef]
- Park, S.D.; Bang, I.C. Experimental study of a universal CHF enhancement mechanism in nanofluids using hydrodynamic instability. Int. J. Heat Mass Transf. 2014, 70, 844–850. [Google Scholar] [CrossRef]
- Sefiane, K. On the role of structural disjoining pressure and contact line pinning in critical heat flux enhancement during boiling of nanofluids. Appl. Phys. Lett. 2006, 89, 044106. [Google Scholar] [CrossRef]
- Wen, D. Mechanisms of thermal nanofluids on enhanced critical heat flux (CHF). Int. J. Heat Mass Transf. 2008, 51, 4958–4965. [Google Scholar] [CrossRef]
- Arik, M.; Bar-Cohen, A. Effusivity-based correlation of surface property effects in pool boiling CHF of dielectric liquids. Int. J. Heat Mass Transf. 2003, 46, 3755–3764. [Google Scholar] [CrossRef]




| Name and References | Nanofluids | Concentration | Test Heater | CHF Enhancement |
|---|---|---|---|---|
| Vassallo et al. [19] | water–SiO2 | 0.5 vol.% | NiCr (OD = 1 mm) | 60% |
| Milanova and Kumar [23] | SiO2 in ionic solution of water | 0.5 vol.% | NiCr (OD = 0.32 mm) | 220–320% |
| Kim H. et al. [25,26] | water–TiO2 water–Al2O3 | 10−5–10−1 vol.% | NiCr (OD = 0.2 mm) Ti (OD = 0.25 mm) | 100% 80% 170% |
| Kim S.J. et al. [27] | water–Al2O3 water–ZrO2 | 10−3–10−1 vol.% | SS (OD = 0.381 mm) | 52% 75% |
| Milanova et al. [30] | water–SiO2 water–CeO2 water–Al2O3 | 0.5 vol.% | NiCr (OD = 0.32 mm) | 50% |
| Kim H. et al. [31] | water–TiO2 | 10−5–10−1 vol.% | NiCr (OD = 0.2 mm) | 200% |
| Kim H.D. and Kim M.H. [32] | water–TiO2 water–Al2O3 water–SiO2 water–Ag | 10−5–10−1 vol.% | NiCr (OD = 0.2 mm) | 100% 80% 170%- |
| Kim H.D. et al. [33] | water–TiO2 water–Al2O3 | 10−5–10−1 vol.% | NiCr (OD = 0.2 mm) | 100% |
| Milanova and Kumar [37] | water–SiO2 | 0.5 vol.% | NiCr (OD = 0.32 mm) | 50% |
| Golubovic et al. [38] | water–Al2O3 water–BiO2 | 0.0006–0.01 g/L | NiCr (OD = 0.64 mm) | 50% 33% |
| Kumar and Milanova [40] | single-walled CNT in water withhydrochloric acid | 2 wt.% | NiCr (OD = 0.32 mm) | 300% |
| Kim H. and Kim M. [41] | water–TiO2 water–Al2O3 water–SiO2 | 10−5–10−1 vol.% | NiCr (OD = 0.2 mm) | 80% |
| Park S.D. et al. [50] | water–graphene water–GON water–Al2O3 | 0.001 vol.% | NiCr | 84% 179% 152% |
| Park E.J. et al. [53] | xGnPs oxide xGnPs oxide | 0.005 vol.% | NiCr | enhanced CHF |
| Lee T. et al. [54] | water–magnetite | 1 ppm | Ni–Cr OD = 0.4 mm | 140% to 170% |
| Hiswankar and Kshirsagar [55] | water–ZnO | 0.01 and 0.0001 vol.% | Ni–Cr (OD = 0.4 mm) | 70~80% |
| Park E. et al. [56] | water–Ag water–CuO water Al2O3 | 0.001 vol.% | Ni–Cr | 58% 99% 108% |
| Kole and Dey [57] | water–Cu | 0.5 wt.% | Constantan OD = 70 μm | ~60% |
| He et al. [60] | ZnO–EG–DW (EG–water) | 5.25 wt.% 7.25 wt.% 8.25 wt.% | Ni–Cr (0.5 mm × 5 cm) | 156.7% 161.2% 177.4% |
| Kim J.H. et al. [63] | water–Al2O3 water–RGO water–Al2O3/RGO | (0.0001–0.01 vol.%) (0.00005–0.005 vol.%) 0.0005 vol.% Al2O3 and RGO | Ni–Cr (0.2 × 85) mm | 54% 37% 473% |
| Hwang et al. [64] | water–cellulose | 0.01–0.1 wt.% | Ni–Cr (0.1 × 150) mm |
| Name and References | Nanofluids | Concentration | Test Heater | CHF Enhancement |
|---|---|---|---|---|
| You et al. [18] | water–Al2O3 | 0.001–0.025 g/L | Cu plate (10 × 10 mm) | 200%, (19.9 kPa) |
| Dinh et al. [20] | water–Al2O3 | 37 ppm solution | Ti plate (26.5 × 40 mm) | - |
| Moreno et al. [21] | water–Al2O3 water–ZnO EG–Al2O3 | 0.001–1 g/L | Cu plate 10 × 10 mm | 180% 240% 130% |
| Bang and Chang [22] | water–Al2O3 | 0.5–4 vol.% | plate | 220%—horizontal 160%—vertical |
| Jackson J. et al. [24] | water–Au | 3·10−4 vol.% | Circular plate (11.2 mm) | 250% |
| Kim S.J. et al. [28] | water–SiO2 | 10−3–10−1 vol.% | SS flat plate (5 × 45 mm) | 80% |
| Kashinath [29] | Al2O3 in EG and PG in water | 0.025 g/L | Cu plate (10 × 10 mm) (15 × 15 mm) (20 × 20 mm) | 90% 170% 70% 230% (7.38 kPa) |
| Liu et al. [34] | water–CuO | 0.1–2 wt.% | Cu plate with microgrooves | 50% (~100 kPa) 200% (7.4 kPa) |
| Coursey and Kim [35] | water–Al2O3 ethanol–Al2O3 | 0.001–10 g/L | Glass, Au (0.9 cm2) and Cu (2 cm2) | 40% |
| Liu and Liao L. [36] | CuO SiO2 in water and alcohol with SDBS | 0.2–2 wt.% | Cu disk (OD = 20 mm) | 30% |
| Jo et al. [39] | water–Al2O3 water–Ag | 10−4–10−1 g/L | Cu disk (OD = 10 and 15 mm) | 70% |
| Park K.J. et al. [42] | multiwalled CNT in water with PVP polymer | 10−4–10−2, 0.05 vol.% | Cu plate (9.5 × 9.5 mm) | 200% (19.9 kPa) 140% (19.9 kPa) |
| Kathiravan et al. [44] | Cu in water w/SDS surfactant w/o SDS surfactant | 0.25, 0.5, 1.0 wt.% | plate (30 × 30 mm) | 50% 30% |
| Kwark et al. [45] | water–Al2O3 water–CuO water–diamond | 0.001–1 g/L | Cu plate (10 × 10 mm) | 80% |
| Liu et al. [48] | CNT in water with nitric acid forpH 6.5 | 0.5–4 wt.% | Cu plate (40 × 40 mm) | 60% (100 kPa) 140% (31.2 kPa) 200% (7.4 kPa) |
| Kim H. et al. [49] | water–TiO2 water–Al2O3 | 0.01 vol.% | Cu and Ni discs (OD = 20 mm) | 40% |
| Truong et al. [51] | water–diamond water–Al2O3 water–ZnO | 0.01 vol.% 0.1 vol.% 0.1 vol.% | Cu plate | 11% 35% 35% |
| Kathiravan et al. [52] | water–Cu water–Cu–SDS | 0.25, 0.5, 0.75 wt.% | plate (30 × 30 mm) | 20% 40% 48% |
| Song et al. [58] | water water–SiC | 0.0001, 0.001, 0.01 vol.% | SS plate (10 mm × 50 mm × 0.4 mm; 50 mm × 50 mm × 0.4 mm) | 105% (0.01vol.%) |
| Sarafraz et al. [59] | water–FCNT water–CNT | 0.1 to 0.3 wt.% | discoid Cu heater (0.78 cm2) | Max. 131.1% (0.3 wt.%) |
| Sulaiman et al. [61] | water–TiO2 water–Al2O3 water–SiO2 | 0.04 kg/m3 0.4 kg/m3 1 kg/m3 | Cu block (OD = 20 mm) | 2.5–3 times higher |
| Ham et al. [62] | water–Al2O3 | 0.001–0.1 vol.% | Cu block (Ra = 177.5 nm, Ra = 292.8 nm) | 224.8% 138.5% |
| Kim H. et al. [43] | Al2O3 SiO2 Diamond | 0.001, 0.01, 0.1 vol.% | Sphere SS (D = 9.5 mm), Zircaloy (D = 10 mm) | Quenching |
| Mass Concentration (%) | n (-) | ||
|---|---|---|---|
| Al2O3 | TiO2 | Cu | |
| 0.001 | - | - | 0.0313 |
| 0.01 | 0.0268 | 0.0237 | 0.0291 |
| 0.1 | 0.0273 | 0.0284 | 0.0256 |
| 1 | 0.0361 | 0.0348 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieśliński, J.T.; Ronewicz, K. Burnout Investigation of Small Diameter Tubes Immersed in Nanofluids. Energies 2021, 14, 3888. https://doi.org/10.3390/en14133888
Cieśliński JT, Ronewicz K. Burnout Investigation of Small Diameter Tubes Immersed in Nanofluids. Energies. 2021; 14(13):3888. https://doi.org/10.3390/en14133888
Chicago/Turabian StyleCieśliński, Janusz T., and Katarzyna Ronewicz. 2021. "Burnout Investigation of Small Diameter Tubes Immersed in Nanofluids" Energies 14, no. 13: 3888. https://doi.org/10.3390/en14133888
APA StyleCieśliński, J. T., & Ronewicz, K. (2021). Burnout Investigation of Small Diameter Tubes Immersed in Nanofluids. Energies, 14(13), 3888. https://doi.org/10.3390/en14133888






