A Study on the Corrosion Characteristics of Internal Combustion Engine Materials in Second-Generation Jatropha Curcas Biodiesel
Abstract
:1. Introduction
2. Methodology
2.1. Material Preparation
2.2. Fuel Sample Preparation
2.3. Corrosion Rate Calculation
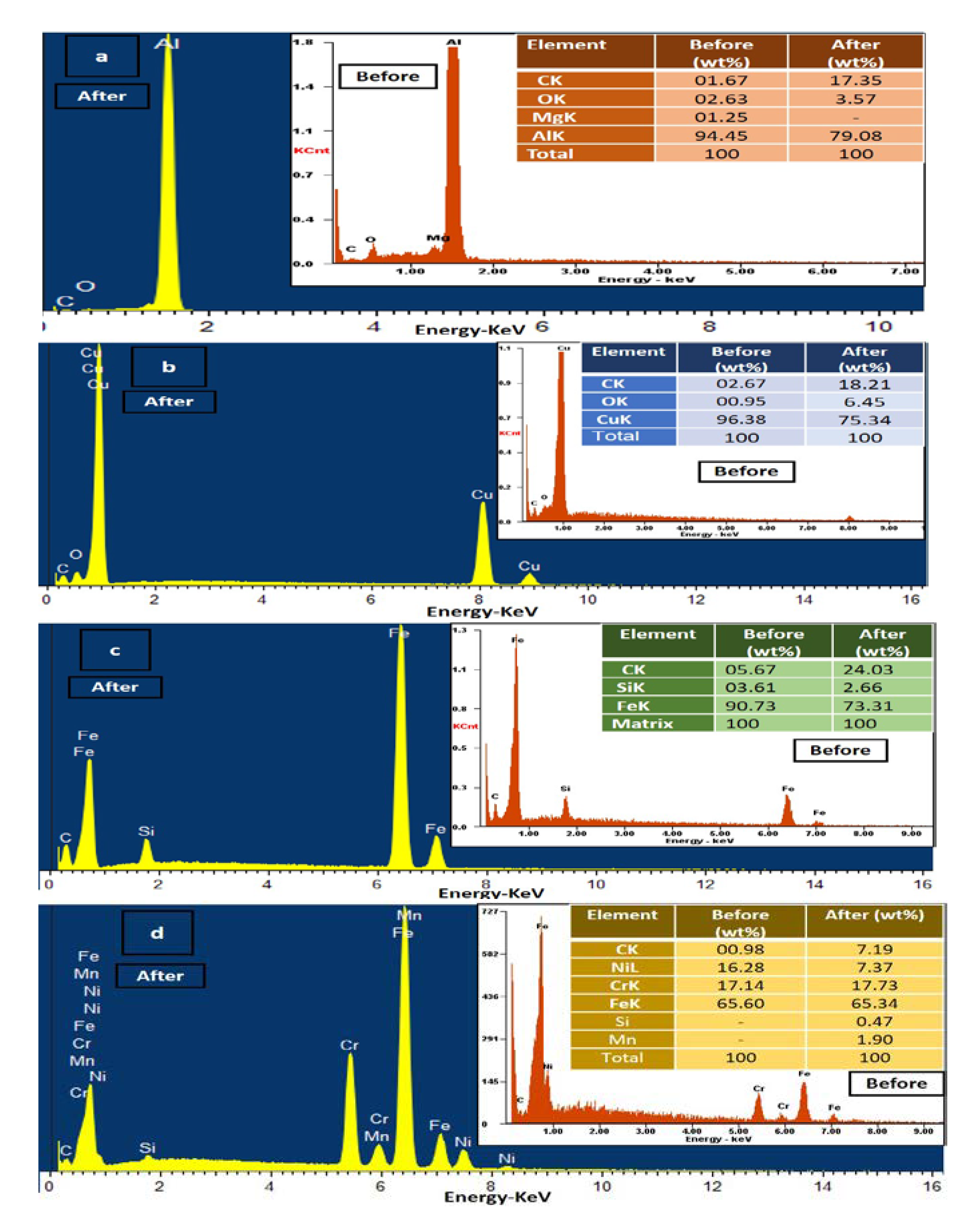
2.4. Surface Morphology Analysis
2.5. Fuel Characterisation after Immersion Test
3. Results and Discussion
3.1. Corrosive Wears Analysis
3.2. Surface Morphology Analysis
3.3. Change in Fuel Composition after the Immersion Test
3.4. Fuel Properties after Immersion Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baena, L.M.; Calderón, J.A. Effects of palm biodiesel and blends of biodiesel with organic acids on metals. Heliyon 2020, 6, e03735. [Google Scholar] [CrossRef]
- Gavhane, R.; Kate, A.; Soudagar, M.E.M.; Wakchaure, V.; Balgude, S.; Rizwanul Fattah, I.; Nik-Ghazali, N.-N.; Fayaz, H.; Khan, T.; Mujtaba, M.; et al. Influence of silica nano-additives on performance and emission characteristics of Soybean biodiesel fuelled diesel engine. Energies 2021, 14, 1489. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Fattah, I.M.R.; Mobarak, H.M. Comparative evaluation of performance and emission characteristics of Moringa oleifera and Palm oil based biodiesel in a diesel engine. Ind. Crop. Prod. 2014, 53, 78–84. [Google Scholar] [CrossRef]
- Mofijur, M.; Atabani, A.E.; Masjuki, H.H.; Kalam, M.A.; Masum, B.M. A study on the effects of promising edible and non-edible biodiesel feedstocks on engine performance and emissions production: A comparative evaluation. Renew. Sustain. Energy Rev. 2013, 23, 391–404. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Masjuki, H.H.; Kalam, M.A.; Bhuiya, M.M.K.; Mehat, H. Comparative tribological investigation of bio-lubricant formulated from a non-edible oil source (Jatropha oil). Ind. Crop. Prod. 2013, 47, 323–330. [Google Scholar] [CrossRef]
- Sorate, K.A.; Bhale, P.V. Biodiesel properties and automotive system compatibility issues. Renew. Sustain. Energy Rev. 2015, 41, 777–798. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Liaquat, A.; Masjuki, H.; Kalam, M.; Mofijur, M. Ignition delay, combustion and emission characteristics of diesel engine fueled with biodiesel. Renew. Sustain. Energy Rev. 2013, 21, 623–632. [Google Scholar] [CrossRef]
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M. A review of the effect of biodiesel on the corrosion behavior of metals/alloys in diesel engines. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2923–2943. [Google Scholar] [CrossRef]
- Singh, B.; Korstad, J.; Sharma, Y.C. A critical review on corrosion of compression ignition (CI) engine parts by biodiesel and biodiesel blends and its inhibition. Renew. Sustain. Energy Rev. 2012, 16, 3401–3408. [Google Scholar] [CrossRef]
- Fazal, M.A.; Jakeria, M.R.; Haseeb, A.S.M.A.; Rubaiee, S. Effect of antioxidants on the stability and corrosiveness of palm biodiesel upon exposure of different metals. Energy 2017, 135, 220–226. [Google Scholar] [CrossRef]
- Fazal, M.A.; Rubaiee, S.; Al-Zahrani, A. Overview of the interactions between automotive materials and biodiesel obtained from different feedstocks. Fuel Process. Technol. 2019, 196, 106178. [Google Scholar] [CrossRef]
- Rocabruno-Valdés, C.I.; Hernández, J.A.; Juantorena, A.U.; Arenas, E.G.; Lopez-Sesenes, R.; Salinas-Bravo, V.M.; González-Rodriguez, J.G. An electrochemical study of the corrosion behaviour of metals in canola biodiesel. Corros. Eng. Sci. Technol. 2018, 53, 153–162. [Google Scholar] [CrossRef]
- Sorate, K.A.; Bhale, P.V. Corrosion Behavior of Automotive Materials with Biodiesel a Different Approach. SAE Int. J. Fuels Lubr. 2018, 11, 147–162. [Google Scholar] [CrossRef]
- Samuel, O.D.; Gulum, M. Mechanical and corrosion properties of brass exposed to waste sunflower oil biodiesel-diesel fuel blends. Chem. Eng. Commun. 2019, 206, 682–694. [Google Scholar] [CrossRef]
- Amaya, A.; Piamba, O.; Olaya, J. Corrosiveness of Palm Biodiesel in Gray Cast Iron Coated by Thermoreactive Diffusion Vanadium Carbide (VC) Coating. Coatings 2019, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Deyab, M.A.; Corrêa, R.G.C.; Mazzetto, S.E.; Dhmees, A.S.; Mele, G. Improving the sustainability of biodiesel by controlling the corrosive effects of soybean biodiesel on aluminum alloy 5052 H32 via cardanol. Ind. Crop. Prod. 2019, 130, 146–150. [Google Scholar] [CrossRef]
- Fraer, R.; Dinh, H.; Proc, K.; McCormick, R.L.; Chandler, K.; Buchholz, B. Operating Experience and Teardown Analysis for Engines Operated on Biodiesel Blends. In Proceedings of the 2005 SAE Commercial Vehicle Engineering Conference, Rosemont, IL, USA, 1–3 November 2005. [Google Scholar]
- Geller, D.P.; Adams, T.T.; Goodrum, J.W.; Pendergrass, J. Storage stability of poultry fat and diesel fuel mixtures: Specific gravity and viscosity. Fuel 2008, 87, 92–102. [Google Scholar] [CrossRef]
- Deshpande, S.; Joshi, A.; Vagge, S.; Anekar, N. Corrosion behavior of nodular cast iron in biodiesel blends. Eng. Fail. Anal. 2019, 105, 1319–1327. [Google Scholar] [CrossRef]
- Fazal, M.A.; Suhaila, N.R.; Haseeb, A.S.M.A.; Rubaiee, S. Sustainability of additive-doped biodiesel: Analysis of its aggressiveness toward metal corrosion. J. Clean. Prod. 2018, 181, 508–516. [Google Scholar] [CrossRef]
- Ahmmad, M.S.; Haji Hassan, M.B.; Kalam, M.A. Comparative corrosion characteristics of automotive materials in Jatropha biodiesel. Int. J. Green Energy 2018, 15, 393–399. [Google Scholar] [CrossRef]
- Cole, G.; Sherman, A. Light weight materials for automotive applications. Mater. Charact. 1995, 35, 3–9. [Google Scholar] [CrossRef]
- Sgroi, M.; Bollito, G.; Saracco, G.; Specchia, S. BIOFEAT: Biodiesel fuel processor for a vehicle fuel cell auxiliary power unit: Study of the feed system. J. Power Sources 2005, 149, 8–14. [Google Scholar] [CrossRef]
- Fazal, M.; Haseeb, A.; Masjuki, H. Comparative corrosive characteristics of petroleum diesel and palm biodiesel for automotive materials. Fuel Process. Technol. 2010, 91, 1308–1315. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Effect of different corrosion inhibitors on the corrosion of cast iron in palm biodiesel. Fuel Process. Technol. 2011, 92, 2154–2159. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering, 3rd ed.; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Biodiesel feasibility study: An evaluation of material compatibility; performance; emission and engine durability. Renew. Sustain. Energy Rev. 2011, 15, 1314–1324. [Google Scholar] [CrossRef]
- Cao, P.; Tremblay, A.Y.; Dube, M.A.; Morse, K. Effect of membrane pore size on the performance of a membrane reactor for biodiesel production. Ind. Eng. Chem. Res. 2007, 46, 52–58. [Google Scholar] [CrossRef]
- Kaul, S.; Saxena, R.; Kumar, A.; Negi, M.; Bhatnagar, A.; Goyal, H.; Gupta, A. Corrosion behavior of biodiesel from seed oils of Indian origin on diesel engine parts. Fuel Process. Technol. 2007, 88, 303–307. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Kalam, M.A.; Masjuki, H.H.; Bhuiya, M.M.K.; Mofijur, M. An experimental investigation into biodiesel stability by means of oxidation and property determination. Energy 2012, 44, 616–622. [Google Scholar] [CrossRef]
- Wood, R.; Hutton, S.; Schiffrin, D. Mass transfer effects of non-cavitating seawater on the corrosion of Cu and 70Cu-30Ni. Corros. Sci. 1990, 30, 1177–1201. [Google Scholar] [CrossRef]
- Keera, S.T.; Elham, E.A.; Taman, A.R. Corrosion of copper metal in distillation process. Anti Corros. Methods Mater. 1998, 45, 252–255. [Google Scholar] [CrossRef]
- Frankel, G. Pitting corrosion of metals a review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Arnvig, P.E.; Davison, R.M. Corrosion Control for Low-Cost Reliability. In Proceedings of the 12th International Corrosion Congress, Houston, TX, USA, 19–24 September 1993; p. 1477. [Google Scholar]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Liao, J.; Fukui, H.; Urakami, T.; Morisaki, H. Effect of biofilm on ennoblement and localized corrosion of stainless steel in fresh dam-water. Corros. Sci. 2010, 52, 1393–1403. [Google Scholar] [CrossRef]
- Lee, W.; Beer, D. Oxygen and pH micro profiles above corrosion mild steel covered with a biofilm. Biofouling 1995, 8, 273–280. [Google Scholar] [CrossRef]
- Street, C.N.; Gibbs, A. Eradication of the corrosion-causing bacterial strains Desulfovibrio vulgaris and Desulfovibrio desulfuricans in planktonic and biofilm form using photo disinfection. Corros. Sci. 2010, 52, 1447–1452. [Google Scholar] [CrossRef]
- Beale, D.J.; Dunn, M.S.; Marney, D. Application of GC–MS metabolic profiling to blue–green water from microbial influenced corrosion in copper pipes. Corros. Sci. 2010, 52, 3140–3145. [Google Scholar] [CrossRef]
- Sherar, B.W.A.; Power, I.M.; Keech, P.G.; Mitlin, S.; Southam, G.; Shoesmith, D.W. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros. Sci. 2011, 53, 955–960. [Google Scholar] [CrossRef]
- Mankowski, G.; Duthil, J.P.; Giusti, A. The pit morphology on copper in chloride- and sulphate-containing solutions. Corros. Sci. 1997, 39, 27–42. [Google Scholar] [CrossRef]
- Norouzi, S.; Eslami, F.; Wyszynski, M.L.; Tsolakis, A. Corrosion effects of RME in blends with ULSD on aluminium and copper. Fuel Process. Technol. 2012, 104, 204–210. [Google Scholar] [CrossRef]
- Evans, U.R. The Corrosion and Oxidation of Metals: Scientific Principles and Practical Applications, 1st ed.; Edward Arnold: London, UK, 1960. [Google Scholar]
- Haseeb, A.S.M.A.; Masjuki, H.H.; Ann, L.J.; Fazal, M.A. Corrosion characteristics of copper and leaded bronze in palm biodiesel. Fuel Process. Technol. 2010, 91, 329–334. [Google Scholar] [CrossRef]
- Huang, T.J.; Tsai, D.H. CO oxidation behavior of copper and copper oxides. Catal. Lett. 2003, 87, 173–178. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.; Kalam, M.; Hazrat, M.; Liaquat, A.; Shahabuddin, M.; Varman, M. Prospects of biodiesel from Jatropha in Malaysia. Renew. Sustain. Energy Rev. 2012, 16, 5007–5020. [Google Scholar] [CrossRef]
- Hu, E.; Xu, Y.; Hu, X.; Pan, L.; Jiang, S. Corrosion behaviors of metals in biodiesel from rapeseed oil and methanol. Renew. Energy 2012, 37, 371–378. [Google Scholar] [CrossRef]
- Wagner, H.; Luther, R.; Mang, T. Lubricant base fluids based on renewable raw materials: Their catalytic manufacture and modification. Appl. Catal. A Gen. 2001, 221, 429–442. [Google Scholar] [CrossRef]
- Wagutu, A.; Chhabra, S.; Thoruwa, C.; Thoruwa, T.; Mahunnah, R. Indigenous oil crops as a source for production of biodiesel in Kenya. Bull. Chem. Soc. Ethiop. 2009, 23, 47660. [Google Scholar] [CrossRef]
- Kamisnki, J.; Kurzydlowski, K.J. Use of impedance spectroscopy on testing corrosion resistance of carbon steel and stainless steel in water–biodiesel configuration. J. Corros. Meas. 2008, 6, 1–5. [Google Scholar]
- Thomas, E.W.; Fuller, R.E.; Terauchi, K. Fluoroelastomer Compatibility with Biodiesel Fuels. In Proceedings of the Commercial Vehicle Engineering Congress & Exhibition Powertrain & Fluid Systems Conference and Exhibition, Rosemont, IL, USA, 29 October–1 November 2007. [Google Scholar]
- Tsuchiya, T.; Shiotani, H.; Goto, S.; Sugiyama, G.; Maeda, A. Japanese Standards for Diesel Fuel Containing 5% FAME Blended Diesel Fuels and its Impact on Corrosion. In Proceedings of the Powertrain & Fluid Systems Conference and Exhibition, Toronto, ON, Canada, 12–16 October 2006. [Google Scholar]
- Hancsók, J.; Bubálik, M.; Beck, Á.; Baladincz, J. Development of multifunctional additives based on vegetable oils for high quality diesel and biodiesel. Chem. Eng. Res. Des. 2008, 86, 793–799. [Google Scholar] [CrossRef]









| Parameters | Stainless Steel (SS) | Cast Iron (CI) | Copper (Cu) | Aluminium (Al) |
|---|---|---|---|---|
| Density (kg/m3) | 754 | 696 | 869 | 269 |
| Brinell Hardness Number | 216 | 159 | 83 | 104 |
| Vickers Hardness Number | 265 | 190 | 97 | 107 |
| Hardness (MPa) | 2599 | 1863 | 951 | 1049 |
| Properties | Unit | B0 | B20 | B30 | B100 |
|---|---|---|---|---|---|
| Density | kg/m3 | 833 | 839 | 842 | 875 |
| Acid value | mg KOH/g | 0.15 | 0.24 | 0.29 | 0.50 |
| FFA content | % | - | 0.04 | 0.06 | 0.20 |
| Water content | mg/kg | - | 100 | 150 | 500 |
| Kinematic viscosity at 40 °C | mm2/s | 3.63 | 3.78 | 3.88 | 4.71 |
| Flash point | °C | 68 | 89 | 107 | 202 |
| Induction period | h | 106 | 85 | 75 | 3.02 |
| Heating value | (MJ/kg) | 45.5 | 44.3 | 43.7 | 39.5 |
| Metal Sample | As-Received Fuels (IP/h) | After the Immersion Test (IP/h) | ||||
|---|---|---|---|---|---|---|
| B0 | B20 | B30 | B0 | B20 | B30 | |
| SS | 106 | 85 | 75 | 42 | 0.60 | 0.35 |
| Al | 106 | 85 | 75 | 96 | 0.75 | 0.30 |
| Ci | 106 | 85 | 75 | 21 | 2.25 | 0.32 |
| Cu | 106 | 85 | 75 | 45 | 0.60 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahabuddin, M.; Mofijur, M.; Shuvho, M.B.A.; Chowdhury, M.A.K.; Kalam, M.A.; Masjuki, H.H.; Chowdhury, M.A. A Study on the Corrosion Characteristics of Internal Combustion Engine Materials in Second-Generation Jatropha Curcas Biodiesel. Energies 2021, 14, 4352. https://doi.org/10.3390/en14144352
Shahabuddin M, Mofijur M, Shuvho MBA, Chowdhury MAK, Kalam MA, Masjuki HH, Chowdhury MA. A Study on the Corrosion Characteristics of Internal Combustion Engine Materials in Second-Generation Jatropha Curcas Biodiesel. Energies. 2021; 14(14):4352. https://doi.org/10.3390/en14144352
Chicago/Turabian StyleShahabuddin, M., M. Mofijur, Md. Bengir Ahmed Shuvho, M. A. K. Chowdhury, M. A. Kalam, H. H. Masjuki, and M. A. Chowdhury. 2021. "A Study on the Corrosion Characteristics of Internal Combustion Engine Materials in Second-Generation Jatropha Curcas Biodiesel" Energies 14, no. 14: 4352. https://doi.org/10.3390/en14144352







