Anion Coordination Improves High-Temperature Performance and Stability of NaPF6-Based Electrolytes for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
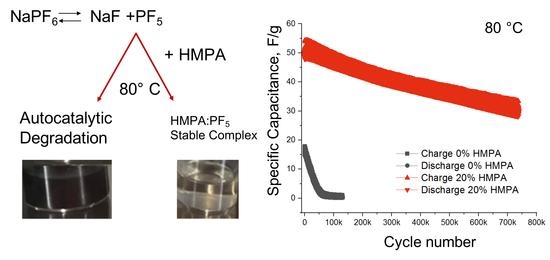
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Hu, M.; Pang, X.; Zhou, Z. Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Ruther, R.E.; Sun, C.-N.; Holliday, A.; Cheng, S.; Delnick, F.M.; Zawodzinski, T.A.; Nanda, J. Stable Electrolyte for High Voltage Electrochemical Double-Layer Capacitors. J. Electrochem. Soc. 2017, 164, A277–A283. [Google Scholar] [CrossRef]
- Balducci, A.; Dugas, R.; Taberna, P.L.; Simon, P.; Pl, D. High temperature carbon—Carbon supercapacitor using ionic liquid as electrolyte. J. Power Sources 2007, 165, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2020, 26, 2219–2251. [Google Scholar] [CrossRef]
- Conte, M. Supercapacitors Technical Requirements for New Applications. Fuel Cells 2010, 10, 806–818. [Google Scholar] [CrossRef] [Green Version]
- Kanchev, H.; Lu, D.; Colas, F.; Lazarov, V.; Francois, B. Energy Management and Operational Planning of a Microgrid with a PV-Based Active Generator for Smart Grid Applications. IEEE Trans. Ind. Electron. 2011, 58, 4583–4592. [Google Scholar] [CrossRef] [Green Version]
- Hung, K.; Masarapu, C.; Ko, T.; Wei, B. Wide-temperature range operation supercapacitors from nanostructured activated carbon fabric. J. Power Sources 2009, 193, 944–949. [Google Scholar] [CrossRef]
- Rafik, F.; Gualous, H.; Gallay, R.; Crausaz, A.; Berthon, A. Frequency, thermal and voltage supercapacitor characterization and modeling. J. Power Sources 2007, 165, 928–934. [Google Scholar] [CrossRef]
- Jian-wen, L.I.U.; Xin-hai, L.I.; Zhi-Xing, W. Preparation and characterization of lithium hexafluorophosphate for lithium-ion battery electrolyte. Trans. Nonferr. Met. Soc. China 2010, 20, 344–348. [Google Scholar] [CrossRef]
- Li, W.; Campion, C.; Lucht, B.L.; Ravdel, B.; Dicarlo, J.; Abraham, K.M. Additives for Stabilizing LiPF 6 -Based Electrolytes Against Thermal Decomposition. J. Electrochem. Soc. 2005, 152, 1361–1365. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Borodin, O.; Behl, W.; Jow, T.R. Oxidative Stability and Initial Decomposition Reactions of Carbonate, Sulfone, and Alkyl Phosphate-Based Electrolytes. J. Phys. Chem. C 2013, 117, 8661–8682. [Google Scholar] [CrossRef]
- Chiba, K.; Ueda, T.; Yamaguchi, Y.; Oki, Y.; Shimodate, F.; Naoi, K. Electrolyte Systems for High Withstand Voltage and Durability I. Linear Sulfones for Electric Double-Layer Capacitors. J. Electrochem. Soc. 2011, 158, A872–A882. [Google Scholar] [CrossRef]
- Brandt, A.; Isken, P.; Lex-Balducci, A.; Balducci, A. Adiponitrile-based electrochemical double layer capacitor. J. Power Sources 2012, 204, 213–219. [Google Scholar] [CrossRef]
- Brandt, A.; Balducci, A. The Influence of Pore Structure and Surface Groups on the Performance of High Voltage Electrochemical Double Layer Capacitors Containing Adiponitrile-Based Electrolyte. J. Electrochem. Soc. 2012, 159, A2053–A2059. [Google Scholar] [CrossRef]
- Morales, D.; Ruther, R.E.; Nanda, J.; Greenbaum, S. Ion transport and association study of glyme-based electrolytes with lithium and sodium salts. Electrochim. Acta 2019, 304, 239–245. [Google Scholar] [CrossRef]
- Yang, H.; Kwon, K.; Devine, T.M.; Evans, J.W. Aluminum Corrosion in Lithium Batteries an Investigation Using the Electrochemical Quartz Crystal Microbalance. J. Electrochem. Soc. 2000, 147, 4399–4407. [Google Scholar] [CrossRef]
- Zhang, S.S.; Jow, T.R. Aluminum corrosion in electrolyte of Li-ion battery. J. Power Sources 2002, 109, 458–464. [Google Scholar] [CrossRef]
- Vali, R.; Janes, A.; Lust, E. Vinylene Carbonate as Co-Solvent for Low-Temperature Mixed Electrolyte Based Supercapacitors. J. Electrochem. Soc. 2016, 163, A851–A857. [Google Scholar] [CrossRef] [Green Version]
- Laheäär, A.; Jänes, A.; Lust, E. NaClO4 and NaPF6 as potential non-aqueous electrolyte salts for electrical double layer capacitor application. Electrochim. Acta 2012, 82, 309–313. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal Decomposition of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, 2327–2334. [Google Scholar] [CrossRef]
- Okamoto, Y. Ab Initio Calculations of Thermal Decomposition Mechanism of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2013, 160, 404–409. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Euler, W.B.; Lucht, B.L.; Ravdel, B.; Dicarlo, J.F.; Gitzendanner, R.; Abraham, K.M. Suppression of Toxic Compounds Produced in the Decomposition of Lithium-Ion Battery Electrolytes. Electrochem. Solid-State Lett. 2004, 7, 194–197. [Google Scholar] [CrossRef]
- Taberna, P.L.; Simon, P.; Fauvarque, J.F. Electrochemical Characteristics and Impedance Spectroscopy Studies of Carbon-Carbon Supercapacitors. J. Electrochem. Soc. 2003, 153, 292–300. [Google Scholar] [CrossRef]
- Bohlen, O.; Kowal, J.; Sauer, D.U. Ageing behaviour of electrochemical double layer capacitors Part I. Experimental study and ageing model. J. Power Sources 2007, 172, 468–475. [Google Scholar] [CrossRef]
- Goren, E.; Chusid, O.; Aurbach, D. The Application of In SITU FTIR Spectroscopy to the Study of Surface Films Formed on Lithium and Noble Metals at Low Potentials in Li Battery Electrolytes. J. Electrochem. Soc. 1991, 138, 6–9. [Google Scholar] [CrossRef]
- Lugez, C.L.; Irikura, K.K.; Jacox, M.E. Experimental and ab initio study of the infrared spectra of ionic species derived from and and trapped in solid neon. J. Chem. Phys. 1998, 108, 8381–8393. [Google Scholar] [CrossRef]
- Wilken, S.; Johansson, P.; Jacobsson, P. Infrared spectroscopy of instantaneous decomposition products of LiPF6-based lithium battery electrolytes. Solid State Sci. 2012, 225, 608–610. [Google Scholar] [CrossRef]
- Lekgoathi, M.D.S.; Vilakazi, B.M.; Wagener, J.B.; Le Roux, J.P.; Moolman, D. Decomposition kinetics of anhydrous and moisture exposed LiPF6 salts by thermogravimetry. J. Fluor. Chem. 2013, 149, 53–56. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyler, J.L.; Sacci, R.L.; Nanda, J. Anion Coordination Improves High-Temperature Performance and Stability of NaPF6-Based Electrolytes for Supercapacitors. Energies 2021, 14, 4409. https://doi.org/10.3390/en14154409
Tyler JL, Sacci RL, Nanda J. Anion Coordination Improves High-Temperature Performance and Stability of NaPF6-Based Electrolytes for Supercapacitors. Energies. 2021; 14(15):4409. https://doi.org/10.3390/en14154409
Chicago/Turabian StyleTyler, J. Landon, Robert L. Sacci, and Jagjit Nanda. 2021. "Anion Coordination Improves High-Temperature Performance and Stability of NaPF6-Based Electrolytes for Supercapacitors" Energies 14, no. 15: 4409. https://doi.org/10.3390/en14154409
APA StyleTyler, J. L., Sacci, R. L., & Nanda, J. (2021). Anion Coordination Improves High-Temperature Performance and Stability of NaPF6-Based Electrolytes for Supercapacitors. Energies, 14(15), 4409. https://doi.org/10.3390/en14154409