A Review on MoS2 Energy Applications: Recent Developments and Challenges
Abstract
:1. Introduction
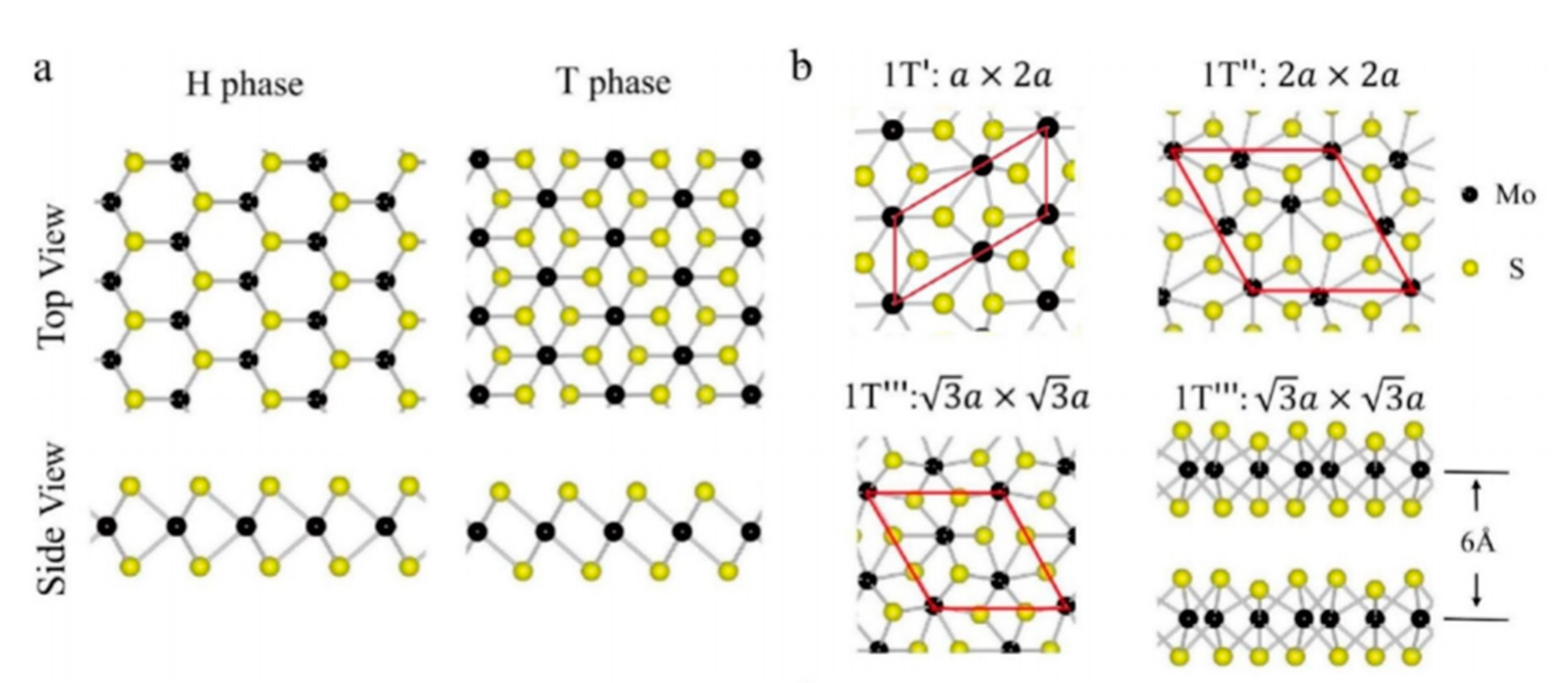
2. Structure and Properties
3. Synthesis
3.1. Lithium Intercalation and Exfoliation
3.2. Hydrothermal and Solvothermal Synthesis
3.3. Other Methods
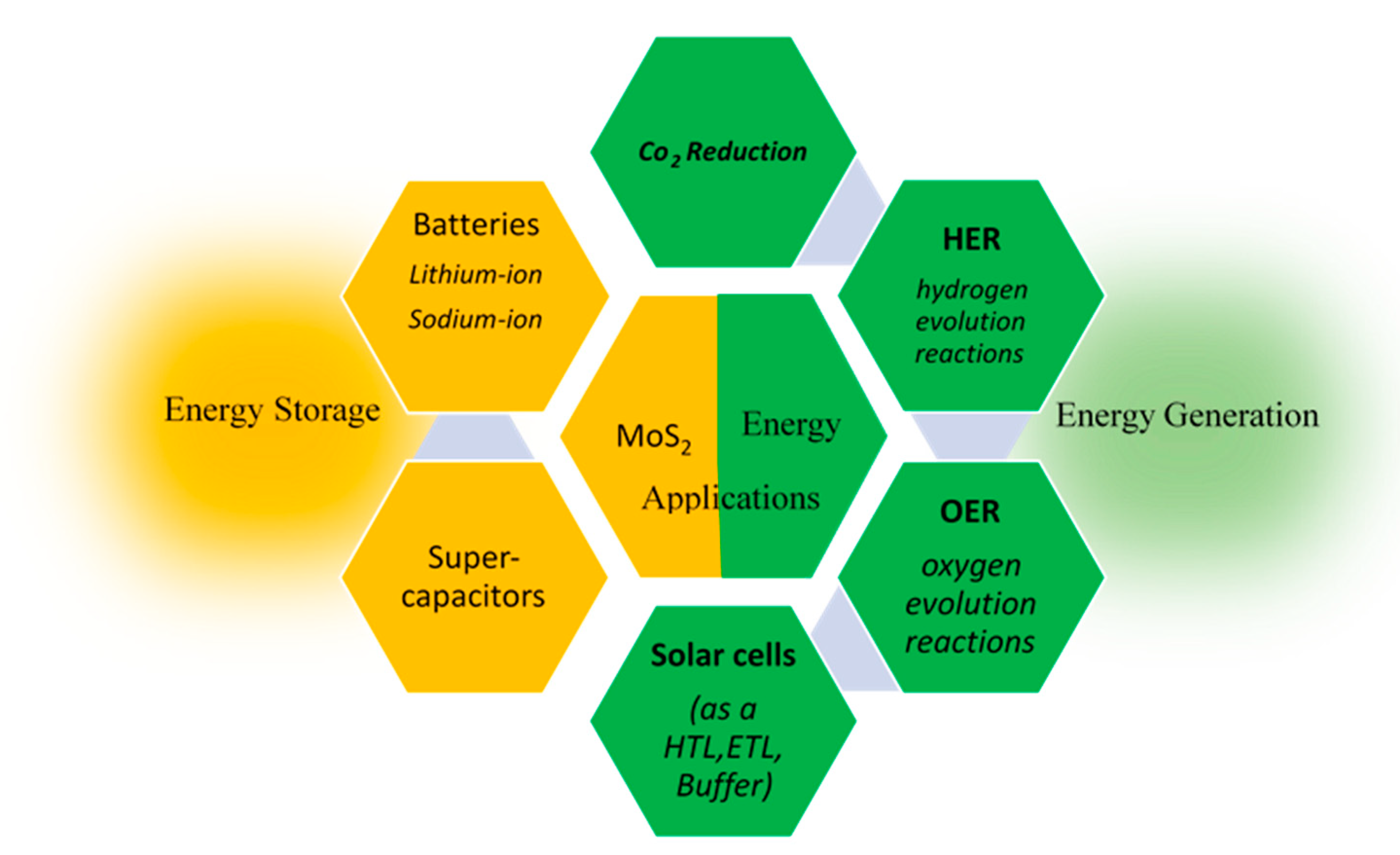
4. Energy Applications
4.1. Energy Storage Applications
4.1.1. Lithium-Ion Batteries (LIB)
4.1.2. Sodium-Ion Batteries (NIB)
4.1.3. Supercapacitors
4.2. Energy Generation Applications
4.2.1. Hydrogen Evolution Reactions (HER)
4.2.2. Oxygen Evolution Reactions (OER)
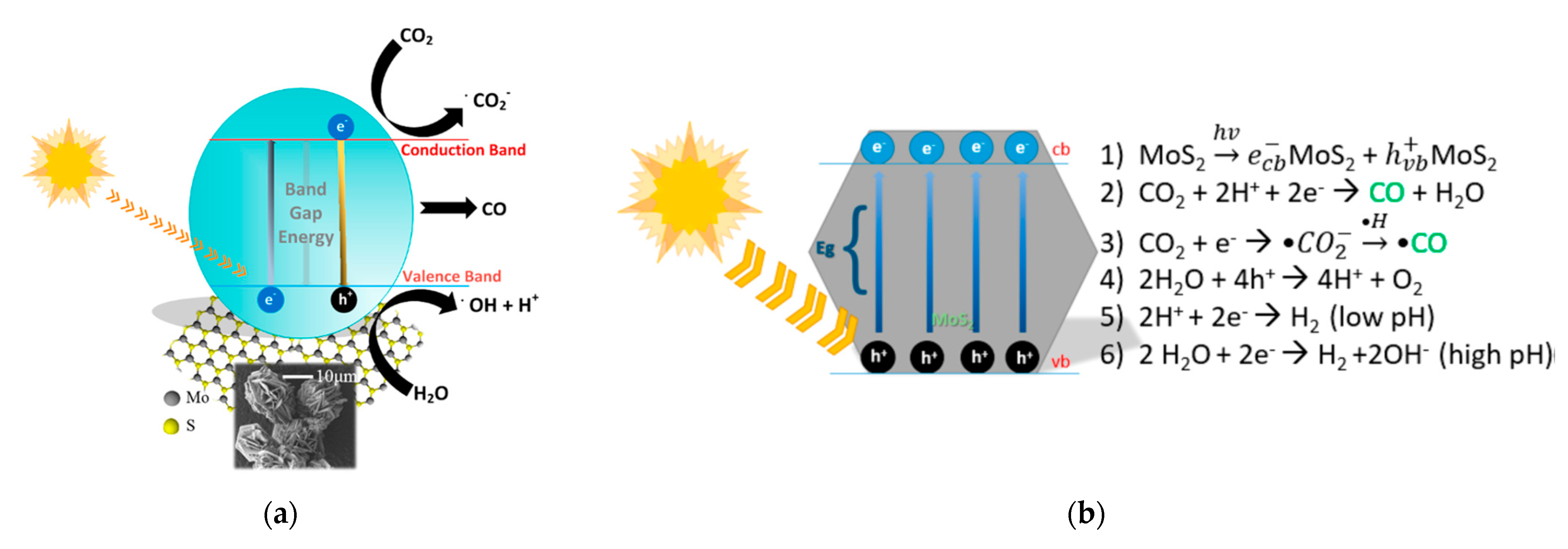
4.2.3. CO2 Reduction
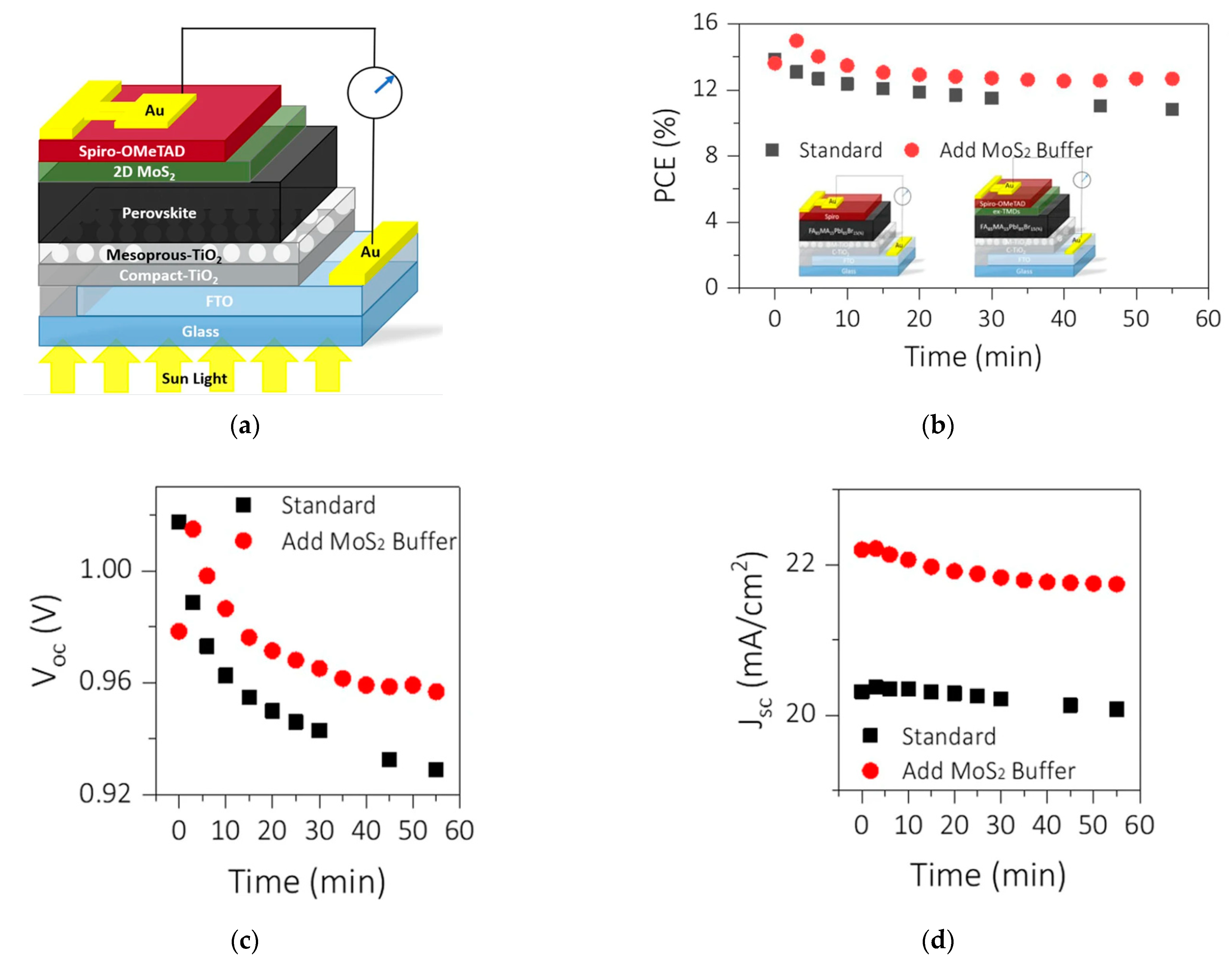
4.2.4. Solar Cells
5. Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Kim, H.-Y.; Lotya, M.; Coleman, J.N.; Kim, G.-T.; Duesberg, G.S. Electrical Characteristics of Molybdenum Disulfide Flakes Produced by Liquid Exfoliation. Adv. Mater. 2011, 23, 4178–4182. [Google Scholar] [CrossRef]
- Chang, K.; Hai, X.; Pang, H.; Zhang, H.; Shi, L.; Liu, G.; Liu, H.; Zhao, G.; Li, M.; Ye, J. Targeted Synthesis of 2H- and 1T-Phase MoS2 Monolayers for Catalytic Hydrogen Evolution. Adv. Mater. 2016, 28, 10033–10041. [Google Scholar] [CrossRef]
- Hersam, M.C. Emerging Device Applications for Two-Dimensional Nanomaterial Heterostructures. In Proceedings of the 2015 73rd Annual Device Research Conference (DRC), Columbus, OH, USA, 21–24 June 2015; IEEE: Columbus, OH, USA, 2015; p. 209. [Google Scholar]
- Zhao, G.-Y.; Deng, H.; Tyree, N.; Guy, M.; Lisfi, A.; Peng, Q.; Yan, J.-A.; Wang, C.; Lan, Y. Recent Progress on Irradiation-Induced Defect Engineering of Two-Dimensional 2H-MoS2 Few Layers. Appl. Sci. 2019, 9, 678. [Google Scholar] [CrossRef] [Green Version]
- del Alamo, J.A. Nanometer-Scale III–V CMOS. In Proceedings of the 2016 Compound Semiconductor Week (CSW) Includes 28th International Conference on Indium Phosphide & Related Materials (IPRM) & 43rd International Symposium on Compound Semiconductors (ISCS), Toyama, Japan, 26–30 June 2016; IEEE: Toyama, Japan, 2016; p. 1. [Google Scholar]
- Hijazi, A.; Moutaouakil, A.E. Graphene and MoS2 Structures for THz Applications. In Proceedings of the 2019 44th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Paris, France, 1–6 September 2019; IEEE: Paris, France, 2019; pp. 1–2. [Google Scholar]
- Choudhary, N.; Patel, M.D.; Park, J.; Sirota, B.; Choi, W. Synthesis of Large Scale MoS2 for Electronics and Energy Applications. J. Mater. Res. 2016, 31, 824–831. [Google Scholar] [CrossRef]
- Li, Y.; Chang, K.; Sun, Z.; Shangguan, E.; Tang, H.; Li, B.; Sun, J.; Chang, Z. Selective Preparation of 1T- and 2H-Phase MoS2 Nanosheets with Abundant Monolayer Structure and Their Applications in Energy Storage Devices. ACS Appl. Energy Mater. 2020, 3, 998–1009. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Chan, K.; Tsai, C.; Nørskov, J.K. How Doped MoS2 Breaks Transition-Metal Scaling Relations for CO2 Electrochemical Reduction. ACS Catal. 2016, 6, 4428–4437. [Google Scholar] [CrossRef]
- Gupta, D.; Chauhan, V.; Kumar, R. A Comprehensive Review on Synthesis and Applications of Molybdenum Disulfide (MoS2) Material: Past and Recent Developments. Inorg. Chem. Commun. 2020, 121, 108200. [Google Scholar] [CrossRef]
- Nalwa, H.S. A Review of Molybdenum Disulfide (MoS2) Based Photodetectors: From Ultra-Broadband, Self-Powered to Flexible Devices. RSC Adv. 2020, 10, 30529–30602. [Google Scholar] [CrossRef]
- Yadav, V.; Roy, S.; Singh, P.; Khan, Z.; Jaiswal, A. 2D MoS2 -Based Nanomaterials for Therapeutic, Bioimaging, and Biosensing Applications. Small 2019, 15, 1803706. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, X.; Guo, W.; Zhao, M.; Fan, X.; Dong, Y.; Xu, C.; Deng, J.; Fu, Y. Synthesis Methods of Two-Dimensional MoS2: A Brief Review. Crystals 2017, 7, 198. [Google Scholar] [CrossRef]
- Krishnan, U.; Kaur, M.; Singh, K.; Kumar, M.; Kumar, A. A Synoptic Review of MoS2: Synthesis to Applications. Superlattices Microstruct. 2019, 128, 274–297. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Yang, L.; Liu, P.; Li, J.; Xiang, B. Two-Dimensional Material Molybdenum Disulfides as Electrocatalysts for Hydrogen Evolution. Catalysts 2017, 7, 285. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Que, W. Molybdenum Disulfide Nanomaterials: Structures, Properties, Synthesis and Recent Progress on Hydrogen Evolution Reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Winer, W.O. Molybdenum Disulfide as a Lubricant: A Review of the Fundamental Knowledge. Wear 1967, 10, 422–452. [Google Scholar] [CrossRef] [Green Version]
- Joly-Pottuz, L.; Dassenoy, F.; Belin, M.; Vacher, B.; Martin, J.M.; Fleischer, N. Ultralow-Friction and Wear Properties of IF-WS2 under Boundary Lubrication. Tribol. Lett. 2005, 18, 477–485. [Google Scholar] [CrossRef]
- Yadgarov, L.; Petrone, V.; Rosentsveig, R.; Feldman, Y.; Tenne, R.; Senatore, A. Tribological Studies of Rhenium Doped Fullerene-like MoS2 Nanoparticles in Boundary, Mixed and Elasto-Hydrodynamic Lubrication Conditions. Wear 2013, 297, 1103–1110. [Google Scholar] [CrossRef]
- Sgroi, M.; Gili, F.; Mangherini, D.; Lahouij, I.; Dassenoy, F.; Garcia, I.; Odriozola, I.; Kraft, G. Friction Reduction Benefits in Valve-Train System Using IF-MoS2 Added Engine Oil. Tribol. Trans. 2015, 58, 207–214. [Google Scholar] [CrossRef]
- Sgroi, M.F.; Asti, M.; Gili, F.; Deorsola, F.A.; Bensaid, S.; Fino, D.; Kraft, G.; Garcia, I.; Dassenoy, F. Engine Bench and Road Testing of an Engine Oil Containing MoS2 Particles as Nano-Additive for Friction Reduction. Tribol. Int. 2017, 105, 317–325. [Google Scholar] [CrossRef]
- Song, I.; Park, C.; Choi, H.C. Synthesis and Properties of Molybdenum Disulphide: From Bulk to Atomic Layers. RSC Adv. 2015, 5, 7495–7514. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Peng, R.; Du, H.; Shen, Y.; Li, Y.; Li, J.; Dong, G. The Application of Nano-MoS2 Quantum Dots as Liquid Lubricant Additive for Tribological Behavior Improvement. Nanomaterials 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahouij, I.; Dassenoy, F.; de Knoop, L.; Martin, J.-M.; Vacher, B. In Situ TEM Observation of the Behavior of an Individual Fullerene-Like MoS2 Nanoparticle in a Dynamic Contact. Tribol. Lett. 2011, 42, 133–140. [Google Scholar] [CrossRef]
- Cizaire, L.; Vacher, B.; Le Mogne, T.; Martin, J.M.; Rapoport, L.; Margolin, A.; Tenne, R. Mechanisms of Ultra-Low Friction by Hollow Inorganic Fullerene-like MoS2 Nanoparticles. Surf. Coat. Technol. 2002, 160, 282–287. [Google Scholar] [CrossRef]
- Lahouij, I.; Vacher, B.; Martin, J.-M.; Dassenoy, F. IF-MoS2 Based Lubricants: Influence of Size, Shape and Crystal Structure. Wear 2012, 296, 558–567. [Google Scholar] [CrossRef]
- Dai, Z.; Jin, W.; Grady, M.; Sadowski, J.T.; Dadap, J.I.; Osgood, R.M.; Pohl, K. Surface Structure of Bulk 2H-MoS2(0001) and Exfoliated Suspended Monolayer MoS2: A Selected Area Low Energy Electron Diffraction Study. Surf. Sci. 2017, 660, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhu, H. Two-Dimensional MoS2: Properties, Preparation, and Applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Toh, R.J.; Sofer, Z.; Luxa, J.; Sedmidubský, D.; Pumera, M. 3R Phase of MoS2 and WS2 Outperforms the Corresponding 2H Phase for Hydrogen Evolution. Chem. Commun. 2017, 53, 3054–3057. [Google Scholar] [CrossRef] [Green Version]
- Terrones, H.; López-Urías, F.; Terrones, M. Novel Hetero-Layered Materials with Tunable Direct Band Gaps by Sandwiching Different Metal Disulfides and Diselenides. Sci. Rep. 2013, 3, 1549. [Google Scholar] [CrossRef]
- Kadantsev, E.S.; Hawrylak, P. Electronic Structure of a Single MoS2 Monolayer. Solid State Commun. 2012, 152, 909–913. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Dumcenco, D.O.; Huang, Y.-S.; Suenaga, K. Atomic Mechanism of the Semiconducting-to-Metallic Phase Transition in Single-Layered MoS2. Nat. Nanotechnol. 2014, 9, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kappera, R.; Voiry, D.; Yalcin, S.E.; Branch, B.; Gupta, G.; Mohite, A.D.; Chhowalla, M. Phase-Engineered Low-Resistance Contacts for Ultrathin MoS2 Transistors. Nat. Mater. 2014, 13, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Shan, X.; Wu, Y.; Zhao, J.; Lu, X. Laser Thinning and Patterning of MoS2 with Layer-by-Layer Precision. Sci. Rep. 2017, 7, 15538. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.; Kim, J.H.; Zhao, J.; Seok, J.; Keum, D.H.; Baik, J.; Choe, D.-H.; Chang, K.J.; Suenaga, K.; et al. Phase Patterning for Ohmic Homojunction Contact in MoTe2. Science 2015, 349, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Liu, Y.; Pan, Y.; Zhu, H.; Zhao, J.; Zeng, L.; Liu, Z.; Liu, C. Targeted Bottom-up Synthesis of 1T-Phase MoS2 Arrays with High Electrocatalytic Hydrogen Evolution Activity by Simultaneous Structure and Morphology Engineering. Nano Res. 2018, 11, 4368–4379. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K.; Wang, C.; Zhang, Y.; Chen, S.; Wu, C.; Vasileff, A.; Qiao, S.-Z.; Song, L. Hierarchical 1T-MoS2 Nanotubular Structures for Enhanced Supercapacitive Performance. J. Mater. Chem. A 2017, 5, 23704–23711. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, Y.; Han, Y.; Li, J.; Yang, L.; Benamara, M.; Chen, L.; Zhu, H. Two-Dimensional Water-Coupled Metallic MoS2 with Nanochannels for Ultrafast Supercapacitors. Nano Lett. 2017, 17, 1825–1832. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Gong, S.; Lv, F.; Wang, W.; Li, Y.; Luo, M.; Xing, Y.; Wang, Q.; Guo, S. Co-Doped 1T-MoS2 Nanosheets Embedded in N, S-Doped Carbon Nanobowls for High-Rate and Ultra-Stable Sodium-Ion Batteries. Nano Res. 2019, 12, 2218–2223. [Google Scholar] [CrossRef]
- Geng, X.; Jiao, Y.; Han, Y.; Mukhopadhyay, A.; Yang, L.; Zhu, H. Freestanding Metallic 1T MoS2 with Dual Ion Diffusion Paths as High Rate Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1702998. [Google Scholar] [CrossRef]
- Tang, W.; Wang, X.; Xie, D.; Xia, X.; Gu, C.; Tu, J. Hollow Metallic 1T MoS2 Arrays Grown on Carbon Cloth: A Freestanding Electrode for Sodium Ion Batteries. J. Mater. Chem. A 2018, 6, 18318–18324. [Google Scholar] [CrossRef]
- Shang, C.; Fang, Y.Q.; Zhang, Q.; Wang, N.Z.; Wang, Y.F.; Liu, Z.; Lei, B.; Meng, F.B.; Ma, L.K.; Wu, T.; et al. Superconductivity in the Metastable 1 T’ and 1 T’’’ Phases of MoS2 Crystals. Phys. Rev. B 2018, 98, 184513. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Liu, J.; Fu, L.; Li, J. Quantum Spin Hall Effect in Two-Dimensional Transition Metal Dichalcogenides. Science 2014, 346, 1344–1347. [Google Scholar] [CrossRef] [Green Version]
- Kappera, R.; Voiry, D.; Yalcin, S.E.; Jen, W.; Acerce, M.; Torrel, S.; Branch, B.; Lei, S.; Chen, W.; Najmaei, S.; et al. Metallic 1T Phase Source/Drain Electrodes for Field Effect Transistors from Chemical Vapor Deposited MoS2. APL Mater. 2014, 2, 092516. [Google Scholar] [CrossRef] [Green Version]
- Coogan, Á.; Gun’ko, Y.K. Solution-Based “Bottom-up” Synthesis of Group VI Transition Metal Dichalcogenides and Their Applications. Mater. Adv. 2021, 2, 146–164. [Google Scholar] [CrossRef]
- Kaushik, S.; Tiwari, U.K.; Choubey, R.K.; Singh, K.; Sinha, R.K. Study of Sonication Assisted Synthesis of Molybdenum Disulfide (MoS2) Nanosheets. Mater. Today Proc. 2020, 21, 1969–1975. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Thenuwara, A.C.; Patra, A.; Aulin, Y.V.; Tran, T.M.; Chakraborty, H.; Borguet, E.; Klein, M.L.; Perdew, J.P.; Strongin, D.R. Effect of Intercalated Metals on the Electrocatalytic Activity of 1T-MoS2 for the Hydrogen Evolution Reaction. ACS Energy Lett. 2018, 3, 7–13. [Google Scholar] [CrossRef]
- Muratore, C.; Hu, J.J.; Wang, B.; Haque, M.A.; Bultman, J.E.; Jespersen, M.L.; Shamberger, P.J.; McConney, M.E.; Naguy, R.D.; Voevodin, A.A. Continuous Ultra-Thin MoS2 Films Grown by Low-Temperature Physical Vapor Deposition. Appl. Phys. Lett. 2014, 104, 261604. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Liu, Y.; Halim, U.; Ding, M.; Liu, Y.; Wang, Y.; Jia, C.; Chen, P.; Duan, X.; Wang, C.; et al. Solution-Processable 2D Semiconductors for High-Performance Large-Area Electronics. Nature 2018, 562, 254–258. [Google Scholar] [CrossRef]
- Choi, S.H.; Stephen, B.; Park, J.-H.; Lee, J.S.; Kim, S.M.; Yang, W.; Kim, K.K. Water-Assisted Synthesis of Molybdenum Disulfide Film with Single Organic Liquid Precursor. Sci. Rep. 2017, 7, 1983. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Kang, W.; Liu, J.; Zhang, C. Synergistic Exfoliation of MoS2 by Ultrasound Sonication in a Supercritical Fluid Based Complex Solvent. Nanoscale Res. Lett. 2019, 14, 317. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Bark, H.; Oh, I.-K.; Ryu, G.H.; Lee, Z.; Kim, H.; Cho, J.H.; Ahn, J.-H.; Lee, C. Synthesis of Wafer-Scale Uniform Molybdenum Disulfide Films with Control over the Layer Number Using a Gas Phase Sulfur Precursor. Nanoscale 2014, 6, 2821. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kang, M.-A.; Kim, S.H.; Lee, Y.; Song, W.; Myung, S.; Lee, S.S.; Lim, J.; An, K.-S. Large-Scale Growth and Simultaneous Doping of Molybdenum Disulfide Nanosheets. Sci. Rep. 2016, 6, 24054. [Google Scholar] [CrossRef] [Green Version]
- Wypych, F.; Schöllhorn, R. 1T-MoS2, a New Metallic Modification of Molybdenum Disulfide. J. Chem. Soc. Chem. Commun. 1992, 1386–1388. [Google Scholar] [CrossRef]
- Heising, J.; Kanatzidis, M.G. Exfoliated and Restacked MoS2 and WS2: Ionic or Neutral Species? Encapsulation and Ordering of Hard Electropositive Cations. J. Am. Chem. Soc. 1999, 121, 11720–11732. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-Layer Semiconducting Nanosheets: High-Yield Preparation and Device Fabrication. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef]
- Xiang, T.; Fang, Q.; Xie, H.; Wu, C.; Wang, C.; Zhou, Y.; Liu, D.; Chen, S.; Khalil, A.; Tao, S.; et al. Vertical 1T-MoS2 Nanosheets with Expanded Interlayer Spacing Edged on a Graphene Frame for High Rate Lithium-Ion Batteries. Nanoscale 2017, 9, 6975–6983. [Google Scholar] [CrossRef]
- Jiao, Y.; Mukhopadhyay, A.; Ma, Y.; Yang, L.; Hafez, A.M.; Zhu, H. Ion Transport Nanotube Assembled with Vertically Aligned Metallic MoS2 for High Rate Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702779. [Google Scholar] [CrossRef]
- Wu, M.; Zhan, J.; Wu, K.; Li, Z.; Wang, L.; Geng, B.; Wang, L.; Pan, D. Metallic 1T MoS2 Nanosheet Arrays Vertically Grown on Activated Carbon Fiber Cloth for Enhanced Li-Ion Storage Performance. J. Mater. Chem. A 2017, 5, 14061–14069. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Zhu, J.; Zhang, C.; Liu, T. Self-Templated Growth of Vertically Aligned 2H-1T MoS2 for Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 31702–31708. [Google Scholar] [CrossRef]
- Liu, Q.; Shang, Q.; Khalil, A.; Fang, Q.; Chen, S.; He, Q.; Xiang, T.; Liu, D.; Zhang, Q.; Luo, Y.; et al. In Situ Integration of a Metallic 1T-MoS2/CdS Heterostructure as a Means to Promote Visible-Light-Driven Photocatalytic Hydrogen Evolution. ChemCatChem 2016, 8, 2614–2619. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Wu, X.; Song, L. Gram-Scale Aqueous Synthesis of Stable Few-Layered 1T-MoS2: Applications for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, N.; Guo, Y.; Yang, J.; Wang, J.; Wang, F.; Sun, J.; Xu, H.; Liu, Z.-H.; Jiang, R. Metallic-Phase MoS2 Nanopetals with Enhanced Electrocatalytic Activity for Hydrogen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 13435–13442. [Google Scholar] [CrossRef]
- Geng, X.; Sun, W.; Wu, W.; Chen, B.; Al-Hilo, A.; Benamara, M.; Zhu, H.; Watanabe, F.; Cui, J.; Chen, T. Pure and Stable Metallic Phase Molybdenum Disulfide Nanosheets for Hydrogen Evolution Reaction. Nat. Commun. 2016, 7, 10672. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Ao, K.; Lv, P.; Wei, Q. MoS2 Coexisting in 1T and 2H Phases Synthesized by Common Hydrothermal Method for Hydrogen Evolution Reaction. Nanomaterials 2019, 9, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasani, A.; Le, Q.V.; Tekalgne, M.; Choi, M.-J.; Lee, T.H.; Jang, H.W.; Kim, S.Y. Direct Synthesis of Two-Dimensional MoS2 on p-Type Si and Application to Solar Hydrogen Production. NPG Asia Mater. 2019, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Chi, Z.; Zhao, J.; Zhang, Y.; Yu, H.; Yu, H. The Fabrication of Atomically Thin-MoS2 Based Photoanodes for Photoelectrochemical Energy Conversion and Environment Remediation: A Review. Green Energy Environ. 2021, S246802572100090X. [Google Scholar] [CrossRef]
- Jiao, Y.; Hafez, A.M.; Cao, D.; Mukhopadhyay, A.; Ma, Y.; Zhu, H. Metallic MoS2 for High Performance Energy Storage and Energy Conversion. Small 2018, 14, 1800640. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Kruse, P. Editors’ Choice—Review—Conductive Forms of MoS2 and Their Applications in Energy Storage and Conversion. J. Electrochem. Soc. 2020, 167, 126517. [Google Scholar] [CrossRef]
- Li, Z.; Zhan, X.; Zhu, W.; Qi, S.; Braun, P.V. Carbon-Free, High-Capacity and Long Cycle Life 1D–2D NiMoO4 Nanowires/Metallic 1T MoS2 Composite Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2019, 11, 44593–44600. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, J.; Cui, J.; Yao, S.; Ihsan-Ul-Haq, M.; Mubarak, N.; Quattrocchi, E.; Ciucci, F.; Kim, J.-K. Dual-Phase MoS2 as a High-Performance Sodium-Ion Battery Anode. J. Mater. Chem. A 2020, 8, 2114–2122. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, Q.; Zheng, F.; Liu, Y.; Li, Y.; Ou, X.; Xiong, X.; Yang, C.; Liu, M. Construction of MoS2/C Hierarchical Tubular Heterostructures for High-Performance Sodium Ion Batteries. ACS Nano 2018, 12, 12578–12586. [Google Scholar] [CrossRef]
- Cao, L.; Yang, S.; Gao, W.; Liu, Z.; Gong, Y.; Ma, L.; Shi, G.; Lei, S.; Zhang, Y.; Zhang, S.; et al. Direct Laser-Patterned Micro-Supercapacitors from Paintable MoS2 Films. Small 2013, 9, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Kumar, S.; Agarwal, K.; Soni, K.; Ramana Gedela, V.; Ghosh, K. Three-Dimensional Graphene with MoS2 Nanohybrid as Potential Energy Storage/Transfer Device. Sci. Rep. 2017, 7, 9458. [Google Scholar] [CrossRef] [Green Version]
- Manuraj, M.; Kavya Nair, K.V.; Unni, K.N.N.; Rakhi, R.B. High Performance Supercapacitors Based on MoS2 Nanostructures with near Commercial Mass Loading. J. Alloy. Compd. 2020, 819, 152963. [Google Scholar] [CrossRef]
- Nardekar, S.S.; Krishnamoorthy, K.; Pazhamalai, P.; Sahoo, S.; Mariappan, V.K.; Kim, S.-J. Exceptional Interfacial Electrochemistry of Few-Layered 2D MoS2 Quantum Sheets for High Performance Flexible Solid-State Supercapacitors. J. Mater. Chem. A 2020, 8, 13121–13131. [Google Scholar] [CrossRef]
- Zhan, C.; Liu, W.; Hu, M.; Liang, Q.; Yu, X.; Shen, Y.; Lv, R.; Kang, F.; Huang, Z.-H. High-Performance Sodium-Ion Hybrid Capacitors Based on an Interlayer-Expanded MoS2/RGO Composite: Surpassing the Performance of Lithium-Ion Capacitors in a Uniform System. NPG Asia Mater. 2018, 10, 775–787. [Google Scholar] [CrossRef]
- Su, J.; Pei, Y.; Yang, Z.; Wang, X. Ab Initio Study of Graphene-like Monolayer Molybdenum Disulfide as a Promising Anode Material for Rechargeable Sodium Ion Batteries. RSC Adv. 2014, 4, 43183–43188. [Google Scholar] [CrossRef]
- Kühne, M.; Börrnert, F.; Fecher, S.; Ghorbani-Asl, M.; Biskupek, J.; Samuelis, D.; Krasheninnikov, A.V.; Kaiser, U.; Smet, J.H. Reversible Superdense Ordering of Lithium between Two Graphene Sheets. Nature 2018, 564, 234–239. [Google Scholar] [CrossRef]
- Chepkasov, I.V.; Ghorbani-Asl, M.; Popov, Z.I.; Smet, J.H.; Krasheninnikov, A.V. Alkali Metals inside Bi-Layer Graphene and MoS2: Insights from First-Principles Calculations. Nano Energy 2020, 75, 104927. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T Phase MoS2 Nanosheets as Supercapacitor Electrode Materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Recent Advances in Carbon-Based Supercapacitors. Mater. Adv. 2020, 1, 945–966. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, C.; Zhao, W.; Zhang, Y.; Srimuk, P.; Presser, V.; Feng, G. Molecular Understanding of Charge Storage in MoS2 Supercapacitors with Ionic Liquids. Energy Environ. Mater. 2021, eem2.12147. [Google Scholar] [CrossRef]
- Balat, M. Potential Importance of Hydrogen as a Future Solution to Environmental and Transportation Problems. Int. J. Hydrog. Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Gao, M.-R.; Chan, M.K.Y.; Sun, Y. Edge-Terminated Molybdenum Disulfide with a 9.4-Å Interlayer Spacing for Electrochemical Hydrogen Production. Nat. Commun. 2015, 6, 7493. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Li, M.; Luo, J.; Wu, B.; Lai, L. The H2O Dissociation and Hydrogen Evolution Performance of Monolayer MoS2 Containing Single Mo Vacancy: A Theoretical Study. IEEE Trans. Nanotechnol. 2020, 19, 163–167. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Ye, K.; Li, M.; Luo, J.; Wu, B.; Lai, L. Activating Inert Basal Plane of MoS2 for H2O Dissociation and HER via Formation of Vacancy Defects: A DFT Study. In Proceedings of the 2019 IEEE 19th International Conference on Nanotechnology (IEEE-NANO), Macao, China, 22–26 July 2019; IEEE: Macao, China, 2019; pp. 48–53. [Google Scholar]
- Li, J.; Joseph, T.; Ghorbani-Asl, M.; Kolekar, S.; Krasheninnikov, A.V.; Batzill, M. Mirror Twin Boundaries in MoSe2 Monolayers as One Dimensional Nanotemplates for Selective Water Adsorption. Nanoscale 2021, 13, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Lai, L.; Li, M.; Luo, J.; Wu, B.; Ren, Z. Strain Effect on the Hydrogen Evolution Reaction of V Mo -SLMoS2. IEEE Trans. Nanotechnol. 2020, 19, 192–196. [Google Scholar] [CrossRef]
- Gao, M.-R.; Liang, J.-X.; Zheng, Y.-R.; Xu, Y.-F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.-H. An Efficient Molybdenum Disulfide/Cobalt Diselenide Hybrid Catalyst for Electrochemical Hydrogen Generation. Nat. Commun. 2015, 6, 5982. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Zhang, S.; Dong, X.; Song, Y.; Cai, T.; Liu, Y. Cracked Monolayer 1T MoS2 with Abundant Active Sites for Enhanced Electrocatalytic Hydrogen Evolution. Catal. Sci. Technol. 2017, 7, 718–724. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Jia, X.; Xu, B.; Lin, H.; Yang, H.; Song, L.; Wang, X. Amorphous Nickel-Cobalt Complexes Hybridized with 1T-Phase Molybdenum Disulfide via Hydrazine-Induced Phase Transformation for Water Splitting. Nat. Commun. 2017, 8, 15377. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Lu, Y. Direct Growth of MoS2 Microspheres on Ni Foam as a Hybrid Nanocomposite Efficient for Oxygen Evolution Reaction. Small 2016, 12, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 Quantum Dots as Efficient Catalyst Materials for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1683–1689. [Google Scholar] [CrossRef]
- Asadi, M.; Kumar, B.; Behranginia, A.; Rosen, B.A.; Baskin, A.; Repnin, N.; Pisasale, D.; Phillips, P.; Zhu, W.; Haasch, R.; et al. Robust Carbon Dioxide Reduction on Molybdenum Disulphide Edges. Nat. Commun. 2014, 5, 4470. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Kim, J.; Do, J.Y.; Seo, M.W.; Kang, M. Carbon Dioxide Photoreduction on the Bi2S3/MoS2 Catalyst. Catalysts 2019, 9, 998. [Google Scholar] [CrossRef] [Green Version]
- Meier, A.J.; Garg, A.; Sutter, B.; Kuhn, J.N.; Bhethanabotla, V.R. MoS2 Nanoflowers as a Gateway for Solar-Driven CO2 Photoreduction. ACS Sustain. Chem. Eng. 2019, 7, 265–275. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct Human Health Risks of Increased Atmospheric Carbon Dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- He, J.; Janáky, C. Recent Advances in Solar-Driven Carbon Dioxide Conversion: Expectations versus Reality. ACS Energy Lett. 2020, 5, 1996–2014. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hasanuzzaman, M. A Review of Global Current Techniques and Evaluation Methods of Photocatalytic CO2 Reduction. In Proceedings of the 5th IET International Conference on Clean Energy and Technology (CEAT2018), Kuala, Lumpur, 5–6 September 2018; Institution of Engineering and Technology: Kuala Lumpur, Malaysia, 2018; p. 6. [Google Scholar]
- Ueckerdt, F.; Bauer, C.; Dirnaichner, A.; Everall, J.; Sacchi, R.; Luderer, G. Potential and Risks of Hydrogen-Based e-Fuels in Climate Change Mitigation. Nat. Clim. Chang. 2021, 11, 384–393. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, X.; Liu, Y.; Qiao, J.; Zhou, X.-D.; Xu, N.; Malcombe, J.L.; Yi, J.; Zhang, J. Metal Chalcogenide-Associated Catalysts Enabling CO2 Electroreduction to Produce Low-Carbon Fuels for Energy Storage and Emission Reduction: Catalyst Structure, Morphology, Performance, and Mechanism. J. Mater. Chem. A 2021, 9, 2526–2559. [Google Scholar] [CrossRef]
- Yin, J.; Jin, J.; Lin, H.; Yin, Z.; Li, J.; Lu, M.; Guo, L.; Xi, P.; Tang, Y.; Yan, C. Optimized Metal Chalcogenides for Boosting Water Splitting. Adv. Sci. 2020, 7, 1903070. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Li, X.; Wang, Y.; Li, B.; Yang, L.; Zhao, N.; Liu, M.; Wang, X.; Yu, Y.; Liu, J.-M. Reaction Mechanisms for Reduction of CO2 to CO on Monolayer MoS2. Appl. Surf. Sci. 2020, 499, 143964. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Yang, W.; Wang, T.-B.; Deng, X.-H.; Liu, J.-T. Broadband Perfect Light Trapping in the Thinnest Monolayer Graphene-MoS2 Photovoltaic Cell: The New Application of Spectrum-Splitting Structure. Sci. Rep. 2016, 6, 20955. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, M.; Palummo, M.; Grossman, J.C. Extraordinary Sunlight Absorption and One Nanometer Thick Photovoltaics Using Two-Dimensional Monolayer Materials. Nano Lett. 2013, 13, 3664–3670. [Google Scholar] [CrossRef]
- Choudhary, S.; Garg, A.K. Enhanced Absorption in MoS2/Hg0.33Cd0.66 Te Heterostructure for Application in Solar Cell Absorbers. IEEE Trans. Nanotechnol. 2019, 18, 989–994. [Google Scholar] [CrossRef]
- Abouelkhair, H.M.; Orlovskaya, N.A.; Peale, R.E. Growth of MoS2 Thin Films with Microdome Texture as Omnidirectional Light Trap for Solar Cell Applications. In Proceedings of the 2017 IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017; IEEE: Washington, DC, USA, 2017; pp. 2324–2329. [Google Scholar]
- Lin, S.; Li, X.; Wang, P.; Xu, Z.; Zhang, S.; Zhong, H.; Wu, Z.; Xu, W.; Chen, H. Interface Designed MoS2/GaAs Heterostructure Solar Cell with Sandwich Stacked Hexagonal Boron Nitride. Sci. Rep. 2015, 5, 15103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, M.; Dey, M.; Alam, S.; Das, N.K.; Matin, M.A.; Amin, N. Study of Molybdenum Sulphide as a Novel Buffer Layer for CZTS Solar Cells. In Proceedings of the 2016 3rd International Conference on Electrical Engineering and Information Communication Technology (ICEEICT), Dhaka, Bangladesh, 22–24 September 2016; IEEE: Dhaka, Bangladesh, 2016; pp. 1–4. [Google Scholar]
- Singh, R.; Giri, A.; Pal, M.; Thiyagarajan, K.; Kwak, J.; Lee, J.-J.; Jeong, U.; Cho, K. Perovskite Solar Cells with an MoS2 Electron Transport Layer. J. Mater. Chem. A 2019, 7, 7151–7158. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Nabi, J.; Siddique, S.; Awan, H.T.A.; Haider, S.S.; Sulman, M. Role of Graphene and Transition Metal Dichalcogenides as Hole Transport Layer and Counter Electrode in Solar Cells. Int. J. Energy Res. 2020, 44, 1464–1487. [Google Scholar] [CrossRef]
- Capasso, A.; Del Rio Castillo, A.E.; Najafi, L.; Pellegrini, V.; Bonaccorso, F.; Matteocci, F.; Cina, L.; Di Carlo, A. Spray Deposition of Exfoliated MoS2 Flakes as Hole Transport Layer in Perovskite-Based Photovoltaics. In Proceedings of the 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO), Rome, Italy, 27–30 July 2015; IEEE: Rome, Italy, 2015; pp. 1138–1141. [Google Scholar]
- Xu, H.; Xin, L.; Liu, L.; Pang, D.; Jiao, Y.; Cong, R.; Yu, W. Large Area MoS2/Si Heterojunction-Based Solar Cell through Sol-Gel Method. Mater. Lett. 2019, 238, 13–16. [Google Scholar] [CrossRef]
- Liang, M.; Ali, A.; Belaidi, A.; Hossain, M.I.; Ronan, O.; Downing, C.; Tabet, N.; Sanvito, S.; EI-Mellouhi, F.; Nicolosi, V. Improving Stability of Organometallic-Halide Perovskite Solar Cells Using Exfoliation Two-Dimensional Molybdenum Chalcogenides. npj 2D Mater. Appl. 2020, 4, 40. [Google Scholar] [CrossRef]
- Abd Malek, N.A.; Alias, N.; Md Saad, S.K.; Abdullah, N.A.; Zhang, X.; Li, X.; Shi, Z.; Rosli, M.M.; Tengku Abd Aziz, T.H.; Umar, A.A.; et al. Ultra-Thin MoS2 Nanosheet for Electron Transport Layer of Perovskite Solar Cells. Opt. Mater. 2020, 104, 109933. [Google Scholar] [CrossRef]
- Shi, S.; Sun, Z.; Hu, Y.H. Synthesis, Stabilization and Applications of 2-Dimensional 1T Metallic MoS2. J. Mater. Chem. A 2018, 6, 23932–23977. [Google Scholar] [CrossRef]
- Cha, E.; Kim, D.K.; Choi, W. Advances of 2D MoS2 for High-Energy Lithium Metal Batteries. Front. Energy Res. 2021, 9, 645403. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Abroshan, H.; Zhang, L.; Kim, J.K.; Zhang, J.; Guo, J.; Siahrostami, S.; Zheng, X. Enhancing Catalytic Activity of MoS2 Basal Plane S-Vacancy by Co Cluster Addition. ACS Energy Lett. 2018, 3, 2685–2693. [Google Scholar] [CrossRef]
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Yu, S.-H. Nanostructured Metal Chalcogenides: Synthesis, Modification, and Applications in Energy Conversion and Storage Devices. Chem. Soc. Rev. 2013, 42, 2986. [Google Scholar] [CrossRef]
- Lau, T.H.M.; Foord, J.S.; Tsang, S.C.E. 2D Molybdenum Disulphide Nanosheets Incorporated with Single Heteroatoms for the Electrochemical Hydrogen Evolution Reaction. Nanoscale 2020, 12, 10447–10455. [Google Scholar] [CrossRef]
- Li, X.L.; Li, T.C.; Huang, S.; Zhang, J.; Pam, M.E.; Yang, H.Y. Controllable Synthesis of Two-Dimensional Molybdenum Disulfide (MoS2) for Energy-Storage Applications. ChemSusChem 2020, 13, 1379–1391. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Pang, H.; Xue, H.; Yu, Y. MoS2 -Based Nanocomposites for Electrochemical Energy Storage. Adv. Sci. 2017, 4, 1600289. [Google Scholar] [CrossRef]
- Kamila, S.; Mohanty, B.; Samantara, A.K.; Guha, P.; Ghosh, A.; Jena, B.; Satyam, P.V.; Mishra, B.K.; Jena, B.K. Highly Active 2D Layered MoS2 -RGO Hybrids for Energy Conversion and Storage Applications. Sci. Rep. 2017, 7, 8378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, F.; Zhou, W.; Xu, Y.; Du, Y.; Guan, C.; Huang, W. Recent Developments of Advanced Micro-Supercapacitors: Design, Fabrication and Applications. npj Flex Electron. 2020, 4, 31. [Google Scholar] [CrossRef]
- Chaojian, H.; Bo, L.; Qingwei, L.; Lijun, Y.; Yang, W.; Zhan, Y.; Lixin, D. Plasmon-Enhanced Photovoltaic Characteristics of Black Phosphorus-MoS2 Heterojunction. IEEE Open J. Nanotechnol. 2021, 2, 41–51. [Google Scholar] [CrossRef]
- Arora, Y.; Shah, A.P.; Battu, S.; Maliakkal, C.B.; Haram, S.; Bhattacharya, A.; Khushalani, D. Nanostructured MoS2/BiVO4 Composites for Energy Storage Applications. Sci. Rep. 2016, 6, 36294. [Google Scholar] [CrossRef] [PubMed]
- Moutaouakil, A.E.; Kang, H.-C.; Handa, H.; Fukidome, H.; Suemitsu, T.; Sano, E.; Suemitsu, M.; Otsuji, T. Room Temperature Logic Inverter on Epitaxial Graphene-on-Silicon Device. Jpn. J. Appl. Phys. 2011, 50, 070113. [Google Scholar] [CrossRef] [Green Version]
- Moutaouakil, A.E. Two-Dimensional Electronic Materials for Terahertz Applications: Linking the Physical Properties with Engineering Expertise. In Proceedings of the 2018 6th International Renewable and Sustainable Energy Conference (IRSEC), Rabat, Morocco, 5–8 December 2018; IEEE: Rabat, Morocco, 2018; pp. 1–4. [Google Scholar]
- Moutaouakil, A.E.; Watanabe, T.; Haibo, C.; Komori, T.; Nishimura, T.; Suemitsu, T.; Otsuji, T. Spectral Narrowing of Terahertz Emission from Super-Grating Dual-Gate Plasmon-Resonant High-Electron Mobility Transistors. J. Phys. Conf. Ser. 2009, 193, 012068. [Google Scholar] [CrossRef]
- Moutaouakil, A.E.; Suemitsu, T.; Otsuji, T.; Coquillat, D.; Knap, W. Room Temperature Terahertz Detection in High-Electron-Mobility Transistor Structure Using InAlAs/InGaAs/InP Material Systems. In Proceedings of the 35th International Conference on Infrared, Millimeter, and Terahertz Waves, Rome, Italy, 5–10 September 2010; IEEE: Rome, Italy, 2010; pp. 1–2. [Google Scholar]
- Moutaouakil, A.E.; Komori, T.; Horiike, K.; Suemitsu, T.; Otsuji, T. Room Temperature Intense Terahertz Emission from a Dual Grating Gate Plasmon-Resonant Emitter Using InAlAs/InGaAs/InP Material Systems. IEICE Trans. Electron. 2010, E93.C, 1286–1289. [Google Scholar] [CrossRef]
- El Moutaouakil, A.; Suemitsu, T.; Otsuji, T.; Videlier, H.; Boubanga-Tombet, S.-A.; Coquillat, D.; Knap, W. Device Loading Effect on Nonresonant Detection of Terahertz Radiation in Dual Grating Gate Plasmon-Resonant Structure Using InGaP/InGaAs/GaAs Material Systems. Phys. Status Solidi C 2011, 8, 346–348. [Google Scholar] [CrossRef]
- Tiouitchi, G.; Ali, M.A.; Benyoussef, A.; Hamedoun, M.; Lachgar, A.; Kara, A.; Ennaoui, A.; Mahmoud, A.; Boschini, F.; Oughaddou, H.; et al. Efficient Production of Few-Layer Black Phosphorus by Liquid-Phase Exfoliation. R. Soc. Open Sci. 2020, 7, 201210. [Google Scholar] [CrossRef] [PubMed]
- Moutaouakil, A.E.; Fukidome, H.; Otsuji, T. Investigation of Terahertz Properties in Graphene Ribbons. In Proceedings of the 2020 45th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Buffalo, NY, USA, 8–13 November 2020; IEEE: Buffalo, NY, USA, 2020; pp. 1–2. [Google Scholar]
- Moutaouakil, A.E.; Suemitsu, T.; Otsuji, T.; Coquillat, D.; Knap, W. Nonresonant Detection of Terahertz Radiation in High-Electron-Mobility Transistor Structure Using InAlAs/InGaAs/InP Material Systems at Room Temperature. J. Nanosci. Nanotechnol. 2012, 12, 6737–6740. [Google Scholar] [CrossRef]

| Synthesis Technique | Specifications | Application | References |
|---|---|---|---|
| Solvothermal | Vertical 1T-MoS2 nanosheets interlayer spacing = 9.8 Å | Lithium-ion battery Capacity = 666 mA h g−1 at current density = 3500 mA g−1 | [61] |
| Solvothermal | 1D metallic MoS2 nanotube | Lithium-ion battery Capacity = 1100 mA h g−1 at current density = 5000 mA g−1 and capacity = 589 mA h g−1 at a high current density = 20,000 mA g−1 | [62] |
| Solvothermal | 1T-MoS2 nanosheet arrays | Lithium-ion battery Reversible specific capacity of 1789 mA h g−1 at 0.1 A g−1 and a retained capacity of 853 mA h g−1 after 140 cycles at 1 A g−1 | [63] |
| Solvothermal | 1T-MoS2 | HER Low potential of 203 mV at 10 mA cm−2, Tafel slope = 60 mV dec−1 | [64] |
| Solvothermal | STable 1T-MoS2 slabs grown on CdS nanorods 1T-MoS2@CdS | Photocatalytic HER 39 times better photocatalytic activity when compared to bare CdS | [65] |
| Hydrothermal | Stabilized 1T-MoS2 layers Mo–Mo bond length = 2.72 Å | Hydrogen evolution 21 times higher than pure CdS and 3 times higher than annealed CdS: 2H-MoS2 | [66] |
| Hydrothermal | Metallic MoS2 nanopetals | (HER) Overpotential = 210 mV at current density = 10 mA cm−2 and a Tafel slope of 44 mV dec−1 | [67] |
| Hydrothermal | Pure and stable metallic MoS2 nanosheets | HER Current density of 10 mA cm−2 Overpotential = 175 mV Tafel slope = 41 mV dec−1 | [68] |
| Hydrothermal followed by solvothermal method | Both 1T and 2H phases | HER Overpotential = 180 mV Tafel slope = 88 mV dec−1 | [69] |
| Battery Type | MoS2 Phase | Structure | Capacity | References |
|---|---|---|---|---|
| Lithium-ion | 1T (Metallic) | Nanotube-like MoS2 over graphene | Discharge capacity = 666 mA h g−1 at current density = 3500 mA g−1 | [61] |
| Lithium-ion | 1T (Metallic) | MoS2 over carbon cloth | Reversible specific capacity = 1789 mA h g−1 at 0.1 Ag−1 Retained capacity = 853 mA h g−1 after 140 cycles at 1 Ag−1 | [63] |
| Lithium-ion | 1T (Metallic) | 1T MoS2 + (NiMoO4) | Charged mass capacity = 940.1 mA h g−1 Discharged mass capacity = 941.6 mA h g−1 | [74] |
| Lithium-ion | 1T (Metallic) | Pure MoS2 | Specific capacity ≈ 935 mA h g−1 for 200 cycles at 5 A g−1 can be increased to 1150 mA h g−1 | [62] |
| Sodium-ion | 1T (Metallic) | MoS2-graphene-MoS2 | Capacity of 175 mA h g−1 at a high current density of 2 A g−1 Reverse capacity of ≈313 mA h g−1 at low current density of 50 mA g−1. Stabilizes at current density = 313 mA h g−1 after 200 cycles | [43] |
| Sodium-ion | 2H and 1T MoS2 | Dual phase of 2H and 1T MoS2 | Capacity = 300 mA h g−1 after 200 cycles, and coulombic efficiency = 99% | [75] |
| Sodium-ion | 2H phase transfers to 1T through chemical reactions | MoS2 and amorphous carbon (C) | Capacity = 563.5 mA h g−1 at 0.2 A g−1 Coulombic efficiency = 86.6% Cyclic stability = 484.9 mA h g−1 at 2 A g−1 | [76] |
| Supercapacitor | 2D MoS2 | Spraying MoS2 nanosheets on Si/SiO2 | Area capacitance = 8 mF cm−2, and volumetric capacitance = 178 F cm−3 | [77] |
| Supercapacitor | Nanoflower-like MoS2 structure | 3D-graphene/MoS2 nanohybrid | Dimensions 23.6 × 22.4 × 0.6 mm3 Specific capacitance (Csp) = 58 F g−1, energy density of 24.59 W h Kg−1, and power density of 8.8 W Kg−1 with operating window of 2.7 V (−1.5 to +1.2 V) | [78] |
| Supercapacitor | Brush-like arrangement MoS2 | MoS2 nanowires over Ni foam | The high mass loading of MoS2 (30 mg cm−2) retains 92% of maximum capacitance after 9000 charge–discharge cycles at 5 A g−1 | [79] |
| Supercapacitor | MoS2 QSs | Exfoliated MoS2 QSs lateral size (5–10 nm) | Capacitance = 162 F g−1 Energy density = 14.4 W h kg−1 | [80] |
| Hybrid Supercapacitor | N-3DG and 3D-IEMoS2@G | Prepared using solvothermal process | Energy density = 140 W h kg−1 at 630 W kg−1, and 43 W h kg−1 at power density of 103 kW kg−1 Lifecycle over 10,000 | [81] |
| Type of Reaction | Catalyst Used | Specification | References |
|---|---|---|---|
| HER | (MoS2/CoSe2) | Tafel slope = 36 mV dec−1 Onset potential = −11 mV Exchange current density = 7.3 × 10−2 mA cm−2 | [95] |
| HER | 1T MoS2 | Overpotential = 156 mV, at 10 mA cm−2 Tafel slope = 42.7 mV dec−1 | [96] |
| HER/OER | Amorphous Ni–Co complexes hybridized with 1T MoS2 | Overpotentials = 70 mV HER and 235 mV for OER at 10 mA cm−2 Tafel slope = 38.1 to 45.7 mV dec−1 | [97] |
| OER | Rhombohedral MoS2 microspheres over conductive Ni | Overpotential ≈ 310 mV Tafel slope ≈ 105 mV dec−1 | [98] |
| OER | MoS2 quantum dots (MSQDs) | Overpotential = 280 mV Tafel slope = 39 mV dec−1 | [99] |
| CO2 reduction | Vertically aligned MoS2 nanoflakes (2H and 1T phases coexist) | Overpotential = 54 mV Reduction current density = 130 mA cm−2 at −0.764 V | [100] |
| CO2 reduction | p–n junction Bi2S3/MoS2 composite | Photocatalytic CO2 reduction 20 times higher than single catalysts under visible light irradiation | [101] |
| CO2 reduction | 3R MoS2 nanoflower powder | Synthesized using CVD CO production < 0.01 μmol-gcat−1 hr−1 at 25 °C which is negligible | [102] |
| Structure | Role of MoS2 | Enhanced Property | References |
|---|---|---|---|
| Graphene-MoS2 Wedge-shaped microcavity | Enhance the cell performance | Enhance the light absorbance to above 90% | [110] |
| (Hg0.33 Cd0.66 Te) and monolayer MoS2 | Enhance the cell performance | Shift the cell absorbance to visible light range | [112] |
| Microdome texture on MoS2 thin film | Enhance the cell performance | Decreases reflections and traps light for incident angles (0–50) | [113] |
| MoS2/GaAs over boron nitride | Enhance the cell performance | PCE increased to 9.03% | [114] |
| MoS2 spray coating over perovskite cells | HTL | PCE = 3.9% | [118] |
| 5 monolayer MoS2 nanosheets onto indium tin oxide ITO substrate | ETL | Jsc = 16.24 mA cm−2 Voc = 0.56 V (fill-factor) FF = 0.37 PCE = 3.36% | [121] |
| ZnO-MoS2-CZTS | Buffer | Jsc = 29.42 mA cm−2 Voc = 1.01 V FF = 0.574 Efficiency = 17.03% | [115] |
| Organometallic-halide perovskite solar cell | Buffer | Jsc ≈ 22 mA cm−2 Voc ≈ 0.96 V FF ≈ 0.6 PCE = 14.9% | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samy, O.; El Moutaouakil, A. A Review on MoS2 Energy Applications: Recent Developments and Challenges. Energies 2021, 14, 4586. https://doi.org/10.3390/en14154586
Samy O, El Moutaouakil A. A Review on MoS2 Energy Applications: Recent Developments and Challenges. Energies. 2021; 14(15):4586. https://doi.org/10.3390/en14154586
Chicago/Turabian StyleSamy, Omnia, and Amine El Moutaouakil. 2021. "A Review on MoS2 Energy Applications: Recent Developments and Challenges" Energies 14, no. 15: 4586. https://doi.org/10.3390/en14154586
APA StyleSamy, O., & El Moutaouakil, A. (2021). A Review on MoS2 Energy Applications: Recent Developments and Challenges. Energies, 14(15), 4586. https://doi.org/10.3390/en14154586







