Effects of Ambient Pressure on Burning Characteristics of Gasoline: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Environmental Parameters
3.2. HRR
3.3. Flame Height
3.4. Smoke Release Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.N.; Wu, X.Q.; Park, Y.G.; Zhang, T.H.; Huang, X.Y.; Xiao, F.; Usmani, A. Perspectives of big experimental database and artificial intelligence in tunnel fire research. Tunn. Undergr. Space Technol. 2021, 108, 25. [Google Scholar] [CrossRef]
- Lang, Z.H.; Liu, H.; Meng, N.; Wang, H.N.; Wang, H.; Kong, F.Y. Mapping the knowledge domains of research on fire safety—An informetrics analysis. Tunn. Undergr. Space Technol. 2021, 108, 18. [Google Scholar] [CrossRef]
- Himoto, K. Conceptual framework for quantifying fire resilience? A new perspective on fire safety performance of buildings. Fire Saf. J. 2021, 120, 8. [Google Scholar] [CrossRef]
- Yan, G.F.; Wang, M.N.; Yu, L.; Tian, Y.; Guo, X.H. Study of smoke movement characteristics in tunnel fires in high-altitude areas. Fire Mater. 2020, 44, 65–75. [Google Scholar] [CrossRef]
- Shah, H.R.; Wang, K.; Lang, X.Q.; Wang, J.W.; Wang, J.J.; Fang, J.; Zhang, Y.M.; Aldossary, O.M. Comprehensive Assimilation of Fire Suppression Modeling and Simulation of Radiant Fire by Water and Its Synergistic Effects with Carbon Dioxide. Energies 2020, 13, 5850. [Google Scholar] [CrossRef]
- Bae, S.; Choi, J.H.; Ryou, H.S. Modification of Interaction Forces between Smoke and Evacuees. Energies 2020, 13, 4177. [Google Scholar] [CrossRef]
- Zimny, M.; Antosiewicz, P.; Krajewski, G.; Burdzy, T.; Krasuski, A.; Wegrzynski, W. Several Problems with Froude-Number Based Scale Modeling of Fires in Small Compartments. Energies 2019, 12, 3625. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Zuo, C.; Xiong, Y.Y.; Wang, K.H.; Shi, J.K.; Chen, Z.N.; Lu, X.X. An experimental study of the self-extinction mechanism of fire in tunnels. Tunn. Undergr. Space Technol. 2021, 109, 16. [Google Scholar] [CrossRef]
- Wu, E.; Huang, R.; Wu, L.; Shen, X.; Li, Z.Y. Numerical Study on the Influence of Altitude on Roof Temperature in Mine Fires. IEEE Access 2020, 8, 102855–102866. [Google Scholar] [CrossRef]
- Wang, X.Y.; Fleischmann, C.; Spearpoint, M. Applying the FDS pyrolysis model to predict heat release rate in small-scale forced ventilation tunnel experiments. Fire Saf. J. 2020, 112, 12. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Wang, H.L.; Bi, H.Q.; Liu, X.X.; Gou, Q.L. Heat release rate of high-speed train fire in railway tunnels. Tunn. Undergr. Space Technol. 2020, 105, 13. [Google Scholar] [CrossRef]
- Shi, C.L.; Zhong, M.H.; Chen, C.K.; Jiao, W.B.; Li, J.; Zhang, Y.L.; Zhang, L.; Li, Y.; He, L. Metro train carriage combustion behaviors—Full-scale experiment study. Tunn. Undergr. Space Technol. 2020, 104, 12. [Google Scholar] [CrossRef]
- Wang, T.Y.; Tsai, K.C. Effects of Time to Unactuate Air Conditioning on Fire Growth. Energies 2021, 14, 3100. [Google Scholar] [CrossRef]
- Chen, L.; Tang, F.; Pang, H.P. Ceiling heat flux and downward received radiation heat flux induced by weak and relative strong fire plume in ventilation tunnels. Appl. Therm. Eng. 2020, 169, 8. [Google Scholar] [CrossRef]
- Carvel, R.O.; Beard, A.N.; Jowitt, P.W. The influence of longitudinal ventilation systems on fires in tunnels. Tunn. Undergr. Space Technol. 2001, 16, 3–21. [Google Scholar] [CrossRef]
- Ingason, H.; Li, Y.Z. Model scale tunnel fire tests with longitudinal ventilation. Fire Saf. J. 2010, 45, 371–384. [Google Scholar] [CrossRef]
- Li, H.H.; Liu, H.Q.; Liu, J.H.; Ge, J.W.; Tang, F. Spread and burning characteristics of continuous spill fires in a tunnel. Tunn. Undergr. Space Technol. 2021, 109, 7. [Google Scholar] [CrossRef]
- Yun, H.S.; Nam, D.G.; Hwang, C.H. An Experimental Study on the Fire Spread Rate and Separation Distance between Facing Stores in Passage-Type Traditional Markets. Energies 2020, 13, 4458. [Google Scholar] [CrossRef]
- Craig, M.; Asim, T. Numerical Investigations on the Propagation of Fire in a Railway Carriage. Energies 2020, 13, 4999. [Google Scholar] [CrossRef]
- Carvel, R.O.; Beard, A.N.; Jowitt, P.W.; Drysdale, D.D. The influence of tunnel geometry and ventilation on the heat release rate of a fire. Fire Technol. 2004, 40, 5–26. [Google Scholar] [CrossRef]
- Park, Y.; Ryu, J.; Ryou, H.S. Experimental Study on the Fire-Spreading Characteristics and Heat Release Rates of Burning Vehicles Using a Large-Scale Calorimeter. Energies 2019, 12, 1465. [Google Scholar] [CrossRef] [Green Version]
- Carvel, R.O.; Beard, A.N.; Jowitt, P.W. A Bayesian estimation of the effect of forced ventilation on a pool fire in a tunnel. Civ. Eng. Environ. Syst. 2001, 18, 279–302. [Google Scholar] [CrossRef]
- Cheng, H.; Hadjisophocleous, G.V. The modeling of fire spread in buildings by Bayesian network. Fire Saf. J. 2009, 44, 901–908. [Google Scholar] [CrossRef]
- Cheng, H.; Hadjisophocleous, G.V. Dynamic modeling of fire spread in building. Fire Saf. J. 2011, 46, 211–224. [Google Scholar] [CrossRef]
- Zhang, S.G.; Cheng, X.D.; Yao, Y.Z.; Zhu, K.; Li, K.Y.; Lu, S.; Zhang, R.F.; Zhang, H.P. An experimental investigation on blockage effect of metro train on the smoke back-layering in subway tunnel fires. Appl. Therm. Eng. 2016, 99, 214–223. [Google Scholar] [CrossRef]
- Yao, Y.Z.; Li, Y.Z.; Lonnermark, A.; Ingason, H.; Cheng, X.D. Study of tunnel fires during construction using a model scale tunnel. Tunn. Undergr. Space Technol. 2019, 89, 50–67. [Google Scholar] [CrossRef]
- Yao, Y.Z.; Li, Y.Z.; Ingason, H.; Cheng, X.D. The characteristics of under-ventilated pool fires in both model and medium-scale tunnels. Tunn. Undergr. Space Technol. 2019, 87, 27–40. [Google Scholar] [CrossRef]
- Yao, Y.Z.; Li, Y.Z.; Ingason, H.; Cheng, X.D. Scale effect of mass loss rates for pool fires in an open environment and in tunnels with wind. Fire Saf. J. 2019, 105, 41–50. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, H.C. A water requirements estimation model for fire suppression: A study based on integrated uncertainty analysis. Fire Technol. 2005, 41, 5–24. [Google Scholar] [CrossRef]
- Kobes, M.; Helsloot, I.; de Vries, B.; Post, J.G. Building safety and human behaviour in fire: A literature review. Fire Saf. J. 2010, 45, 1–11. [Google Scholar] [CrossRef]
- Wong, L.T.; Lau, S.W. A fire safety evaluation system for prioritizing fire improvements in old high-rise buildings in Hong Kong. Fire Technol. 2007, 43, 233–249. [Google Scholar] [CrossRef]
- Tomar, M.S.; Khurana, S. Impact of passive fire protection on heat release rates in road tunnel fire: A review. Tunn. Undergr. Space Technol. 2019, 85, 149–159. [Google Scholar] [CrossRef]
- Seike, M.; Kawabata, N.; Hasegawa, M.; Tanaka, H. Heat release rate and thermal fume behavior estimation of fuel cell vehicles in tunnel fires. Int. J. Hydrogen Energy 2019, 44, 26597–26608. [Google Scholar] [CrossRef]
- Fernandez-Alaiz, F.; Castanon, A.M.; Gomez-Fernandez, F.; Bernardo-Sanchez, A.; Bascompta, M. Determination and Fire Analysis of Gob Characteristics Using CFD. Energies 2020, 13, 5274. [Google Scholar] [CrossRef]
- Seike, M.; Kawabata, N.; Hasegawa, M.; Kobayashi, T. The retarding effect of fixed barriers on smoke propagation in tunnel fires. Tunn. Undergr. Space Technol. 2019, 85, 100–113. [Google Scholar] [CrossRef]
- Ma, G.F.; Wu, Z.J. BIM-based building fire emergency management: Combining building users’ behavior decisions. Autom. Constr. 2020, 109, 16. [Google Scholar] [CrossRef]
- Xu, Z.S.; Zhao, J.M.; Liu, Q.L.; Chen, H.G.; Liu, Y.H.; Geng, Z.Y.; He, L. Experimental investigation on smoke spread characteristics and smoke layer height in tunnels. Fire Mater. 2019, 43, 303–309. [Google Scholar] [CrossRef]
- Ye, K.; Zhou, X.D.; Zheng, Y.; Liu, H.; Tang, X.; Cao, B.; Huang, Y.B.; Chen, Y.Q.; Yang, L.Z. Estimating the longitudinal maximum gas temperature attenuation of ceiling jet flows generated by strong fire plumes in an urban utility tunnel. Int. J. Therm. Sci. 2019, 142, 434–448. [Google Scholar] [CrossRef]
- Ye, K.; Zhou, X.D.; Yang, L.Z.; Tang, X.; Zheng, Y.; Cao, B.; Peng, Y.; Liu, H.; Ni, Y. A Multi-Scale Analysis of the Fire Problems in an Urban Utility Tunnel. Energies 2019, 12, 1976. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.H.; Xiao, J.Z.; Gardoni, P.; Hu, K.X. Probabilistic Analysis of Building Fire Severity Based on Fire Load Density Models. Fire Technol. 2019, 55, 1349–1375. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yan, Z.G.; Zhu, H.H.; Shen, Y.; Guo, Q.H.; Guo, Q.C. Experimental investigation of pedestrian evacuation using an extra-long steep-slope evacuation path in a high altitude tunnel fire. Sust. Cities Soc. 2019, 46, 14. [Google Scholar] [CrossRef]
- Wang, M.N.; Yan, G.F.; Yu, L.; Xie, W.Q.; Dai, Y. Effects of different artificial oxygen-supply systems on migrants’ physical and psychological reactions in high-altitude tunnel construction. Build. Environ. 2019, 149, 458–467. [Google Scholar] [CrossRef]
- Khieu, H.T.; Lee, Y.M.; Kim, J.T.; Ryou, H.S. Numerical Study of the Effects of the Jet Fan Speed, Heat Release Rate and Aspect Ratio on Smoke Movement in Tunnel Fires. Energies 2020, 13, 1206. [Google Scholar] [CrossRef] [Green Version]
- Gomez, R.S.; Porto, T.R.N.; Magalhaes, H.L.F.; Santos, A.C.Q.; Viana, V.H.V.; Gomes, K.C.; Lima, A.G.B. Thermo-Fluid Dynamics Analysis of Fire Smoke Dispersion and Control Strategy in Buildings. Energies 2020, 13, 6000. [Google Scholar] [CrossRef]
- Li, C.; Yang, R.; Yao, Y.; Tao, Z.X.; Zhu, P.; Zhang, H. Effects of static pressure, pressurization, and depressurization on n-heptane pool fires in an airplane cargo compartment. Fire Mater. 2019, 43, 266–276. [Google Scholar] [CrossRef]
- Yan, Z.-G.; Guo, Q.-H.; Zhu, H.-H. Full-scale experiments on fire characteristics of road tunnel at high altitude. Tunn. Undergr. Space Technol. 2017, 66, 134–146. [Google Scholar] [CrossRef]
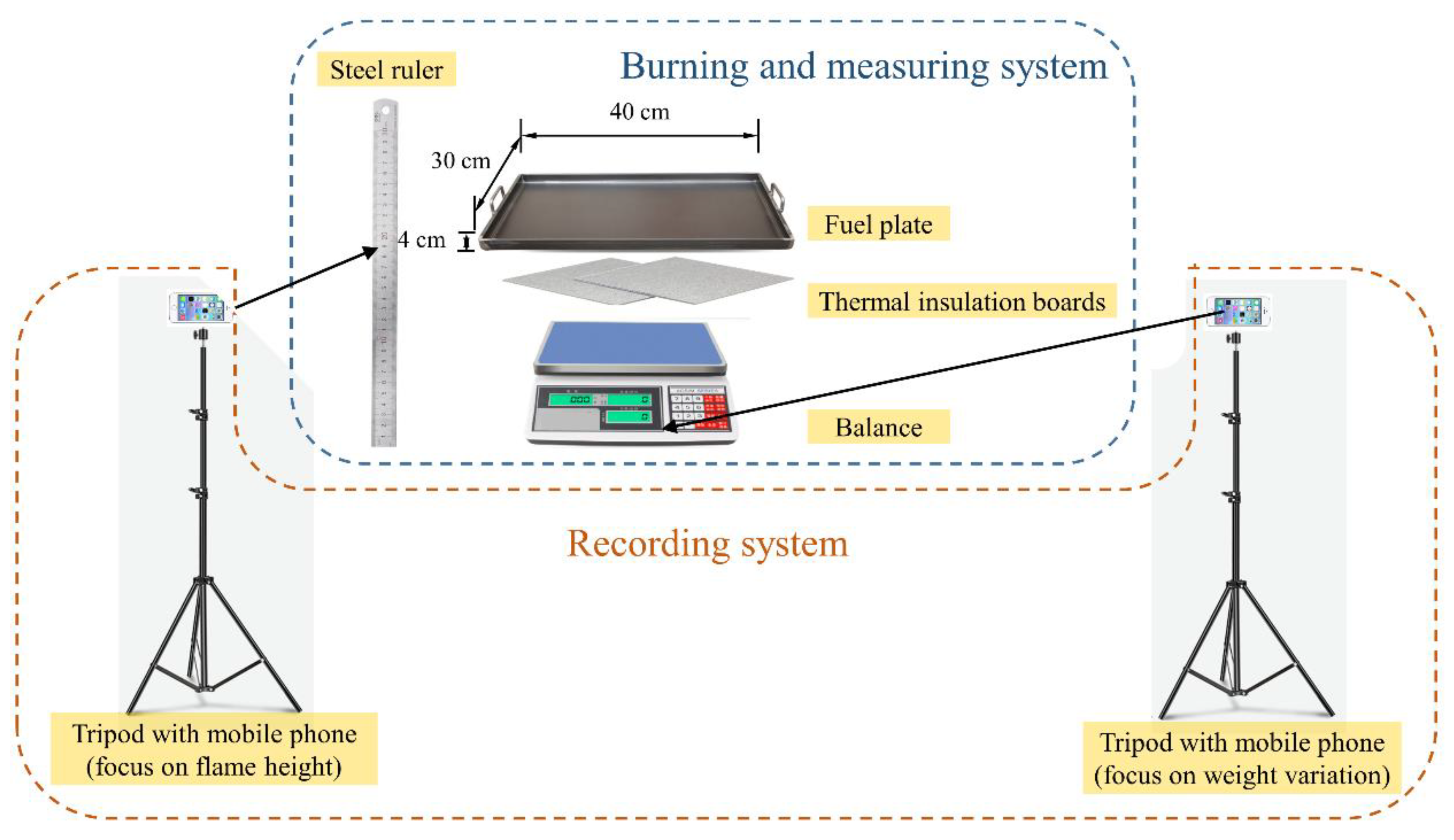






| Sites | Altitude (m) |
|---|---|
| Chengdu, Sichuan | 500 |
| Kunming, Yunnan | 1900 |
| Lhasa, Tibet | 4150 |
| Environmental Parameters | Device Type | Working Method | Range | Resolution |
|---|---|---|---|---|
| Ambient pressure | DYM3 | Elastic displacement method | 50–110 kPa | 0.01 kPa |
| Temperature | GM1362 | Temperature sensor | −30–70 °C | 0.1 °C |
| Humidity | GM8901 | Humidity sensor | 10–95% | 1% |
| Oxygen volume percentage | BHe4S | Electrochemistry | 0–25% | 0.1% |
| Experimental Sites | Altitude (m) | Ambient Pressure (kPa) | Temperature (°C) | Humidity (%) | Oxygen Volume Percentage (%) |
|---|---|---|---|---|---|
| Chengdu, Sichuan | 500 | 96.7 | 24.5 | 81.3 | 20.9 |
| Kunming, Yunnan | 1950 | 81.1 | 24.3 | 78.3 | 20.9 |
| Lhasa, Tibet | 4150 | 61.6 | 22.4 | 26.4 | 20.5 |
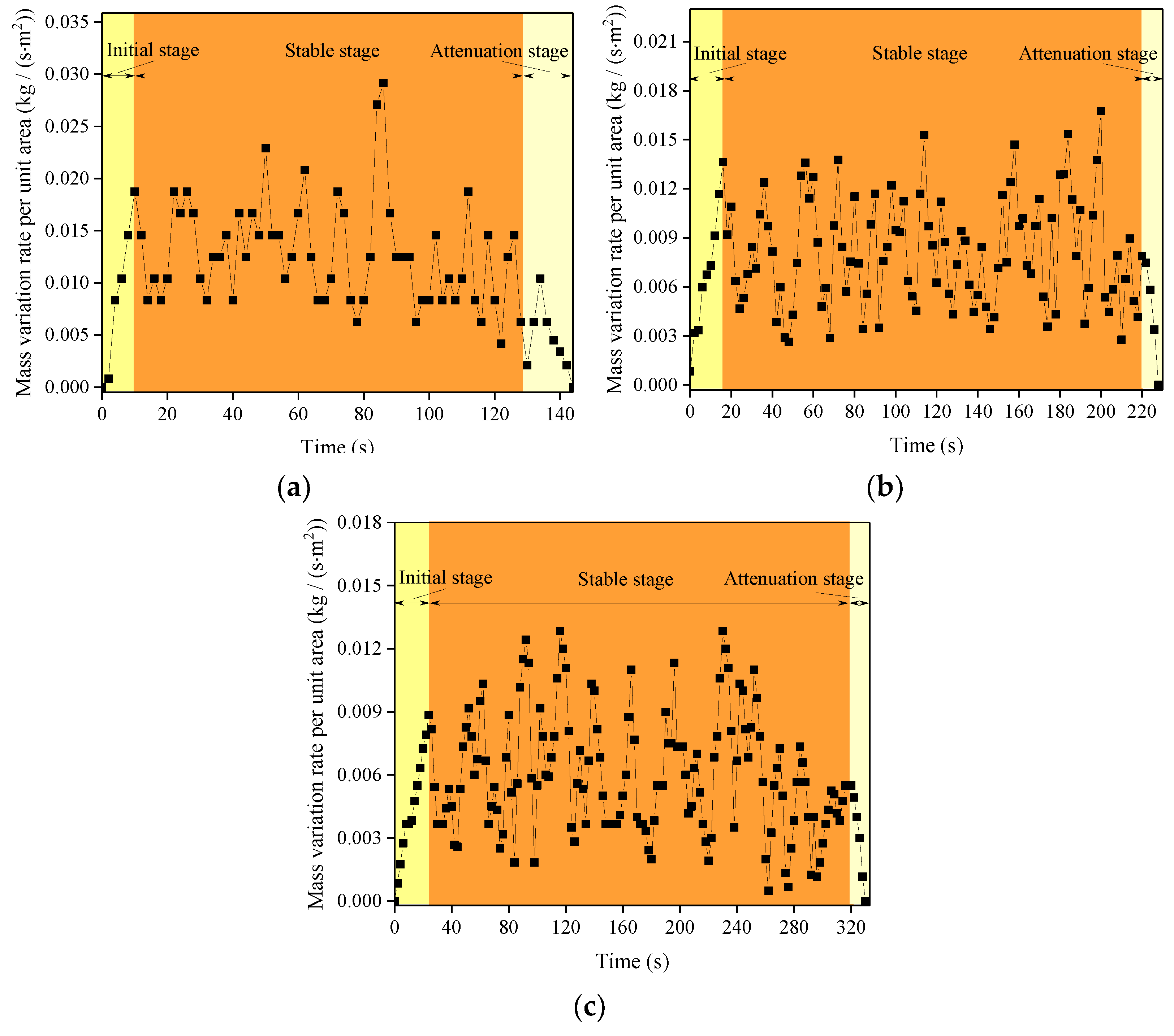
| Experimental Sites | Initial Stage (s) | Stable Stage (s) | Attenuation Stage (s) |
|---|---|---|---|
| Chengdu, Sichuan | 11 | 126 | 7 |
| Kunming, Yunnan | 17 | 204 | 11 |
| Lhasa, Tibet | 25 | 288 | 15 |
| Experimental Sites | Ambient Pressure (kPa) | Mass Loss Rate Per Unit Area (kg/(m2 s)) | HRR (kW) |
|---|---|---|---|
| Chengdu, Sichuan | 96.7 | 0.0125 | 68 |
| Kunming, Yunnan | 81.1 | 0.0090 | 49 |
| Lhasa, Tibet | 61.6 | 0.0063 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, P.; Wang, M.; Chen, Z.; Yan, G.; Yan, T.; Han, C.; Wang, A. Effects of Ambient Pressure on Burning Characteristics of Gasoline: A Pilot Study. Energies 2021, 14, 4627. https://doi.org/10.3390/en14154627
Qin P, Wang M, Chen Z, Yan G, Yan T, Han C, Wang A. Effects of Ambient Pressure on Burning Characteristics of Gasoline: A Pilot Study. Energies. 2021; 14(15):4627. https://doi.org/10.3390/en14154627
Chicago/Turabian StyleQin, Pengcheng, Mingnian Wang, Zhanwen Chen, Guanfeng Yan, Tao Yan, Changling Han, and Anmin Wang. 2021. "Effects of Ambient Pressure on Burning Characteristics of Gasoline: A Pilot Study" Energies 14, no. 15: 4627. https://doi.org/10.3390/en14154627
APA StyleQin, P., Wang, M., Chen, Z., Yan, G., Yan, T., Han, C., & Wang, A. (2021). Effects of Ambient Pressure on Burning Characteristics of Gasoline: A Pilot Study. Energies, 14(15), 4627. https://doi.org/10.3390/en14154627





