Characterization of Potential-Induced Degradation and Recovery in CIGS Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of CIGS Solar Cells
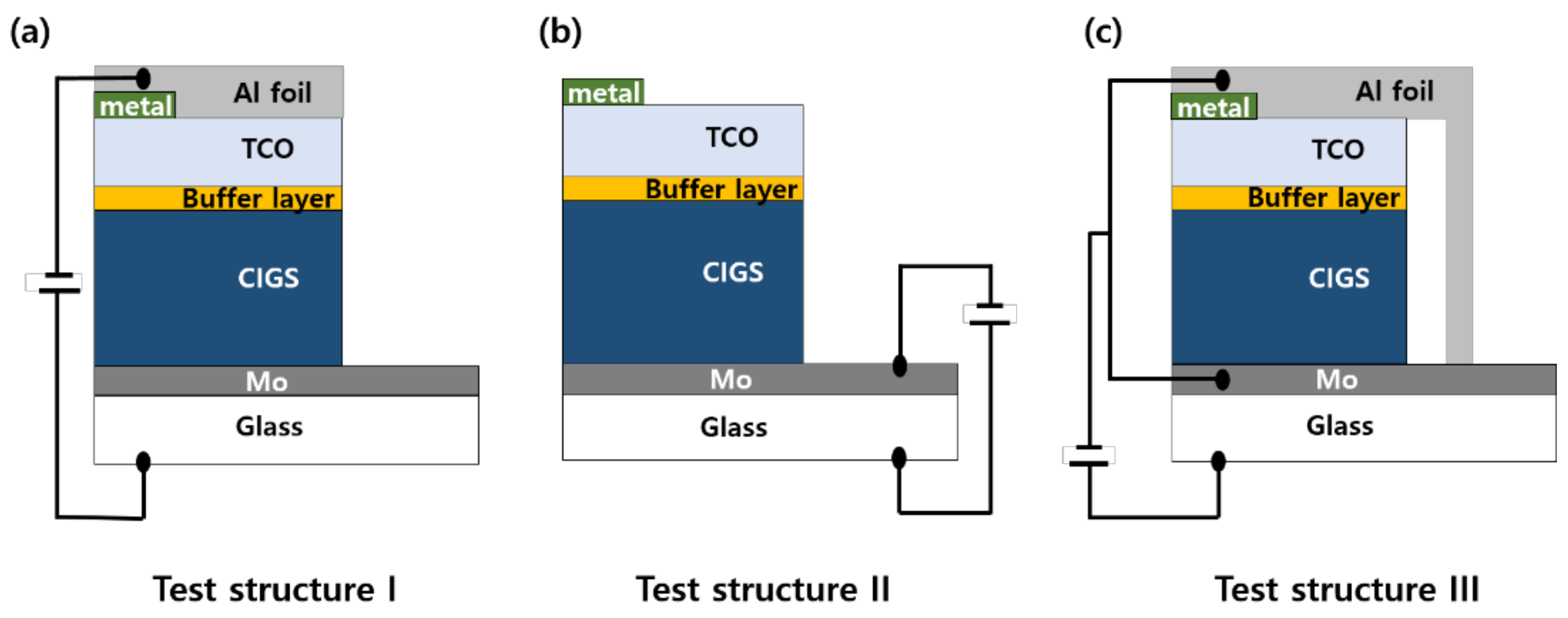
2.2. Design of the Voltage Application Structure for Potential-Induced Degradation
2.3. Potential-Induced Degradation and Recovery
2.4. Characterization
3. Results
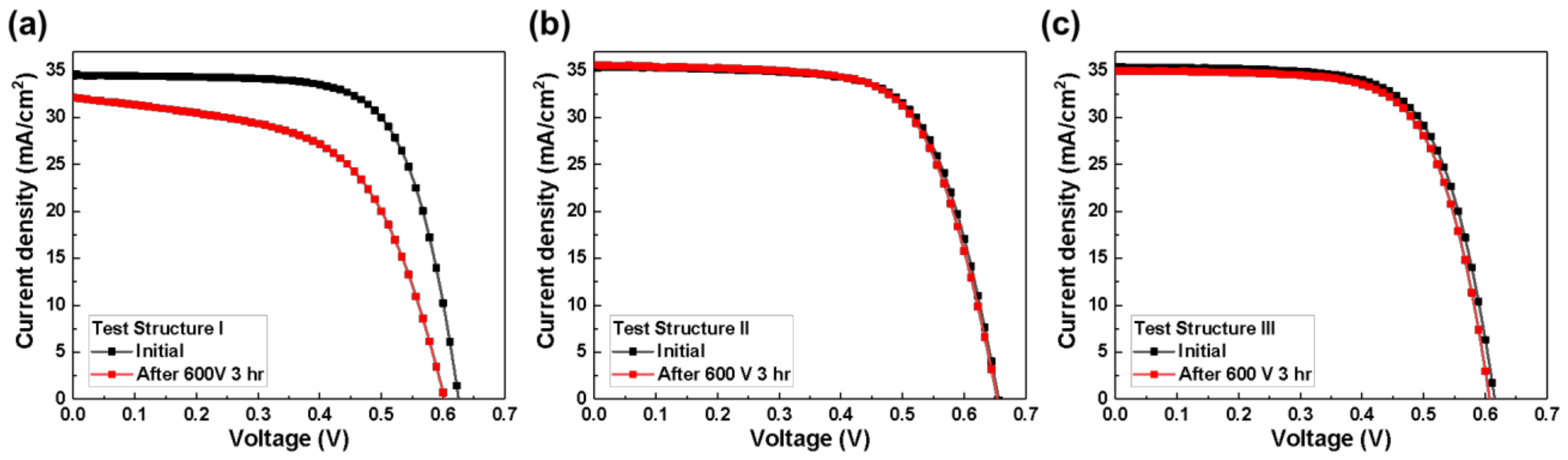
3.1. Design of the Voltage Application Structure for the PID Experiment by Short-Term Biasing Test
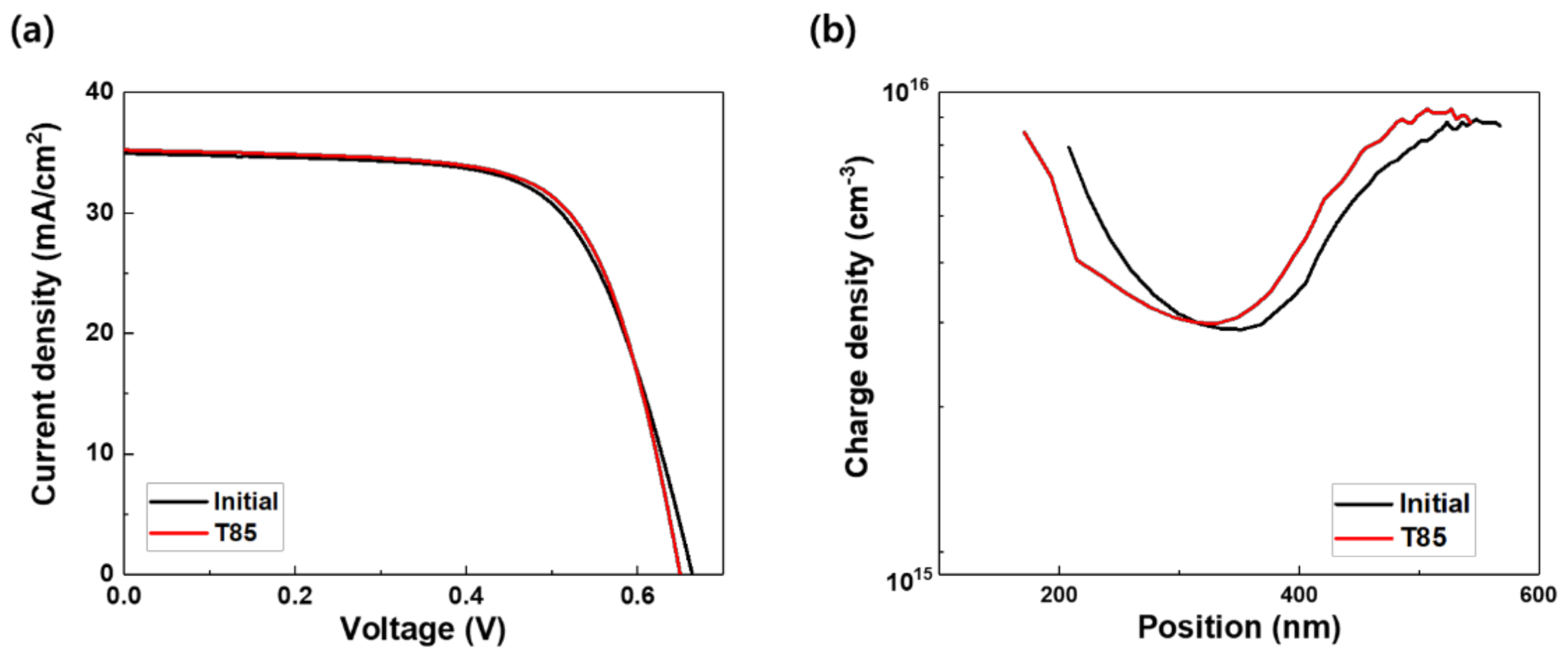
3.2. Potential-Induced Degradation and Recovery
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, G.; Al-Jassim, M.M.; Metzger, W.; Glunz, S.W.; Verlinden, P.; Gang, X.; Mansfield, L.; Stanbery, B.J.; Zhu, K.; Yan, Y. The 2020 photovoltaic technologies roadmap. J. Phys. D Appl. Phys. 2020, 53, 493001. [Google Scholar] [CrossRef]
- Köntges, M.; Kurtz, S.; Packard, C.; Jahn, U.; Berger, K.A.; Kato, K. Performance and Reliability of Photovoltaic Systems: Subtask 3.2: Review of Failures of Photovoltaic Modules: IEA PVPS Task 13: External Final Report IEA-PVPS; International Energy Agency, Photovoltaic Power Systems Programme: Paris, France, 2014. [Google Scholar]
- Schmidt, J.; Werner, F.; Veith, B.; Zielke, D.; Bock, R.; Tiba, V.; Poodt, P.; Roozeboom, F.; Li, A.; Cuevas, A. Industrially Relevant Al2O3 Deposition Techniques for the Surface Passivation of Si Solar Cells. In Proceedings of the 25th European Photovoltaic Solar Energy Conference, Valencia, Spain, 6–10 September 2010. [Google Scholar]
- Pingel, S.; Frank, O.; Winkler, M.; Daryan, S.; Geipel, T.; Hoehne, H.; Berghold, J. Potential induced degradation of solar cells and panels. In Proceedings of the 2010 35th IEEE Photovoltaic Specialists Conference, Honolulu, HI, USA, 20–25 June 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 2817–2822. [Google Scholar]
- Berghold, J.; Koch, S.; Pingel, S.; Janke, S.; Ukar, A.; Grunow, P.; Shioda, T. PID: From material properties to outdoor performance and quality control counter measures. In Reliability of Photovoltaic Cells, Modules, Components, and Systems VIII; International Society for Optics and Photonics: Bellingham, WA, USA, 2015; p. 95630A. [Google Scholar]
- Bauer, J.; Naumann, V.; Großer, S.; Hagendorf, C.; Schütze, M.; Breitenstein, O. On the mechanism of potential-induced degradation in crystalline silicon solar cells. Phys. Status Solidi (RRL) Rapid Res. Lett. 2012, 6, 331–333. [Google Scholar] [CrossRef]
- Naumann, V.; Lausch, D.; Großer, S.; Werner, M.; Swatek, S.; Hagendorf, C.; Bagdahn, J. Microstructural Analysis of Crystal Defects Leading to Potential-Induced Degradation (PID) of Si Solar Cells. Energy Procedia 2013, 33, 76–83. [Google Scholar] [CrossRef]
- Lausch, D.; Naumann, V.; Graff, A.; Hähnel, A.; Breitenstein, O.; Hagendorf, C.; Bagdahn, J. Sodium Outdiffusion from Stacking Faults as Root Cause for the Recovery Process of Potential-induced Degradation (PID). Energy Procedia 2014, 55, 486–493. [Google Scholar] [CrossRef]
- Naumann, V.; Lausch, D.; Hähnel, A.; Bauer, J.; Breitenstein, O.; Graff, A.; Werner, M.; Swatek, S.; Großer, S.; Bagdahn, J. Explanation of potential-induced degradation of the shunting type by Na decoration of stacking faults in Si solar cells. Sol. Energy Mater. Sol. Cells 2014, 120, 383–389. [Google Scholar] [CrossRef]
- Bae, S.; Oh, W.; Lee, K.D.; Kim, S.; Kim, H.; Park, N.; Chan, S.I.; Park, S.; Kang, Y.; Lee, H.S. Potential induced degradation of n-type crystalline silicon solar cells with p+front junction. Energy Sci. Eng. 2017, 5, 30–37. [Google Scholar] [CrossRef]
- Hara, K.; Ogawa, K.; Okabayashi, Y.; Matsuzaki, H.; Masuda, A. Influence of surface structure of n-type single-crystalline Si solar cells on potential-induced degradation. Sol. Energy Mater. Sol. Cells 2017, 166, 132–139. [Google Scholar] [CrossRef]
- Kronik, L.; Cahen, D.; Schock, H.W. Effects of sodium on polycrystalline Cu (In, Ga) Se2 and its solar cell performance. Adv. Mater. 1998, 10, 31–36. [Google Scholar] [CrossRef]
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of heavy alkali elements in Cu(In,Ga)Se 2 solar cells with efficiencies up to 22.6%. Phys. Status Solidi (RRL) Rapid Res. Lett. 2016, 10, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Malitckaya, M.; Komsa, H.-P.; Havu, V.; Puska, M. Effect of Alkali Metal Atom Doping on the CuInSe2-Based Solar Cell Absorber. J. Phys. Chem. C 2017, 121, 15516–15528. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, P.; Bissig, B.; Pianezzi, F.; Avancini, E.; Hagendorfer, H.; Keller, D.; Fuchs, P.; Döbeli, M.; Vigo, C.; Crivelli, P. Features of KF and NaF Postdeposition Treatments of Cu(In,Ga)Se2 Absorbers for High Efficiency Thin Film Solar Cells. Chem. Mater. 2015, 27, 5755–5764. [Google Scholar] [CrossRef]
- Fjällström, V.; Salomé, P.; Hultqvist, A.; Edoff, M.; Jarmar, T.; Aitken, B.; Zhang, K.; Fuller, K.; Williams, C.K. Potential-Induced Degradation of CuIn1−xGax Se2 Thin Film Solar Cells. IEEE J. Photovolt. 2013, 3, 1090–1094. [Google Scholar] [CrossRef]
- Fjällström, V.; Szaniawski, P.; Vermang, B.; Salome, P.M.; Rostvall, F.; Zimmermann, U.; Edoff, M. Recovery After Potential-Induced Degradation of CuIn1−xGaxSe2 Solar Cells with CdS and Zn (O, S) Buffer Layers. IEEE J. Photovolt. 2015, 5, 664–669. [Google Scholar] [CrossRef]
- Alonso-Garcia, M.C.; Hacke, P.; Glynn, S.; Muzzillo, C.P.; Mansfield, L.M. Analysis of Potential-Induced Degradation in Soda-Lime Glass and Borosilicate-Glass Cu(In,Ga)Se2 Samples. IEEE J. Photovolt. 2018, 9, 331–338. [Google Scholar] [CrossRef]
- Muzzillo, C.P.; Glynn, S.; Hacke, P.; Moutinho, H.R.; Young, M.R.; Teeter, G.; Repins, I.L.; Mansfield, L.M. Potential-Induced Degradation of Cu (In, Ga) Se 2 Solar Cells: Alkali Metal Drift and Diffusion Effects. IEEE J. Photovolt. 2018, 8, 1337–1342. [Google Scholar] [CrossRef]
- Harvey, S.P.; Guthrey, H.; Muzzillo, C.P.; Teeter, G.; Mansfield, L.; Hacke, P.; Johnston, S.; Al-Jassim, M. Investigating PID Shunting in Polycrystalline CIGS Devices via Multi-Scale, Multi-Technique Characterization. IEEE J. Photovolt. 2019, 9, 559–564. [Google Scholar] [CrossRef]
- IEC TS 62804–1: 2015. Photovoltaic (PV) Modules-Test Methods for the Detection of Potential-Induced Degradation-Part 1; IEC: London, UK, 2015. [Google Scholar]
- Boulhidja, S.; Mellit, A.; Voswinckel, S. Potential-induced degradation test on CIGS photovoltaic modules. In Proceedings of the 2017 5th International Conference on Electrical Engineering-Boumerdes (ICEE-B), Boumerdes, Algeria, 29–31 October 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–4. [Google Scholar]
- Boulhidja, S.; Mellit, A.; Voswinckel, S.; Lughi, V.; Ciocia, A.; Spertino, F.; Massi Pavan, A. Experimental Evidence of PID Effect on CIGS Photovoltaic Modules. Energies 2020, 13, 537. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, K.; Tomita, H.; Schmitz, D.; Tokuda, S.; Ogawa, K.; Shibata, H.; Masuda, A. Accelerated Outdoor PID Testing of CIGS Modules and Comparison with Indoor PID Tests. In Proceedings of the 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa, HI, USA, 10–15 June 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1320–1323. [Google Scholar]
- Sakurai, K.; Tomita, H.; Tokuda, S.; Schmitz, D.; Ogawa, K.; Shibata, H.; Masuda, A. Rapid Recovery of CIGS Solar Cells from PID Stress with Light Soaking. In Proceedings of the 2019 IEEE 46th Photovoltaic Specialists Conference (PVSC), Chicago, IL, USA, 16–21 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2000–2002. [Google Scholar]
- Voswinckel, S.; Mikolajick, T.; Wesselak, V. Influence of the active leakage current pathway on the potential induced degradation of CIGS thin film solar modules. Sol. Energy 2020, 197, 455–461. [Google Scholar] [CrossRef]
- Voswinckel, S.; Wesselak, V.; Münter, S.; Gerstenberg, L. Correlation of Leakage Current Pathways and Potential Induced Degradation of CIGS Thin Film Solar Modules. In Proceedings of the ISES Solar World Conference 2017 and the IEA SHC Solar Heating and Cooling Conference for Buildings and Industry, Abu Dhabi, United Arab Emirates, 29 October–2 November 2017. [Google Scholar]
- Contreras, M.A.; Hacke, P.; Repins, I. Development of Cu (In, Ga) Se2 test coupons for potential induced degradation studies. In Proceedings of the 2016 IEEE 43rd Photovoltaic Specialists Conference (PVSC), Portland, OR, USA, 5–10 June 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 857–862. [Google Scholar]
- Salomon, O.; Hempel, W.; Kiowski, O.; Lotter, E.; Witte, W.; Ferati, A.; Schneikart, A.; Kaune, G.; Schäffler, R.; Becker, M. Influence of Molybdenum Back Contact on the PID Effect for Cu(In,Ga)Se2 Solar Cells. Coatings 2019, 9, 794. [Google Scholar] [CrossRef] [Green Version]
- Muzzillo, C.P.; Glynn, S.; Hacke, P.; Moutinho, H.R.; Young, M.R.; Repins, I.L.; Mansfield, L.M. Potential-Induced Degradation Depends on Leakage Current and Light/Electrical Bias in Cu(In,Ga)Se2 Devices. IEEE J. Photovolt. 2019, 9, 1852–1856. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Jonai, S.; Hara, K.; Komaki, H.; Shimizu-Kamikawa, Y.; Shibata, H.; Niki, S.; Kawakami, Y.; Masuda, A. Potential-induced degradation of Cu (In, Ga) Se2 photovoltaic modules. Jpn. J. Appl. Phys. 2015, 54, 08KC13. [Google Scholar] [CrossRef]
- Fjällström, V. Potential-Induced Degradation and Possibilities for Recovery of CuIn1−xGaxSe2 Thin Film Solar Cells. Ph.D. Thesis, Uppsala Universitet, Uppsala, Sweden, 2015. [Google Scholar]
- Mo, C.B.; Park, S.J.; Bae, S.; Lim, M.-H.; Nam, J.; Kim, D.; Yang, J.; Suh, D.; Min, B.K.; Kim, D. Impact of buffer layer process and Na on shunt paths of monolithic series-connected CIGSSe thin film solar cells. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Green, M.A. Solar cells: Operating principles, technology, and system applications. Englewood Cliffs 1982, 5, 85–102. [Google Scholar]
- Osterwald, C.; McMahon, T.; del Cueto, J. Electrochemical corrosion of SnO2:F transparent conducting layers in thin-film photovoltaic modules. Sol. Energy Mater. Sol. Cells 2003, 79, 21–33. [Google Scholar] [CrossRef]
- McMahon, T. Accelerated testing and failure of thin-film PV modules. Prog. Photovolt. Res. Appl. 2004, 12, 235–248. [Google Scholar] [CrossRef]
- Kosyachenko, L.; Savchuk, A.; Grushko, E. Dependence of efficiency of thin-film CdS/CdTe solar cell on parameters of absorber layer and barrier structure. Thin Solid Films 2009, 517, 2386–2391. [Google Scholar] [CrossRef]
- Breitenstein, O.; Altermatt, P.; Ramspeck, K.; Green, M.; Zhao, J.; Schenk, A. Interpretation of the commonly observed IV characteristics of c-Si cells having ideality factor larger than two. In Proceedings of the 2006 IEEE 4th World Conference on Photovoltaic Energy Conference, Waikoloa, HI, USA, 7–12 May 2006; IEEE: Piscataway, NJ, USA, 2006; pp. 879–884. [Google Scholar]
- Contreras, M.A.; Tuttle, J.; Gabor, A.; Tennant, A.; Ramanathan, K.; Asher, S.; Franz, A.; Keane, J.; Wang, L.; Scofield, J. High efficiency Cu (In, Ga) Se/sub 2/-based solar cells: Processing of novel absorber structures. In Proceedings of the 1994 IEEE 1st World Conference on Photovoltaic Energy Conversion-WCPEC (A Joint Conference of PVSC, PVSEC and PSEC), Waikoloa, HI, USA, 5–9 December 1994; IEEE: Piscataway, NJ, USA, 1994; pp. 68–75. [Google Scholar]
- Wang, H.; Zhang, Y.; Kou, X.; Cai, Y.; Liu, W.; Yu, T.; Pang, J.; Li, C.; Sun, Y. Effect of substrate temperature on the structural and electrical properties of CIGS films based on the one-stage co-evaporation process. Semicond. Sci. Technol. 2010, 25, 055007. [Google Scholar] [CrossRef]
- Lindahl, J.; Zimmermann, U.; Szaniawski, P.; Törndahl, T.; Hultqvist, A.; Salomé, P.; Platzer-Björkman, C.; Edoff, M. Inline Cu (In, Ga) Se2 Co-evaporation for high-efficiency solar cells and modules. IEEE J. Photovolt. 2013, 3, 1100–1105. [Google Scholar] [CrossRef]
- Rudmann, D.; Brémaud, D.; da Cunha, A.; Bilger, G.; Strohm, A.; Kaelin, M.; Zogg, H.; Tiwari, A. Sodium incorporation strategies for CIGS growth at different temperatures. Thin Solid Films 2005, 480, 55–60. [Google Scholar] [CrossRef]
- Caballero, R.; Kaufmann, C.; Eisenbarth, T.; Cancela, M.; Hesse, R.; Unold, T.; Eicke, A.; Klenk, R.; Schock, H. The influence of Na on low temperature growth of CIGS thin film solar cells on polyimide substrates. Thin Solid Films 2009, 517, 2187–2190. [Google Scholar] [CrossRef]
- Pianezzi, F.; Reinhard, P.; Chirilă, A.; Bissig, B.; Nishiwaki, S.; Buecheler, S.; Tiwari, A.N. Unveiling the effects of post-deposition treatment with different alkaline elements on the electronic properties of CIGS thin film solar cells. Phys. Chem. Chem. Phys. 2014, 16, 8843–8851. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, J.; Ye, Q.; Luo, K.; Jiang, Y.; Liao, C.; Ouyang, L.; Zhuang, D.; Mei, J.; Lau, W. The role of Na incorporation in the low-temperature processed CIGS thin film solar cells using post deposition treatment. J. Alloys Compd. 2016, 658, 12–18. [Google Scholar] [CrossRef]





| Test Structure I | Test Structure II | Test Structure III | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Unit | Initial | After PID | Initial | After PID | Initial | After PID |
| Eff | % | 15.1 | 11.1 | 15.8 | 15.6 | 14.9 | 14.5 |
| JSC | mA/cm2 | 34.5 | 32.1 | 35.4 | 35.6 | 35.4 | 35.0 |
| VOC | mV | 625 | 603 | 656 | 654 | 615 | 607 |
| FF | % | 69.8 | 57.5 | 68.0 | 67.1 | 68.2 | 68.2 |
| RS | Ohm | 5.9 | 9.9 | 3.0 | 3.2 | 6.1 | 5.6 |
| Rsh | Ohm | 3120 | 347 | 671 | 672 | 2320 | 2650 |
| Unit | Initial | PID | Recovery 12 Days | |
|---|---|---|---|---|
| Eff | % | 15.4 | 9.3 | 14.7 |
| JSC | mA/cm2 | 34.6 | 35.2 | 35.2 |
| VOC | mV | 656 | 487 | 618 |
| FF | % | 67.9 | 54.0 | 67.6 |
| Unit | Initial | PID | Recovery 12 Days | ||
|---|---|---|---|---|---|
| Rs | Light I-V | Ohm | 2.9 | 3.8 | 2.6 |
| Sun-shaded | Ohm | 2.1 | 3.2 | 1.7 | |
| Rsh | Light I-V | Ohm | 538 | 201 | 460 |
| Sun-shaded | Ohm | 796 | 279 | 550 | |
| n-factor | - | 1.4 | 1.7 | 1.6 | |
| Unit | Initial | PID | Recovery 12 Days | Δ PID | Δ Recovery | |
|---|---|---|---|---|---|---|
| FFideal | % | 83.8 | 80.0 | 83.1 | −4.8 | +3.8 |
| FFn | % | 79.2 | 71.7 | 76.9 | −10.4 | +6.8 |
| FFs | % | 70.0 | 54.1 | 69.1 | −29.4 | +21.8 |
| FFsh | % | 77.6 | 68.9 | 74.9 | −12.6 | +8.0 |
| FFcal | % | 68.7 | 52.5 | 67.5 | −30.9 | +22.2 |
| FFlight I–V | % | 67.9 | 54.0 | 67.6 | −25.9 | +20.2 |
| Unit | Initial | PID | Recovery 12 Days | |
|---|---|---|---|---|
| J01 | mA/cm2 | 6.9 × 10−4 | 3.1 × 10−3 | 1.1 × 10−3 |
| J02 | mA/cm2 | 0.165 | 0.106 | 0.197 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Bae, S.; Park, S.J.; Gwak, J.; Yun, J.; Kang, Y.; Kim, D.; Eo, Y.-J.; Lee, H.-S. Characterization of Potential-Induced Degradation and Recovery in CIGS Solar Cells. Energies 2021, 14, 4628. https://doi.org/10.3390/en14154628
Lee S, Bae S, Park SJ, Gwak J, Yun J, Kang Y, Kim D, Eo Y-J, Lee H-S. Characterization of Potential-Induced Degradation and Recovery in CIGS Solar Cells. Energies. 2021; 14(15):4628. https://doi.org/10.3390/en14154628
Chicago/Turabian StyleLee, Solhee, Soohyun Bae, Se Jin Park, Jihye Gwak, JaeHo Yun, Yoonmook Kang, Donghwan Kim, Young-Joo Eo, and Hae-Seok Lee. 2021. "Characterization of Potential-Induced Degradation and Recovery in CIGS Solar Cells" Energies 14, no. 15: 4628. https://doi.org/10.3390/en14154628
APA StyleLee, S., Bae, S., Park, S. J., Gwak, J., Yun, J., Kang, Y., Kim, D., Eo, Y.-J., & Lee, H.-S. (2021). Characterization of Potential-Induced Degradation and Recovery in CIGS Solar Cells. Energies, 14(15), 4628. https://doi.org/10.3390/en14154628








