Hydrocarbon Toxicity towards Hydrogenotrophic Methanogens in Oily Waste Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Toxicity Assays with Pure Methanogenic Cultures and Hexadecane
2.3. Toxicity Assessment with Granular Sludge and a Real Complex Mixture of Hydrocarbons
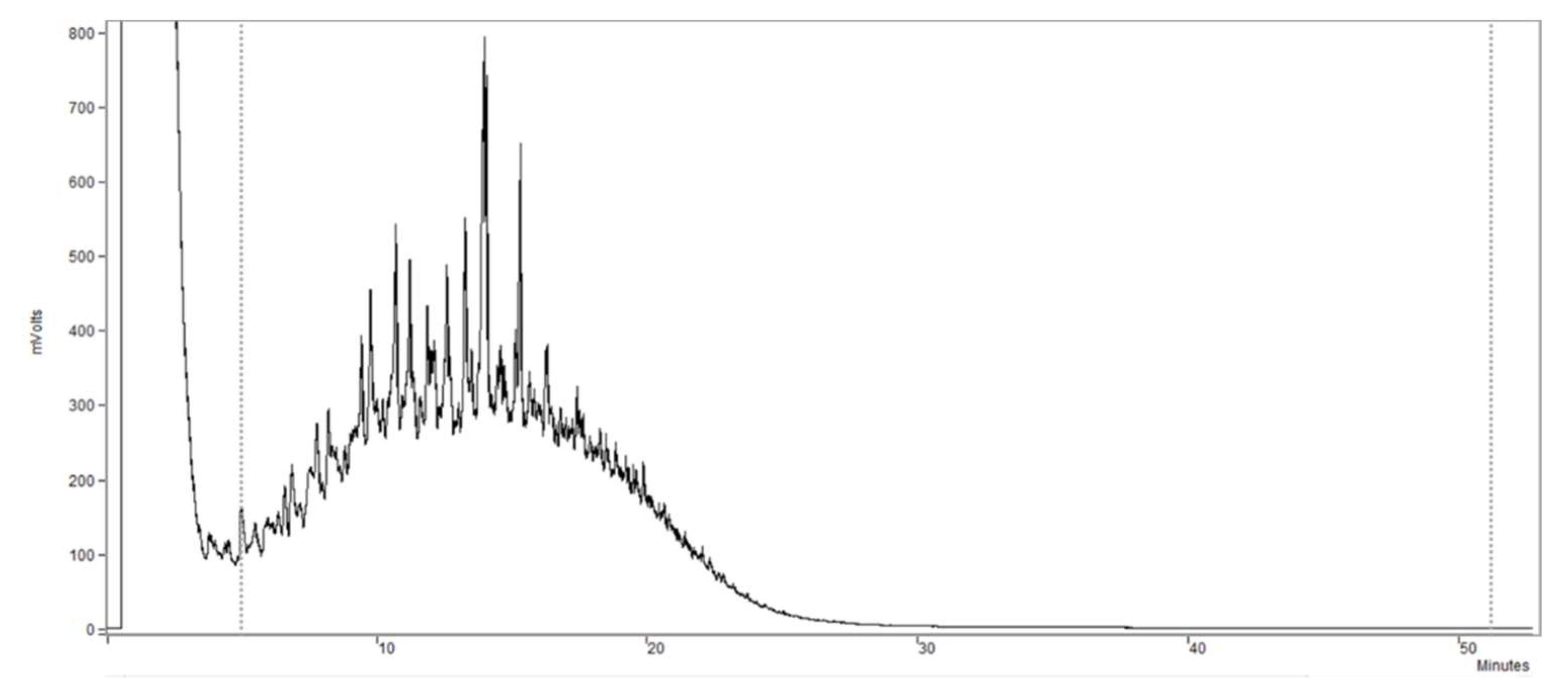
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Toxicity Assays with Pure Methanogenic Cultures and Hexadecane
3.2. Toxicity Assessment with Granular Sludge and a Real Complex Mixture of Hydrocarbons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fortune Global 500. Available online: https://fortune.com/global500/ (accessed on 24 May 2021).
- Hentati, O.; Lachhab, R.; Ayadi, M.; Ksibi, M. Toxicity assessment for petroleum-contaminated soil using terrestrial invertebrates and plant bioassays. Environ. Monit. Assess. 2013, 185, 2989–2998. [Google Scholar] [CrossRef]
- Meslé, M.; Dromart, G.; Oger, P. Microbial methanogenesis in subsurface oil and coal. Res. Microbiol. 2013, 164, 959–972. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, B.; Thanyamanta, W.; Hawboldt, K.; Zhang, B.; Liu, B. Offshore produced water management: A review of current practice and challenges in harsh/Arctic environments. Mar. Pollut. Bull. 2016, 104, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Krivoruchko, A.V.; Ivshina, I.B. Advanced bioreactor treatments of hydrocarbon-containing wastewater. Appl. Sci. 2020, 10, 831. [Google Scholar] [CrossRef] [Green Version]
- Zengler, K.; Richnow, H.H.; Rosselló-Mora, R.; Michaelis, W.; Widdel, F. Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 1999, 401, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Fedorak, P.M.; Foght, J.M. Biodegradation of short-chain n-alkanes in oil sands tailings under methanogenic conditions. Environ. Sci. Technol. 2006, 40, 5459–5464. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Gao, C.-X.; Mbadinga, S.M.; Zhou, L.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Characterization of an alkane-degrading methanogenic enrichment culture from production water of an oil reservoir after 274 days of incubation. Int. Biodeterior. Biodegrad. 2011, 65, 444–450. [Google Scholar] [CrossRef]
- Grishchenkov, V.G.; Townsend, R.T.; McDonald, T.J.; Autenrieth, R.L.; Bonner, J.S.; Boronin, A.M. Degradation of petroleum hydrocarbons by facultative anaerobic bacteria under aerobic and anaerobic conditions. Process Biochem. 2000, 35, 889–896. [Google Scholar] [CrossRef]
- Townsend, G.T.; Prince, R.C.; Suflita, J.M. Anaerobic oxidation of crude oil hydrocarbons by the resident microorganisms of a contaminated anoxic aquifer. Environ. Sci. Technol. 2003, 37, 5213–5218. [Google Scholar] [CrossRef]
- Abbasian, F.; Lockington, R.; Mallavarapu, M.; Naidu, R. A Comprehensive review of aliphatic hydrocarbon biodegradation by bacteria. Appl. Biochem. Biotechnol. 2015, 176, 670–699. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.; Richnow, H.H.; Vogt, C.; Treude, T.; Krüger, M. Methanogenic hydrocarbon degradation: Evidence from field and laboratory studies. Microb. Physiol. 2016, 26, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Dolfing, J.; Larter, S.R.; Head, I.M. Thermodynamic constraints on methanogenic crude oil biodegradation. ISME J. 2008, 2, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gieg, L.M.; Fowler, S.J.; Berdugo-Clavijo, C. Syntrophic biodegradation of hydrocarbon contaminants. Curr. Opin. Biotechnol. 2014, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.E.L.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev. 2013, 37, 384–406. [Google Scholar] [CrossRef] [PubMed]
- Sieber, J.R.; McInerney, M.J.; Gunsalus, R.P. Genomic insights into syntrophy: The paradigm for anaerobic metabolic cooperation. Annu. Rev. Microbiol. 2012, 66, 429–452. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Sherry, A.; Grant, R.; Aitken, C.; Jones, M.; Head, I.; Gray, N. Volatile hydrocarbons inhibit methanogenic crude oil degradation. Front. Microbiol. 2014, 5, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, J.; Liu, X.; Hu, Z. Substrate interactions during anaerobic biodegradation of BTEX by the mixed cultures under nitrate reducing conditions. J. Hazard. Mater. 2008, 158, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Martinez, M.F.; Kelessidou, N.; Law, Z.; Gardiner, J.; Stephens, G. Effect of solvents on obligately anaerobic bacteria. Anaerobe 2008, 14, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Colleran, E.; Concannon, F.; Golden, T.; Geoghegan, F.; Crumlish, B.; Killilea, E.; Henry, M.; Coates, J. Use of methanogenic activity tests to characterize anaerobic sludges, screen for anaerobic biodegradability and determine toxicity thresholds against individual anaerobic trophic groups and species. Water Sci. Technol. 1992, 25, 31–40. [Google Scholar] [CrossRef]
- Pries, F.; van der Ploeg, J.R.; Dolfing, J.; Janssen, D.B. Degradation of halogenated aliphatic compounds: The role of adaptation. FEMS Microbiol. Rev. 1994, 15, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Rodríguez, N.; Amils, R. Effect of chlorinated aliphatic hydrocarbons on the acetoclastic methanogenic activity of granular sludge. Appl. Microbiol. Biotechnol. 1997, 47, 324–328. [Google Scholar] [CrossRef]
- Yu, Z.; Smith, G.B. Inhibition of methanogenesis by C1- and C2-polychlorinated aliphatic hydrocarbons. Environ. Toxicol. Chem. 2000, 19, 2212–2217. [Google Scholar] [CrossRef]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Weber, F.J.; de Bont, J.A.M. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta-Rev. Biomembr. 1996, 1286, 225–245. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Van Dijk, J.B.; Dijkema, C.; Plugge, C.M. Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl. Environ. Microbiol. 1993, 59, 1114–1119. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.M.; Mota Vieira, J.A.; Álvares Pereira, R.M.; Pereira, M.A.; Mota, M. Effect of lipids and oleic acid on biomass development in anaerobic fixed-bed reactors. Part I: Biofilm growth and activity. Water Res. 2001, 35, 255–263. [Google Scholar] [CrossRef] [Green Version]
- APHA; AWWA; WPCF. Standard Methods for the Examination of water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Siddique, T.; Rutherford, P.M.; Arocena, J.M.; Thring, R.W. A proposed method for rapid and economical extraction of petroleum hydrocarbons from contaminated soils. Can. J. Soil Sci. 2006, 86, 725–728. [Google Scholar] [CrossRef]
- Method 3510C: Separatory Funnel Liquid-Liquid Extraction. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3510c.pdf (accessed on 15 June 2021).
- Paulo, A.M.S.; Salvador, A.F.; Alves, J.I.; Castro, R.; Langenhoff, A.A.M.; Stams, A.J.M.; Cavaleiro, A.J. Enhancement of methane production from 1-hexadecene by additional electron donors. Microb. Biotechnol. 2017, 11, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, A.L.; Debenedetti, P.G.; Panagiotopoulos, A.Z. Solubility and molecular conformations of n-alkane chains in water. J. Phys. Chem. B 2009, 113, 6405–6414. [Google Scholar] [CrossRef]
- Pereda, S.; Awan, J.A.; Mohammadi, A.H.; Valtz, A.; Coquelet, C.; Brignole, E.A.; Richon, D. Solubility of hydrocarbons in water: Experimental measurements and modeling using a group contribution with association equation of state (GCA-EoS). Fluid Phase Equilib. 2009, 275, 52–59. [Google Scholar] [CrossRef]
- Vega, L.F.; Llovell, F.; Blas, F.J. Capturing the solubility minima of n-alkanes in water by Soft-SAFT. J. Phys. Chem. B 2009, 113, 7621–7630. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.-K.-H.; de Hemptinne, J.-C.; Lugo, R.; Ferrando, N.; Passarello, J.-P. Hydrogen solubility in hydrocarbon and oxygenated organic compounds. J. Chem. Eng. Data 2016, 61, 19–34. [Google Scholar] [CrossRef]
- Tolls, J.; van Dijk, J.; Verbruggen, E.J.M.; Hermens, J.L.M.; Loeprecht, B.; Schüürmann, G. Aqueous solubility—Molecular size relationships: A mechanistic case study using C10- to C19-alkanes. J. Phys. Chem. A 2002, 106, 2760–2765. [Google Scholar] [CrossRef]
- Coates, M.; Connell, D.W.; Barron, D.M. Aqueous solubility and octan-1-ol-water partition coefficients of aliphatic hydrocarbons. Environ. Sci. Technol. 1985, 19, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Pires, O.C.; Mota, M.; Alves, M.M. Anaerobic biodegradation of oleic and palmitic acids: Evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol. Bioeng. 2005, 92, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.M.; Pereira, M.A.; Sousa, D.Z.; Cavaleiro, A.J.; Picavet, M.; Smidt, H.; Stams, A.J. Waste lipids to energy: How to optimize methane production from long-chain fatty acids (LCFA). Microb. Biotechnol. 2009, 2, 538–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, Y.; Nishihara, M.; Morii, H.; Akagawa-Matsushita, M. Ether polar lipids of methanogenic bacteria: Structures, comparative aspects, and biosyntheses. Microbiol. Rev. 1993, 57, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Hanford, M.J.; Peeples, T.L. Archaeal tetraether lipids: Unique structures and applications. Appl. Biochem. Biotechnol. 2002, 97, 45–62. [Google Scholar] [CrossRef]
- Whitman, W.B.; Bowen, T.L.; Boone, D.R. The methanogenic bacteria. In The Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 3, pp. 165–207. [Google Scholar] [CrossRef]
- Klingl, A. S-layer and cytoplasmic membrane—Exceptions from the typical archaeal cell wall with a focus on double membranes. Front. Microbiol. 2014, 5, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, S.-V.; Meyer, B.H. The archaeal cell envelope. Nat. Rev. Microbiol. 2011, 9, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J.; Graham, L.L. Surface layers of bacteria. Microbiol. Rev. 1991, 55, 684–705. [Google Scholar] [CrossRef]



| Methanogen | Hexadecane (mM) | λ (d) | P (mM) | Rm (mM d−1) | R2 | Inhibition (%) |
|---|---|---|---|---|---|---|
| M. formicicum | 0 | 5.5 ± 0.1 | 17.8 ± 0.6 | 10.9 ± 1.0 a | 0.984 | - |
| 1 | 5.1 ± 0.1 | 16.7 ± 0.7 | 10.2 ± 0.6 a | 0.994 | 6 ± 1 | |
| 5 | 5.5 ± 0.1 | 16.5 ± 0.3 | 10.6 ± 0.6 a | 0.995 | 3 ± 0 | |
| 15 | 5.1 ± 0.1 | 17.3 ± 0.8 | 5.2 ± 0.3 b | 0.993 | 52 ± 6 | |
| 30 | 5.2 ± 0.1 | 17.2 ± 1.0 | 5.0 ± 0.3 b | 0.984 | 54 ± 6 | |
| M. hungatei | 0 | 1.7 ± 0.1 | 18.2 ± 0.3 | 11.1 ± 0.8 a | 0.992 | - |
| 1 | 2.2 ± 0.3 | 17.1 ± 0.7 | 12.5 ± 2.6 a | 0.979 | 0 | |
| 5 | 1.6 ± 0.1 | 17.1 ± 0.4 | 10.5 ± 1.1 a | 0.987 | 4 ± 1 | |
| 15 | 1.7 ± 0.1 | 16.9 ± 0.3 | 9.9 ± 0.7 b | 0.994 | 10 ± 1 | |
| 30 | 1.6 ± 0.1 | 17.3 ± 0.6 | 8.1 ± 0.8 b | 0.987 | 27 ± 3 |
| Methanogen | Hexadecane (mM) | H2 Consumed (mM) | Maximum Cumulative CH4 Production (mM) | CH4 Yield (%) |
|---|---|---|---|---|
| M. formicicum | 0 | 68 ± 2 | 17 ± 1 | 100 ± 11 |
| 1 | 66 ± 1 | 16 ± 0 | 96 ± 2 | |
| 5 | 65 ± 1 | 16 ± 0 | 99 ± 2 | |
| 15 | 65 ± 1 | 15 ± 0 | 94 ± 1 | |
| 30 | 64 ± 1 | 14 ± 0 | 91 ± 4 | |
| M. hungatei | 0 | 71 ± 4 | 18 ± 0 | 105 ± 3 |
| 1 | 67 ± 1 | 19 ± 0 | 117 ± 0 | |
| 5 | 67 ± 1 | 18 ± 0 | 110 ± 2 | |
| 15 | 67 ± 1 | 17 ± 0 | 103 ± 18 | |
| 30 | 67 ± 1 | 17 ± 0 | 101 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, B.P.; Martins, V.; Martins, G.; Castro, A.R.; Alves, M.M.; Pereira, M.A.; Cavaleiro, A.J. Hydrocarbon Toxicity towards Hydrogenotrophic Methanogens in Oily Waste Streams. Energies 2021, 14, 4830. https://doi.org/10.3390/en14164830
Morais BP, Martins V, Martins G, Castro AR, Alves MM, Pereira MA, Cavaleiro AJ. Hydrocarbon Toxicity towards Hydrogenotrophic Methanogens in Oily Waste Streams. Energies. 2021; 14(16):4830. https://doi.org/10.3390/en14164830
Chicago/Turabian StyleMorais, Bruno P., Valdo Martins, Gilberto Martins, Ana Rita Castro, Maria Madalena Alves, Maria Alcina Pereira, and Ana J. Cavaleiro. 2021. "Hydrocarbon Toxicity towards Hydrogenotrophic Methanogens in Oily Waste Streams" Energies 14, no. 16: 4830. https://doi.org/10.3390/en14164830
APA StyleMorais, B. P., Martins, V., Martins, G., Castro, A. R., Alves, M. M., Pereira, M. A., & Cavaleiro, A. J. (2021). Hydrocarbon Toxicity towards Hydrogenotrophic Methanogens in Oily Waste Streams. Energies, 14(16), 4830. https://doi.org/10.3390/en14164830







