Experimental Study on Heat Transfer and Adsorption Cooling Performance of MIL-101/Few Layer Graphene Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MIL-101/FLG Composites
2.2. Characterization Methods of MIL-101/FLG Composites
2.3. Measurement of Thermophysical Properties of MIL-101/FLG Composites
2.4. Determination of Adsorption and Desorption Performance of MIL-101/FLG Composite
2.5. Determination Method of Adsorption Cooling Performance of MIL-101/FLG-Water Working Pairs
3. Results and Discussion
3.1. Thermal Conductivity of MIL-101/FLG Composites
3.2. Adsorption and Desorption Performance of MIL-101/FLG Composite
3.3. Temperature Characteristic Curves of MIL-101/FLG Adsorber in Adsorption Cooling Process
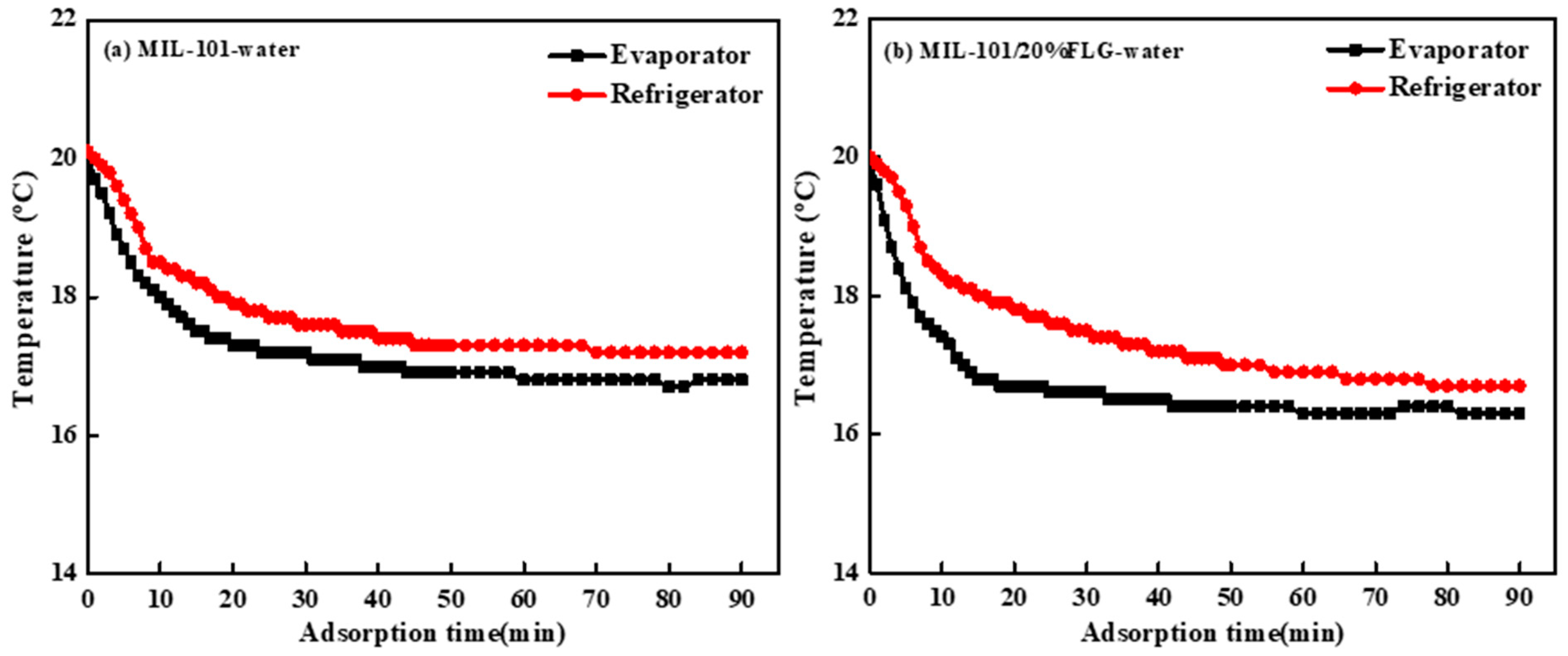
3.3.1. Temperature Characteristic Curves during Adsorption Process
3.3.2. Heating Curves in the Desorption Process
3.3.3. Cooling Rate
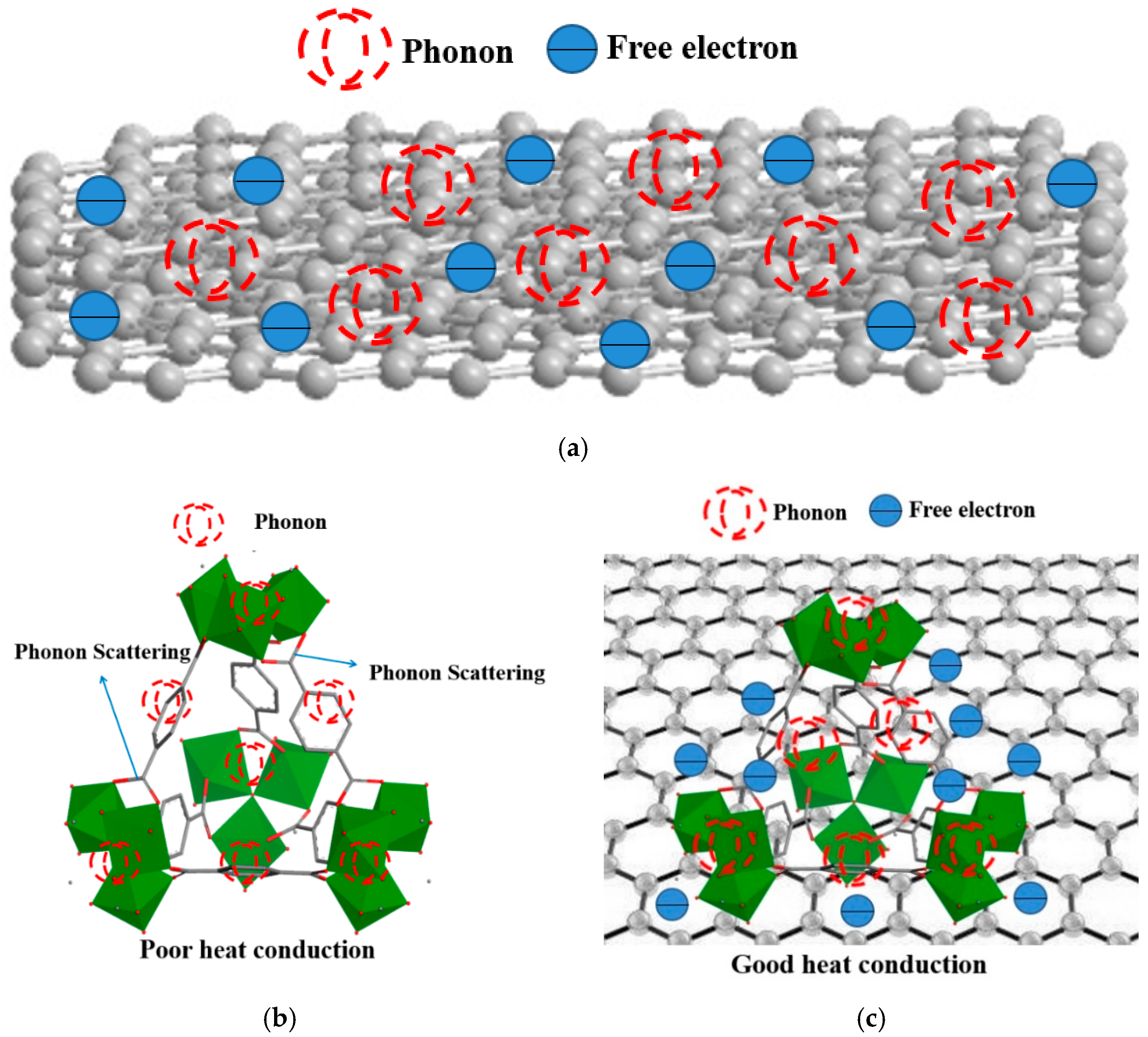
3.3.4. Heat Transfer Mechanism
3.4. Adsorption Cooling Performance of MIL-101/20%FLG-Water Working Pair
3.4.1. Working Condition Parameters
Evaporation Temperature
Adsorption Time
Desorption Time
3.4.2. Adsorption Cooling Performance Comparison of MIL-101/20%FLG-Water and MIL-101-Water Working Pairs
3.5. Adsorption Cooling Cyclic Stability and Characterization of MIL-101/20%FLG Composite
3.5.1. Cyclic Stability
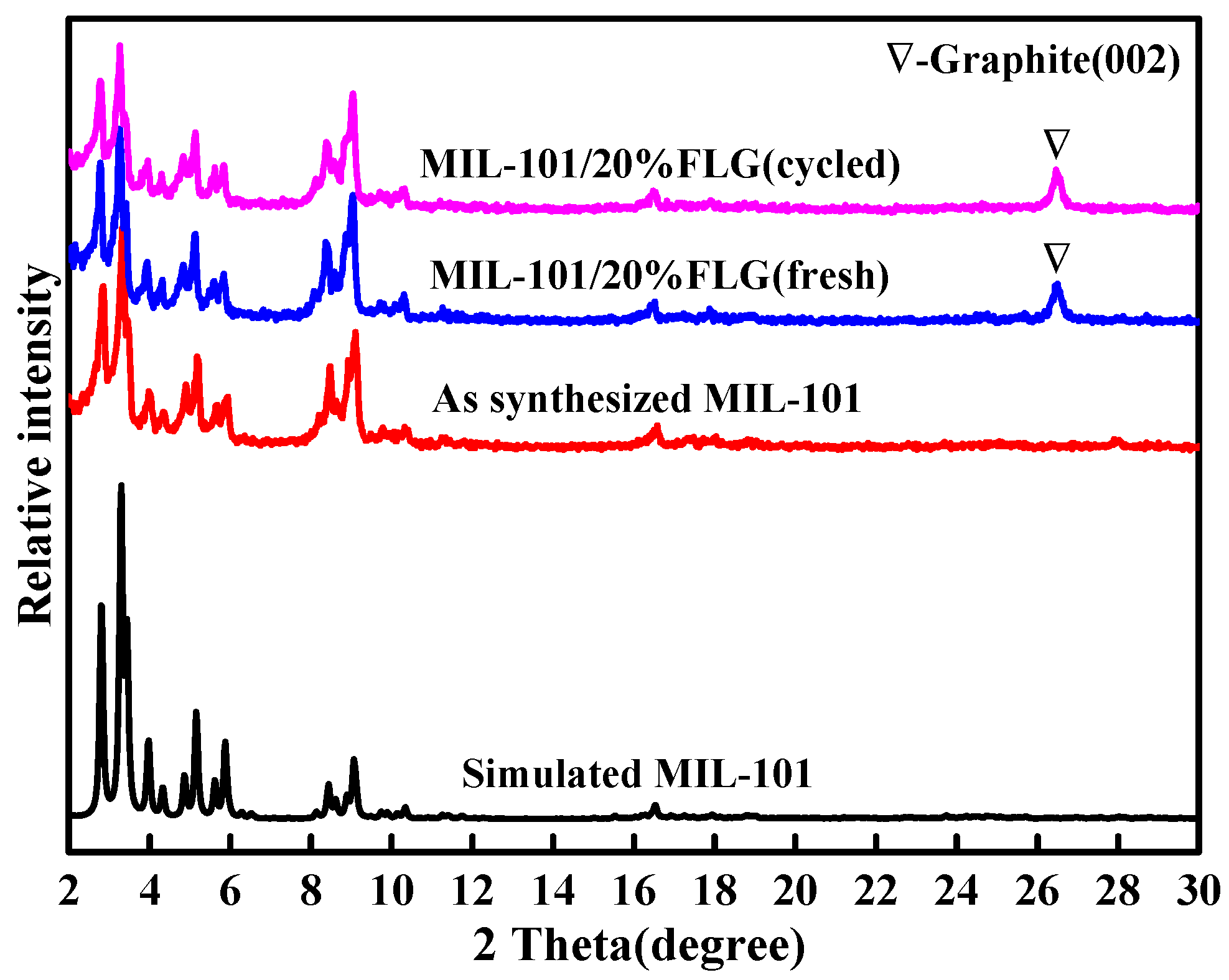
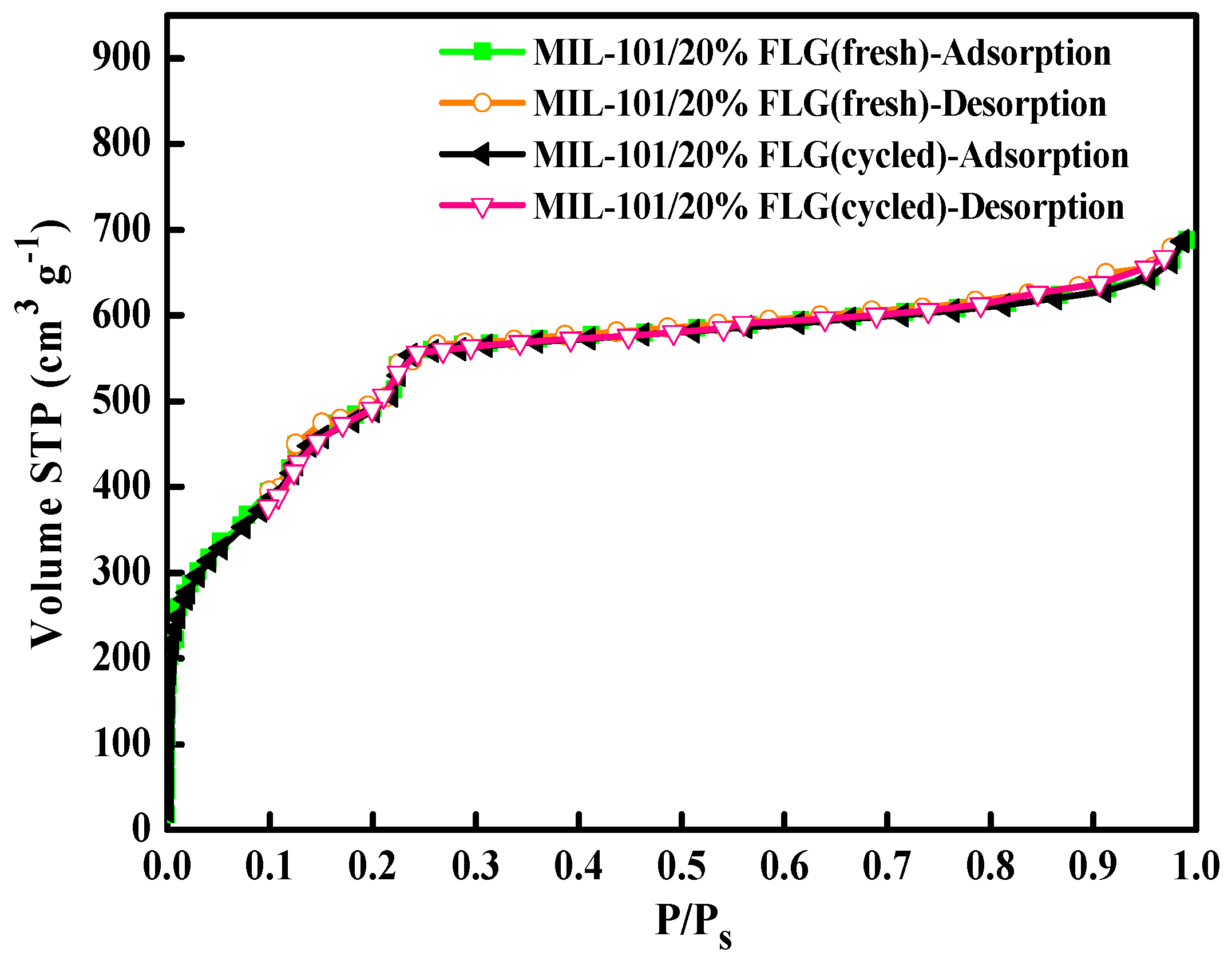
3.5.2. Characterization of Cycled MIL-101/20%FLG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| SCP | specific cooling power [W kg−1] |
| COP | coefficient of performance |
| Qc | cooling capacity per unit of cycle time [W] |
| Δmad | cyclic water amount [kg] |
| ΔHe | latent heat of vaporization [kJ kg−1] |
| m | mass [kg] |
| Qevap | evaporative cooling capacity [kJ] |
| Qinput | heat absorbed during desorption process [kJ] |
| hd | desorption enthalpy [kJ kg−1] |
| T | temperature of adsorber [°C] |
| Δt | cycle time [s] |
| wt | mass fraction [%] |
| y | result calculated from the measured data |
| vi | measured data |
| ∆vi | measurement error |
| P | vapor pressure [Pa] |
| Ed | desorption activation energy [kJ mol−1] |
| φ | heating rate [°C min−1] |
| R | gas constant [8.314 J mol−1 °C−1] |
| ∆x | cyclic water amount per unit mass of adsorbents [kg kg−1] |
| Subscript: | |
| ads | adsorbent |
| c | capacity |
| s | saturated |
| d | desorption |
| M | Middle |
| evap | evaporation |
References
- Alahmer, A.; Ajib, S. Solar cooling technologies: State of art and perspectives. Energy Convers. Manag. 2020, 214, 112896. [Google Scholar] [CrossRef]
- Allouhi, A.; Kousksou, T.; Jamil, A.; El Rhafiki, T.; Mourad, Y.; Zeraouli, Y. Optimal working pairs for solar adsorption cooling applications. Energy 2015, 79, 235–247. [Google Scholar] [CrossRef]
- Choudhury, B.; Saha, B.; Chatterjee, P.K.; Sarkar, J.P. An overview of developments in adsorption refrigeration systems towards a sustainable way of cooling. Appl. Energy 2013, 104, 554–567. [Google Scholar] [CrossRef]
- Deng, J.; Wang, R.; Han, G. A review of thermally activated cooling technologies for combined cooling, heating and power systems. Prog. Energy Combust. Sci. 2011, 37, 172–203. [Google Scholar] [CrossRef]
- Shabir, F.; Sultan, M.; Miyazaki, T.; Saha, B.B.; Askalany, A.; Ali, D.I.; Zhou, Y.; Ahmad, R.; Shamshiri, R.R. Recent updates on the adsorption capacities of adsorbent-adsorbate pairs for heat transformation applications. Renew. Sustain. Energy Rev. 2019, 119, 109630. [Google Scholar] [CrossRef]
- Wang, J.; Hu, E.; Blazewicz, A.; Ezzat, A.W. Simulation of accumulated performance of a solar thermal powered adsorption refrigeration system with daily climate conditions. Energy 2018, 165, 487–498. [Google Scholar] [CrossRef]
- Habib, K.; Saha, B.; Koyama, S. Study of various adsorbent–refrigerant pairs for the application of solar driven adsorption cooling in tropical climates. Appl. Therm. Eng. 2014, 72, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Zhang, X.; Li, X.; Shi, W.; Wang, B. Comparisons of different working pairs and cycles on the performance of absorption heat pump for heating and domestic hot water in cold regions. Appl. Therm. Eng. 2012, 48, 349–358. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Xia, Z. Experimental performance of a silica gel–water adsorption chiller. Appl. Therm. Eng. 2005, 25, 359–375. [Google Scholar] [CrossRef]
- Manila, M.R.; Mitra, S.; Dutta, P. Studies on dynamics of two-stage air cooled water/silica gel adsorption system. Appl. Therm. Eng. 2020, 178, 115552. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Wang, H.; Sun, H.; Wang, R. Experimental investigation of an adsorption air-conditioner using silica gel-water working pair. Sol. Energy 2019, 185, 64–71. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Wang, R. Experimental study of an adsorption chiller for extra low temperature waste heat utilization. Appl. Therm. Eng. 2019, 163, 114341. [Google Scholar] [CrossRef]
- Wang, D.C.; Wu, J.Y.; Xia, Z.Z.; Zhai, H.; Wang, R.Z.; Dou, W.D. Study of a novel silica gel-water ad-sorption chiller. Part II. Experimental study. Int. J. Refrig. 2005, 28, 1084–1091. [Google Scholar] [CrossRef]
- Younes, M.M.; El-Sharkawy, I.I.; Kabeel, A.E.; Uddin, K.; Pal, A.; Mitra, S.; Thu, K.; Saha, B.B. Synthesis and characterization of silica gel composite with polymer binders for adsorption cooling applications. Int. J. Refrig. 2018, 98, 161–170. [Google Scholar] [CrossRef]
- Brancato, V.; Frazzica, A. Characterisation and comparative analysis of zeotype water adsorbents for heat transformation applications. Sol. Energy Mater. Sol. Cells 2018, 180, 91–102. [Google Scholar] [CrossRef]
- Du, S.W.; Li, X.H.; Yuan, Z.X.; Du, C.X.; Wang, W.C.; Liu, Z.B. Performance of solar adsorption re-frigeration in system of SAPO-34 and ZSM-5 zeolite. Sol. Energy 2016, 138, 98–104. [Google Scholar] [CrossRef]
- Teo, H.W.B.; Chakraborty, A.; Han, B. Water adsorption on CHA and AFI types zeolites: Modelling and investigation of adsorption chiller under static and dynamic conditions. Appl. Therm. Eng. 2017, 127, 35–45. [Google Scholar] [CrossRef]
- Myat, A.; Choon, N.K.; Thu, K.; Kim, Y.-D. Experimental investigation on the optimal performance of Zeolite–water adsorption chiller. Appl. Energy 2013, 102, 582–590. [Google Scholar] [CrossRef]
- Wang, D.; Xia, Z.; Wu, J. Design and performance prediction of a novel zeolite–water adsorption air conditioner. Energy Convers. Manag. 2006, 47, 590–610. [Google Scholar] [CrossRef]
- AL-Dadah, R.; Mahmoud, S.; Elsayed, E.; Youssef, P.; Al-Mousawi, F. Metal-organic framework mate-rials for adsorption heat pumps. Energy 2020, 190, 116356. [Google Scholar] [CrossRef]
- Al-Mousawi, F.N.; Al-Dadah, R.; Mahmoud, S. Low grade heat driven adsorption system for cooling and power generation using advanced adsorbent materials. Energy Convers. Manag. 2016, 126, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Ehrenmann, J.; Henninger, S.K.; Janiak, C. Water adsorption characteristics of MIL-101 for heat-transformation applications of MOFs. Eur. J. Inorg. Chem. 2011, 4, 471–474. [Google Scholar] [CrossRef]
- Henninger, S.K.; Habib, H.A.; Janiak, C. MOFs as Adsorbents for Low Temperature Heating and Cooling Applications. J. Am. Chem. Soc. 2009, 131, 2776–2777. [Google Scholar] [CrossRef]
- Solovyeva, M.V.; Gordeeva, L.G.; Krieger, T.A.; Aristov, Y.I. MOF-801 as a promising material for ad-sorption cooling: Equilibrium and dynamics of water adsorption. Energ. Convers. Manag. 2018, 174, 356–363. [Google Scholar] [CrossRef]
- He, F.; Nagano, K.; Togawa, J. Experimental study and development of a low-cost 1 kW adsorption chiller using composite adsorbent based on natural mesoporous material. Energy 2020, 209, 118365. [Google Scholar] [CrossRef]
- He, Z.; Bai, Y.; Huang, H.; Li, J.; Huhetaoli; Kobayashi, N.; Osaka, Y.; Deng, L. Study on the performance of compact adsorption chiller with vapor valves. Appl. Therm. Eng. 2017, 126, 37–42. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.W.; Tian, Y.C. Numerical and experimental investigation of multi-halide chemisorp-tion system for exhaust gas heat recycling. Appl. Therm. Eng. 2021, 194, 117–118. [Google Scholar] [CrossRef]
- Vasta, S.; Freni, A.; Sapienza, A.; Costa, F.; Restuccia, G. Development and lab-test of a mobile adsorption air-conditioner. Int. J. Refrig. 2012, 35, 701–708. [Google Scholar] [CrossRef]
- Zimmermann, S.; Meijer, I.; Tiwari, M.; Paredes, S.; Michel, B.; Poulikakos, D. Aquasar: A hot water cooled data center with direct energy reuse. Energy 2012, 43, 237–245. [Google Scholar] [CrossRef]
- Laurenz, E.; Füldner, G.; Schnabel, L.; Schmitz, G. A Novel Approach for the Determination of Sorption Equilibria and Sorption Enthalpy Used for MOF Aluminium Fumarate with Water. Energies 2020, 13, 3003. [Google Scholar] [CrossRef]
- Elsayed, E.; Al-Dadah, R.; Mahmoud, S.; Anderson, P.A.; Elsayed, A.; Youssef, P.G. CPO-27(Ni), alu-minium fumarate and MIL-101(Cr) MOF materials for adsorption water desalination. Desalination 2017, 406, 25–36. [Google Scholar] [CrossRef]
- Khutia, A.; Rammelberg, H.U.; Schmidt, T.; Henninger, S.K.; Janiak, C. Water sorption cycle meas-urements on functionalized MIL-101Cr for heat transformation application. Chem. Mater. 2013, 25, 790–798. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, E.; Wang, H.Y.; Anderson, P.A.; Al-Dadah, R.; Mahmoud, S.; Navarro, H.; Ding, Y.L.; Bowen, J. Development of MIL-101(Cr)/GrO composites for adsorption heat pump applications. Micropor. Mesopor. Mat. 2017, 244, 180–191. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Tu, Y.D.; Pan, Q.; Palash, M.; Saha, B.B.; Aristov, Y.I.; Wang, R.Z. Metal-organic frameworks for energy conversion and water harvesting: A bridge between thermal engineering and material science. Nano Energy 2021, 84, 105946. [Google Scholar] [CrossRef]
- Rui, Z.; Li, Q.; Cui, Q.; Wang, H.; Chen, H.; Yao, H. Adsorption Refrigeration Performance of Shaped MIL-101-Water Working Pair. Chin. J. Chem. Eng. 2014, 22, 570–575. [Google Scholar] [CrossRef]
- Ma, L.; Rui, Z.; Wu, Q.; Yang, H.; Yin, Y.; Liu, Z.; Cui, Q.; Wang, H. Performance evaluation of shaped MIL-101–ethanol working pair for adsorption refrigeration. Appl. Therm. Eng. 2015, 95, 223–228. [Google Scholar] [CrossRef]
- Xu, Z.; Yin, Y.; Shao, J.; Liu, Y.; Zhang, L.; Cui, Q.; Wang, H. Study on heat transfer and cooling performance of copper foams cured MIL-101 adsorption unit tube. Energy 2019, 191, 116302. [Google Scholar] [CrossRef]
- Chen, H.J.; Cui, Q.; Gu, C.H.; Yao, H.Q. Heat conduction enhancement of adsorbents in adsorption refrigeration system. J. Nanjing Tech Univ. 2004, 26, 13–18. [Google Scholar]
- Hu, P.; Yao, J.J.; Chen, Z.S. Analysis for composite zeolite/foam aluminum–water mass recovery ad-sorption refrigeration system driven by engine exhaust heat. Energ. Convers. Manag. 2009, 50, 255–261. [Google Scholar] [CrossRef]
- Mohammed, R.H.; Mesalhy, O.; Elsayed, M.L.; Chow, L.C. Performance enhancement of adsorption beds with silica-gel particles packed in aluminum foams. Int. J. Refrig. 2019, 104, 201–212. [Google Scholar] [CrossRef]
- Chan, K.; Chao, C.Y.; Wu, C. Measurement of properties and performance prediction of the new MWCNT-embedded zeolite 13X/CaCl2 composite adsorbents. Int. J. Heat Mass Transf. 2015, 89, 308–319. [Google Scholar] [CrossRef]
- Eun, T.H.; Song, H.K.; Han, J.H.; Lee, K.H.; Kim, J.N. Enhancement of heat and mass transfer in silica-expanded graphite composite blocks for adsorption heat pumps: Part I. Characterization of the composite blocks. Int. J. Refrig. 2000, 23, 64–73. [Google Scholar] [CrossRef]
- Eun, T.H.; Song, H.K.; Han, J.H.; Lee, K.H.; Kim, J.N. Enhancement of heat and mass transfer in silica-expanded graphite composite blocks for adsorption heat pumps. Part II. Cooling system using the composite blocks. Int. J. Refrig. 2000, 23, 74–81. [Google Scholar] [CrossRef]
- Wu, W.D.; Wang, C.; Meng, X.W.; Zhang, H. Physical properties and refrigeration performance of compound adsorbent composed of additive and zeolite molecular sieve. Chem. Ind. Eng. Pro. 2016, 35, 692–699. [Google Scholar]
- Bahremand, H.; Ahmadi, M.; Bahrami, M. Analytical modeling of oscillatory heat transfer in coated sorption beds. Int. J. Heat Mass Transf. 2018, 121, 1–9. [Google Scholar] [CrossRef]
- Bahrehmand, H.; Khajehpour, M.; Bahrami, M. Finding optimal conductive additive content to enhance the performance of coated sorption beds: An experimental study. Appl. Therm. Eng. 2018, 143, 308–315. [Google Scholar] [CrossRef]
- Pal, A.; Uddin, K.; Rocky, K.A.; Thu, K.; Saha, B.B. CO2 adsorption onto activated carbon–graphene composite for cooling applications. Int. J. Refrig. 2019, 106, 558–569. [Google Scholar] [CrossRef]
- Pal, A.; Uddin, K.; Thu, K.; Saha, B.B. Activated carbon and graphene nanoplatelets based novel composite for performance enhancement of adsorption cooling cycle. Energy Convers. Manag. 2018, 180, 134–148. [Google Scholar] [CrossRef]
- Yang, S.; Kim, H.; Narayanan, S.; McKay, I.S.; Wang, E.N. Dimensionality effects of carbon-based thermal additives for microporous adsorbents. Mater. Des. 2015, 85, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Nika, D.L.; Balandin, A.A. Phonons and thermal transport in graphene and graphene-based materials. Rep. Prog. Phys. 2017, 80, 036502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.K.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Ghosh, S.; Bao, W.; Nika, D.L.; Subrina, S.; Pokatilov, E.P.; Lau, C.N.; Balandin, A.A. Dimensional crossover of thermal transport in few-layer graphene. Nat. Mater. 2010, 9, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Phononics of Graphene and Related Materials. ACS Nano 2020, 14, 5170–5178. [Google Scholar] [CrossRef]
- Ma, L.; Yang, H.; Wu, Q.; Yin, Y.; Liu, Z.; Cui, Q.; Wang, H. Study on adsorption refrigeration performance of MIL-101-isobutane working pair. Energy 2015, 93, 786–794. [Google Scholar] [CrossRef]
- Yin, Y.; Shao, J.; Zhang, L.; Cui, Q.; Wang, H. Study on heat conduction and adsorption/desorption characteristic of MIL-101/few layer graphene composite. J. Porous Mater. 2021, 28, 1197–1213. [Google Scholar] [CrossRef]
- Gustafsson, S.E. Transient plane source techniques for thermal conductivity and thermal diffusivity measurements of solid materials. Rev. Sci. Instrum. 1991, 62, 797–804. [Google Scholar] [CrossRef]
- Al-Ajlan, S.A. Measurements of thermal properties of insulation materials by using transient plane source technique. Appl. Therm. Eng. 2006, 26, 2184–2191. [Google Scholar] [CrossRef]
- Cui, Q.; Tao, G.; Chen, H.; Guo, X.; Yao, H. Environmentally benign working pairs for adsorption refrigeration. Energy 2005, 30, 261–271. [Google Scholar] [CrossRef]
- Wang, R.Z.; Wang, L.W.; Wu, J.Y. Theory and its Application of Adsorption Refrigeration; China Science Publishing House: Beijing, China, 2007. [Google Scholar]
- Liu, Z.; Zhao, B.; Zhu, L.; Lou, F.; Yan, J. Performance of MIL-101(Cr)/Water Working Pair Adsorption Refrigeration System Based on a New Type of Adsorbent Filling Method. Materials 2020, 13, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
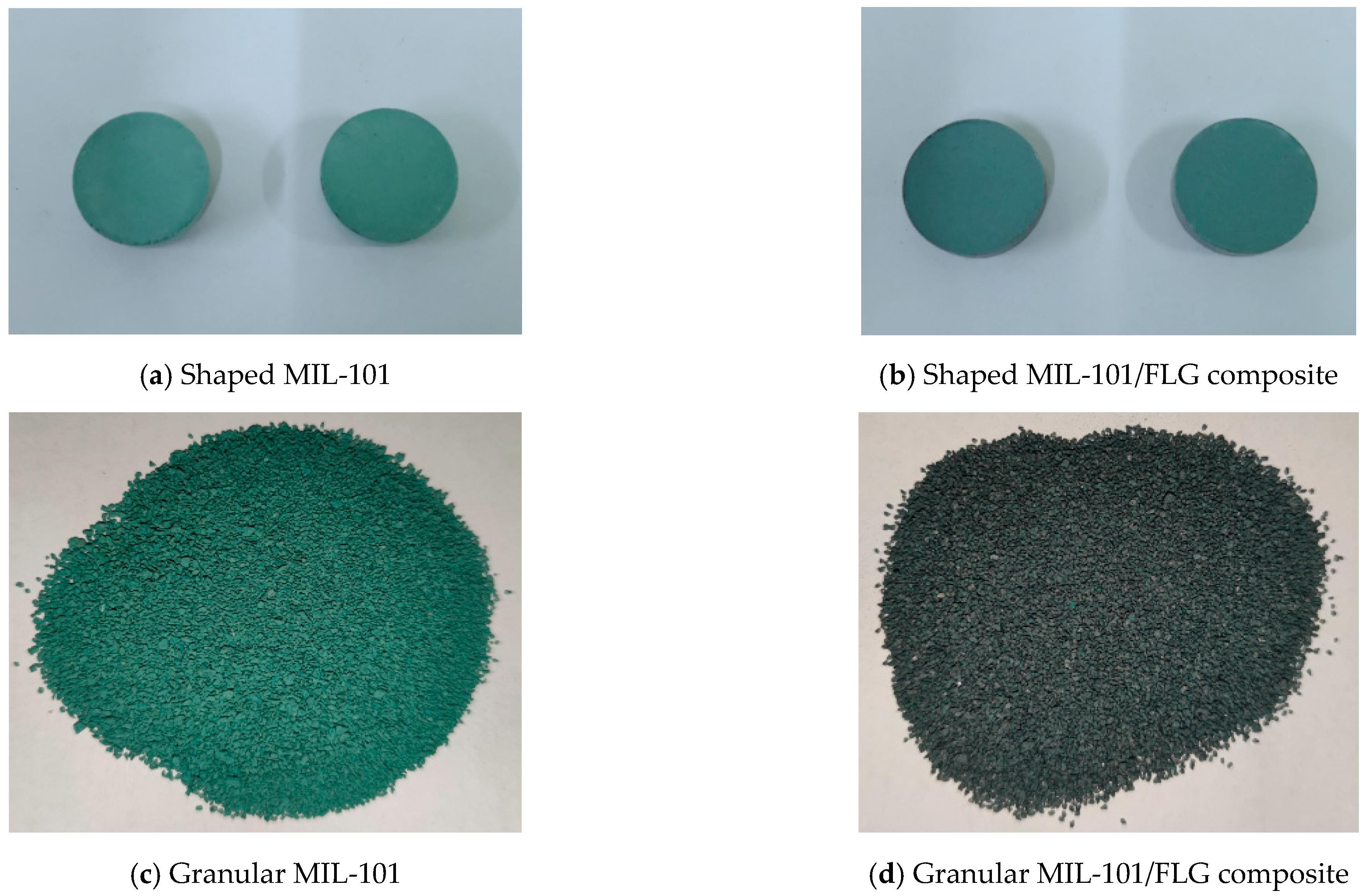
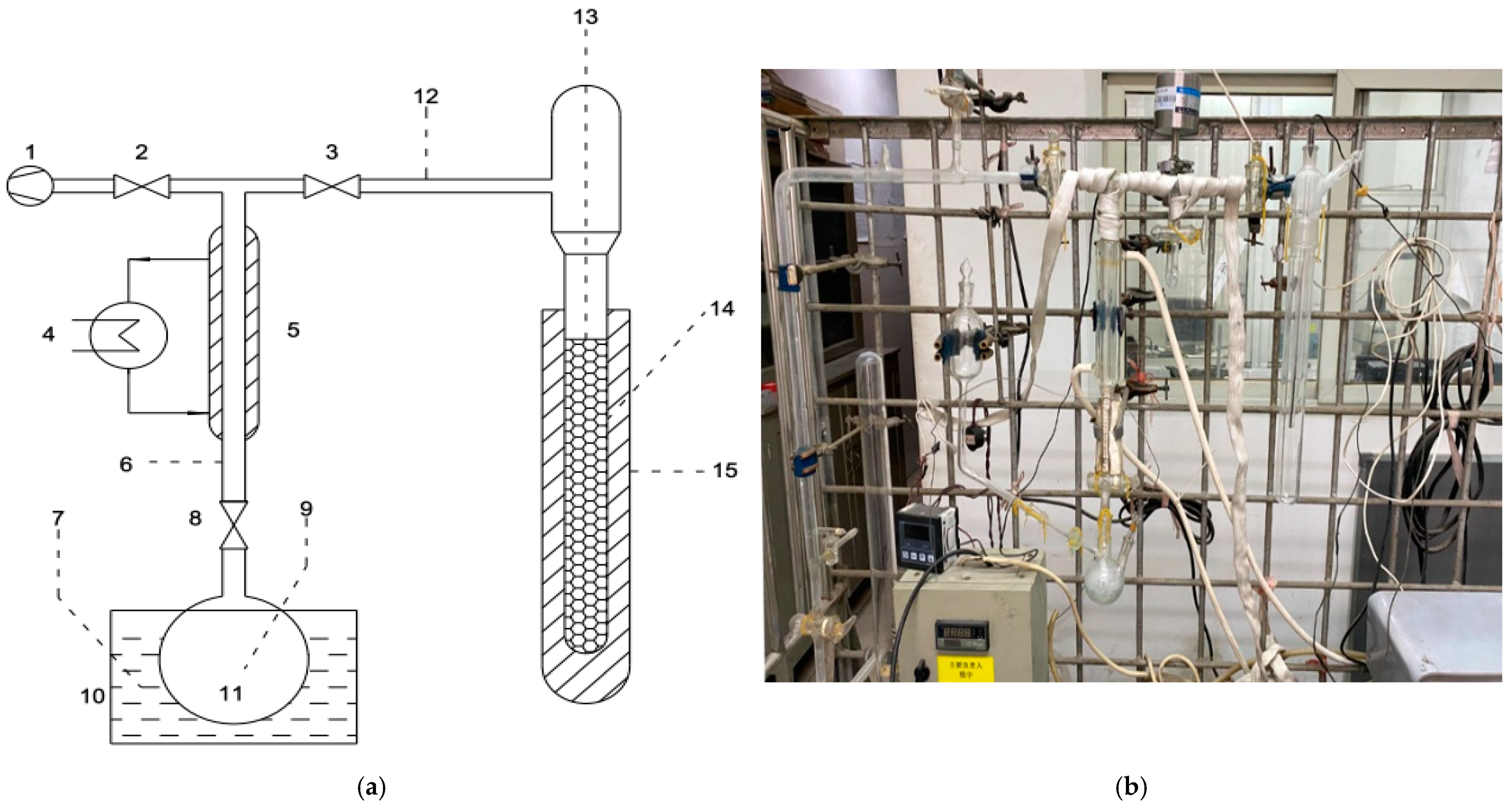

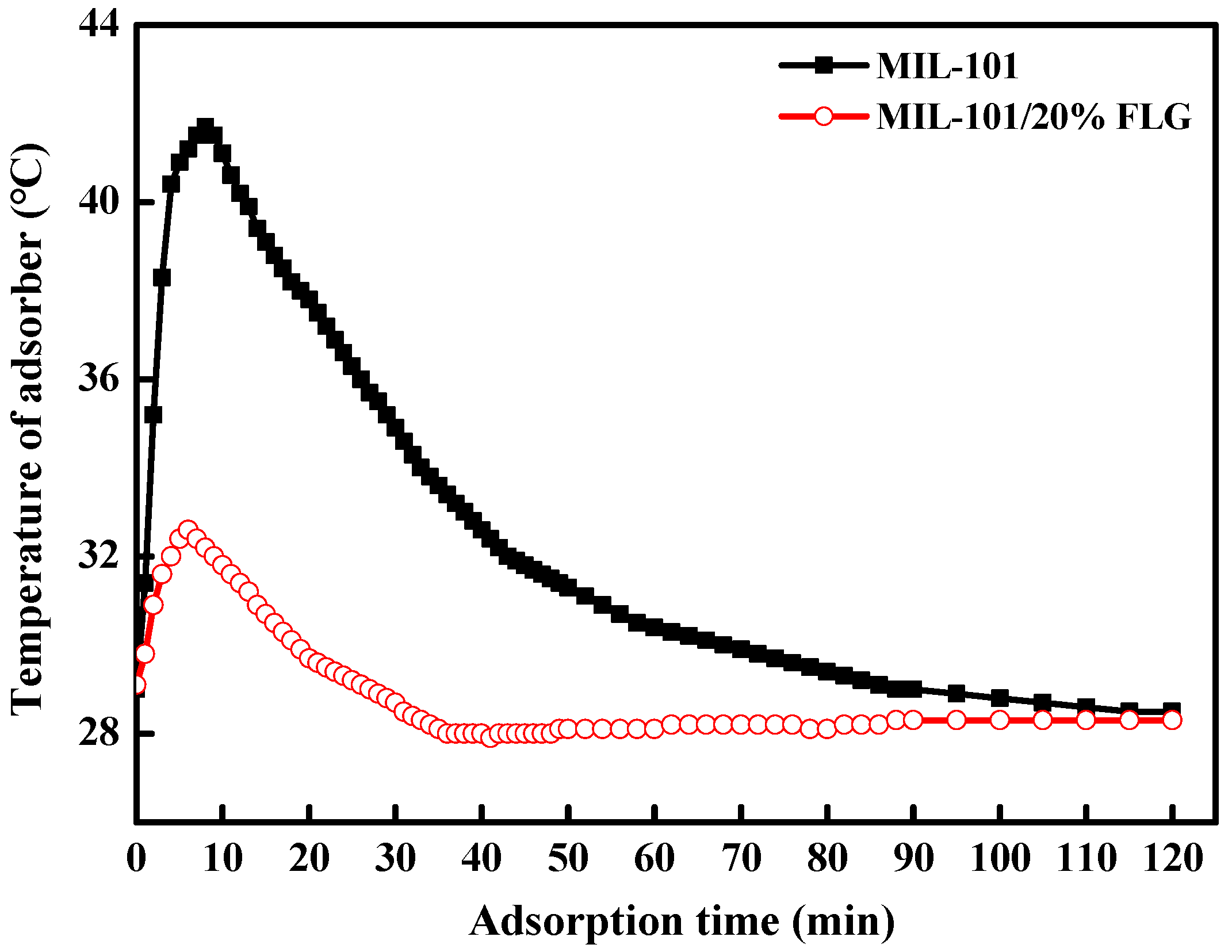

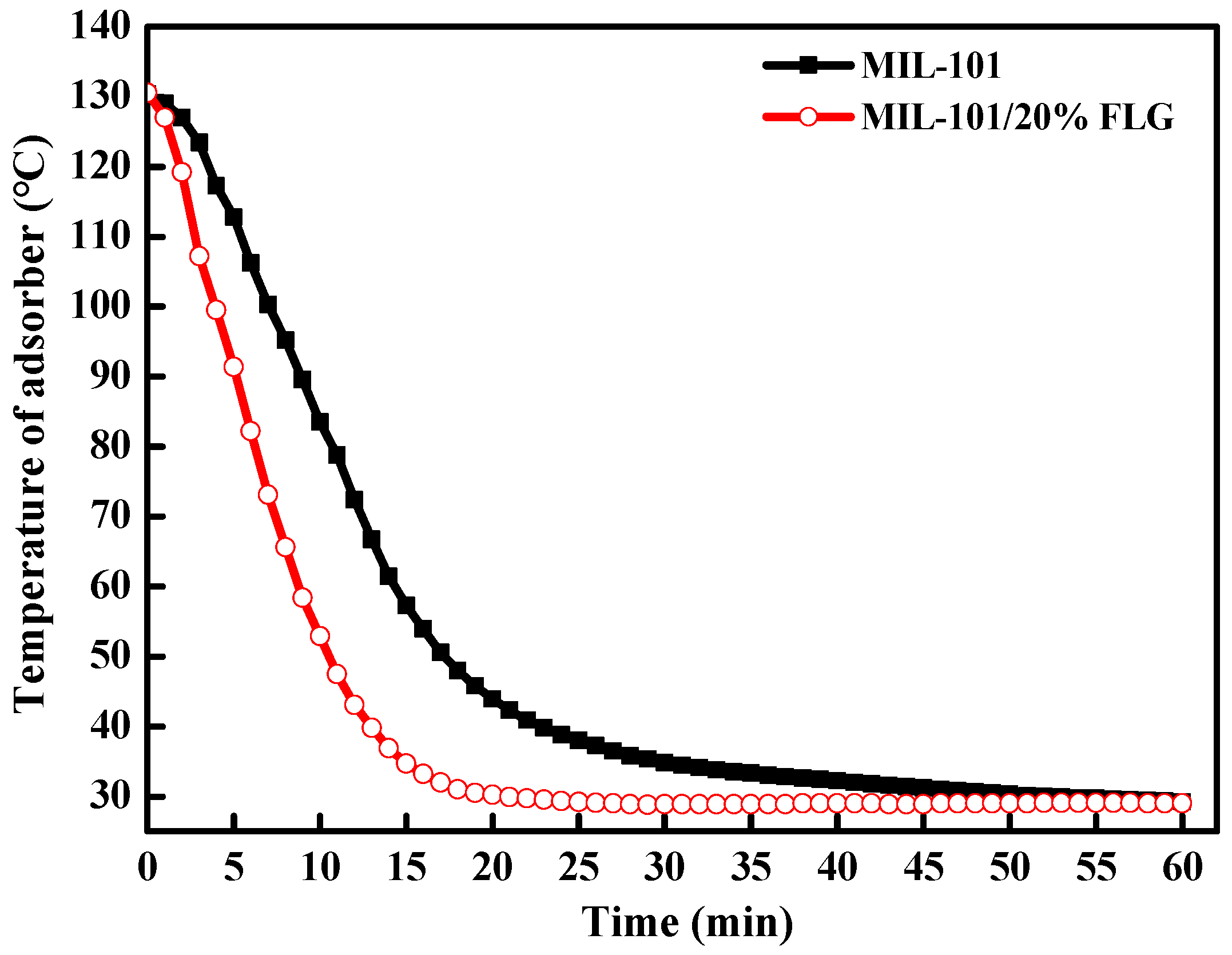



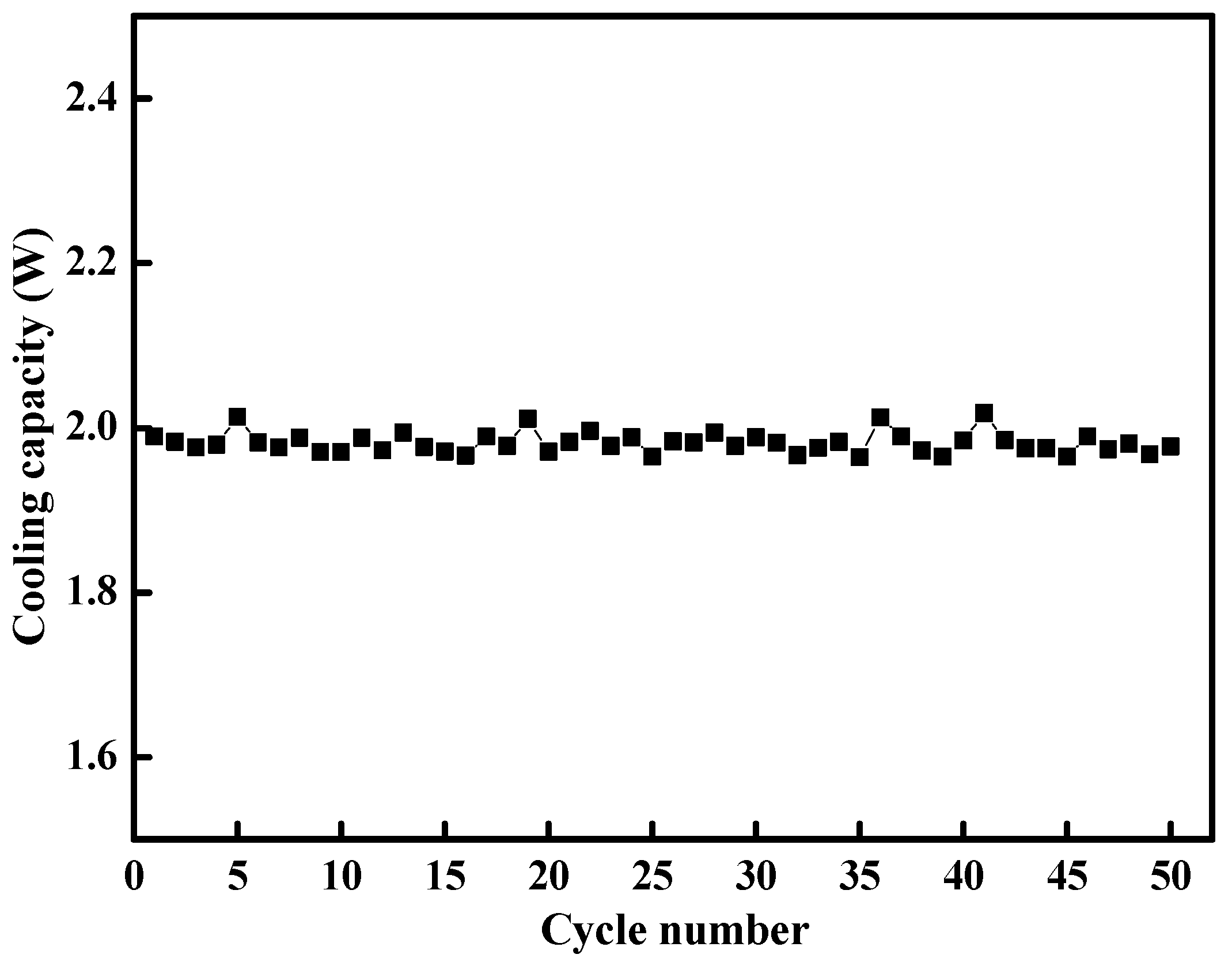
| Working Pair | Working Conditions | Cooling Capacity, W | SCP, W kg−1 | COP |
|---|---|---|---|---|
| MIL-101-water | Ta = 30 °C, Te = 16–20 °C, Td = 70 °C, Tc = 30 °C Ta = 30 °C, Te = 16–20 °C, Td = 90 °C, Tc = 30 °C | 1.16 1.37 | 46.3 54.8 | 0.126 0.141 |
| MIL-101/20%FLG-water | Ta = 30 °C, Te = 16–20 °C, Td = 70 °C, Tc = 30 °C Ta = 30 °C, Te = 16–20 °C, Td = 90 °C, Tc = 30 °C | 1.77 1.98 | 72.2 81.0 | 0.187 0.202 |
| Sample | BET Surface Area, m2 g−1 | Total Pore Volume, cm3 g−1 | Mesopore Volume, cm3 g−1 | Micropore Volume, cm3 g−1 |
|---|---|---|---|---|
| MIL-101/20%FLG (fresh) | 1558 | 0.85 | 0.41 | 0.44 |
| MIL-101/20%FLG (cycled) | 1552 | 0.84 | 0.39 | 0.45 |
| MIL-101 [37] | 2047 | 1.12 | 0.56 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Shao, J.; Zhang, L.; Cui, Q.; Wang, H. Experimental Study on Heat Transfer and Adsorption Cooling Performance of MIL-101/Few Layer Graphene Composite. Energies 2021, 14, 4970. https://doi.org/10.3390/en14164970
Yin Y, Shao J, Zhang L, Cui Q, Wang H. Experimental Study on Heat Transfer and Adsorption Cooling Performance of MIL-101/Few Layer Graphene Composite. Energies. 2021; 14(16):4970. https://doi.org/10.3390/en14164970
Chicago/Turabian StyleYin, Yu, Junpeng Shao, Lin Zhang, Qun Cui, and Haiyan Wang. 2021. "Experimental Study on Heat Transfer and Adsorption Cooling Performance of MIL-101/Few Layer Graphene Composite" Energies 14, no. 16: 4970. https://doi.org/10.3390/en14164970






