Improved Microbial Fuel Cell Performance by Engineering E. coli for Enhanced Affinity to Gold
Abstract
:1. Introduction
2. Materials and Methods
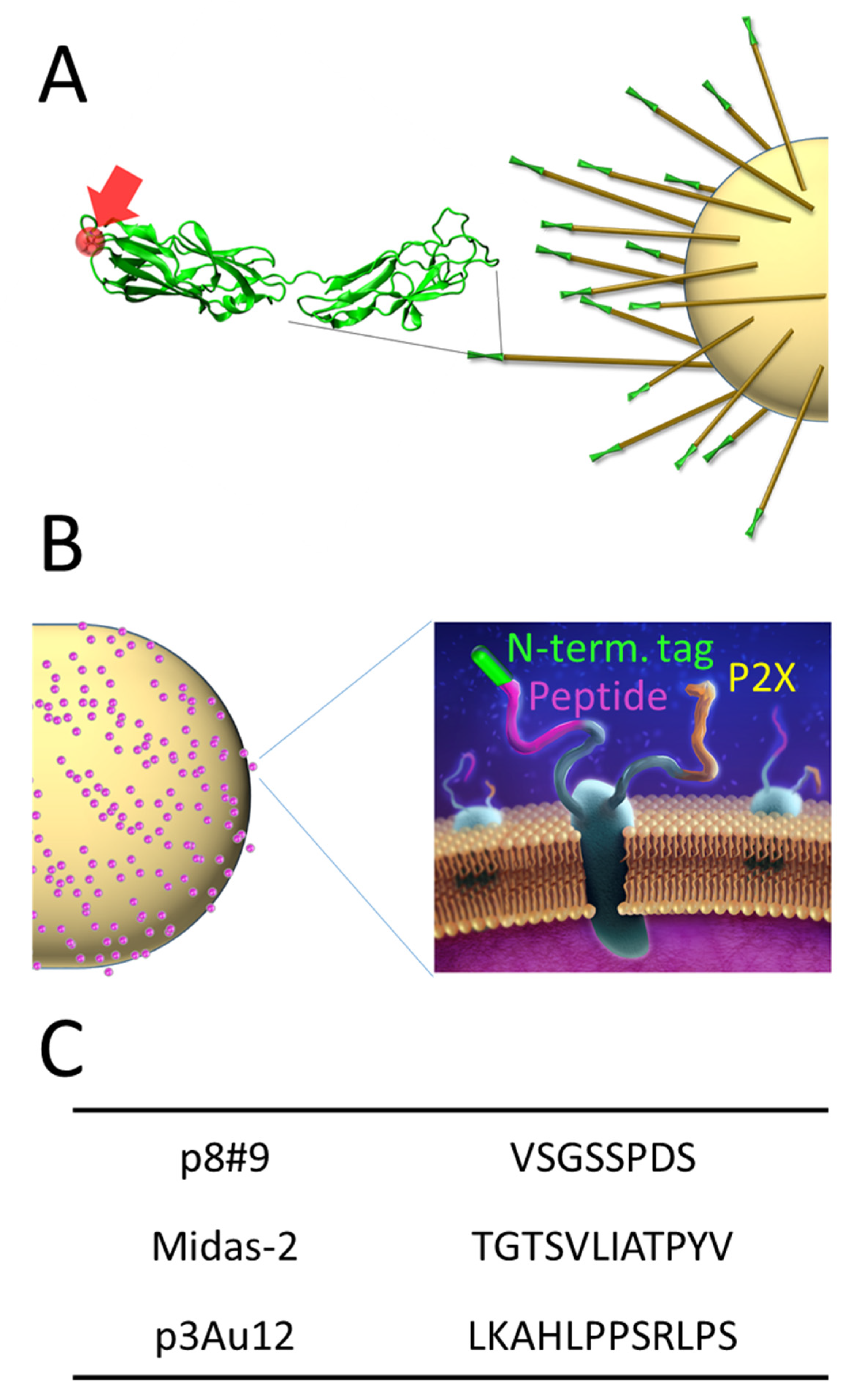
2.1. Scaffolds and Cell Culturing
2.2. Gold-Binding Spot Assay
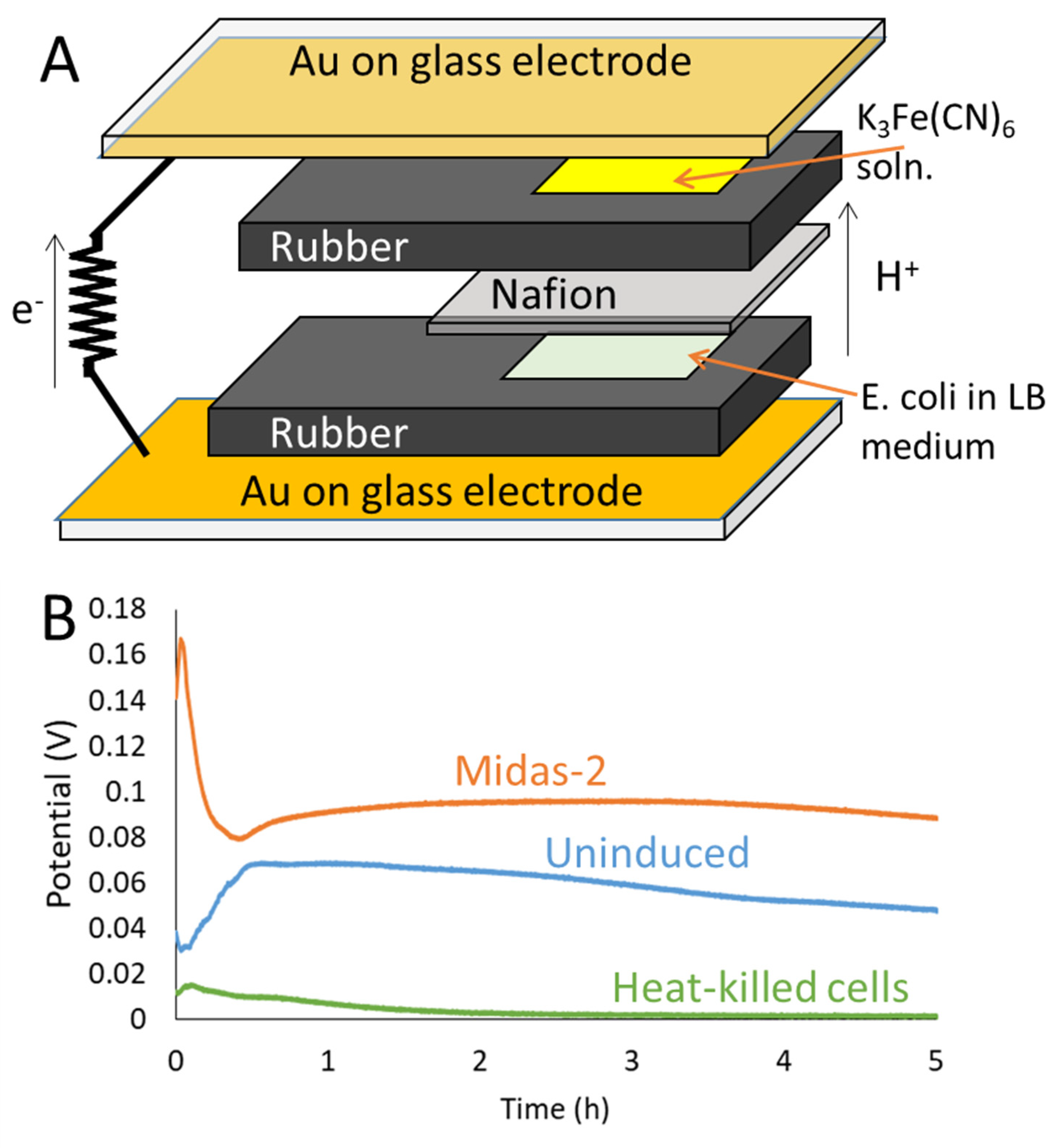
2.3. Microbial Fuel Cells (MFCs)
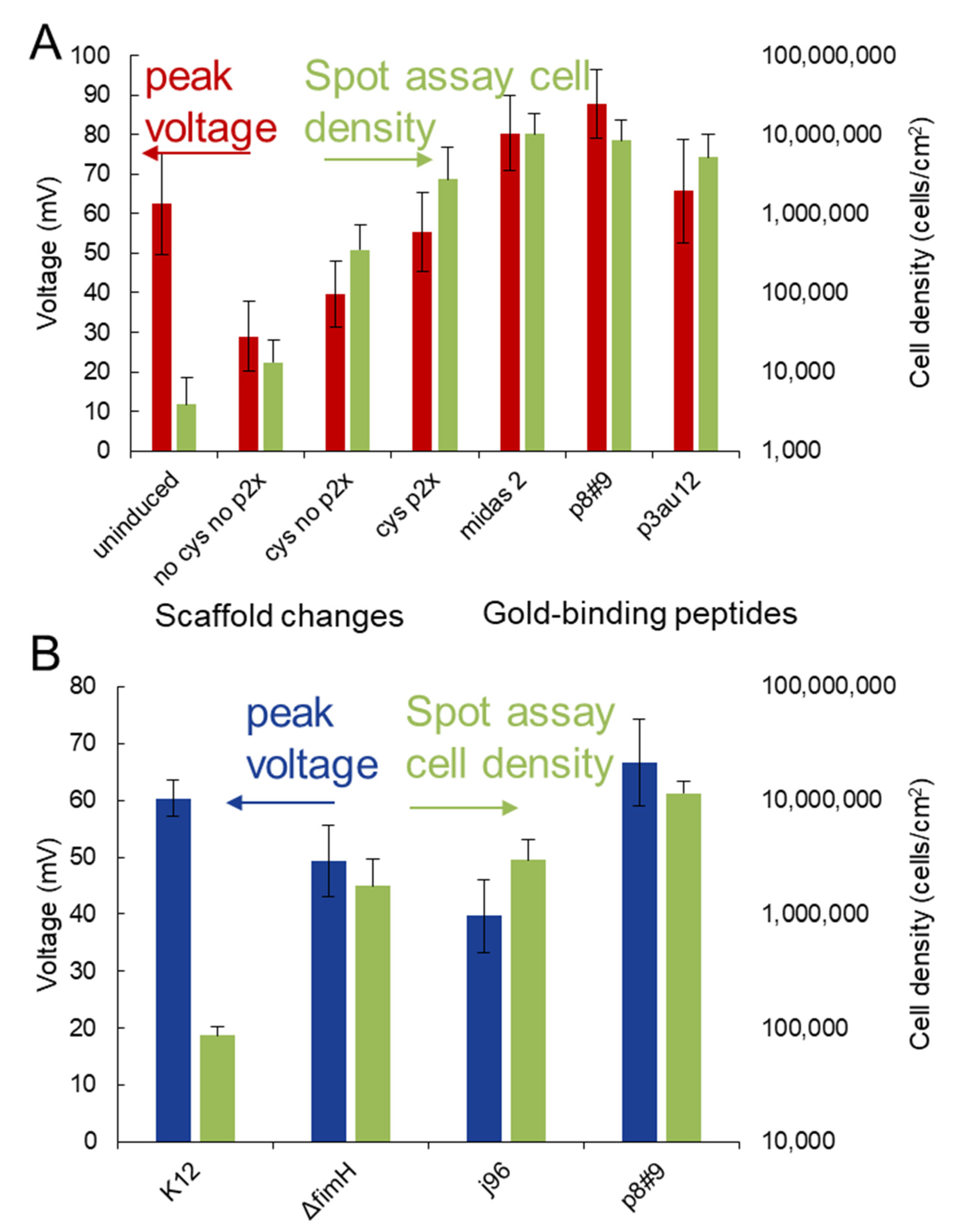
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Feng, Y.; Ryser, M.D.; Zhu, K.; Herschlag, G.; Cao, C.; Marusak, K.; Zauscher, S.; You, L. Programmable assembly of pressure sensors using pattern-forming bacteria. Nat. Biotechnol. 2017, 35, 1087. [Google Scholar] [CrossRef]
- Feinberg, A.W. Biological Soft Robotics. Annu. Rev. Biomed. Eng. 2015, 17, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Martel, S. Bacterial microsystems and microrobots. Biomed. Microdevices 2012, 14, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, J.P.; Idso, M.N.; Hussain, S.; Junk, M.J.N.; Fsher, J.M.; Phan, D.D.; Han, S.; Chmelka, B.F. Functionally Active Membrane Proteins Incorporated in Mesostructured Silica Films. J. Am. Chem. Soc. 2018, 140, 3892–3906. [Google Scholar] [CrossRef] [PubMed]
- Halma, M.; Mousty, C.; Forano, C.; Sancelme, M.; Besse-Hoggan, P.; Prevot, V. Bacteria encapsulated in layered double hydroxides: Towards an efficient bionanohybrid for pollutant degradation. Colloids Surf. B Biointerfaces 2015, 126, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Petrova, O.E.; Sauer, K. Sticky situations: Key components that control bacterial surface attachment. J. Bacteriol. 2012, 194, 2413–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozovičar, K.; Bratkovič, T. Evolving a Peptide: Library Platforms and Diversification Strategies. Int. J. Mol. Sci. 2020, 21, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Care, A.; Bergquist, P.L.; Sunna, A. Solid-binding peptides: Smart tools for nanobiotechnology. Trends Biotechnol. 2015, 33, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, A.B.; Miller, K.P.H.; Belcher, A.M.; Schmidt, C.E. Biomaterials functionalization using a novel peptide that selectively binds to a conducting polymer. Nat. Mater. 2005, 4, 496. [Google Scholar] [CrossRef]
- Brown, S. Metal-recognition by repeating polypeptides. Nat. Biotechnol. 1997, 15, 269. [Google Scholar] [CrossRef]
- Adams, B.L.; Hurley, M.M.; Jahnke, J.P.; Stratis-Cullum, D.N. Functional and Selective Bacterial Interfaces Using Cross-Scaffold Gold Binding Peptides. JOM 2015, 67, 2483–2493. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Humphreys, E.S.; Chung, S.-Y.; Delduco, D.F.; Lustig, S.R.; Wang, H.; Parker, K.N.; Rizzo, N.W.; Subramoney, S.; Chiang, Y.-M.; et al. Peptides with selective affinity for carbon nanotubes. Nat. Mater. 2003, 2, 196. [Google Scholar] [CrossRef]
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.E.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169. [Google Scholar] [CrossRef]
- Oh, D.; Qi, J.; Lu, Y.-C.; Zhang, Y.; Shao-Horn, Y.; Belcher, A.M. Biologically enhanced cathode design for improved capacity and cycle life for lithium-oxygen batteries. Nat. Commun. 2013, 4, 2756. [Google Scholar] [CrossRef] [Green Version]
- Seker, U.O.; Demir, H.V. Material binding peptides for nanotechnology. Molecules 2011, 16, 1426–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, C.R.; Hayamizu, Y.; Yazici, H.; Gresswell, C.; Khatayevich, D.; Tamerler, C.; Sarikaya, M. Controlling Self-Assembly of Engineered Peptides on Graphite by Rational Mutation. ACS Nano 2012, 6, 1648–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Sarkes, D.A.; Rice, J.J.; Hurley, M.M.; Fu, A.J.; Stratis-Cullum, D.N. Living Bacteria–Nanoparticle Hybrids Mediated through Surface-Displayed Peptides. Langmuir 2018, 34, 5837–5848. [Google Scholar] [CrossRef]
- Du, J.; Catania, C.; Bazan, G.C. Modification of Abiotic–Biotic Interfaces with Small Molecules and Nanomaterials for Improved Bioelectronics. Chem. Mater. 2014, 26, 686–697. [Google Scholar] [CrossRef]
- Ross, D.E.; Flynn, J.M.; Baron, D.B.; Gralnick, J.A.; Bond, D.R. Towards electrosynthesis in shewanella: Energetics of reversing the mtr pathway for reductive metabolism. PLoS ONE 2011, 6, e16649. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Wallack, M.J.; Kim, K.-Y.; He, W.; Feng, Y.; Saikaly, P.E. Assessment of Microbial Fuel Cell Configurations and Power Densities. Environ. Sci. Technol. Lett. 2015, 2, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Venkata Mohan, S.; Velvizhi, G.; Annie Modestra, J.; Srikanth, S. Microbial fuel cell: Critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew. Sustain. Energy Rev. 2014, 40, 779–797. [Google Scholar] [CrossRef]
- Ali, J.; Wang, L.; Waseem, H.; Sharif, H.M.A.; Djellabi, R.; Zhang, C.; Pan, G. Bioelectrochemical recovery of silver from wastewater with sustainable power generation and its reuse for biofouling mitigation. J. Clean. Prod. 2019, 235, 1425–1437. [Google Scholar] [CrossRef]
- Tremblay, P.-L.; Zhang, T. Electrifying microbes for the production of chemicals. Front. Microbiol. 2015, 6, 201. [Google Scholar] [CrossRef] [Green Version]
- Tschirhart, T.; Kim, E.; McKay, R.; Ueda, H.; Wu, H.-C.; Pottash, A.E.; Zargar, A.; Negrete, A.; Shiloach, J.; Payne, G.F.; et al. Electronic control of gene expression and cell behaviour in Escherichia coli through redox signalling. Nat. Commun. 2017, 8, 14030. [Google Scholar] [CrossRef] [Green Version]
- Terrell, J.L.; Tschirhart, T.; Jahnke, J.P.; Stephens, K.; Liu, Y.; Dong, H.; Hurley, M.M.; Pozo, M.; McKay, R.; Tsao, C.Y.; et al. Bioelectronic control of a microbial community using surface-assembled electrogenetic cells to route signals. Nat. Nanotechnol. 2021, 16, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, A.; Choi, S. A stackable, two-chambered, paper-based microbial fuel cell. Biosens. Bioelectron. 2016, 83, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Sund, C.; McMasters, S.; Crittenden, S.; Harrell, L.; Sumner, J. Effect of electron mediators on current generation and fermentation in a microbial fuel cell. Appl. Microbiol. Biotechnol. 2007, 76, 561–568. [Google Scholar] [CrossRef]
- Garner, L.E.; Park, J.; Dyar, S.M.; Chworos, A.; Sumner, J.J.; Bazan, G.C. Modification of the Optoelectronic Properties of Membranes via Insertion of Amphiphilic Phenylenevinylene Oligoelectrolytes. J. Am. Chem. Soc. 2010, 132, 10042–10052. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, J.P.; Dong, H.; Sarkes, D.A.; Sumner, J.J.; Stratis-Cullum, D.N.; Hurley, M.M. Peptide-mediated binding of gold nanoparticles to E. coli for enhanced microbial fuel cell power generation. MRS Commun. 2019, 9, 904–909. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, S.K.; Shin, I.H.; Jeong, Y.J. Electricity production in biofuel cell using modified graphite electrode with Neutral Red. Biotechnol. Lett. 2000, 22, 1301–1304. [Google Scholar] [CrossRef]
- Popov, A.L.; Kim, J.R.; Dinsdale, R.M.; Esteves, S.R.; Guwy, A.J.; Premier, G.C. The effect of physico-chemically immobilized methylene blue and neutral red on the anode of microbial fuel cell. Biotechnol. Bioprocess Eng. 2012, 17, 361–370. [Google Scholar] [CrossRef]
- Crittenden, S.R.; Sund, C.J.; Sumner, J.J. Mediating Electron Transfer from Bacteria to a Gold Electrode via a Self-Assembled Monolayer. Langmuir 2006, 22, 9473–9476. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.-C.; Dong, X.-C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and Monolithic Anode Based on Polyaniline Hybridized Three-Dimensional Graphene for High-Performance Microbial Fuel Cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef]
- Amir, L.; Carnally, S.A.; Rayo, J.; Rosenne, S.; Melamed Yerushalmi, S.; Schlesinger, O.; Meijler, M.M.; Alfonta, L. Surface Display of a Redox Enzyme and its Site-Specific Wiring to Gold Electrodes. J. Am. Chem. Soc. 2013, 135, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.M.; Albers, A.E.; Malley, K.R.; Londer, Y.Y.; Cohen, B.E.; Helms, B.A.; Weigele, P.; Groves, J.T.; Ajo-Franklin, C.M. Engineering of a synthetic electron conduit in living cells. Proc. Natl. Acad. Sci. USA 2010, 107, 19213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, A.L.; Bond, D.R.; Gralnick, J.A. Electrochemical Analysis of Shewanella oneidensis Engineered to Bind Gold Electrodes. ACS Synth. Biol. 2013, 2, 93–101. [Google Scholar] [CrossRef]
- Causa, F.; Della Moglie, R.; Iaccino, E.; Mimmi, S.; Marasco, D.; Scognamiglio, P.L.; Battista, E.; Palmieri, C.; Cosenza, C.; Sanguigno, L.; et al. Evolutionary screening and adsorption behavior of engineered M13 bacteriophage and derived dodecapeptide for selective decoration of gold interfaces. J. Colloid Interface Sci. 2013, 389, 220–229. [Google Scholar] [CrossRef]
- Kim, J.; Rheem, Y.; Yoo, B.; Chong, Y.; Bozhilov, K.N.; Kim, D.; Sadowsky, M.J.; Hur, H.-G.; Myung, N.V. Peptide-mediated shape- and size-tunable synthesis of gold nanostructures. Acta Biomater. 2010, 6, 2681–2689. [Google Scholar] [CrossRef]
- Huang, Y.; Chiang, C.-Y.; Lee, S.K.; Gao, Y.; Hu, E.L.; Yoreo, J.D.; Belcher, A.M. Programmable Assembly of Nanoarchitectures Using Genetically Engineered Viruses. Nano Lett. 2005, 5, 1429–1434. [Google Scholar] [CrossRef]
- Nam, K.T.; Kim, D.-W.; Yoo, P.J.; Chiang, C.-Y.; Meethong, N.; Hammond, P.T.; Chiang, Y.-M.; Belcher, A.M. Virus-Enabled Synthesis and Assembly of Nanowires for Lithium Ion Battery Electrodes. Science 2006, 312, 885–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, J.J.; Schohn, A.; Bessette, P.H.; Boulware, K.T.; Daugherty, P.S. Bacterial display using circularly permuted outer membrane protein OmpX yields high affinity peptide ligands. Protein Sci. 2006, 15, 825–836. [Google Scholar] [CrossRef]
- Rice, J.J.; Daugherty, P.S. Directed evolution of a biterminal bacterial display scaffold enhances the display of diverse peptides. Protein Eng. Des. Sel. 2008, 21, 435–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrell, J.L.; Dong, H.; Holthoff, E.L.; Small, M.C.; Sarkes, D.A.; Hurley, M.M.; Stratis-Cullum, D.N. Investigation of engineered bacterial adhesins for opportunity to interface cells with abiotic materials. In Proceedings of the Smart Biomedical and Physiological Sensor Technology XIII, Baltimore, MD, USA, 17–21 April 2016; p. 986308. [Google Scholar]
- Adams, B.L.; Finch, A.S.; Hurley, M.M.; Sarkes, D.A.; Stratis-Cullum, D.N. Genetically Engineered Peptides for Inorganics: Study of an Unconstrained Bacterial Display Technology and Bulk Aluminum Alloy. Adv. Mater. 2013, 25, 4585–4591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomfjeld, I.C.; McClain, M.S.; Eisenstein, B.I. Type 1 fimbriae mutants of Escherichia coli K12: Characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol. Microbiol. 1991, 5, 1439–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokurenko, E.V.; Chesnokova, V.; Doyle, R.J.; Hasty, D.L. Diversity of the Escherichia coli type 1 fimbrial lectin. Differential binding to mannosides and uroepithelial cells. J. Biol. Chem. 1997, 272, 17880–17886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemm, P.; Jørgensen, B.J.; van Die, I.; de Ree, H.; Bergmans, H. The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli, cloning and genetic organization. Mol. Gen. Genet. MGG 1985, 199, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Li, L.; Cho, Y.; de Figueiredo, P.; Han, A. Microfabricated Microbial Fuel Cell Arrays Reveal Electrochemically Active Microbes. PLoS ONE 2009, 4, e6570. [Google Scholar] [CrossRef]
- Sund, C.J.; Wong, M.S.; Sumner, J.J. Mitigation of the effect of catholyte contamination in microbial fuel cells using a wicking air cathode. Biosens. Bioelectron. 2009, 24, 3144–3147. [Google Scholar] [CrossRef]
- Sarkes, D.A.; Jahnke, J.P.; Stratis-Cullum, D.N. Semi-automated Biopanning of Bacterial Display Libraries for Peptide Affinity Reagent Discovery and Analysis of Resulting Isolates. JoVE 2017, e56061. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Arya, S.K.; Glass, N.; Hanifi-Moghaddam, P.; Naidoo, R.; Szymanski, C.M.; Tanha, J.; Evoy, S. Bacteriophage tailspike proteins as molecular probes for sensitive and selective bacterial detection. Biosens. Bioelectron. 2010, 26, 131–138. [Google Scholar] [CrossRef]
- Hong, N.; Cheng, L.; Wei, B.; Chen, C.; He, L.L.; Kong, D.; Ceng, J.; Cui, H.-F.; Fan, H. An electrochemical DNA sensor without electrode pre-modification. Biosens. Bioelectron. 2017, 91, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Choi, S. Microscale microbial fuel cells: Advances and challenges. Biosens. Bioelectron. 2015, 69, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Chen, X.; Thomas, A.W.; Catania, C.; Kirchhofer, N.D.; Garner, L.E.; Han, A.; Bazan, G.C. Conjugated Oligoelectrolytes Increase Power Generation in E. coli Microbial Fuel Cells. Adv. Mater. 2013, 25, 1593–1597. [Google Scholar] [CrossRef]
- Hou, H.; Li, L.; Ceylan, C.U.; Haynes, A.; Cope, J.; Wilkinson, H.H.; Erbay, C.; de Figueiredo, P.; Han, A. A microfluidic microbial fuel cell array that supports long-term multiplexed analyses of electricigens. Lab Chip 2012, 12, 4151–4159. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, J.; Terrell, J.; Smith, A.; Cheng, X.; Stratis-Cullum, D. Influences of Adhesion Variability on the “Living” Dynamics of Filamentous Bacteria in Microfluidic Channels. Molecules 2016, 21, 985. [Google Scholar] [CrossRef] [PubMed]
- Wehrens, M.; Ershov, D.; Rozendaal, R.; Walker, N.; Schultz, D.; Kishony, R.; Levin, P.A.; Tans, S.J. Size Laws and Division Ring Dynamics in Filamentous Escherichia coli cells. Curr. Biol. 2018, 28, 972–979.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, V.B.; Du, J.; Chen, X.; Thomas, A.W.; Kirchhofer, N.D.; Garner, L.E.; Maw, M.T.; Poh, W.H.; Hinks, J.; Wuertz, S.; et al. Improving charge collection in Escherichia coli-carbon electrode devices with conjugated oligoelectrolytes. Phys. Chem. Chem. Phys. 2013, 15, 5867–5872. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, J.M.; Yadav, A.; Ghosh, P.C.; Adeloju, S.B. Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens. Bioelectron. 2017, 90, 558–576. [Google Scholar] [CrossRef]


| Paper | Approach | Electrode | Enhancement |
|---|---|---|---|
| [29] | Gold-binding peptide + nanoparticles | gold(111) surface | 4.0 |
| [30] | Redox mediator (Neutral Red) immobilized on electrode | Graphite | 4.8 |
| [31] | Redox mediator (Methylene Blue) immobilized on electrode | carbon felt | 2.1 |
| [33] | Conductive Polymer (PANI) | carbon cloth | 2.0 |
| carbon felt | 13 | ||
| nickel foam | 3.2 | ||
| graphene foam | 60 | ||
| [34] | Enzyme engineering & gold nanoparticles | Gold | 8.0 |
| [36] | Gold-binding peptide | gold(111) surface | 1.4 |
| This work | Gold-binding peptide | gold wire | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahnke, J.P.; Sarkes, D.A.; Liba, J.L.; Sumner, J.J.; Stratis-Cullum, D.N. Improved Microbial Fuel Cell Performance by Engineering E. coli for Enhanced Affinity to Gold. Energies 2021, 14, 5389. https://doi.org/10.3390/en14175389
Jahnke JP, Sarkes DA, Liba JL, Sumner JJ, Stratis-Cullum DN. Improved Microbial Fuel Cell Performance by Engineering E. coli for Enhanced Affinity to Gold. Energies. 2021; 14(17):5389. https://doi.org/10.3390/en14175389
Chicago/Turabian StyleJahnke, Justin P., Deborah A. Sarkes, Jessica L. Liba, James J. Sumner, and Dimitra N. Stratis-Cullum. 2021. "Improved Microbial Fuel Cell Performance by Engineering E. coli for Enhanced Affinity to Gold" Energies 14, no. 17: 5389. https://doi.org/10.3390/en14175389
APA StyleJahnke, J. P., Sarkes, D. A., Liba, J. L., Sumner, J. J., & Stratis-Cullum, D. N. (2021). Improved Microbial Fuel Cell Performance by Engineering E. coli for Enhanced Affinity to Gold. Energies, 14(17), 5389. https://doi.org/10.3390/en14175389







