A Review of Recent Developments and Advanced Applications of High-Temperature Polymer Electrolyte Membranes for PEM Fuel Cells
Abstract
:1. Introduction
2. Desirable Properties of PEMs
3. Improving Proton Conductivity of the Membranes
3.1. Effects of Dopants and Additives
3.2. Effect of Molecular Weight
3.3. Polymer Composites
3.4. Other Important Parameters
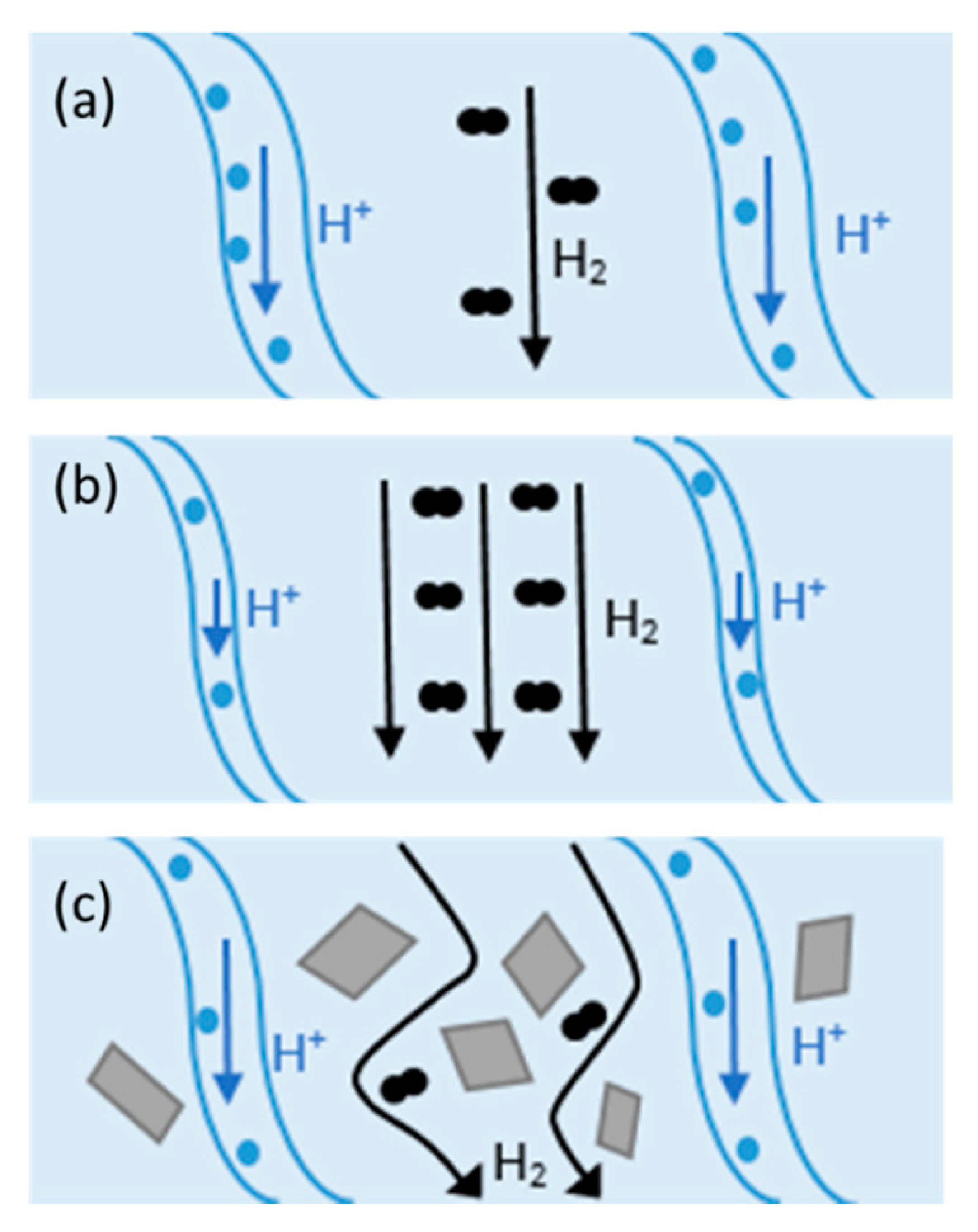
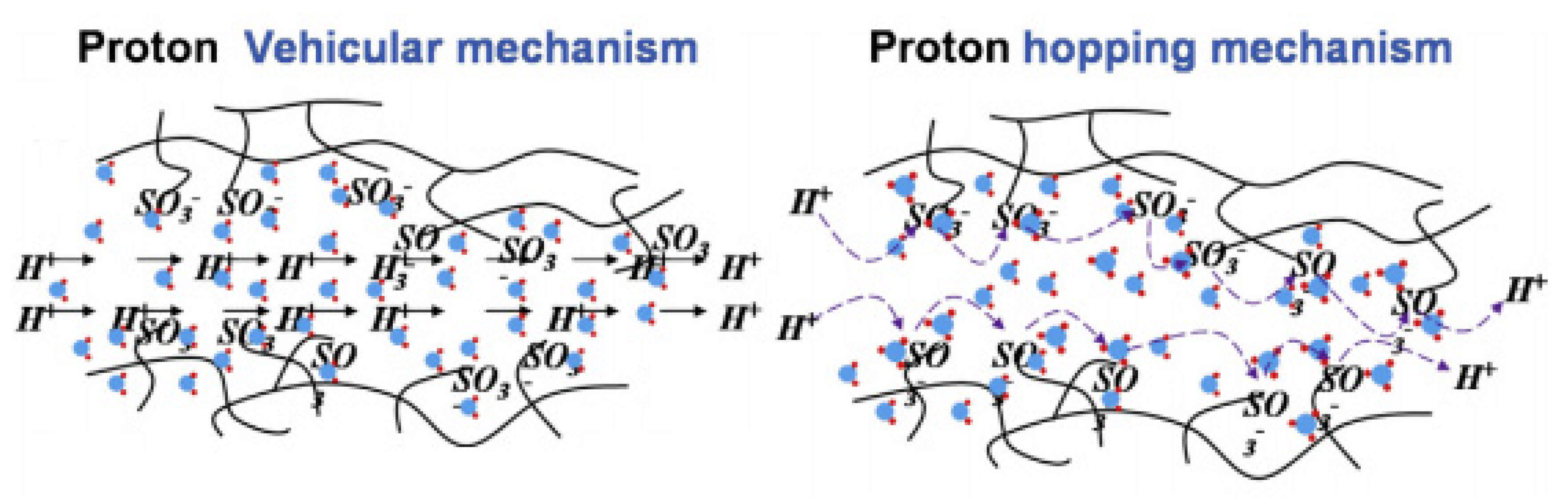
4. Proton Conduction Mechanism
5. Polymer Electrolyte Membrane Types
5.1. PEM Types
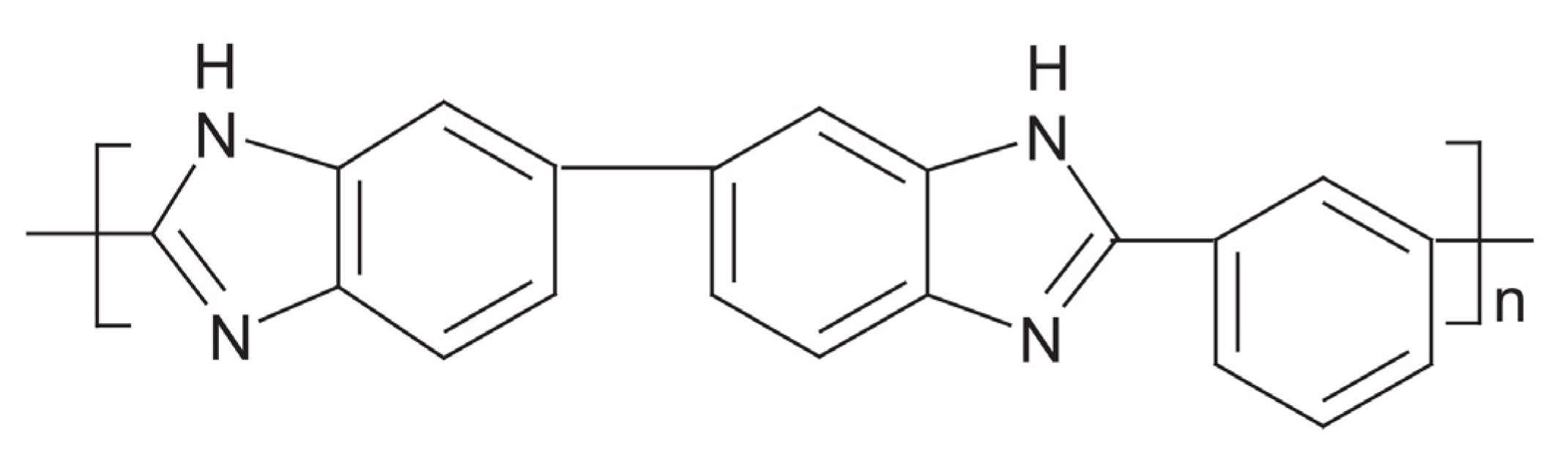
5.2. High-Temperature PEMs Based on PBI
6. Degradation and Fuel Cell Performance
7. Experimental Characterization
7.1. In Situ Characterisation
7.2. Ex Situ Characterization
7.3. In-Plane and Through-Plane Techniques
7.4. The Two-Probe Electrodes and Four-Probe Electrodes Cells
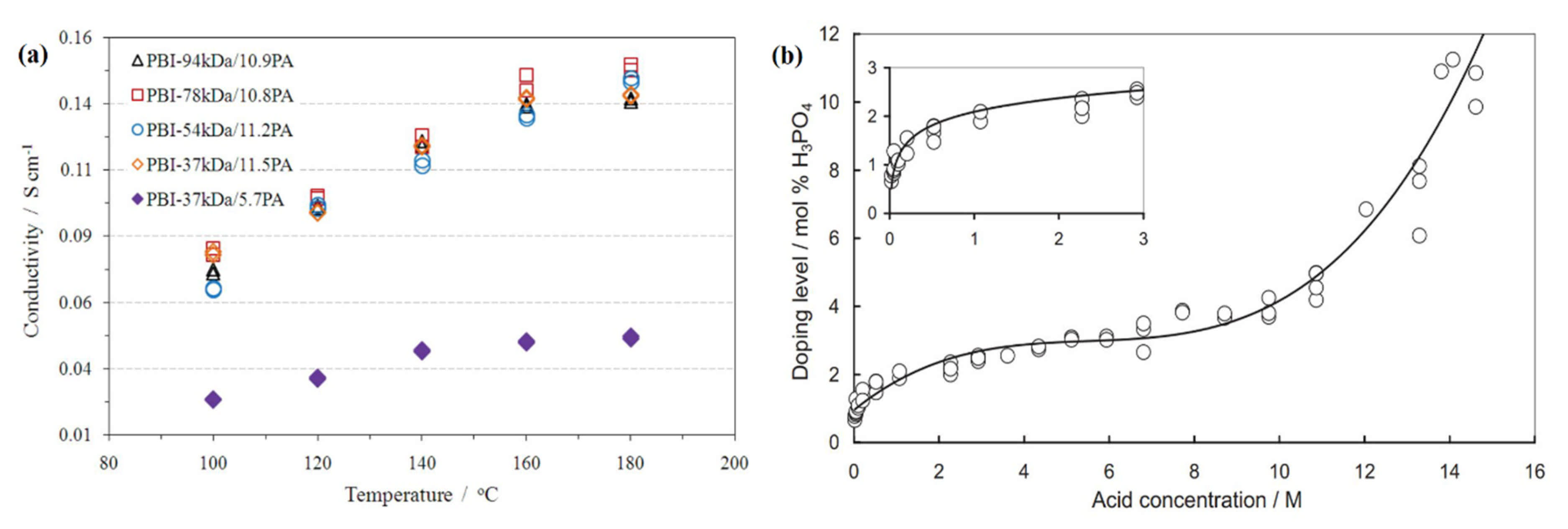
7.5. Proton Conductivity and PA Doping Level
7.6. Thermal, Chemical, and Mechanical Stability
8. Challenges, Economic Viability, and Future Research Direction of High-Temperature PEM Fuel Cells
8.1. Current Challenges
8.2. Economic Viability and the Prospect of HT-PEM Fuel Cells
8.3. Advanced Application and Future Research Direction
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, C.; Lee, S.; Gu, B.; Chang, I.; Cho, G.Y.; Baek, J.D.; Cha, S.W. A Study of Anode-Supported Solid Oxide Fuel Cell Modeling and Optimization Using Neural Network and Multi-Armed Bandit Algorithm. Energies 2020, 13, 1621. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Horri, B.A. Progress in Material Development for Low-Temperature Solid Oxide Fuel Cells: A Review. Energies 2021, 14, 1280. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Wang, D.; Zhang, J. Fundamentals and Challenges of Lithium Ion Batteries at Temperatures between −40 and 60 °C. Adv. Energy Mater. 2020, 10, 1904152. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Doetsch, C.; Pohlig, A. The Use of Flow Batteries in Storing Electricity for National Grids; Elsevier Ltd.: Oxford, UK, 2020; ISBN 9780081028865. [Google Scholar]
- Clemente, A.; Costa-Castelló, R. Redox flow batteries: A literature review oriented to automatic control. Energies 2020, 13, 4514. [Google Scholar] [CrossRef]
- Shri Prakash, B.; Pavitra, R.; Senthil Kumar, S.; Aruna, S.T. Electrolyte bi-layering strategy to improve the performance of an intermediate temperature solid oxide fuel cell: A review. J. Power Sources 2018, 381, 136–155. [Google Scholar] [CrossRef]
- Da Silva, F.S.; de Souza, T.M. Novel materials for solid oxide fuel cell technologies: A literature review. Int. J. Hydrogen Energy 2017, 42, 26020–26036. [Google Scholar] [CrossRef] [Green Version]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Non-humidified fuel cells using a deep eutectic solvent (DES) as the electrolyte within a polymer electrolyte membrane (PEM): The effect of water and counterions. Phys. Chem. Chem. Phys. 2020, 22, 2917–2929. [Google Scholar] [CrossRef] [PubMed]
- Beydaghi, H.; Bagheri, A.; Salarizadeh, P.; Kashefi, S.; Hooshyari, K.; Amoozadeh, A.; Shamsi, T.; Bonaccorso, F.; Pellegrini, V. Enhancing the Performance of Poly(phthalazinone ether ketone)-Based Membranes Using a New Type of Functionalized TiO2 with Superior Proton Conductivity. Ind. Eng. Chem. Res. 2020, 59, 6589–6599. [Google Scholar] [CrossRef]
- Kumar, A.; Hong, J.-W.; Yun, Y.; Bhardwaj, A.; Song, S.-J. The role of surface lattice defects of CeO2−δ nanoparticles as a scavenging redox catalyst in polymer electrolyte membrane fuel cells. J. Mater. Chem. A 2020, 8, 26023–26034. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Salarizadeh, P.; Bageri, A. Advanced nanocomposite membranes based on sulfonated polyethersulfone: Influence of nanoparticles on PEMFC performance. J. Iran. Chem. Soc. 2019, 16, 1617–1629. [Google Scholar] [CrossRef]
- Jayakumar, A. An Assessment on Polymer Electrolyte Membrane Fuel Cell Stack Components. In Applied Physical Chemistry with Multidisciplinary Approaches; Haghi, A.K., Balköse, D., Thomas, S., Eds.; Apple Academic Press Inc.: Oakville, ON, Canada, 2018; pp. 23–49. ISBN 9781771886062. [Google Scholar]
- Beydaghi, H.; Javanbakht, M.; Bagheri, A.; Ghafarian-Zahmatkesh, H.; Hooshyari, K. Preparation and Characterization of Electrocatalyst Nanoparticles for Direct Methanol Fuel Cell Applications Using β-D-glucose as Protection Agent. Iran. J. Hydrog. Fuel Cell 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Jannelli, E.; Minutillo, M.; Perna, A. Analyzing microcogeneration systems based on LT-PEMFC and HT-PEMFC by energy balances. Appl. Energy 2013, 108, 82–91. [Google Scholar] [CrossRef]
- Ma, K.B.; Kwak, D.H.; Han, S.B.; Park, H.S.; Kim, D.H.; Won, J.E.; Kwon, S.H.; Kim, M.C.; Moon, S.H.; Park, K.W. Direct Ethanol Fuel Cells with Superior Ethanol-Tolerant Nonprecious Metal Cathode Catalysts for Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2018, 6, 7609–7618. [Google Scholar] [CrossRef]
- Velayutham, P.; Sahu, A.K.; Parthasarathy, S. A Nafion-ceria composite membrane electrolyte for reduced methanol crossover in direct methanol fuel cells. Energies 2017, 10, 259. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, N.J.; Waltman, L. VOSviewer Manual; Univeristeit Leiden: Leiden, The Netherlands, 2013. [Google Scholar]
- Zhou, Z.; Zholobko, O.; Wu, X.-F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2020, 14, 135. [Google Scholar] [CrossRef]
- Moradi, M.; Moheb, A.; Javanbakht, M.; Hooshyari, K. Experimental study and modeling of proton conductivity of phosphoric acid doped PBI-Fe2TiO5 nanocomposite membranes for using in high temperature proton exchange membrane fuel cell (HT-PEMFC). Int. J. Hydrogen Energy 2016, 41, 2896–2910. [Google Scholar] [CrossRef]
- Tian, D.; Gu, T.; Yellamilli, S.N.; Bae, C. Phosphoric acid-doped ion-pair coordinated PEMs with broad relative humidity tolerance. Energies 2020, 13, 1924. [Google Scholar] [CrossRef] [Green Version]
- Üregen, N.; Pehlivanoğlu, K.; Özdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2636–2647. [Google Scholar] [CrossRef]
- Attaran, A.M. Fabrication and Characterization of Poly Vinyl Alcohol/ Poly Vinyl Pyrrolidone/MnTiO3 Nanocomposite Membranes for PEM Fuel Cells. Iran. J. Energy Environ. 2013, 4, 86–90. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Askari, M.B.; Mohammadi, M.; Hooshyari, K. Electrocatalytic performance of CeO2-decorated rGO as an anode electrocatalyst for the methanol oxidation reaction. J. Phys. Chem. Solids 2020, 142, 109442. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Liu, Z.; Peng, J.; Shi, C.; Hu, W.; Jiang, Z.; Liu, B. Arylether-type polybenzimidazoles bearing benzimidazolyl pendants for high-temperature proton exchange membrane fuel cells. J. Power Sources 2018, 393, 99–107. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Seo, K.; Ghorpade, R.V.; Nam, K.H.; Han, H. High temperature anhydrous proton exchange membranes based on chemically-functionalized titanium/polybenzimidazole composites for fuel cells. Mater. Lett. 2020, 263, 127167. [Google Scholar] [CrossRef]
- Berber, M.R.; Nakashima, N. Bipyridine-based polybenzimidazole membranes with outstanding hydrogen fuel cell performance at high temperature and non-humidifying conditions. J. Memb. Sci. 2019, 591, 117354. [Google Scholar] [CrossRef]
- Hosseinabadi, P.; Hooshyari, K.; Javanbakht, M.; Enhessari, M. Synthesis and optimization of nanocomposite membranes based on SPEEK and perovskite nanoparticles for polymer electrolyte membrane fuel cells. New J. Chem. 2019, 43, 16232–16245. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Javanbakht, M.; Pourmahdian, S.; Hazer, M.S.A.; Hooshyari, K.; Askari, M.B. Novel proton exchange membranes based on proton conductive sulfonated PAMPS/PSSA-TiO2 hybrid nanoparticles and sulfonated poly (ether ether ketone) for PEMFC. Int. J. Hydrogen Energy 2019, 44, 3099–3114. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Enhessari, M.; Beydaghi, H. Novel PVA/La2Ce2O7 hybrid nanocomposite membranes for application in proton exchange membrane fuel cells. Iran. J. Hydrog. Fuel Cell Iran. J. Hydrog. Fuel Cell IJHFC J. 2014, 2, 105–112. [Google Scholar]
- Attaran, A.M.; Javanbakht, M.; Hooshyari, K.; Enhessari, M. New proton conducting nanocomposite membranes based on poly vinyl alcohol/poly vinyl pyrrolidone/BaZrO3 for proton exchange membrane fuel cells. Solid State Ionics 2015, 269, 98–105. [Google Scholar] [CrossRef]
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite membranes for high temperature PEM fuel cells and electrolysers: A critical review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, M.T.D.; Jensen, J.O.; Cleemann, L.N.; Li, Q. Durability Issues and Status of PBI-Based Fuel Cells. In High Temperature Polymer Electrolyte Membrane Fuel Cells. Approaches, Status, and Perspectives; Li, Q., Aili, D., Hjuler, H.A., Jensen, J.O., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 487–509. ISBN 9783319170824. [Google Scholar]
- Leykin, A.Y.; Askadskii, A.A.; Vasilev, V.G.; Rusanov, A.L. Dependence of some properties of phosphoric acid doped PBIs on their chemical structure. J. Memb. Sci. 2010, 347, 69–74. [Google Scholar] [CrossRef]
- Kang, Y.; Zou, J.; Sun, Z.; Wang, F.; Zhu, H.; Han, K.; Yang, W.; Song, H.; Meng, Q. Polybenzimidazole containing ether units as electrolyte for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2013, 38, 6494–6502. [Google Scholar] [CrossRef]
- Farrokhi, M.; Abdollahi, M. Enhancing medium/high temperature proton conductivity of poly(benzimidazole)-based proton exchange membrane via blending with poly(vinyl imidazole-co-vinyl phosphonic acid) copolymer: Proton conductivity-copolymer microstructure relationship. Eur. Polym. J. 2020, 131, 109691. [Google Scholar] [CrossRef]
- Bouchet, R.; Siebert, E. Proton conduction in acid doped polybenzimidazole. Solid State Ionics 1999, 118, 287–299. [Google Scholar] [CrossRef]
- Luo, H.; Vaivars, G.; Agboola, B.; Mu, S.; Mathe, M. Anion exchange membrane based on alkali doped poly (2,5-benzimidazole) for fuel cell. Solid State Ionics 2012, 208, 52–55. [Google Scholar] [CrossRef]
- Lin, A.J.; Yan, X.; He, G.; Chen, W.; Zhen, D.; Li, T.; Ma, L.; Wu, X. Thermoplastic interpenetrating polymer networks based on polybenzimidazole and poly (1, 2-dimethy-3-allylimidazolium) for anion exchange membranes. Electrochim. Acta 2017, 257, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Fuel Cells and Hydrogen Production a Volume in the Encyclopedia of Sustainability Science and Technology, 2nd ed.; Lipman, T.E.; Weber, A.Z. (Eds.) Springer: Berkeley, CA, USA, 2019; ISBN 9781493977888. [Google Scholar]
- Escorihuela, J.; García-Bernabé, A.; Compañ, V. A deep insight into different acidic additives as doping agents for enhancing proton conductivity on polybenzimidazole membranes. Polymers 2020, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Schechter, A.; Savinell, R.F. Imidazole and 1-methyl imidazole in phosphoric acid doped polybenzimidazole, electrolyte for fuel cells. Solid State Ionics 2002, 147, 181–187. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Berg, R.W.; Hjuler, H.A.; Bjerrum, N.J. Water uptake and acid doping of polybenzimidazoles as electrolyte membranes for fuel cells. Solid State Ionics 2004, 168, 177–185. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Memb. Sci. 2003, 226, 169–184. [Google Scholar] [CrossRef]
- Mader, J.A.; Benicewicz, B.C. Sulfonated polybenzimidazoles for high temperature PEM fuel cells. Macromolecules 2010, 43, 6706–6715. [Google Scholar] [CrossRef]
- Li, X.; Ma, H.; Wang, P.; Liu, Z.; Peng, J.; Hu, W.; Jiang, Z.; Liu, B.; Guiver, M.D. Highly Conductive and Mechanically Stable Imidazole-Rich Cross-Linked Networks for High-Temperature Proton Exchange Membrane Fuel Cells. Chem. Mater. 2020, 32, 1182–1191. [Google Scholar] [CrossRef]
- Jones, D.J.; Rozière, J. Recent advances in the functionalisation of polybenzimidazole and polyetherketone for fuel cell applications. J. Memb. Sci. 2001, 185, 41–58. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sánchez, E.M.; Romero, P.G. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef]
- Zaidi, S.M.J.; Mikhailenko, S.D.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S. Proton conducting composite membranes from polyether ether ketone and heteropolyacids for fuel cell applications. J. Memb. Sci. 2000, 173, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Park, K.T.; Jung, U.H.; Choi, D.W.; Chun, K.; Lee, H.M.; Kim, S.H. ZrO2–SiO2/Nafion® composite membrane for polymer electrolyte membrane fuel cells operation at high temperature and low humidity. J. Power Sources 2008, 177, 247–253. [Google Scholar] [CrossRef]
- Hooshyari, K.; Khanamiri, S.N.; Salarizadeh, P.; Beydaghi, H. Nanocomposite Membranes with High Fuel Cell Performance Based on Sulfonated Poly (1,4-phenylene ether ether sulfone) and Ytterbium/Yttrium Doped-Perovskite Nanoparticles. J. Electrochem. Soc. 2019, 166, F976–F989. [Google Scholar] [CrossRef]
- Berber, M.R.; Nakashima, N. Tailoring Different Molecular Weight Phenylene-Polybenzimidazole Membranes with Remarkable Oxidative Stability and Conductive Properties for Higherature Polymer Electrolyte Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 46269–46277. [Google Scholar] [CrossRef] [PubMed]
- Berber, M.R. Molecular Weight Impact of Poly(2,5-Benzimidazole) Polymer on Film Conductivity, Ion Exchange Capacity, Acid Retention Capability, and Oxidative Stability. Front. Energy Res. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Yang, J.S.; Cleemann, L.N.; Steenberg, T.; Terkelsen, C.; Li, Q.F.; Jensen, J.O.; Hjuler, H.A.; Bjerrum, N.J.; He, R.H. High molecular weight polybenzimidazole membranes for high temperature PEMFC. Fuel Cells 2014, 14, 7–15. [Google Scholar] [CrossRef]
- Schaffer, J.V.; Lupatini, K.N.; Machado, B.; Silva, E.S.; Ferracin, R.J.; Alves, H.J. Parameters effect on proton conductivity to obtain chitosan membranes for use as electrolytes in PEMFC. Int. J. Energy Res. 2018, 42, 1381–1385. [Google Scholar] [CrossRef]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite polymers development and application for polymer electrolyte membrane technologies—A review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef] [Green Version]
- Adjemian, K.T.; Dominey, R.; Krishnan, L.; Ota, H.; Majsztrik, P.; Zhang, T.; Mann, J.; Kirby, B.; Gatto, L.; Velo-Simpson, M.; et al. Function and Characterization of Metal Oxide−Nafion Composite Membranes for Elevated-Temperature H2/O2 PEM Fuel Cells. Chem. Mater. 2006, 18, 2238–2248. [Google Scholar] [CrossRef]
- Wei, Y.; Qian, T.; Liu, J.; Guo, X.; Gong, Q.; Liu, Z.; Tian, B.; Qiao, J. Novel composite Nafion membranes modified with copper phthalocyanine tetrasulfonic acid tetrasodium salt for fuel cell application. J. Mater. 2019, 5, 252–257. [Google Scholar] [CrossRef]
- Muthuraja, P.; Prakash, S.; Shanmugam, V.M.; Radhakrsihnan, S.; Manisankar, P. Novel perovskite structured calcium titanate-PBI composite membranes for high-temperature PEM fuel cells: Synthesis and characterizations. Int. J. Hydrogen Energy 2018, 43, 4763–4772. [Google Scholar] [CrossRef]
- Ioana-Maria, N.; Aurora, J.; Victoria, C.; Cristian, B. Advanced polymeric materials based on PBI/SiO2 composite with high-performances designated for PEM-fuel cells. In Proceedings of the 2017 Electric Vehicles International Conference, Bucharest, Romania, 5–6 October 2017; pp. 1–6. [Google Scholar]
- Li, X.; Ke, C.; Qu, S.; Li, J.; Shao, Z.; Yi, B. High Temperature PEM Fuel Cells Based on Nafion®/SiO2 Composite Membrane, Energy Storage in the Emerging Era of Smart Grids. In Energy Storage in the Emerging Era of Smart Grids; Carbone, R., Ed.; IntechOpen: Reggio Calabria, Italy, 2011; pp. 279–298. ISBN 978-953-307-269-2. [Google Scholar]
- He, Y.; Wang, D.; Li, Q.; Huang, L.; Bao, H. Composite Polymer Electrolyte Membranes based on Nafion and Modified PVDF Electrospun Nanofiber Mats. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 677–681. [Google Scholar] [CrossRef]
- Yang, J.; Shen, P.K.; Varcoe, J.; Wei, Z. Nafion/polyaniline composite membranes specifically designed to allow proton exchange membrane fuel cells operation at low humidity. J. Power Sources 2009, 189, 1016–1019. [Google Scholar] [CrossRef]
- Trogadas, P.; Parrondo, J.; Ramani, V. Degradation mitigation in polymer electrolyte membranes using cerium oxide as a regenerative free-radical scavenger. Electrochem. Solid-State Lett. 2008, 11, 113–116. [Google Scholar] [CrossRef]
- Di Noto, V.; Boaretto, N.; Negro, E.; Pace, G. New inorganic-organic proton conducting membranes based on Nafion and hydrophobic fluoroalkylated silica nanoparticles. J. Power Sources 2010, 195, 7734–7742. [Google Scholar] [CrossRef]
- Giffin, G.A.; Piga, M.; Lavina, S.; Navarra, M.A.; D’Epifanio, A.; Scrosati, B.; Noto, V. Di Characterization of sulfated-zirconia/Nafion® composite membranes for proton exchange membrane fuel cells. J. Power Sources 2012, 198, 66–75. [Google Scholar] [CrossRef]
- Saccà, A.; Gatto, I.; Carbone, A.; Pedicini, R.; Passalacqua, E. ZrO2-Nafion composite membranes for polymer electrolyte fuel cells (PEFCs) at intermediate temperature. J. Power Sources 2006, 163, 47–51. [Google Scholar] [CrossRef]
- He, D.; Tang, H.; Kou, Z.; Pan, M.; Sun, X.; Zhang, J.; Mu, S. Engineered Graphene Materials: Synthesis and Applications for Polymer Electrolyte Membrane Fuel Cells. Adv. Mater. 2017, 29, 1601741. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton Conductivity of Composite Polyelectrolyte Membranes with Metal-Organic Frameworks for Fuel Cell Applications. Adv. Mater. Interfaces 2019, 6, 1801146. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Chen, C.; Ahn, W.S. Chromium terephthalate metal-organic framework MIL-101: Synthesis, functionalization, and applications for adsorption and catalysis. RSC Adv. 2014, 4, 52500–52525. [Google Scholar] [CrossRef]
- Anahidzade, N.; Abdolmaleki, A.; Dinari, M.; Firouz Tadavani, K.; Zhiani, M. Metal-organic framework anchored sulfonated poly(ether sulfone) as a high temperature proton exchange membrane for fuel cells. J. Memb. Sci. 2018, 565, 281–292. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.; Wang, D.; Li, Z.; Peng, X.; Liu, C.; Zheng, P. Metal Organic Frameworks Modified Proton Exchange Membranes for Fuel Cells. Front. Chem. 2020, 8, 694. [Google Scholar] [CrossRef]
- Kitao, T.; Zhang, Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of MOFs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef]
- Rao, Z.; Feng, K.; Tang, B.; Wu, P. Construction of well interconnected metal-organic framework structure for effectively promoting proton conductivity of proton exchange membrane. J. Memb. Sci. 2017, 533, 160–170. [Google Scholar] [CrossRef]
- Donnadio, A.; Narducci, R.; Casciola, M.; Marmottini, F.; D’Amato, R.; Jazestani, M.; Chiniforoshan, H.; Costantino, F. Mixed Membrane Matrices Based on Nafion/UiO-66/SO3H-UiO-66 Nano-MOFs: Revealing the Effect of Crystal Size, Sulfonation, and Filler Loading on the Mechanical and Conductivity Properties. ACS Appl. Mater. Interfaces 2017, 9, 42239–42246. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wiersum, A.D.; Llewellyn, P.L.; Guillerm, V.; Serre, C.; Maurin, G. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: A computational exploration. Chem. Commun. 2011, 47, 9603–9605. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, M.R.; Islamoglu, T.; Garibay, S.J.; Hupp, J.T.; Farha, O.K. Room Temperature Synthesis of UiO-66 and Thermal Modulation of Densities of Defect Sites. Chem. Mater. 2017, 29, 1357–1361. [Google Scholar] [CrossRef]
- Patel, H.A.; Mansor, N.; Gadipelli, S.; Brett, D.J.L.; Guo, Z. Superacidity in Nafion/MOF Hybrid Membranes Retains Water at Low Humidity to Enhance Proton Conduction for Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 30687–30691. [Google Scholar] [CrossRef] [Green Version]
- Rao, Z.; Tang, B.; Wu, P. Proton Conductivity of Proton Exchange Membrane Synergistically Promoted by Different Functionalized Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 22597–22603. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Zheng, L.; Shang, J.; Liu, X.; Yu, R.; Shui, J. Sequential Synthesis and Active-Site Coordination Principle of Precious Metal Single-Atom Catalysts for Oxygen Reduction Reaction and PEM Fuel Cells. Adv. Energy Mater. 2020, 10, 2000689. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, F.; Feng, W.; Zou, X.; Zhao, C.; Na, H.; Liu, C.; Sun, F.; Zhu, G. From metal-organic framework (MOF) to MOF-polymer composite membrane: Enhancement of low-humidity proton conductivity. Chem. Sci. 2013, 4, 983–992. [Google Scholar] [CrossRef]
- Horike, S.; Umeyama, D.; Inukai, M.; Itakura, T.; Kitagawa, S. Coordination-network-based ionic plastic crystal for anhydrous proton conductivity. J. Am. Chem. Soc. 2012, 134, 7612–7615. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, J.; Zhang, B.; Wang, L. Effect of chemical structure and degree of branching on the stability of proton exchange membranes based on sulfonated polynaphthylimides. Polymers 2020, 12, 652. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, N. A property trend study of polybenzimidazole using molecular modeling. Polym. Eng. Sci. 1994, 34, 434–437. [Google Scholar] [CrossRef]
- Sun, R.; Xia, Z.; Yang, C.; Jing, F.; Wang, S.; Sun, G. Experimental measurement of proton conductivity and electronic conductivity of membrane electrode assembly for proton exchange membrane fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 912–917. [Google Scholar] [CrossRef]
- Sana, B.; Koyilapu, R.; Dineshkumar, S.; Muthusamy, A.; Jana, T. High temperature PEMs developed from the blends of Polybenzimidazole and poly(azomethine-ether). J. Polym. Res. 2019, 26. [Google Scholar] [CrossRef]
- Aaron, D.; Yiacoumi, S.; Tsouris, C. Effects of proton-exchange membrane fuel-cell operating conditions on charge transfer resistances measured by electrochemical impedance spectroscopy. Sep. Sci. Technol. 2008, 43, 2307–2320. [Google Scholar] [CrossRef]
- Paul, D.K.; McCreery, R.; Karan, K. Proton Transport Property in Supported Nafion Nanothin Films by Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2014, 161, F1395–F1402. [Google Scholar] [CrossRef] [Green Version]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compañ, V. Recent progress in the development of composite membranes based on polybenzimidazole for high temperature proton exchange membrane (PEM) fuel cell applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Li, Q.; Yin, Q.; Zheng, Y.S.; Sui, Z.J.; Zhou, X.G.; Chen, D.; Zhu, Y.A. Insights into Hydrogen Transport Behavior on Perovskite Surfaces: Transition from the Grotthuss Mechanism to the Vehicle Mechanism. Langmuir 2019, 35, 9962–9969. [Google Scholar] [CrossRef]
- Luduena, G.A.; Kuhne, T.D.; Sebastiani, D. Comment on “Mixed Grotthuss and vehicle transport mechanism in proton conducting polymers from Ab initio molecular dynamics simulations”. Chem. Mater. 2011, 23, 1424–1429. [Google Scholar] [CrossRef]
- Singha, S.; Koyilapu, R.; Dana, K.; Jana, T. Polybenzimidazole-Clay Nanocomposite Membrane for PEM fuel cell: Effect of organomodifier structure. Polymer 2019, 167, 13–20. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; Wang, J.; Zhang, T.; Shi, B.; Liu, J.; Cao, S. Enhanced anhydrous proton conductivity of polymer electrolyte membrane enabled by facile ionic liquid-based hoping pathways. J. Memb. Sci. 2015, 476, 136–147. [Google Scholar] [CrossRef]
- Liu, Z.; Tsou, Y.M.; Calundann, G.; De Castro, E. New process for high temperature polybenzimidazole membrane production and its impact on the membrane and the membrane electrode assembly. J. Power Sources 2011, 196, 1055–1060. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A review of polymer-nanocomposite electrolyte membranes for fuel cell application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Hogarth, W.H.J.; Diniz Da Costa, J.C.; Lu, G.Q. Solid acid membranes for high temperature (>140 ° C) proton exchange membrane fuel cells. J. Power Sources 2005, 142, 223–237. [Google Scholar] [CrossRef]
- Lafitte, B.; Jannasch, P. On the Prospects for Phosphonated Polymers as Proton-Exchange Fuel Cell Membranes. In Advances in Fuel Cells; Kreuer, K.-D., Van Nguyen, T., Eds.; Elsevier: Lund, Sweden, 2007; Volume 1, pp. 119–185. ISBN 9780080453941. [Google Scholar]
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in proton conductors for fuel-cell applications: Simulations, elementary reactions, and phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Z.; Fu, Y.; Manthiram, A. Novel blend membranes based on acid-base interactions for fuel cells. Polymers 2012, 4, 1627–1644. [Google Scholar] [CrossRef]
- Yamada, M.; Honma, I. Anhydrous proton conducting polymer electrolytes based on poly(vinylphosphonic acid)-heterocycle composite material. Polymer 2005, 46, 2986–2992. [Google Scholar] [CrossRef]
- Fontanella, J.J.; Wintersgill, M.C.; Wainright, J.S.; Savinell, R.F.; Litt, M. High pressure electrical conductivity studies of acid doped polybenzimidazole. Electrochim. Acta 1998, 43, 1289–1294. [Google Scholar] [CrossRef]
- Fu, Y.-Z.; Manthiram, A. Nafion–Imidazole–H3PO4 Composite Membranes for Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2007, 154, B8. [Google Scholar] [CrossRef]
- Jung, H.Y.; Park, J.K. Blend membranes based on sulfonated poly(ether ether ketone) and poly(vinylidene fluoride) for high performance direct methanol fuel cell. Electrochim. Acta 2007, 52, 7464–7468. [Google Scholar] [CrossRef]
- Du, C.; Zhao, B.; Chen, X.B.; Birbilis, N.; Yang, H. Effect of water presence on choline chloride-2urea ionic liquid and coating platings from the hydrated ionic liquid. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pei, P.; Wang, M.; Chen, D.; Ren, P.; Zhang, L. Key technologies for polymer electrolyte membrane fuel cell systems fueled impure hydrogen. Prog. Nat. Sci. Mater. Int. 2020, 30, 751–763. [Google Scholar] [CrossRef]
- Schenk, A.; Cermenek, B.; Hacker, V. Other polymer electrolyte fuel cells. In Fuel Cells and Hydrogen: From Fundamentals to Applied Research; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 91–115. ISBN 9780128114599. [Google Scholar]
- Wan, Z.; Chang, H.; Shu, S.; Wang, Y.; Tang, H. A review on cold start of proton exchange membrane fuel cells. Energies 2014, 7, 3179–3203. [Google Scholar] [CrossRef] [Green Version]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Yang, C.; Costamagna, P.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Approaches and technical challenges to high temperature operation of proton exchange membrane fuel cells. J. Power Sources 2001, 103, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.A.; Sulong, A.B.; Loh, K.S.; Majlan, E.H.; Husaini, T.; Rosli, R.E. Acid doped polybenzimidazoles based membrane electrode assembly for high temperature proton exchange membrane fuel cell: A review. Int. J. Hydrogen Energy 2017, 42, 9156–9179. [Google Scholar] [CrossRef]
- Branco, C.M.; El-kharouf, A.; Du, S. Materials for Polymer Electrolyte Membrane Fuel Cells (PEMFCs): Electrolyte Membrane, Gas Diffusion Layers and Bipolar Plates; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; ISBN 9780128157329. [Google Scholar]
- Soltanimehr, S.; Rezaei, F.; Rahimpour, M.R. Impact assessment of exhaust gas emissions from cogeneration PEMFC systems. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–64. ISBN 9780128178072. [Google Scholar]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)-A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Adibi, M. Novel composite membranes based on PBI and dicationic ionic liquids for high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2016, 205, 142–152. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Reimer, U.; Ehlert, J.; Janßen, H.; Lehnert, W. Water distribution in high temperature polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2016, 41, 1837–1845. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Bagheri, A.; Beydaghi, H.; Hooshyari, K. Enhanced properties of SPEEK with incorporating of PFSA and barium strontium titanate nanoparticles for application in DMFCs. Int. J. Energy Res. 2019, 43, 4840–4853. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Effect of deep eutectic solvents hydrogen bond acceptor on the anhydrous proton conductivity of Nafion membrane for fuel cell applications. J. Memb. Sci. 2020, 605, 118116. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Naji, L.; Enhessari, M. Nanocomposite proton exchange membranes based on Nafion containing Fe2TiO5 nanoparticles in water and alcohol environments for PEMFC. J. Memb. Sci. 2014, 454, 74–81. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Potential use of deep eutectic solvents (DESs) to enhance anhydrous proton conductivity of Nafion 115® membrane for fuel cell applications. J. Memb. Sci. 2020, 611, 118217. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Li, X.; Peng, J.; Hu, W.; Liu, B. Toward enhanced conductivity of high-temperature proton exchange membranes: Development of novel PIM-1 reinforced PBI alloy membranes. Chem. Commun. 2019, 55, 6491–6494. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Lee, S.; Ghorpade, R.V.; Konovalova, A.; Jang, J.H.; Kim, H.J.; Han, J.; Henkensmeier, D.; Han, H. Polybenzimidazole (PBI-OO) based composite membranes using sulfophenylated TiO2 as both filler and crosslinker, and their use in the HT-PEM fuel cell. J. Memb. Sci. 2018, 560, 11–20. [Google Scholar] [CrossRef]
- Shabanikia, A.; Javanbakht, M.; Amoli, H.S.; Hooshyari, K.; Enhessari, M. Novel nanocomposite membranes based on polybenzimidazole and Fe2TiO5 nanoparticles for proton exchange membrane fuel cells. Ionics 2015, 21, 2227–2236. [Google Scholar] [CrossRef]
- Shabanikia, A.; Javanbakht, M.; Amoli, H.S.; Hooshyari, K.; Enhessari, M. Polybenzimidazole/strontium cerate nanocomposites with enhanced proton conductivity for proton exchange membrane fuel cells operating at high temperature. Electrochim. Acta 2015, 154, 370–378. [Google Scholar] [CrossRef]
- Chen, J.C.; Hsiao, Y.R.; Liu, Y.C.; Chen, P.Y.; Chen, K.H. Polybenzimidazoles containing heterocyclic benzo[c]cinnoline structure prepared by sol-gel process and acid doping level adjustment for high temperature PEMFC application. Polymer 2019, 182, 121814. [Google Scholar] [CrossRef]
- Hooshyari, K.; Rezania, H.; Vatanpour, V.; Salarizadeh, P.; Askari, M.B.; Beydaghi, H.; Enhessari, M. High temperature membranes based on PBI/sulfonated polyimide and doped-perovskite nanoparticles for PEM fuel cells. J. Memb. Sci. 2020, 612, 118436. [Google Scholar] [CrossRef]
- Ogawa, T.; Kamiguchi, K.; Tamaki, T.; Imai, H.; Yamaguchi, T. Differentiating Grotthuss proton conduction mechanisms by nuclear magnetic resonance spectroscopic analysis of frozen samples. Anal. Chem. 2014, 86, 9362–9366. [Google Scholar] [CrossRef]
- Asadi Tashvigh, A.; Luo, L.; Chung, T.S.; Weber, M.; Maletzko, C. Performance enhancement in organic solvent nanofiltration by double crosslinking technique using sulfonated polyphenylsulfone (sPPSU) and polybenzimidazole (PBI). J. Memb. Sci. 2018, 551, 204–213. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.T.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Shabanikia, A.; Enhessari, M. Fabrication BaZrO3/PBI-based nanocomposite as a new proton conducting membrane for high temperature proton exchange membrane fuel cells. J. Power Sources 2015, 276, 62–72. [Google Scholar] [CrossRef]
- Vilčiauskas, L.; Tuckerman, M.E.; Bester, G.; Paddison, S.J.; Kreuer, K.D. The mechanism of proton conduction in phosphoric acid. Nat. Chem. 2012, 4, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, H.; Kim, D.K.; Lee, W.J.; Choi, I.; Kim, H.J.; Kim, S.K. Performance deterioration and recovery in high-temperature polymer electrolyte membrane fuel cells: Effects of deliquescence of phosphoric acid. Int. J. Hydrogen Energy 2020, 45, 32844–32855. [Google Scholar] [CrossRef]
- Korte, C.; Conti, F.; Wackerl, J.; Lehnert, W. Phosphoric acid and its interactions with polybenzimidazole-type Polymers. In High Temperature Polymer Electrolyte Membrane Fuel Cells; Springer: Cham, Switzerland, 2016; pp. 169–194. ISBN 9783319170824. [Google Scholar]
- Maity, S.; Singha, S.; Jana, T. Low acid leaching PEM for fuel cell based on polybenzimidazole nanocomposites with protic ionic liquid modified silica. Polymer 2015, 66, 76–85. [Google Scholar] [CrossRef]
- Sana, B.; Jana, T. Polybenzimidazole composite with acidic surfactant like molecules: A unique approach to develop PEM for fuel cell. Eur. Polym. J. 2016, 84, 421–434. [Google Scholar] [CrossRef]
- Rajabi, Z.; Javanbakht, M.; Hooshyari, K.; Badiei, A.; Adibi, M. High temperature composite membranes based on polybenzimidazole and dendrimer amine functionalized SBA-15 mesoporous silica for fuel cells. New J. Chem. 2020, 44, 5001–5018. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Adibi, M. Novel composite membranes based on dicationic ionic liquid and polybenzimidazole mixtures as strategy for enhancing thermal and electrochemical properties of proton exchange membrane fuel cells applications at high temperature. Int. J. Hydrogen Energy 2016, 41, 10870–10883. [Google Scholar] [CrossRef]
- Hooshyari, K.; Moradi, M.; Salarizadeh, P. Novel nanocomposite membranes based on PBI and doped-perovskite nanoparticles as a strategy for improving PEMFC performance at high temperatures. Int. J. Energy Res. 2020, 44, 2617–2633. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Wainright, J.S.; Litt, M.H.; Savinell, R.F. Conductivity of PBI Membranes for High-Temperature Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2004, 151, A8. [Google Scholar] [CrossRef]
- Guan, Y.; Pu, H.; Jin, M.; Chang, Z.; Modestov, A.D. Proton conducting membranes based on poly(2,2′-imidazole-5,5′-bibenzimidazole). Fuel Cells 2012, 12, 124–131. [Google Scholar] [CrossRef]
- Molleo, M.A.; Chen, X.; Ploehn, H.J.; Fishel, K.J.; Benicewicz, B.C. High Polymer Content 3,5-Pyridine-Polybenzimidazole Copolymer Membranes with Improved Compressive Properties. Fuel Cells 2014, 14, 16–25. [Google Scholar] [CrossRef]
- Kumar, B.S.; Sana, B.; Unnikrishnan, G.; Jana, T.; Kumar, K.S.S. Polybenzimidazole co-polymers: Their synthesis, morphology and high temperature fuel cell membrane properties. Polym. Chem. 2020, 11, 1043–1054. [Google Scholar] [CrossRef]
- Pingitore, A.T.; Huang, F.; Qian, G.; Benicewicz, B.C. Durable High Polymer Content m/p-Polybenzimidazole Membranes for Extended Lifetime Electrochemical Devices. ACS Appl. Energy Mater. 2019, 2, 1720–1726. [Google Scholar] [CrossRef]
- Jahangiri, S.; Aravi, İ.; Işıkel Şanlı, L.; Menceloğlu, Y.Z.; Özden-Yenigün, E. Fabrication and optimization of proton conductive polybenzimidazole electrospun nanofiber membranes. Polym. Adv. Technol. 2018, 29, 594–602. [Google Scholar] [CrossRef]
- Joseph, D.; Krishnan, N.N.; Henkensmeier, D.; Jang, J.H.; Choi, S.H.; Kim, H.J.; Han, J.; Nam, S.W. Thermal crosslinking of PBI/sulfonated polysulfone based blend membranes. J. Mater. Chem. A 2017, 5, 409–417. [Google Scholar] [CrossRef]
- Mader, J.A.; Benicewicz, B.C. Synthesis and properties of segmented block copolymers of functionalised polybenzimidazoles for high-temperature pem fuel cells. Fuel Cells 2011, 11, 222–237. [Google Scholar] [CrossRef]
- Huang, F.; Pingitore, A.T.; Benicewicz, B.C. Electrochemical Hydrogen Separation from Reformate Using High-Temperature Polybenzimidazole (PBI) Membranes: The Role of Chemistry. ACS Sustain. Chem. Eng. 2020, 8, 6234–6242. [Google Scholar] [CrossRef]
- Hooshyari, K.; Heydari, S.; Javanbakht, M.; Beydaghi, H.; Enhessari, M. Fabrication and performance evaluation of new nanocomposite membranes based on sulfonated poly(phthalazinone ether ketone) for PEM fuel cells. RSC Adv. 2020, 10, 2709–2721. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ma, H.; Shen, Y.; Hu, W.; Jiang, Z.; Liu, B.; Guiver, M.D. Dimensionally-stable phosphoric acid–doped polybenzimidazoles for high-temperature proton exchange membrane fuel cells. J. Power Sources 2016, 336, 391–400. [Google Scholar] [CrossRef]
- Ishihara, T. (Ed.) Perovskite Oxide for Solid Oxide Fuel Cells Fuel Cells and Hydrogen Energy; Springer: Dordrecht, The Netherlands, 2009; ISBN 9780387777078. [Google Scholar]
- Dunne, P.W.; Starkey, C.L.; Munn, A.S.; Tang, S.V.Y.; Luebben, O.; Shvets, I.; Ryder, A.G.; Casamayou-Boucau, Y.; Morrison, L.; Lester, E.H. Bench- and pilot-scale continuous-flow hydrothermal production of barium strontium titanate nanopowders. Chem. Eng. J. 2016, 289, 433–441. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, X.; Aili, D.; Pan, C.; Li, Q.; Fang, J. Poly(imide benzimidazole)s for high temperature polymer electrolyte membrane fuel cells. J. Memb. Sci. 2014, 454, 351–358. [Google Scholar] [CrossRef]
- Maity, S.; Jana, T. Polybenzimidazole block copolymers for fuel cell: Synthesis and studies of block length effects on nanophase separation, mechanical properties, and proton conductivity of PEM. ACS Appl. Mater. Interfaces 2014, 6, 6851–6864. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Fang, M.; Chen, J.; Liu, X.; Yin, B.; Wang, L. Synthesis and preparation of branched block polybenzimidazole membranes with high proton conductivity and single-cell performance for use in high temperature proton exchange membrane fuel cells. J. Memb. Sci. 2020, 602, 117981. [Google Scholar] [CrossRef]
- Suda, T.; Yamazaki, K.; Kawakami, H. Syntheses of sulfonated star-hyperbranched polyimides and their proton exchange membrane properties. J. Power Sources 2010, 195, 4641–4646. [Google Scholar] [CrossRef]
- Pan, H.; Chen, S.; Jin, M.; Chang, Z.; Pu, H. Preparation and properties of sulfonated polybenzimidazole-polyimide block copolymers as electrolyte membranes. Ionics 2018, 24, 1629–1638. [Google Scholar] [CrossRef]
- Skorikova, G.; Rauber, D.; Aili, D.; Martin, S.; Li, Q.; Henkensmeier, D.; Hempelmann, R. Protic ionic liquids immobilized in phosphoric acid-doped polybenzimidazole matrix enable polymer electrolyte fuel cell operation at 200 °C. J. Memb. Sci. 2020, 608, 118188. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Ding, L.; Li, X. Advanced acid-base blend ion exchange membranes with high performance for vanadium flow battery application. J. Memb. Sci. 2018, 553, 25–31. [Google Scholar] [CrossRef]
- Nagamani, C.; Versek, C.; Thorn, M.; Tuominen, M.T.; Thayumanavan, S. Proton conduction in 1H-1,2,3-triazole polymers: Imidazole-like or pyrazole-like? J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1851–1858. [Google Scholar] [CrossRef]
- Ghomari, K.; Boukoussa, B.; Hamacha, R.; Bengueddach, A.; Roy, R.; Azzouz, A. Preparation of dendrimer polyol/mesoporous silica nanocomposite for reversible CO2 adsorption: Effect of pore size and polyol content. Sep. Sci. Technol. 2017, 52, 2421–2428. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kim, N.H.; Lee, J.H. Effects of ionic liquid-functionalized mesoporous silica on the proton conductivity of acid-doped poly(2,5-benzimidazole) composite membranes for high-temperature fuel cells. J. Memb. Sci. 2014, 449, 136–145. [Google Scholar] [CrossRef]
- Bouazizi, N.; Louhichi, S.; Ouargli, R.; Bargougui, R.; Vieillard, J.; Le Derf, F.; Azzouz, A. Cuo-loaded SBA-15@ZnO with improved electrical properties and affinity towards hydrogen. Appl. Surf. Sci. 2017, 404, 146–153. [Google Scholar] [CrossRef]
- Imran, M.A.; He, G.; Wu, X.; Yan, X.; Li, T.; Khan, A.S. Fabrication and characterization of sulfonated polybenzimidazole/sulfonated imidized graphene oxide hybrid membranes for high temperature proton exchange membrane fuel cells. J. Appl. Polym. Sci. 2019, 136, 1–13. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di, V. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ionics 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Cai, Y.; Yue, Z.; Xu, S. A novel polybenzimidazole composite modified by sulfonated graphene oxide for high temperature proton exchange membrane fuel cells in anhydrous atmosphere. J. Appl. Polym. Sci. 2017, 134, 44986. [Google Scholar] [CrossRef]
- Nambi Krishnan, N.; Konovalova, A.; Aili, D.; Li, Q.; Park, H.S.; Jang, J.H.; Kim, H.J.; Henkensmeier, D. Thermally crosslinked sulfonated polybenzimidazole membranes and their performance in high temperature polymer electrolyte fuel cells. J. Memb. Sci. 2019, 588, 117218. [Google Scholar] [CrossRef]
- Wang, S.; Sun, P.; Li, Z.; Liu, G.; Yin, X. Comprehensive performance enhancement of polybenzimidazole based high temperature proton exchange membranes by doping with a novel intercalated proton conductor. Int. J. Hydrogen Energy 2018, 43, 9994–10003. [Google Scholar] [CrossRef]
- Sun, P.; Li, Z.; Jin, L.; Yang, Y.; Wang, S.; Yin, X.; Wang, Y. Pre-Oxidized Acrylic Fiber Reinforced Ferric Sulfophenyl Phosphate-Doped Polybenzimidazole-Based High-Temperature Proton Exchange Membrane. Macromol. Mater. Eng. 2017, 302, 1600468. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Montero, Á.; Sahuquillo, Ó.; Giménez, E.; Compañ, V. Ionic liquid composite polybenzimidazol membranes for high temperature PEMFC applications. Polymers 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K.; Seo, J.; Nam, K.-H.; Han, H. Polybenzimidazole/Inorganic Composite Membrane with Advanced Performance for High Temperature Polymer Electrolyte Membrane Fuel Cells. Polym. Polym. Compos. 2017, 38, 87–95. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Li, X.; Xu, R.; Zhong, J.; Chen, R.; Gu, X. Synthesis and characterization of polybenzimidazole/α-zirconium phosphate composites as proton exchange membrane. Polym. Eng. Sci. 2016, 56, 622–628. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Lu, S.; Kuang, H.; Bradley, J.; De Marco, R.; Aili, D.; Li, Q.; Cui, C.Q.; Jiang, S.P. High CO tolerance of new SiO2 doped phosphoric acid/polybenzimidazole polymer electrolyte membrane fuel cells at high temperatures of 200–250 °C. Int. J. Hydrogen Energy 2018, 22487–22499. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Gao, L.; Wang, J.; Xu, Y.; He, R. Novel composite membranes of triazole modified graphene oxide and polybenzimidazole for high temperature polymer electrolyte membrane fuel cell applications. RSC Adv. 2015, 5, 101049–101054. [Google Scholar] [CrossRef]
- Yang, J.; Gao, L.; Wang, J.; Xu, Y.; Liu, C.; He, R. Strengthening Phosphoric Acid Doped Polybenzimidazole Membranes with Siloxane Networks for Using as High Temperature Proton Exchange Membranes. Macromol. Chem. Phys. 2017, 218, 1–10. [Google Scholar] [CrossRef]
- Yang, J.; Aili, D.; Li, Q.; Cleemann, L.N.; Jensen, J.O.; Bjerrum, N.J.; He, R. Covalently cross-linked sulfone polybenzimidazole membranes with poly(vinylbenzyl chloride) for fuel cell applications. ChemSusChem 2013, 6, 275–282. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Liu, F.; Wang, D.; Li, J.; Mao, T.; Liu, G.; Wang, X.; Xu, J.; Wang, Z. Base-acid doped polybenzimidazole with high phosphoric acid retention for HT-PEMFC applications. J. Memb. Sci. 2020, 596, 117722. [Google Scholar] [CrossRef]
- Eguizábal, A.; Lemus, J.; Roda, V.; Urbiztondo, M.; Barreras, F.; Pina, M.P. Nanostructured electrolyte membranes based on zeotypes, protic ionic liquids and porous PBI membranes: Preparation, characterization and MEA testing. Int. J. Hydrogen Energy 2012, 37, 7221–7234. [Google Scholar] [CrossRef]
- Özdemir, Y.; Üregen, N.; Devrim, Y. Polybenzimidazole based nanocomposite membranes with enhanced proton conductivity for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2648–2657. [Google Scholar] [CrossRef]
- Aili, D.; Allward, T.; Alfaro, S.M.; Hartmann-Thompson, C.; Steenberg, T.; Hjuler, H.A.; Li, Q.; Jensen, J.O.; Stark, E.J. Polybenzimidazole and sulfonated polyhedral oligosilsesquioxane composite membranes for high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2014, 140, 182–190. [Google Scholar] [CrossRef]
- Barati, S.; Abdollahi, M.; Mehdipourghazi, M.; Khoshandam, B. High temperature proton exchange porous membranes based on polybenzimidazole/ lignosulfonate blends: Preparation, morphology and physical and proton conductivity properties. Int. J. Hydrogen Energy 2019, 44, 30440–30453. [Google Scholar] [CrossRef]
- Barati, S.; Abdollahi, M.; Khoshandam, B.; Mehdipourghazi, M. Highly proton conductive porous membranes based on polybenzimidazole/ lignin blends for high temperatures proton exchange membranes: Preparation, characterization and morphology- proton conductivity relationship. Int. J. Hydrogen Energy 2018, 43, 19681–19690. [Google Scholar] [CrossRef]
- Hazarika, M.; Jana, T. Novel proton exchange membrane for fuel cell developed from blends of polybenzimidazole with fluorinated polymer. Eur. Polym. J. 2013, 49, 1564–1576. [Google Scholar] [CrossRef]
- Li, Q.; Pan, C.; Jensen, J.O.; Noye, P.; Bjerrum, N.J. Cross-Linked Polybenzimidazole Membranes for Fuel Cells. Chem. Mater. 2007, 19, 350–352. [Google Scholar] [CrossRef]
- Chang, Z.; Pu, H.; Wan, D.; Liu, L.; Yuan, J.; Yang, Z. Chemical oxidative degradation of Polybenzimidazole in simulated environment of fuel cells. Polym. Degrad. Stab. 2009, 94, 1206–1212. [Google Scholar] [CrossRef]
- LaConti, A.B.; Liu, H.; Mittelsteadt, C.; McDonald, R.C. Polymer Electrolyte Membrane Degradation Mechanisms in Fuel Cells—Findings Over the Past 30 Years and Comparison with Electrolyzers. ECS Trans. 2006, 1, 199–219. [Google Scholar] [CrossRef]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal Stability of Proton Conducting Acid Doped Polybenzimidazole in Simulated Fuel Cell Environments. J. Electrochem. Soc. 1996, 143, 1225–1232. [Google Scholar] [CrossRef]
- Jaffe, M.; Haider, M.I.; Menczel, J.; Rafalko, J. Thermal characterization of high performance PBI and 6F polymers and their alloys. Polym. Eng. Sci. 1992, 32, 1236–1241. [Google Scholar] [CrossRef]
- Wang, J.T.; Savinell, R.F.; Wainright, J.; Litt, M.; Yu, H. A H2/O2 fuel cell using acid doped polybenzimidazole as polymer electrolyte. Electrochim. Acta 1996, 41, 193–197. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Yousfi-Steiner, N.; Moçotéguy, P.; Candusso, D.; Hissel, D. A review on polymer electrolyte membrane fuel cell catalyst degradation and starvation issues: Causes, consequences and diagnostic for mitigation. J. Power Sources 2009, 194, 130–145. [Google Scholar] [CrossRef]
- Akita, T.; Taniguchi, A.; Maekawa, J.; Siroma, Z.; Tanaka, K.; Kohyama, M.; Yasuda, K. Analytical TEM study of Pt particle depositiaon in the proton-exchange membrane of a membrane-electrode-assembly. J. Power Sources 2006, 159, 461–467. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Wang, Z.; Yao, X.; Wei, Y.; Kang, Y. Mitigating Metal Dissolution and Redeposition of Pt-Co Catalysts in PEM Fuel Cells: Impacts of Structural Ordering and Particle Size Mitigating Metal Dissolution and Redeposition of Pt-Co Catalysts in PEM Fuel Cells: Impacts of Structural Ordering and P. J. Electrochem. Soc. 2020, 167, 064520. [Google Scholar] [CrossRef]
- Borup, R.L.; Davey, J.R.; Garzon, F.H.; Wood, D.L.; Inbody, M.A. PEM fuel cell electrocatalyst durability measurements. J. Power Sources 2006, 163, 76–81. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Xing, D.; Shao, Z.G. The stability of Pt/C catalyst in H3PO4/PBI PEMFC during high temperature life test. J. Power Sources 2007, 164, 126–133. [Google Scholar] [CrossRef]
- Galbiati, S.; Baricci, A.; Casalegno, A.; Marchesi, R. Degradation in phosphoric acid doped polymer fuel cells: A 6000 h parametric investigation. Int. J. Hydrogen Energy 2013, 38, 6469–6480. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Baurmeister, J. Properties of high-temperature PEFC Celtec®-P 1000 MEAs in start/stop operation mode. J. Power Sources 2008, 176, 428–434. [Google Scholar] [CrossRef]
- Hartnig, C.; Schmidt, T.J. Simulated start-stop as a rapid aging tool for polymer electrolyte fuel cell electrodes. J. Power Sources 2011, 196, 5564–5572. [Google Scholar] [CrossRef]
- Mack, F.; Heissler, S.; Laukenmann, R.; Zeis, R. Phosphoric acid distribution and its impact on the performance of polybenzimidazole membranes. J. Power Sources 2014, 270, 627–633. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Hu, M.; Cheng, X.; He, K.; Mao, L. A Comparative Study of Using Polarization Curve Models in Proton Exchange Membrane Fuel Cell Degradation Analysis. Energies 2020, 13, 3759. [Google Scholar] [CrossRef]
- Bharath, K.V.S.; Blaabjerg, F.; Haque, A.; Khan, M.A. Model-based data driven approach for fault identification in proton exchange membrane fuel cell. Energies 2020, 13, 3144. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, B.S.; Yoo, S.J.; Kim, S.K.; Hwang, S.J.; Kim, H.J.; Cho, E.; Henkensmeier, D.; Yun, J.W.; Nam, S.W.; et al. Development of a galvanostatic analysis technique as an in-situ diagnostic tool for PEMFC single cells and stacks. Int. J. Hydrogen Energy 2012, 37, 5891–5900. [Google Scholar] [CrossRef]
- Najafi, B.; Bonomi, P.; Casalegno, A.; Rinaldi, F.; Baricci, A. Rapid fault diagnosis of PEM fuel cells through optimal electrochemical impedance spectroscopy tests. Energies 2020, 13, 3643. [Google Scholar] [CrossRef]
- Wang, H.; Macomber, C.; Christ, J.; Bender, G.; Pivovar, B.; Dinh, H.N. Evaluating the Influence of PEMFC System Contaminants on the Performance of Pt Catalyst via Cyclic Voltammetry. Electrocatalysis 2014, 5, 62–67. [Google Scholar] [CrossRef]
- Dobbelaere, T.; Vereecken, P.M.; Detavernier, C. A USB-controlled potentiostat/galvanostat for thin-film battery characterization. HardwareX 2017, 2, 34–49. [Google Scholar] [CrossRef]
- Araya, S.S.; Andreasen, S.J.; Kær, S.K. Parametric sensitivity tests-european polymer electrolyte membrane fuel cell stack test procedures. J. Fuel Cell Sci. Technol. 2014, 11, 061007. [Google Scholar] [CrossRef]
- Pilinski, N.; Rastedt, M.; Wagner, P. Investigation of Phosphoric Acid Distribution in PBI based HT-PEM Fuel Cells. ECS Trans. 2015, 69, 323–335. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Long-term cell degradation mechanism in high-temperature proton exchange membrane fuel cells. J. Power Sources 2012, 210, 366–373. [Google Scholar] [CrossRef]
- Rangel-Cárdenas, A.L.; Koper, G.J.M. Transport in Proton Exchange Membranes for Fuel Cell Applications—A Systematic Non-Equilibrium Approach. Materials 2017, 10, 576. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.H. Polyimide proton exchange membranes. In Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Yang, S.-Y., Ed.; Elsevier Inc.: Beijing, China, 2018; pp. 323–383. ISBN 9780128126400. [Google Scholar]
- Selyanchyn, O.; Selyanchyn, R.; Lyth, S.M. A Review of Proton Conductivity in Cellulosic Materials. Front. Energy Res. 2020, 8, 596164. [Google Scholar] [CrossRef]
- Heimerdinger, P.; Rosin, A.; Danzer, M.A.; Gerdes, T. A novel method for humidity-dependent through-plane impedance measurement for proton conducting polymer membranes. Membranes 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, K. Characterizing Through-Plane and In-Plane Ionic Conductivity of Polymer Electrolyte Membranes. ECS Trans. 2011, 41, 1371–1380. [Google Scholar] [CrossRef]
- Gardner, C.L.; Anantaraman, A.V. Studies on ion-exchange membranes. II. Measurement of the anisotropic conductance of Nafion®. J. Electroanal. Chem. 1998, 449, 209–214. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymeric proton conducting membranes for medium temperature fuel cells (110–160 °C). J. Memb. Sci. 2001, 185, 73–81. [Google Scholar] [CrossRef]
- Xie, Z.; Song, C.; Andreaus, B.; Navessin, T.; Shi, Z.; Zhang, J.; Holdcroft, S. Discrepancies in the Measurement of Ionic Conductivity of PEMs Using Two- and Four-Probe AC Impedance Spectroscopy. J. Electrochem. Soc. 2006, 153, E173. [Google Scholar] [CrossRef]
- Ma, S.; Siroma, Z.; Tanaka, H. Anisotropic Conductivity over In-Plane and Thickness Directions in Nafion-117. J. Electrochem. Soc. 2006, 153, A2274. [Google Scholar] [CrossRef]
- Dippel, T.; Kreuer, K.D.; Lassègues, J.C.; Rodriguez, D. Proton conductivity in fused phosphoric acid; a 1H/31P PFG-NMR and QNS study. Solid State Ionics 1993, 61, 41–46. [Google Scholar] [CrossRef]
- Wannek, C.; Kohnen, B.; Oetjen, H.F.; Lippert, H.; Mergel, J. Durability of ABPBI-based MEAs for high temperature PEMFCs at different operating conditions. Fuel Cells 2008, 8, 87–95. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Influence of the phosphoric acid-doping level in a polybenzimidazole membrane on the cell performance of high-temperature proton exchange membrane fuel cells. J. Power Sources 2009, 189, 943–949. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, J.; Liu, F.; Wang, X.; Chen, H.; Wang, D.; Ni, H.; Wang, Z. Benzimidazole grafted polybenzimidazole cross-linked membranes with excellent PA stability for high-temperature proton exchange membrane applications. Appl. Surf. Sci. 2019, 465, 332–339. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Bach, A.; Jensen, J.O.; Bjerrum, N.J. Physicochemical properties of phosphoric acid doped polybenzimidazole membranes for fuel cells. J. Memb. Sci. 2006, 277, 38–45. [Google Scholar] [CrossRef]
- Garland, N.; Benjamin, T.; Kopasz, J. DOE Fuel Cell Program: Durability Technical Targets and Testing Protocols. ECS Trans. 2019, 11, 923–931. [Google Scholar] [CrossRef]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Greene, D.L. Status and Outlook for the U.S. Non-Automotive Fuel Cell Industry: Impacts of Government Policies and Assessment of Future Opportunities; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2012; ISBN 9781622575589. [Google Scholar]
- Ahluwalia, R.K.; Wang, X.; Kwon, J.; Rousseau, A.; Kalinoski, J.; James, B.; Marcinkoski, J. Performance and cost of automotive fuel cell systems with ultra-low platinum loadings. J. Power Sources 2011, 196, 4619–4630. [Google Scholar] [CrossRef]
- Polymer Fuel Cells–Cost Reduction and Market Potential; Carbon Trust: London, UK, 2012.
- Wilberforce, T.; Olabi, A.G. Performance prediction of proton exchange membrane fuel cells (PEMFC) using adaptive neuro inference system (ANFIS). Sustainability 2020, 12, 4952. [Google Scholar] [CrossRef]
- Rahimi-Esbo, M.; Ranjbar, A.A.; Ramiar, A.; Alizadeh, E.; Aghaee, M. Improving PEM fuel cell performance and effective water removal by using a novel gas flow field. Int. J. Hydrogen Energy 2016, 41, 3023–3037. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. PBI-based polymer membranes for high temperature fuel cells—Preparation, characterization and fuel cell demonstration. Fuel Cells 2004, 4, 147–159. [Google Scholar] [CrossRef]
- Staffell, I.; Green, R. The cost of domestic fuel cell micro-CHP systems. Int. J. Hydrogen Energy 2013, 38, 1088–1102. [Google Scholar] [CrossRef] [Green Version]
- Dyantyi, N.; Parsons, A.; Sita, C.; Pasupathi, S. PEMFC for aeronautic applications: A review on the durability aspects. Open Eng. 2017, 7, 287–302. [Google Scholar] [CrossRef]
- Thampan, T.; Shah, D.; Cook, C.; Novoa, J.; Shah, S. Development and evaluation of portable and wearable fuel cells for soldier use. J. Power Sources 2014, 259, 276–281. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Wolska, J.; Koroniak, H. Polymers application in proton exchange membranes for fuel cells (PEMFCs). Phys. Sci. Rev. 2017, 2, 1–34. [Google Scholar] [CrossRef]
- Ouzounidou, M.; Ipsakis, D.; Voutetakis, S.; Papadopoulou, S.; Seferlis, P. A combined methanol autothermal steam reforming and PEM fuel cell pilot plant unit: Experimental and simulation studies. Energy 2009, 34, 1733–1743. [Google Scholar] [CrossRef]
- Martín, A.J.; Hornés, A.; Martínez-Arias, A.; Daza, L. Recent Advances in Fuel Cells for Transport and Stationary Applications. In Renewable Hydrogen Technologies Production, Purification, Storage, Applications and Safety; Gandía, L.M., Arzamendi, G., DiØguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 361–380. ISBN 9780444563521. [Google Scholar]
- Trencher, G.; Edianto, A. Drivers and Barriers to the Adoption of Fuel Cell Passenger Vehicles and Buses in Germany. Energies 2021, 14, 833. [Google Scholar] [CrossRef]
- Jourdani, M.; Mounir, H.; El Marjani, A. Compilation of factors affecting durability of proton exchange membrane fuel cell (PEMFC). In Proceedings of the 2014 International Renewable and Sustainable Energy Conference, IRSEC 2014, Ouarzazate, Morocco, 17–19 October 2014; pp. 542–547. [Google Scholar]
- Sorlei, I.-S.; Bizon, N.; Thounthong, P.; Varlam, M.; Carcadea, E.; Culcer, M.; Iliescu, M.; Raceanu, M. Fuel Cell Electric Vehicles—A Brief Review of Current Topologies and Energy Management Strategies. Energies 2021, 14, 252. [Google Scholar] [CrossRef]
- McLarty, D.; Brouwer, J.; Ainscough, C. Economic analysis of fuel cell installations at commercial buildings including regional pricing and complementary technologies. Energy Build. 2016, 113, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Milburn, S.; Adamson, K.-A. The Fuel Cell Stack Supply Chain; Pike Research LLC: Boulder, CO, USA, 2012. [Google Scholar]
- Gebert, M.; Höhlein, B.; Stolten, D. Benchmark cost analysis of main PEFC-ionomer membrane solutions. J. Fuel Cell Sci. Technol. 2004, 1, 56–60. [Google Scholar] [CrossRef]


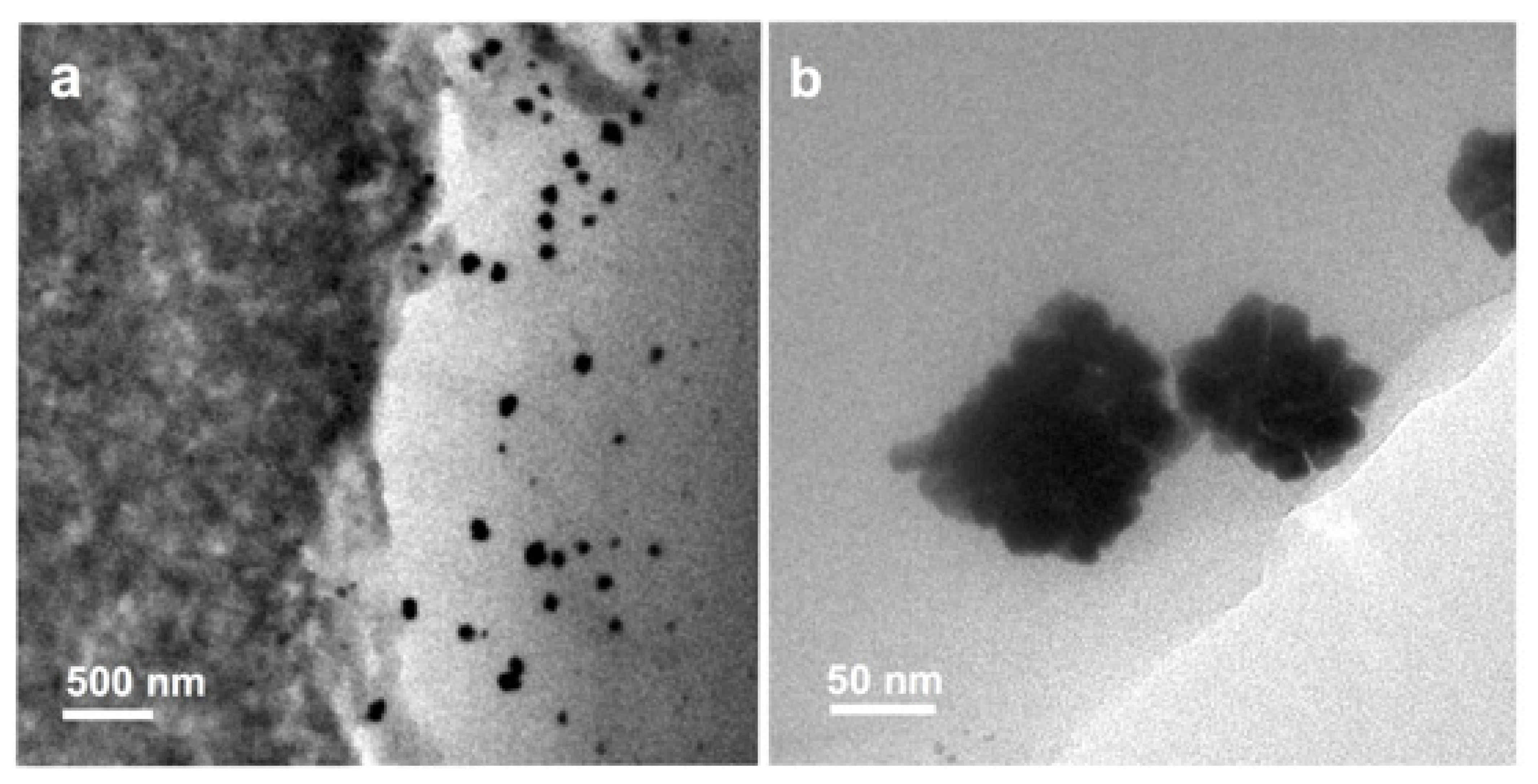
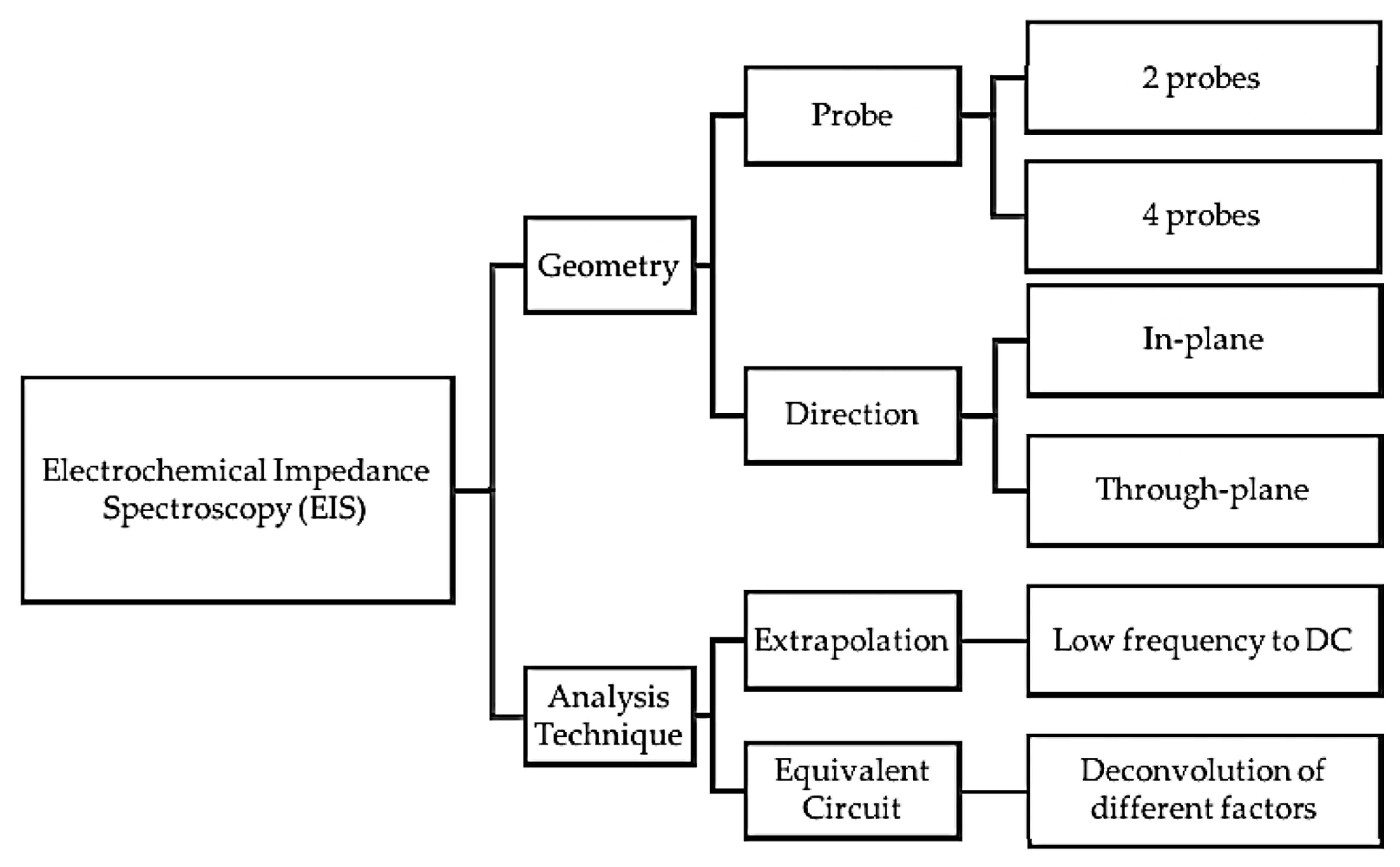

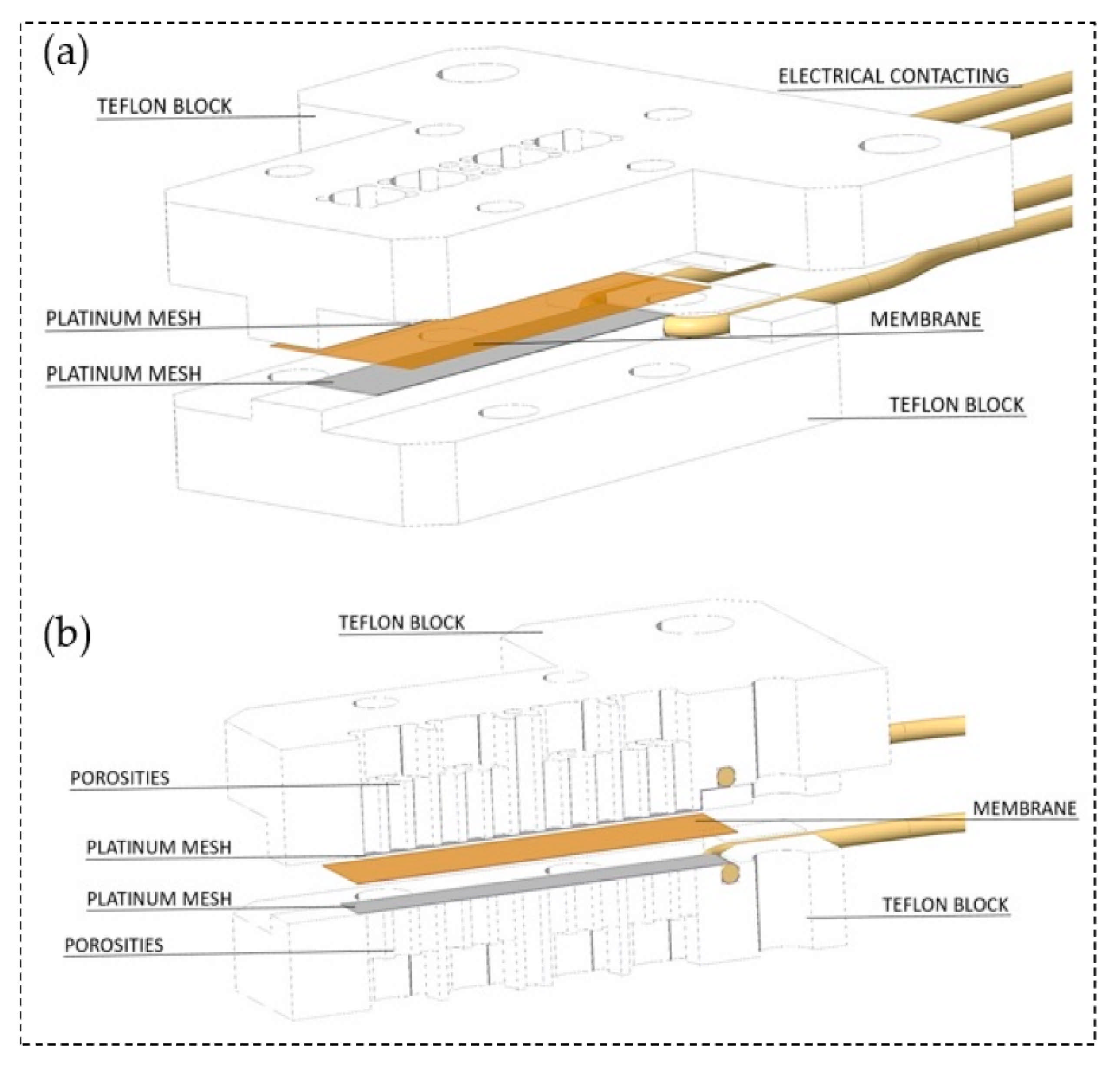
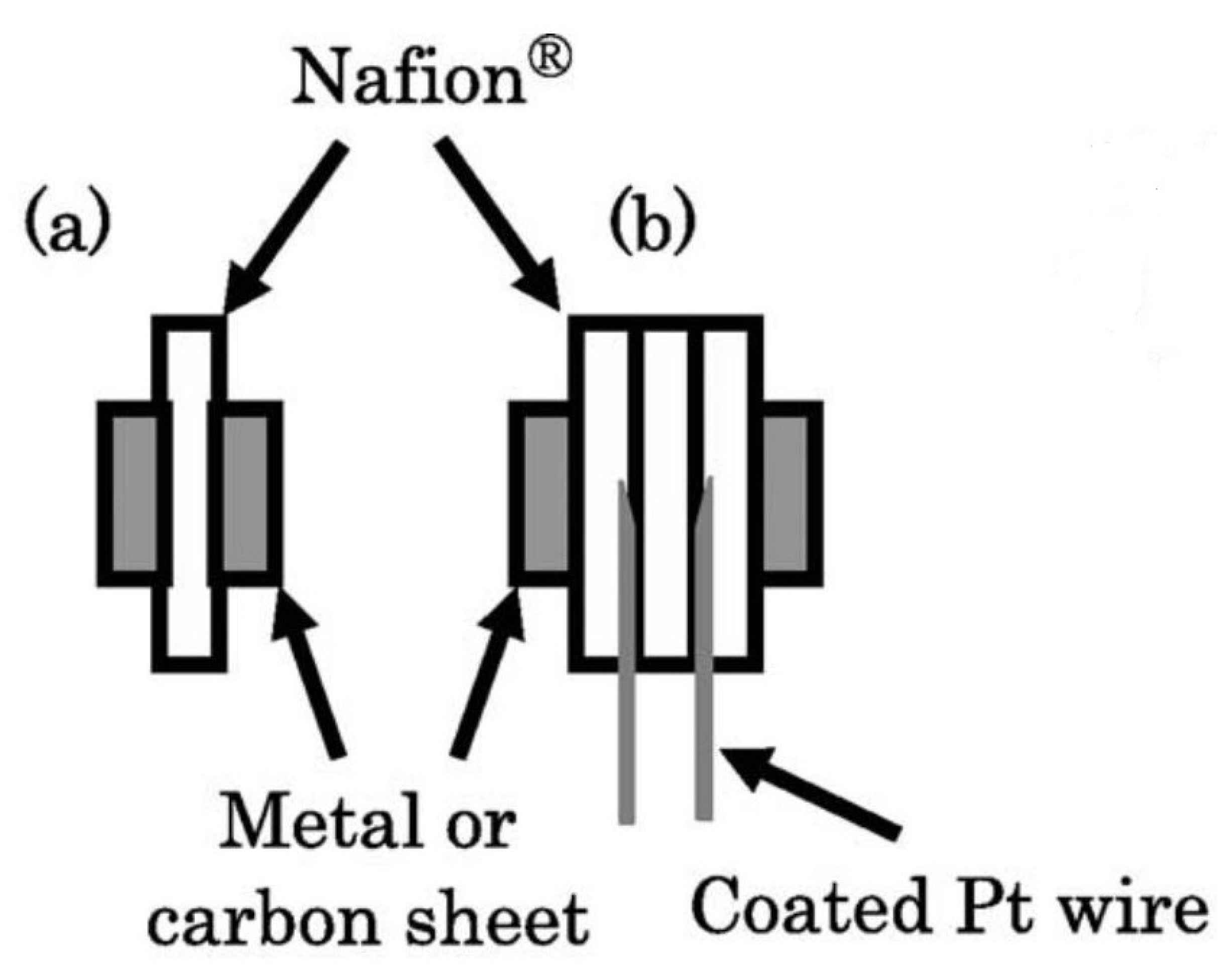
| Item/Factor | LT-PEMs | HT-PEMs |
|---|---|---|
| Membrane | Perfluoro sulfonic/sulfonated based polymer | PBI-based polymer |
| Electrode | Pt-C or Pt alloys | Pt-C or Pt alloys |
| Operating temperature | 70–80 °C | 120–180 °C |
| Efficiency | 40% | 45–50% |
| Operating pressure | Ambient pressure | Ambient pressure |
| Proton carrier | Water | Inorganic acids |
| CO tolerance | <50 ppm | 1–3% by volume |
| Other impurities | Low | Higher |
| Water management | Complex | None |
| Heat management | Complex | None |
| Reaction rate | Low | High |
| Membrane Type | Reinforcement | Temperature (°C) | Proton Conductivity (mS.cm−1) | Ref |
|---|---|---|---|---|
| Sulfonated | -SO3H groups | 180 | 373 | [48] |
| -SO3H groups | 180 | 324 | [151] | |
| Composite | Inorganic Al–Si | 150 | 309.58 | [175] |
| Ionic liquids | 180 | 293 | [162] | |
| Protic ionic liquids | 180 | 293.15 | [162] | |
| Acidic surfactant | 180 | 280 | [140] | |
| Poly(phosphoric acid) | 200 | 255.14 | [152] | |
| Heterocyclic benzo[c]cinnoline | 160 | 251 | [129] | |
| Arylether-type PBI | 200 | 233.81 | [27] | |
| Bulky pendants (phenyl and methylphenyl | 200 | 215 | [154] | |
| Dendrimer amines functionalized SBA-15 mesoporous silica | 180 | 202 | [141] | |
| a-Zirconium phosphate | 160 | 198.29 | [176] | |
| Poly [2,20-(p-oxydiphenylene)-5,50-benzimidazole] | 160 | 188.93 | [38] | |
| SiO2 | 300 | 186.75 | [177] | |
| Graphene oxide | 180 | 170.40 | [24] | |
| Triazole modified graphene oxide | 180 | 135.30 | [178] | |
| Siloxane | 180 | 133.52 | [179] | |
| Branched block PBI | 180 | 156.96 | [159] | |
| Functionalized PBIs | 180 | 152.41 | [114] | |
| Poly(Vinylbenzyl Chloride) | 160 | 109.58 | [180] | |
| Guaternary ammonium groups | 170 | 103.31 | [181] | |
| Zeotypes/protic ionic liquids | 170 | 101.80 | [182] | |
| Sulfophenylated TiO2 | 150 | 94.98 | [29] | |
| Copolymer | 3,5-Pyridine-PBI | 180 | 279.38 | [146] |
| Nanocomposite | Ionic liquid/ nano SiO2 | 180 | 239 | [139] |
| Protic ionic liquid modified silica | 160 | 238.17 | [139] | |
| Titanium dioxide | 180 | 199.52 | [183] | |
| BaZrO3 | 180 | 125.14 | [135] | |
| Clay | 160 | 117.05 | [96] | |
| Ba0.9Sr0.1TiO3 | 180 | 103 | [144] | |
| Fe2TiO5 | 180 | 102.73 | [127] | |
| Blend | Sulfonated polyhedral oligosilsesquioxane composite | 180 | 193.18 | [184] |
| Lignosulfonate | 160 | 186.89 | [185] | |
| Lignosulfonate | 180 | 186 | [186] | |
| Poly(vinylidene fluoride-co-hexafluoro propylene) | 160 | 165 | [187] | |
| Lignin | 160 | 150.18 | [185] | |
| Poly(vinyl imidazole-co-vinyl phosphonic acid) | 160 | 106.32 | [39] |
| Lifetime | Condition | Decay Rate (µV.h−1) | Reference |
|---|---|---|---|
| >1500 h | 160 °C, 0.3 A.cm−2 | 1.5 | [57] |
| >2000 h | 160 °C, 0.3 A.cm−2 | 2.4–6.5 | [112] |
| >2000 h | 180–200 °C | <5 | [146] |
| >2300 h | 180 °C, 0.2 A.cm−2 | 5.2 | [146] |
| 6000 h | 160 °C | 25 | [146] |
| >6000 h | 180 °C | 19 | [200] |
| 18,000 h | 160 °C | 5–6 | [201] |
| 4000 h | 180 °C, 0.2 A.cm−2 | 19 | [202] |
| Reinforcement | T (°C) | Current Density (A.cm−2) | Ref |
|---|---|---|---|
| Sulfophenylated TiO2 | 150 | 0.75 | [139] |
| Poly(vinyl imidazole-co-vinyl phosphonic acid) | 80 | 1.2 | [186] |
| Graphene oxide | 165 | 0.69 | [29] |
| Guaternary ammonium groups | 160 | 0.8 | [182] |
| Branched block PBI | 160 | 1.26 | [181] |
| Protic ionic liquids | 200 | 0.48 | [180] |
| Sulfonated polyhedral oligosilsesquioxane composite | 160 | 0.791 | [132] |
| Heterocyclic benzo[c]cinnoline | 160 | 2.25 | [114] |
| BaZrO3 | 180 | 1.12 | [135] |
| SiO2 | 250 | 0.6 | [178] |
| Bulky pendants (phenyl and methylphenyl | 160 | 0.55 | [24] |
| Arylether-type PBI | 160 | 0.72 | [177] |
| Fe2TiO5 | 180 | 0.76 | [127] |
| Sulfonating | 160 | 0.6 | [151] |
| Melamine-based dendrimer amines functionalized SBA-15 mesoporous silica | 180 | 1.18 | [118] |
| Triazole modified graphene oxide | 180 | 1 | [176] |
| Poly(Vinylbenzyl chloride) | 180 | 0.625 | [154] |
| Ba0.9Sr0.1TiO3 | 180 | 1.24 | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooshyari, K.; Amini Horri, B.; Abdoli, H.; Fallah Vostakola, M.; Kakavand, P.; Salarizadeh, P. A Review of Recent Developments and Advanced Applications of High-Temperature Polymer Electrolyte Membranes for PEM Fuel Cells. Energies 2021, 14, 5440. https://doi.org/10.3390/en14175440
Hooshyari K, Amini Horri B, Abdoli H, Fallah Vostakola M, Kakavand P, Salarizadeh P. A Review of Recent Developments and Advanced Applications of High-Temperature Polymer Electrolyte Membranes for PEM Fuel Cells. Energies. 2021; 14(17):5440. https://doi.org/10.3390/en14175440
Chicago/Turabian StyleHooshyari, Khadijeh, Bahman Amini Horri, Hamid Abdoli, Mohsen Fallah Vostakola, Parvaneh Kakavand, and Parisa Salarizadeh. 2021. "A Review of Recent Developments and Advanced Applications of High-Temperature Polymer Electrolyte Membranes for PEM Fuel Cells" Energies 14, no. 17: 5440. https://doi.org/10.3390/en14175440







