The Effect of Detoxification of Lignocellulosic Biomass for Enhanced Methane Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
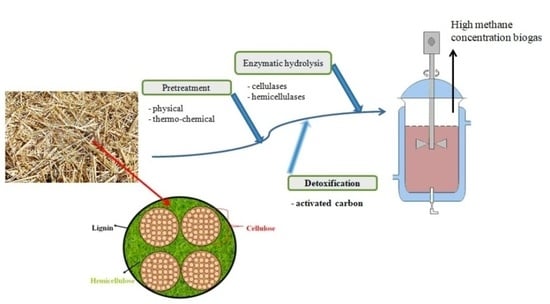
2.2. Chemical and Enzymatic Sequential Pretreatments
2.2.1. Alkaline Thermo-Chemical Pretreatment
2.2.2. Enzymatic Hydrolysis
2.2.3. Detoxification
2.3. Anaerobic Digestion
2.4. Reactor
2.5. Analytical Methods
2.5.1. Total Solids (TS), Volatile Solids (VS), Chemical Oxygen Demand (COD)
2.5.2. Chemical Composition
2.5.3. Composition of the Biogas Produced
3. Results and Discussion
3.1. Chemical Composition
3.2. Characteristics of AD
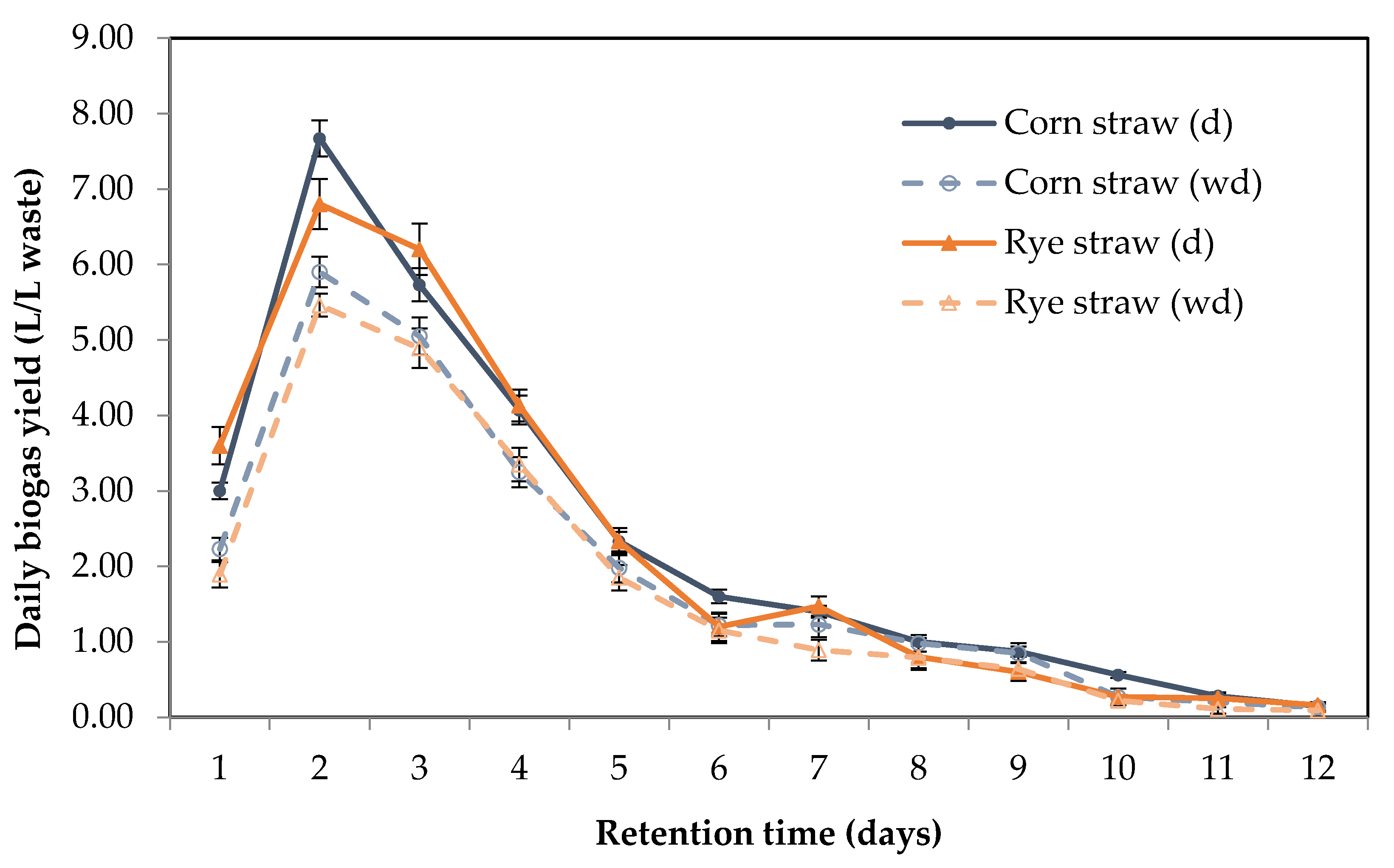
3.2.1. Methane Production
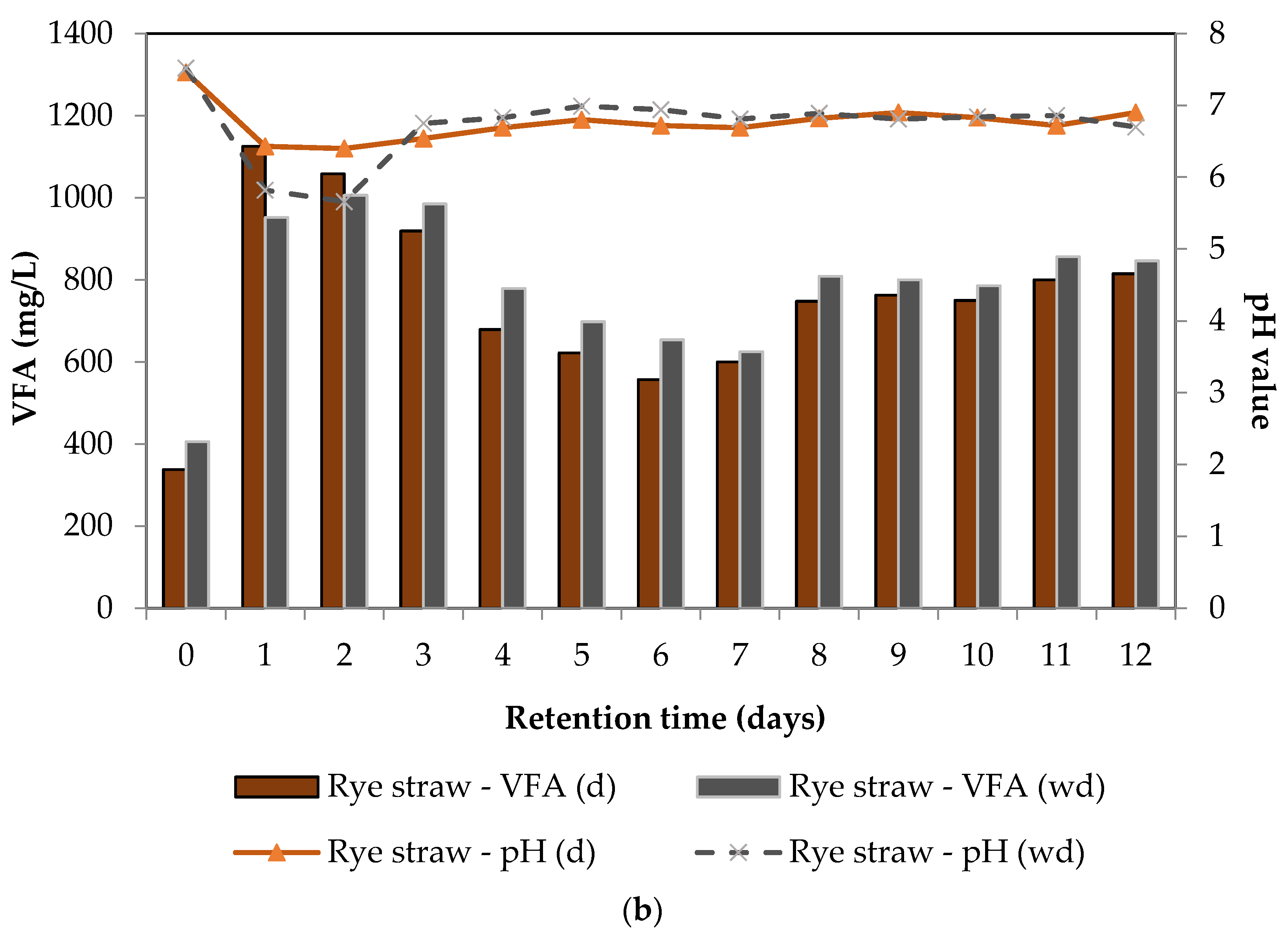
3.2.2. Variation of VFA and pH
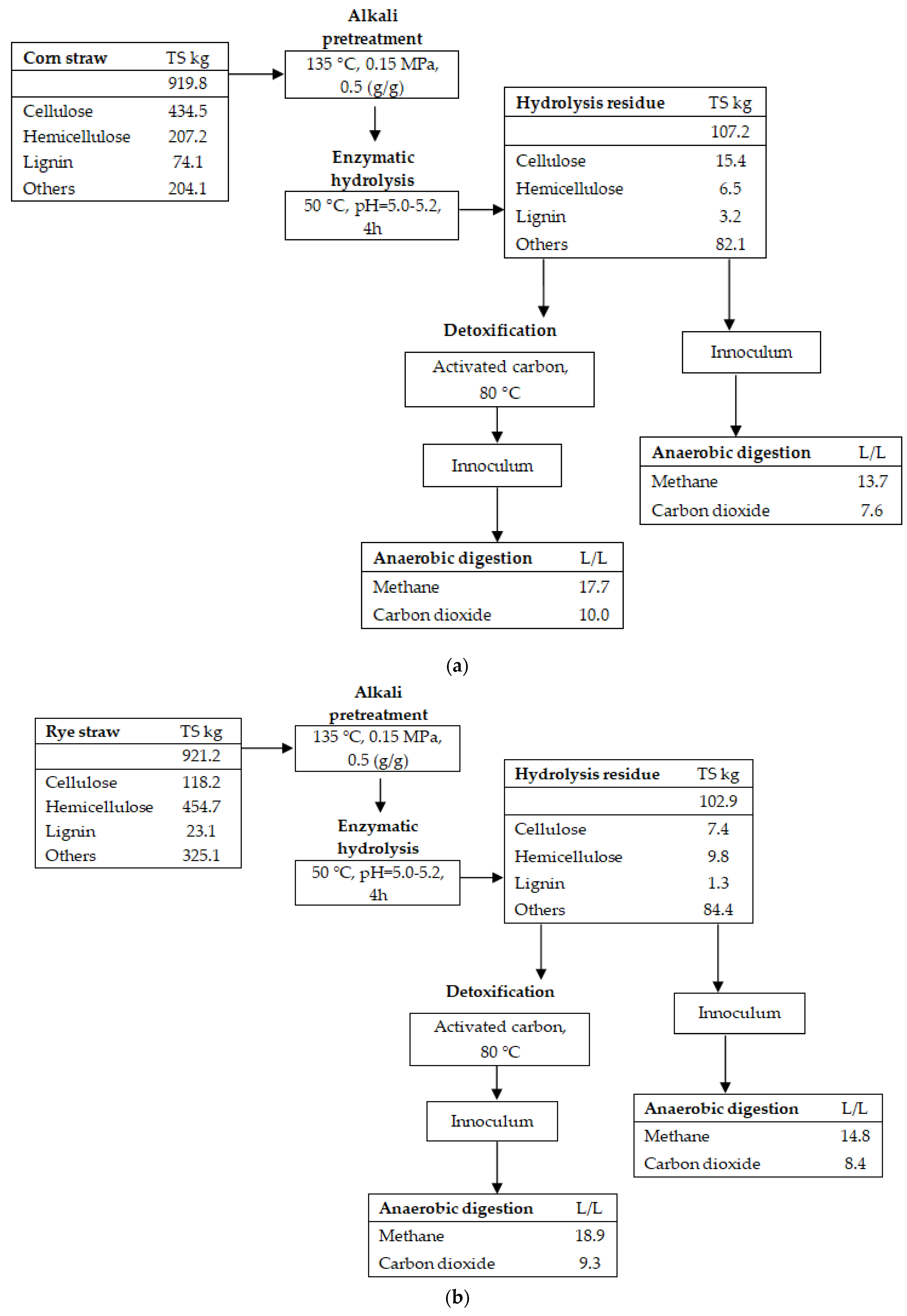
3.3. Mass Balance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [Green Version]
- Kotarska, K.; Dziemianowicz, W.; Świerczyńska, A. Study on enzymatic hydrolysis of polysaccharides in sorghum straw and conversion of monosaccharides to ethanol. Chem. Ind. 2018, 97, 2189–2191. [Google Scholar]
- Azbar, N.; Çetinkaya Dokgöz, F.T.; Keskin, T.; Korkmaz, K.S.; Syed, H.M. Continuous fermentative hydrogen production from cheese whey wastewater under thermophilic anaerobic conditions. Int. J. Hydrogen Energy 2009, 34, 7441–7447. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Thangadurai, D.; Sangeetha, J. Industrial Biotechnology: Sustainable Production and Bioresource Utilization; Apple Academic Press, Inc.: Palm Bay, FL, USA, 2016. [Google Scholar]
- Sukumaran, R.; Surender, V.; Raveendran, S.; Parameswaran, B.; Janu, K.; Valappil, S.; Rajasree, K.P.; Pandey, A. Lignocellulosic ethanol in India: Prospects, challenges and feedstock availability. Bioresour. Technol. 2009, 101, 4826–4833. [Google Scholar] [CrossRef] [PubMed]
- European Parliament, Council of the European Union. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources; European Parliament, Council of the European Union: Brussels, Belgium, 2018. [Google Scholar]
- Hosseini Koupaie, E.; Johnson, T.; Eskicioglu, C. Advanced anaerobic digestion of municipal sludge using a novel and energy-efficient radio frequency pretreatment system. Water Res. 2017, 118, 70–81. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.H. Complex media from processing of agricultural crops for microbial fermentation. Appl. Microbiol. Biotechnol. 2005, 68, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Tovar, F.; Celis, L.B.; Razo-Flores, E.; Alatriste-Mondragón, F. Chemical and enzymatic sequential pretreatment of oat straw for methane production. Bioresour. Technol. 2012, 116, 372–378. [Google Scholar] [CrossRef]
- Baral, N.; Shah, A. Microbial inhibitors: Formation and effects on acetone-butanol-ethanol fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 9151–9172. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethan production-A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Goyal, Y.; Sarkar, A.; Gayen, K. Comparative economic assessment of ABE fermentation based on cellulosic and noncellulosic feedstocks. Appl. Energy 2012, 93, 193–204. [Google Scholar] [CrossRef]
- Qureshi, N.; Ezeji, T.C.; Ebener, J.; Dien, B.S.; Cotta, M.A.; Blaschek, H.P. Butanol production by Clostridium beijerinckii. Part I: Use of acid and enzyme hydrolyzed corn fiber. Bioresour. Technol. 2008, 99, 5915–5922. [Google Scholar] [CrossRef] [PubMed]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanolproducing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of dilutedacid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Tu, M.; Carvin, J.; Wu, Y. Detoxification of biomass hydrolysates with nucleophilic amino acids enhances alcoholic fermentation. Bioresour. Technol. 2015, 186, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pienkos, P.T.; Zhang, M. Role of pretreatment and conditioning processes on toxicity of lignocellulosic biomass hydrolysates. Cellulose 2009, 16, 743–762. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.; Reimann, A.; Nilvebrant, N.O.; Jönsson, L.J. Comparison of different methods for the detoxification of lignocellulose hydrolysates of spruce. Appl. Biochem. Biotechnol. 1999, 77, 91–103. [Google Scholar] [CrossRef]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Comparison of different detoxification methods for steam-exploded poplar wood as a substrate for the bioproduction of ethanol in SHF and SSF. Proc. Biochem. 2004, 39, 1533–1542. [Google Scholar] [CrossRef]
- Gonzàlez-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Gupta, R.; Gautam, S.; Shukla, R.; Kuhad, R.C. Study of charcoal detoxification of acid hydrolysate from corncob and its fermentation to xylitol. J. Environ. Chem. Eng. 2017, 5, 4573–4582. [Google Scholar] [CrossRef]
- Ma, A.H.; Ji, X.; Tian, Z.; Fang, G.; Yang, G. Adsorption removal of inhibiting compounds by modified activated carbon. J. Energy Nat. Resour. 2017, 6, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xia, C.; Lu, M.; Tu, M. Effect of overliming and activated carbon detoxification on inhibitors removal and butanol fermentation of poplar prehydrolysates. Biotechnol. Biofuels 2018, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Akhand, M.; Blancas, A.M. Optimization of NMMO Pretreatment of Straw for Enhanced Biogas Production; University of Borås: Borås, Sweden, 2012; Available online: http://hb.diva-portal.org/smash/get/diva2:1308820/FULLTEXT01.pdf (accessed on 30 April 2019).
- Dahunsi, S.O. Mechanical pretreatment of lignocelluloses for enhanced biogas production: Methane yield prediction from biomass structural components. Bioresour. Technol. 2019, 280, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T.; Kumar, R. Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 2012, 3, 273–282. [Google Scholar] [CrossRef]
- Khor, W.C.; Rabaey, K.; Vervaeren, H. Low temperature calcium hydroxide treatment enhances anaerobic methane production from (extruded) biomass. Bioresour. Technol. 2015, 176, 181–188. [Google Scholar] [CrossRef]
- Dumas, C.; Damasceno, G.S.G.; Barakat, A.; Carrère, H.; Steyer, J.-P.; Rouau, X. Effects of grinding processes on anaerobic digestion of wheat straw. Ind. Crops Prod. 2015, 74, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Michalska, K.; Bizukojć, M.; Ledakowicz, S. Pretreatment of energy crops with sodium hydroxide and cellulolytic enzymes to increase biogas production. Biomass Bioenergy 2015, 80, 213–221. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Angelidaki, I. Effect of reactor configuration on biogas production from wheat straw hydrolysate. Bioresour. Technol. 2009, 100, 6317–6323. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Mazumdar, A. Biogas handbook consolidation of information. In General Information Programme and UNISLST, Pilot ed.; United Nations Educational Scientific & Cultural Organization (UNESCO): Paris, France, 1982. [Google Scholar]
- Gerhardt, M.; Pelenc, V.; Bäuml, M. Application of hydrolytic enzymes in the agricultural biogas production: Results from practical applications in Germany. Biotechnol. J. 2007, 2, 1481–1484. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Kacprzak, A.; Krzystek, L.; Ledakowicz, S.; Matyka, M.; Basińska, A. Comparison of biogas production from energy crops–Canary grass (Phalaris arundinacea L.) and maize (Zea mays). Inż. Ap. Chem. 2013, 52, 330–331. [Google Scholar]
- Yadvika, S.; Sreekrishnan, T.R.; Sangeeta, K.; Vineet, R. Enhancement of biogas production from solid substrates using different techniques––A review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lalak, J.; Kasprzycka, A.; Murat, A.; Paprota, E.M.; Tys, J. Pretreatment methods of lignocellulosic biomass to improve methane fermentation process (a review). Acta Agroph. 2014, 21, 51–62. [Google Scholar]
- Montero, B.; Garcia-Morales, J.L.; Sales, D.; Solera, R. Evolution of microorganisms in thermophilic-dry anaerobic digestion. Bioresour. Technol. 2008, 99, 3233–3243. [Google Scholar] [CrossRef]
- Chen, H.; Han, Y.; Xu, J. Simultaneous saccharification and fermentation of steam exploded wheat straw pretreated with alkaline peroxide. Process Biochem. 2008, 43, 1462–1466. [Google Scholar] [CrossRef]
- Buyukkamaci, N.; Filibeli, A. Volatile fatty acid formation in an anaerobic hybrid reactor. Process Biochem. 2004, 39, 1491–1494. [Google Scholar] [CrossRef]
- Moller, H.B.; Sommer, S.G.; Ahring, B.K. Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure. J. Environ. Qual. 2004, 33, 27–36. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Corn Straw | Rye Straw | Inoculum |
|---|---|---|---|
| TS (%) | 92.1 ± 1.8 | 91.9 ± 0.9 | 6.0 ± 0.1 |
| VS (%) | 93.8 ± 1.3 | 92.7 ± 1.8 | 72.5 ± 1.1 |
| Ash (%) | 6.2 ± 0.1 | 7.3 ± 0.1 | 27.5 ± 0.3 |
| Crude fibre (%) | 38.71 ± 1.51 | 40.41 ± 2.13 | n.d. |
| Chemical Composition (TS%) | Corn Straw | Rye Straw | ||
|---|---|---|---|---|
| Raw | Treated | Raw | Treated | |
| Crude fibre | 38.71 c ± 1.51 | 12.33 a ± 0.30 | 40.41 c ± 2.13 | 15.87 b ± 0.18 |
| NDF | 59.52 b ± 5.10 | 18.44 a ± 0.46 | 71.58 c ± 1.42 | 25.09 a ± 0.29 |
| ADF | 14.11 b ± 0.92 | 8.67 a ± 0.96 | 50.86 d ± 0.32 | 18.57 c ± 0.25 |
| ADL | 2.31 b ± 0.08 | 1.30 a ± 0.02 | 7.41 d ± 0.08 | 3.15 c ± 0.02 |
| Cellulose | 11.80 b ± 0.86 | 7.37 a ± 0.96 | 43.45 d ± 0.40 | 15.42 c ± 0.17 |
| Hemicellulose | 45.40 c ± 4.87 | 9.77 a ± 1.37 | 20.72 b ± 2.05 | 6.52 a ± 0.08 |
| Total lignin | 2.31 b ± 0.08 | 1.30 a ± 0.02 | 7.41 d ± 0.08 | 3.15 c ± 0.02 |
| Other (ash + extractives) | 40.49 b ± 5.18 | 81.56 c ± 4.25 | 28.42 a ± 2.14 | 78.06 c ± 5.07 |
| YD * | - | 70.03 ± 2.51 | - | 65.81 ± 3.19 |
| Raw Material | TS (g/L) | pH | COD (g O2/L) | Tsu (g/L) | VFA (mg/L) |
|---|---|---|---|---|---|
| Corn Straw treated | 102.9 ± 9.8 | 5.1 ± 0.1 | 59.21 ± 7.1 | 33.5 ± 5.8 | 327.3 ± 26.8 |
| Rye Straw treated | 107.2 ± 13.1 | 5.2 ± 0.0 | 63.64 ± 11.2 | 29.6 ± 5.8 | 338.9 ± 19.7 |
| Variants | CH4 Yield (L/L) | CH4 Yield (m3/kg VS) | DOSD (%) |
|---|---|---|---|
| Corn Straw (d) | 18.86 ± 0.91 | 0.31 ± 0.03 | 62.59 ± 3.72 |
| Corn Straw (wd) | 14.80 ± 1.47 | 0.24 ± 0.02 | 59.73 ± 2.64 |
| Rye Straw (d) | 17.69 ± 1.07 | 0.29 ± 0.01 | 62.09 ± 2.88 |
| Rye Straw (wd) | 13.68 ± 0.31 | 0.25 ± 0.03 | 58.40 ± 2.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotarska, K.; Dziemianowicz, W.; Świerczyńska, A. The Effect of Detoxification of Lignocellulosic Biomass for Enhanced Methane Production. Energies 2021, 14, 5650. https://doi.org/10.3390/en14185650
Kotarska K, Dziemianowicz W, Świerczyńska A. The Effect of Detoxification of Lignocellulosic Biomass for Enhanced Methane Production. Energies. 2021; 14(18):5650. https://doi.org/10.3390/en14185650
Chicago/Turabian StyleKotarska, Katarzyna, Wojciech Dziemianowicz, and Anna Świerczyńska. 2021. "The Effect of Detoxification of Lignocellulosic Biomass for Enhanced Methane Production" Energies 14, no. 18: 5650. https://doi.org/10.3390/en14185650