Combining Optical Microscopy and X-ray Computed Tomography Reveals Novel Morphologies and Growth Processes of Methane Hydrate in Sand Pores
Abstract
:1. Introduction
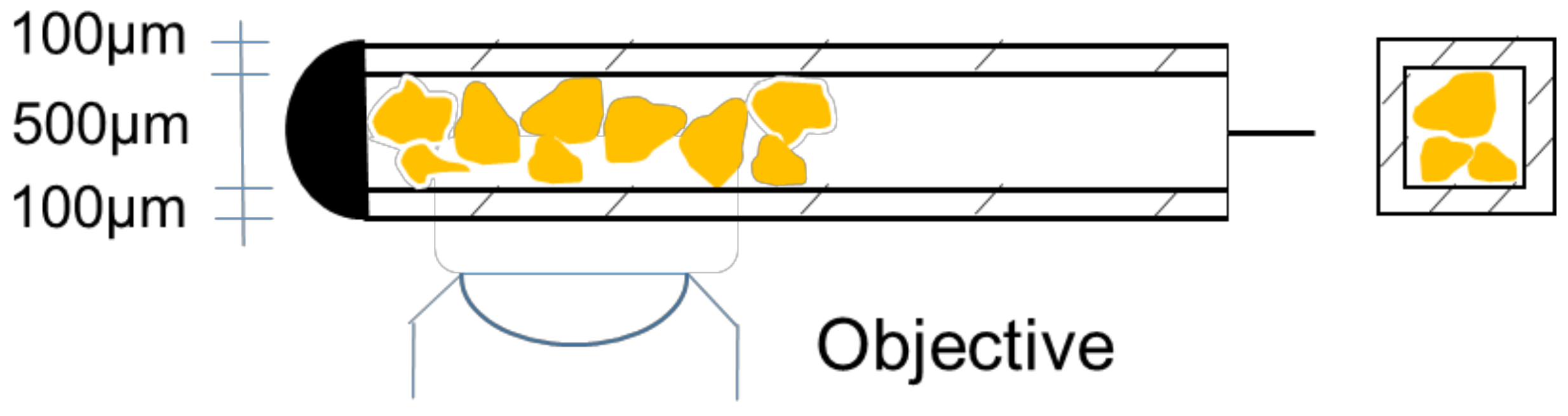
2. Materials and Methods
3. Results
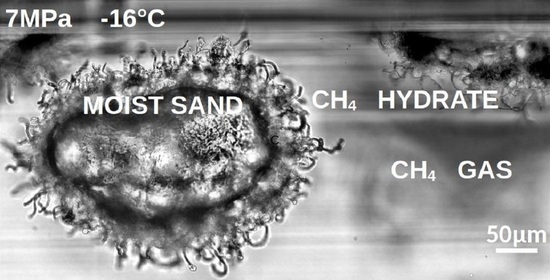
3.1. Early Stages of MH Formation and Growth: Nucleation and Water Migration to Sustain Initial MH Growth
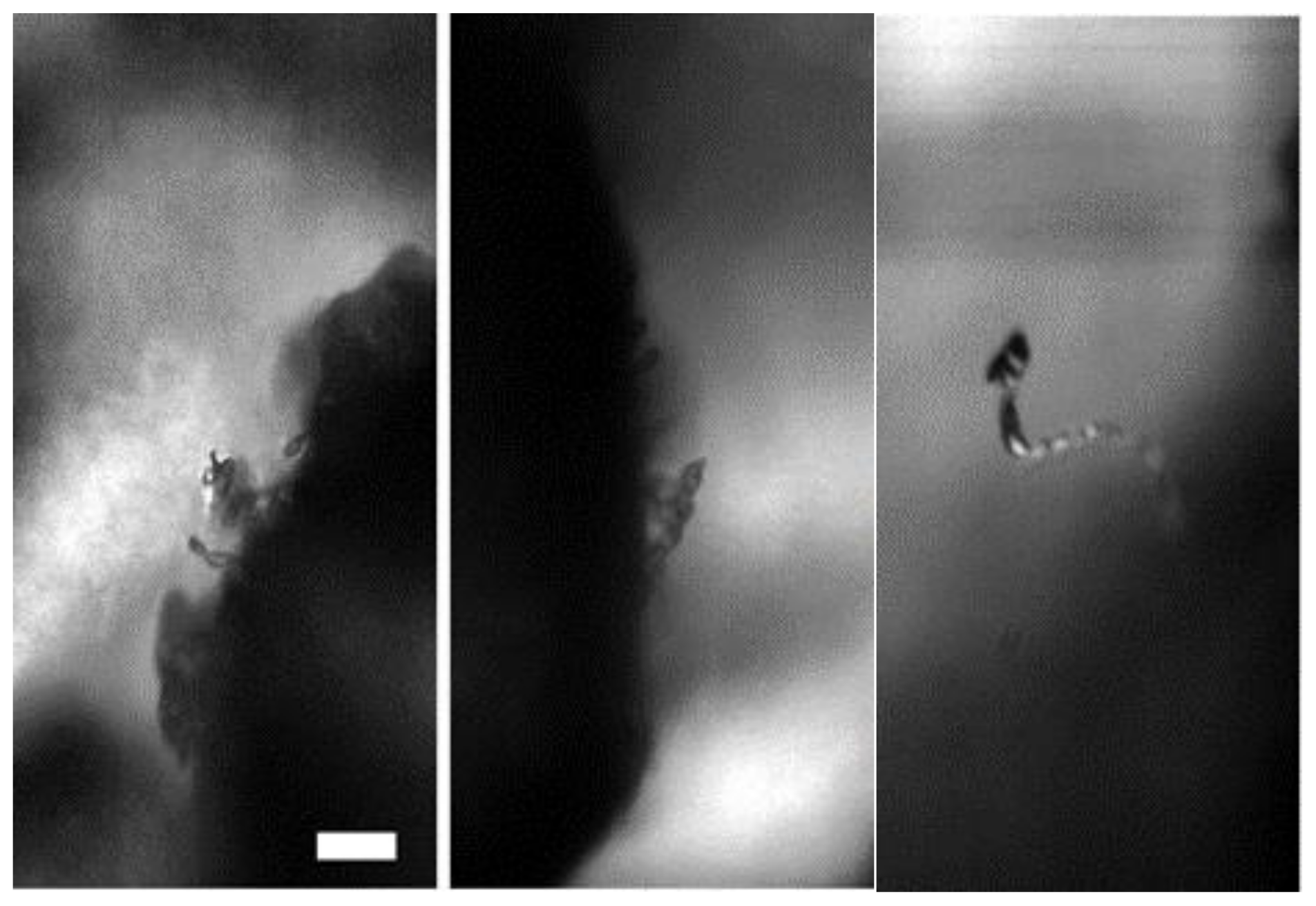
3.2. Growth of Hydrate Filaments
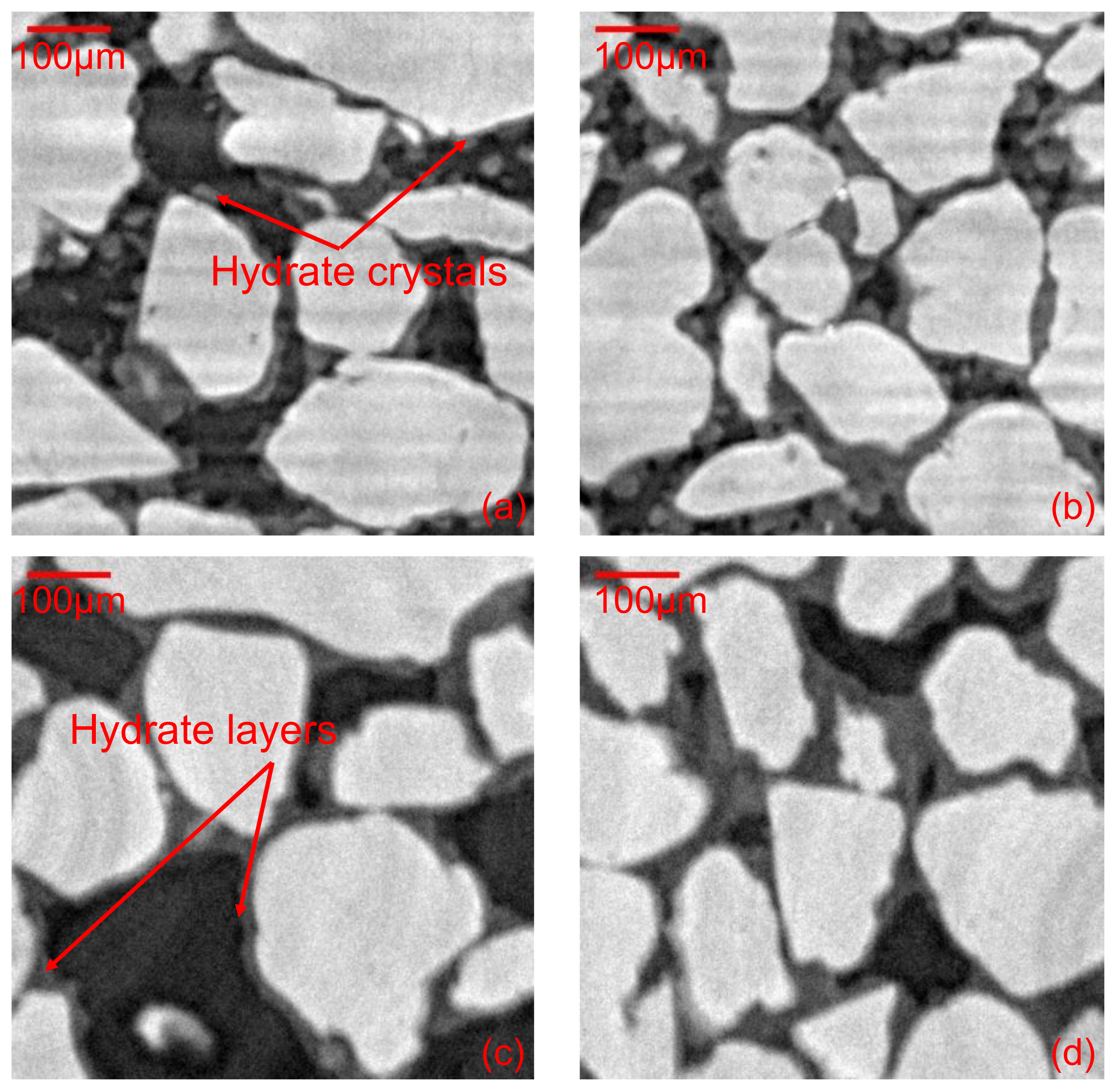
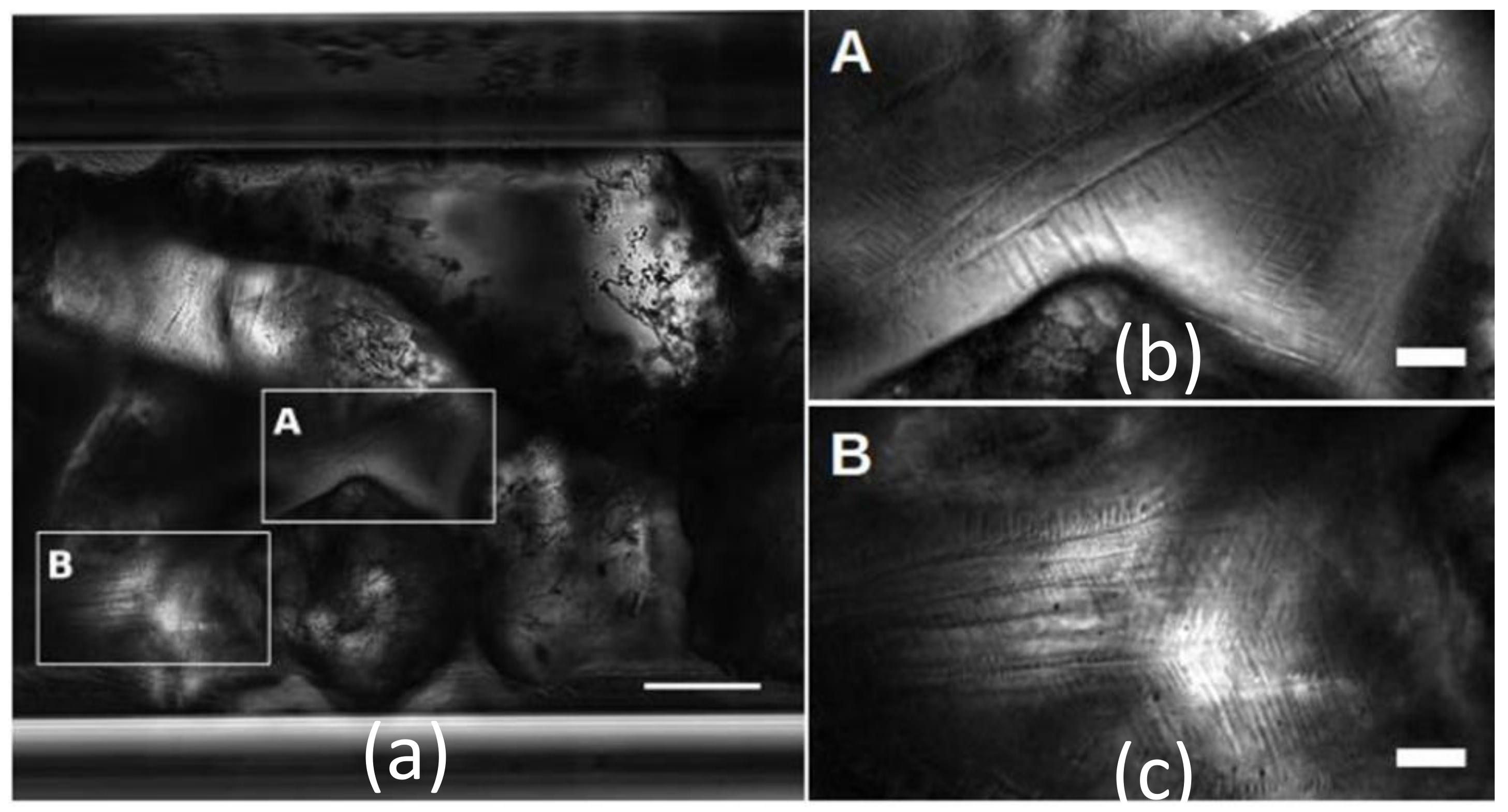
3.3. Later Stages of MH Growth and MH Morphologies
4. Discussion
4.1. Haines Jumps and Proposal for a Cryogenic Suction Mechanism
4.2. A novel Methane Hydrate Morphology: Hollow Filaments
4.3. Methane Hydrate Growth around Sediment Particles and at Their Contacts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collett, T.S.; Johnson, A.H.; Knapp, C.C.; Boswell, R. Natural Gas Hydrates: Energy Resource Potential and Associated Geologic Hazards, AAPG Memoir 89; American Association of Petroleum Geologists: Tulsa, Oklahoma, USA, 2009; Volume 89, pp. 146–219. [Google Scholar] [CrossRef]
- Masui, A.; Kuniyuki, M.; Hironori, H.; Yuji, O.; Kazuo, A. Mechanical Properties of Natural Gas Hydrate Bearing Sediments Retrieved from Eastern Nankai Trough. In Proceedings of the Offshore Technology Conference (OTC), Houston, TX, USA, 5–8 May 2008. [Google Scholar] [CrossRef]
- Dai, S.; Santamarina, J.C.; Waite, W.F.; Kneafsey, T.J. Hydrate morphology: Physical properties of sands with patchy hydrate saturation. J. Geophys. Res. B Solid Earth 2012, 117, B11205. [Google Scholar] [CrossRef] [Green Version]
- Konno, Y.; Yoneda, J.; Egawa, K.; Ito, T.; Jin, Y.; Kida, M.; Suzuki, K.; Fujii, T.; Nagao, J. Permeability of sediment cores from methane hydrate deposit in the Eastern Nankai Trough. Mar. Pet. Geol. 2015, 66, 487–495. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Helgerud, M.B.; Dvorkin, J.; Nur, A. Elastic-wave velocity in marine sediments with gas hydrates: Effective medium modeling. Geophys. Res. Lett. 1999, 26, 2021–2024. [Google Scholar] [CrossRef]
- Dvorkin, J.; Helgerud, M.; Waite, W.; Kirby, S.; Nur, A. Introduction to physical properties and elasticity models. In Natural Gas Hydrate; Max, M.D., Ed.; Springer: Dordrecht, The Netherlands, 2000; pp. 245–260. [Google Scholar] [CrossRef]
- Chand, S.; Minshull, T.A.; Gei, D.; Carcione, J.M. Elastic velocity models for gas-hydrate-bearing sediments—A comparison. Geophys. J. Int. 2004, 159, 573–590. [Google Scholar] [CrossRef] [Green Version]
- Soga, K.; Ng, M.; Lee, S.; Klar, A. Characterisation and engineering properties of methane hydrate soils. Characterisation Eng. Prop. Nat. Soils 2006, 4, 2591–2642. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47, 1–38. [Google Scholar] [CrossRef]
- Uchida, S.; Soga, K.; Yamamoto, K. Critical state soil constitutive model for methane hydrate soil. J. Geophys. Res. Solid Earth 2012, 117, 1–13. [Google Scholar] [CrossRef]
- Sánchez, M.; Gai, X.; Santamarina, J.C. A constitutive mechanical model for gas hydrate bearing sediments incorporating inelastic mechanisms. Comput. Geotech. 2017, 84, 28–46. [Google Scholar] [CrossRef] [Green Version]
- Taleb, F.; Garziglia, S.; Sultan, N. Hydromechanical Properties of Gas Hydrate-Bearing Fine Sediments From In Situ Testing. J. Geophys. Res. Solid Earth 2018, 123, 9615–9634. [Google Scholar] [CrossRef]
- Nguyen-Sy, T.; Tang, A.M.; To, Q.D.; Vu, M.N. A model to predict the elastic properties of gas hydrate-bearing sediments. J. Appl. Geophys. 2019, 169, 154–164. [Google Scholar] [CrossRef]
- Alavoine, A.; Dangla, P.; Pereira, J.M. Fast Fourier transform-based homogenisation of gas hydrate bearing sediments. Géotech. Lett. 2020, 10, 367–376. [Google Scholar] [CrossRef]
- Kerkar, P.B.; Horvat, K.; Jones, K.W.; Mahajan, D. Imaging methane hydrates growth dynamics in porous media using synchrotron X-ray computed tomography. Geochem. Geophys. Geosyst. 2014, 15, 4759–4768. [Google Scholar] [CrossRef]
- Chaouachi, M.; Falenty, A.; Sell, K.; Enzmann, F.; Kersten, M.; Haberthür, D.; Kuhs, W.F. Microstructural evolution of gas hydrates in sedimentary matrices observed with synchrotron X-ray computed tomographic microscopy. Geochem. Geophys. Geosyst. 2015, 16, 1711–1722. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Seol, Y.; Choi, J.H.; Kneafsey, T.J. Pore habit of methane hydrate and its evolution in sediment matrix–Laboratory visualization with phase-contrast micro-CT. Mar. Pet. Geol. 2019, 104, 451–467. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, V.V.; Geser, N.; Dugarov, A.; Duchkov, A.A.; Fokin, M.I.; Arkady Drobchik, N.; Shevchenko, P.D.; De Carlo, F.; Mokso, R. Dynamic in-situ imaging of methane hydrate formation and self-preservation in porous media. Mar. Pet. Geol. 2020, 115, 104234. [Google Scholar] [CrossRef]
- Chen, X.; Espinoza, D.N.; Luo, J.S.; Tisato, N.; Flemings, P.B. Pore-scale evidence of ion exclusion during methane hydrate growth and evolution of hydrate pore-habit in sandy sediments. Mar. Pet. Geol. 2020, 117, 104340. [Google Scholar] [CrossRef]
- Le, T.X.; Aimedieu, P.; Bornert, M.; Chabot, B.; King, A.; Tang, A.M. An experimental investigation on the methane hydrates morphologies and pore habits in sandy sediment by using Synchrotron X-ray Computed Tomography. Mar. Pet. Geol. 2020, 122, 104646. [Google Scholar] [CrossRef]
- Waite, W.; Winters, W.J.; Mason, D.H. Methane hydrate formation in partially water-saturated Ottawa sand. Am. Mineral. 2004, 89, 1202–1207. [Google Scholar] [CrossRef]
- Priest, J.A.; Best, A.I.; Clayton, C.R.I. A laboratory investigation into the seismic velocities of methane gas hydrate-bearing sand. J. Geophys. Res. Solid Earth 2005, 110, 1–13. [Google Scholar] [CrossRef]
- Winters, W.J.; Waite, W.F.; Mason, D.H.; Gilbert, L.Y.; Pecher, I.A. Methane gas hydrate effect on sediment acoustic and strength properties. J. Pet. Sci. Eng. 2007, 56, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Priest, J.A.; Rees, E.V.L.; Clayton, C.R.I. Influence of gas hydrate morphology on the seismic velocities of sands. J. Geophys. Res. Solid Earth 2009, 114, B11205. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Takeya, S.; Hayashi, J.; Nagao, J.; Kamata, Y.; Ebinuma, T.; Narita, H. Structure Analyses of artificial methane hydrate sediments by microfocus X-ray computed tomography. Jpn. J. Appl. Phys. 2004, 43, 5673–5675. [Google Scholar] [CrossRef]
- Jones, K.W.; Feng, H.; Tomov, S.; Winters, W.J.; Prodanoviće, M.; &Mahajan, D. Characterization of methane hydrate host sediments using synchrotron-computed microtomography (CMT). J. Pet. Sci. Eng. 2007, 56, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Takeya, S.; Yoneyama, A.; Ueda, K.; Hyodo, K.; Takeda, T.; Mimachi, H.; Takahashi, M.; Iwasaki, T.; Sano, K.; Yamawaki, H.; et al. Non-destructive imaging of anomalously preserved methane hydrate by phase-contrast X-ray imaging. J. Phys. Chem. C 2011, 115, 16193–16199. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Sun, X.; Liu, W.; Song, Y. Pore-scale 3D morphological modeling and physical characterization of hydrate-bearing sediment based on computed tomography. J. Geophys. Res. Solid Earth 2020, 125. [Google Scholar] [CrossRef]
- Le, T.X.; Aimedieu, P.; Bornert, M.; Chabot, B.; King, A.; Tang, A.M. Experimental development to investigate the grain-scale morphology and pore habit of methane hydrate-bearing sandy sediment by X-ray Computed Tomography. Geotech. Test. J. 2021, 44, 502–519. [Google Scholar] [CrossRef]
- Ta, X.H.; Yun, T.S.; Muhunthan, B.; Kwon, T. Observations of pore-scale growth patterns of carbon dioxide hydrate using X-ray computed microtomography. Geochem. Geophys. Geosyst. 2015, 16, 912–924. [Google Scholar] [CrossRef]
- Davies, S.H.; Sloan, E.D.; Sum, A.K.; Koh, C.A. In-situ studies of the mass transfer mechanism across a methane hydrate film using high-resolution confocal Raman spectroscopy. J. Phys. Chem. C 2010, 114, 1173–1180. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Morphology of methane and carbon dioxide hydrates formed from water droplets. Environ. Energy Eng. AIChE J. 2004, 49, 269–276. [Google Scholar] [CrossRef]
- Lee, J.D.; Song, M.; Susilo, R.; Englezos, P. Dynamics of Methane-Propane Clathrate Hydrate Crystal Growth from Liquid Water with or without the Presence of n-Heptane. Cryst. Growth Des. 2006, 6, 1428–1439. [Google Scholar] [CrossRef]
- Beltrán, J.G.; Servio, P. Morphology investigations of methane-hydrate films formed on a glass surface. Cryst. Growth Des. 2010, 10, 4339–4347. [Google Scholar] [CrossRef]
- Saito, K.; Kishimoto, M.; Tanaka, R.; Ohmura, R. Crystal growth of clathrate hydrate at the interface between hydrocarbon gas mixture and liquid water. Cryst. Growth Des. 2011, 11, 295–301. [Google Scholar] [CrossRef]
- Martinez de Baños, L.M.; Hobeika, N.; Bouriat, P.; Broseta, D.; Enciso, E.; Clément, F.; Brown, R. How do gas hydrates spread on a substrate? Cryst. Growth Des. 2016, 16, 4360–4373. [Google Scholar] [CrossRef]
- Esmail, S.; Beltrán, J.G. Methane hydrate propagation on surfaces of varying wettability. J. Nat. Gas Sci. Eng. 2016, 35, 1535–1543. [Google Scholar] [CrossRef]
- Udegbunam, L.U.; DuQuesnay, J.R.; Osorio, L.; Walker, V.K.; Beltran, J.G. Phase equilibria, kinetics and morphology of methane hydrate inhibited by antifreeze proteins: Application of a novel 3-in-1 method. J. Chem. Therm. 2018, 117, 155–163. [Google Scholar] [CrossRef]
- Stoporev, A.S.; Adamova, T.P.; Manakov, A.Y. Insight into Hydrate Film Growth: Unusual Growth of Methane and the Aqueous Solution of Malonic Acid. Cryst. Growth Des. 2020, 20, 1927–1934. [Google Scholar] [CrossRef]
- Jung, J.-W.; Santamarina, C. Hydrate formation and growth in pores. J. Cryst. Growth 2012, 345, 61–68. [Google Scholar] [CrossRef]
- Touil, A.; Broseta, D.; Hobeika, N.; Brown, R. Roles of Wettability and Supercooling in the Spreading of Cyclopentane Hydrate over a Substrate. Langmuir 2017, 33, 10965–10977. [Google Scholar] [CrossRef]
- Touil, A.; Broseta, D.; Desmedt, A. Gas hydrate crystallization in thin glass capillaries: Roles of supercooling and wettability. Langmuir 2019, 35, 12569–12581. [Google Scholar] [CrossRef] [PubMed]
- Hobeika, N.; Martinez de Baños, M.L.; Bouriat, P.; Broseta, D.; Brown, R. High-Resolution Optical Microscopy of Gas Hydrates. In Gas Hydrates 1: Fundamentals, Characterization and Modeling; Broseta, D., Ruffine, L., Desmedt, A., Eds.; Wiley-ISTE: Hoboken, NJ, USA, 2017; pp. 113–144. [Google Scholar] [CrossRef]
- Atig, D.; Broseta, D.; Pereira, J.M.; Brown, R. Contactless probing of polycrystalline methane hydrate at pore scale suggests weaker tensile properties than thought. Nat. Commun. 2020, 11, 3379. [Google Scholar] [CrossRef] [PubMed]
- Adamova, T.P.; Stoporev, A.S.; Manakov, A.Y. Visual Studies of Methane Hydrate Formation on the Water–Oil Boundaries. Cryst. Growth Des. 2018, 18, 6713–6722. [Google Scholar] [CrossRef]
- Stoporev, A.S.; Semenov, A.P.; Medvedev, V.I.; Sizikova, A.A.; Gushchin, P.A.; Vinokurov, V.A.; Manakov, A.Y. Visual observation of gas hydrates nucleation and growth at a water-organic liquid interface. J. Cryst. Growth 2018, 485, 54–68. [Google Scholar] [CrossRef]
- Ohmura, R.; Shimada, W.; Uchida, T.; Mori, Y.H.; Takeya, S.; Nagao, J.; Minagawa, H.; Ebinuma, T.; Narita, H. Clathrate hydrate crystal growth in liquid water saturated with a hydrate-forming substance: Variations in crystal morphology. Philos. Mag. 2004, 84, 1–16. [Google Scholar] [CrossRef]
- Ohmura, R.; Matsuda, S.; Uchida, T.; Ebinuma, T.; Narita, H. Clathrate Hydrate Crystal Growth in Liquid Water Saturated with a Guest Substance: Observations in a Methane + Water System. Cryst. Growth Des. 2005, 5, 953–957. [Google Scholar] [CrossRef]
- Tohidi, B.; Anderson, R.; Ben Clennell, M.; Burgrass, R.W.; Biderkab, A.B. Visual observation of gas-hydrate formation and dissociation in synthetic porous media by means of glass micromodels. Geology 2001, 29, 867–870. [Google Scholar] [CrossRef]
- Katsuki, D.; Ohymura, R.; Ebinuma, T.; Narita, H. Methane hydrate crystal growth in a porous medium filled with methane-saturated liquid water. Philos. Mag. 2007, 87, 1057–1069. [Google Scholar] [CrossRef]
- Hauge, L.P.; Gauteplass, J.; Høyland, M.D.; Ersland, G.; Kovscek, A.; Fernø, M.A. Pore-level hydrate formation mechanisms using realistic rock structures in high-pressure silicon micromodels. Int. J. Greenh. Gas Control 2016, 53, 178–186. [Google Scholar] [CrossRef]
- Almenningen, S.; Flatlandsmo, J.; Kovscek, A.R.; Ersland, G.; Fernø, M.A. Determination of pore-scale hydrate phase equilibria in sediments using lab-on-a-chip technology. Lab Chip 2017, 17, 4070–4076. [Google Scholar] [CrossRef]
- Pandey, J.S.; Strand, O.; von Solms, N.; Ersland, G.; Almenningen, S. Direct Visualization of CH4/CO2 Hydrate Phase Transitions in Sandstone Pores. Cryst. Growth Des. 2021, 21, 2793–2806. [Google Scholar] [CrossRef]
- Pandey, J.S.; Almmeningen, S.; von Solms, N.; Ersland, G. Pore-Scale Visualization of CH4 Gas Hydrate Dissociation under Permafrost Conditions. Energy Fuels 2021, 35, 1178–1196. [Google Scholar] [CrossRef]
- Schicks, J.M.; Naumann, R.; Erzinger, J.; Hester, K.C.; Koh, C.A.; Sloan, E.D. Phase Transitions in Mixed Gas Hydrates: Experimental Observations versus Calculated Data. J. Phys. Chem. B 2006, 110, 11468–11474. [Google Scholar] [CrossRef] [PubMed]
- David, R.L. CRC Handbook of Chemistry and Physics, 88th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- King, A.; Guignot, N.; Zerbino, P.; Boulard, E.; Desjardins, K.; Bordessoule, M.; Leclerq, N.; Le, S.; Renaud, G.; Cerato, M.; et al. Tomography and Imaging at the PSICHE Beam Line of the SOLEIL Synchrotron. Rev. Sci. Instrum. 2016, 87, 093704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef]
- Chong, Z.R.; Chan, A.H.M.; Babu, P.; Yang, M.; Linga, P. Effect of NaCl on methane hydrate formation and dissociation in porous media. J. Nat. Gas Sci. Eng. 2015, 27, 178–189. [Google Scholar] [CrossRef]
- Ruppel, C.D.; Waite, W.F. Grand challenge: Timescales and processes of methane hydrate formation and breakdown, with application to geologic systems. J. Geophys. Res. Solid Earth 2020, 125, e2018JB016459. [Google Scholar] [CrossRef]
- Le, T.X.; Aimedieu, P.; Bornert, M.; Chabot, B.; Rodts, S.; Tang, A.M. Effect of temperature cycle on mechanical properties of methane hydrate-bearing sediment. Soils Found. 2019, 59, 814–827. [Google Scholar] [CrossRef]
- Le, T.X.; Rodts, S.; Hautemayou, D.; Aimedieu, P.; Bornert, M.; Chabot, B.; Tang, A.M. Kinetics of methane hydrate formation and dissociation in sand sediment. Geomech. Energy Environ. 2020, 23, 100103. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H.R.; Cleall, P.; Li, Y.C.; Harris, C.; Kern-Luetschg, M. Modelling of cryogenic processes in permafrost and seasonally frozen soils. Géotechnique 2009, 59, 173–184. [Google Scholar] [CrossRef]
- Yasuda, K.; Mori, Y.H.; Ohmura, R. Interfacial tension measurements in water-methane system from 278.15 K to 298.15 K and pressures up to 10 MPa. Fluid Phase Equilibria 2016, 413, 170–175. [Google Scholar] [CrossRef]
- Saito, K.; Sum, A.K.; Ohmura, R. Correlation of Hydrate-Film Growth Rate at the Guest/Liquid-Water Interface to Mass Transfer Resistance. Ind. Eng. Chem. Res. 2010, 49, 7102–7103. [Google Scholar] [CrossRef]
- Mochizuki, T.; Mori, Y.H. Simultaneous mass and heat transfer to/from the edge of a clathrate-hydrate film causing its growth along a water/guest-fluid phase boundary. Chem. Eng. Sci. 2017, 171, 61–75. [Google Scholar] [CrossRef]
- Venet, S.; Broseta, D.; Brown, R. A Novel Gas Hydrate Morphology: Massive Hollow Fiber Growth on a Porous Substrate. Cryst. Growh Des. 2021, 21, 3148–3152. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, B.; Xu, L.; Zhang, R.; He, Y.; Wang, F. How porous surfaces influence the nucleation and growth of methane hydrates. Fuel 2021, 291, 120142. [Google Scholar] [CrossRef]










| Run | Aqueous Phase | Initial Gravimetric Moisture Content (%) | Temperature (°C) | Duration (h) |
|---|---|---|---|---|
| 1 | 3.5 wt% KI | 15 | 1–2 | 22 |
| 2 | Tap water | 10 | 2–3 | 18 |
| 3 | Tap water | 10 | 3–4 | 21 |
| 4 | Tap water | 15 | 2–3 | 7 |
| 5 | Deionized water | - | −16 to −5 (1st formation) 4 (2nd formation) | 3 |
| 6 | Deionized water | - | 2 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.X.; Bornert, M.; Brown, R.; Aimedieu, P.; Broseta, D.; Chabot, B.; King, A.; Tang, A.M. Combining Optical Microscopy and X-ray Computed Tomography Reveals Novel Morphologies and Growth Processes of Methane Hydrate in Sand Pores. Energies 2021, 14, 5672. https://doi.org/10.3390/en14185672
Le TX, Bornert M, Brown R, Aimedieu P, Broseta D, Chabot B, King A, Tang AM. Combining Optical Microscopy and X-ray Computed Tomography Reveals Novel Morphologies and Growth Processes of Methane Hydrate in Sand Pores. Energies. 2021; 14(18):5672. https://doi.org/10.3390/en14185672
Chicago/Turabian StyleLe, Thi Xiu, Michel Bornert, Ross Brown, Patrick Aimedieu, Daniel Broseta, Baptiste Chabot, Andrew King, and Anh Minh Tang. 2021. "Combining Optical Microscopy and X-ray Computed Tomography Reveals Novel Morphologies and Growth Processes of Methane Hydrate in Sand Pores" Energies 14, no. 18: 5672. https://doi.org/10.3390/en14185672