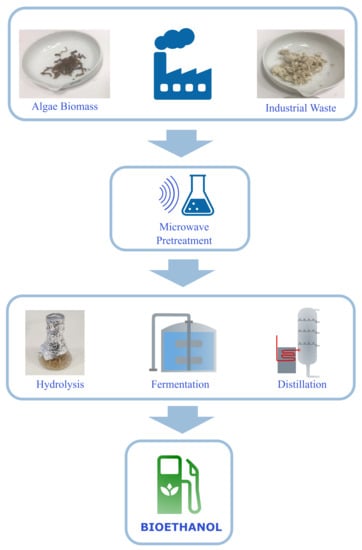
Microwave Assisted Alkaline Pretreatment of Algae Waste in the Production of Cellulosic Bioethanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Raw Material
2.3. Microwave Pretreatment
2.4. Acid Hydrolysis
2.5. Fermentation
2.6. Analytical Methods
2.6.1. Reducing Sugars and Ethanol
2.6.2. HPLC Analysis
2.6.3. Calculations
3. Results and Discussion
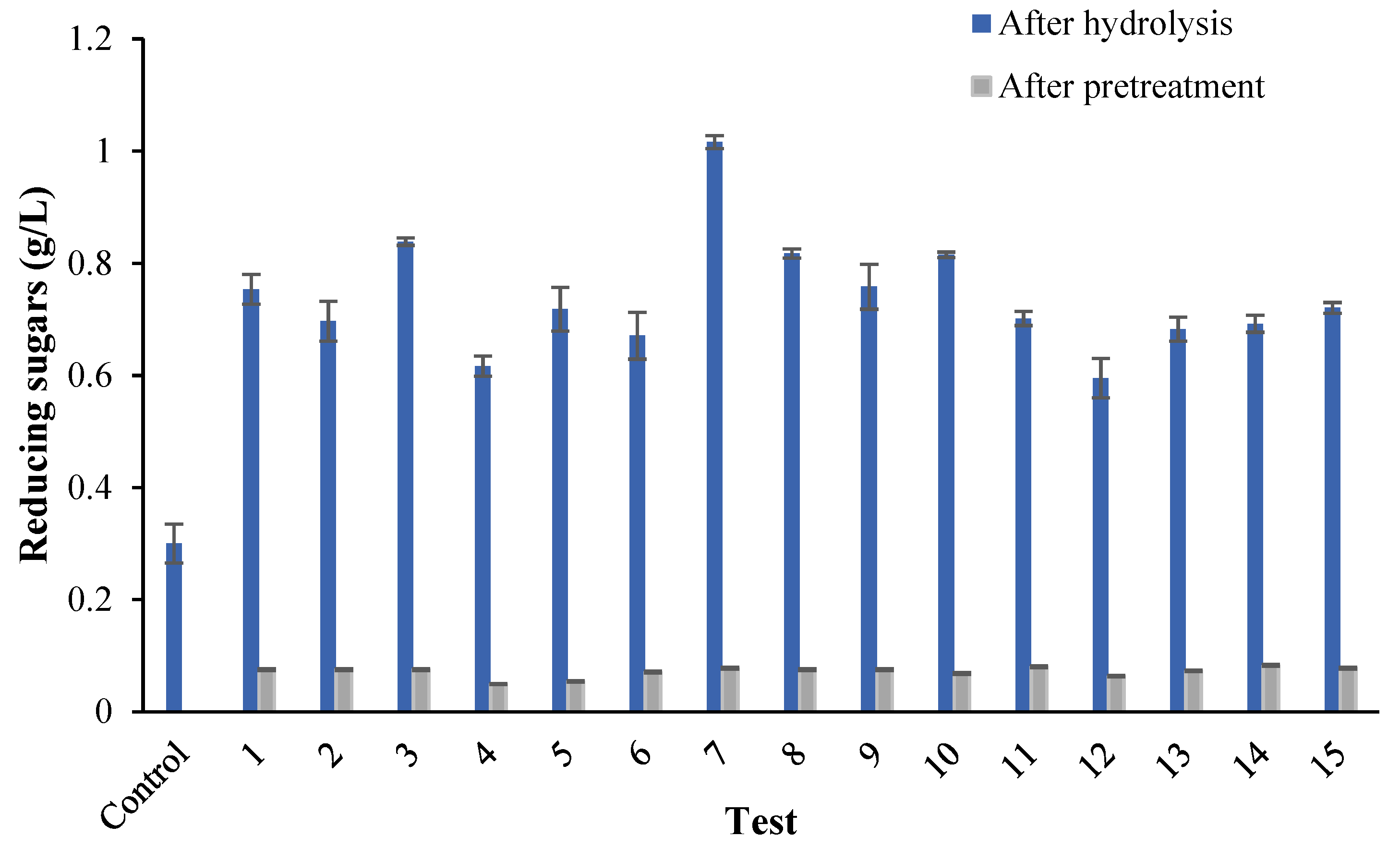
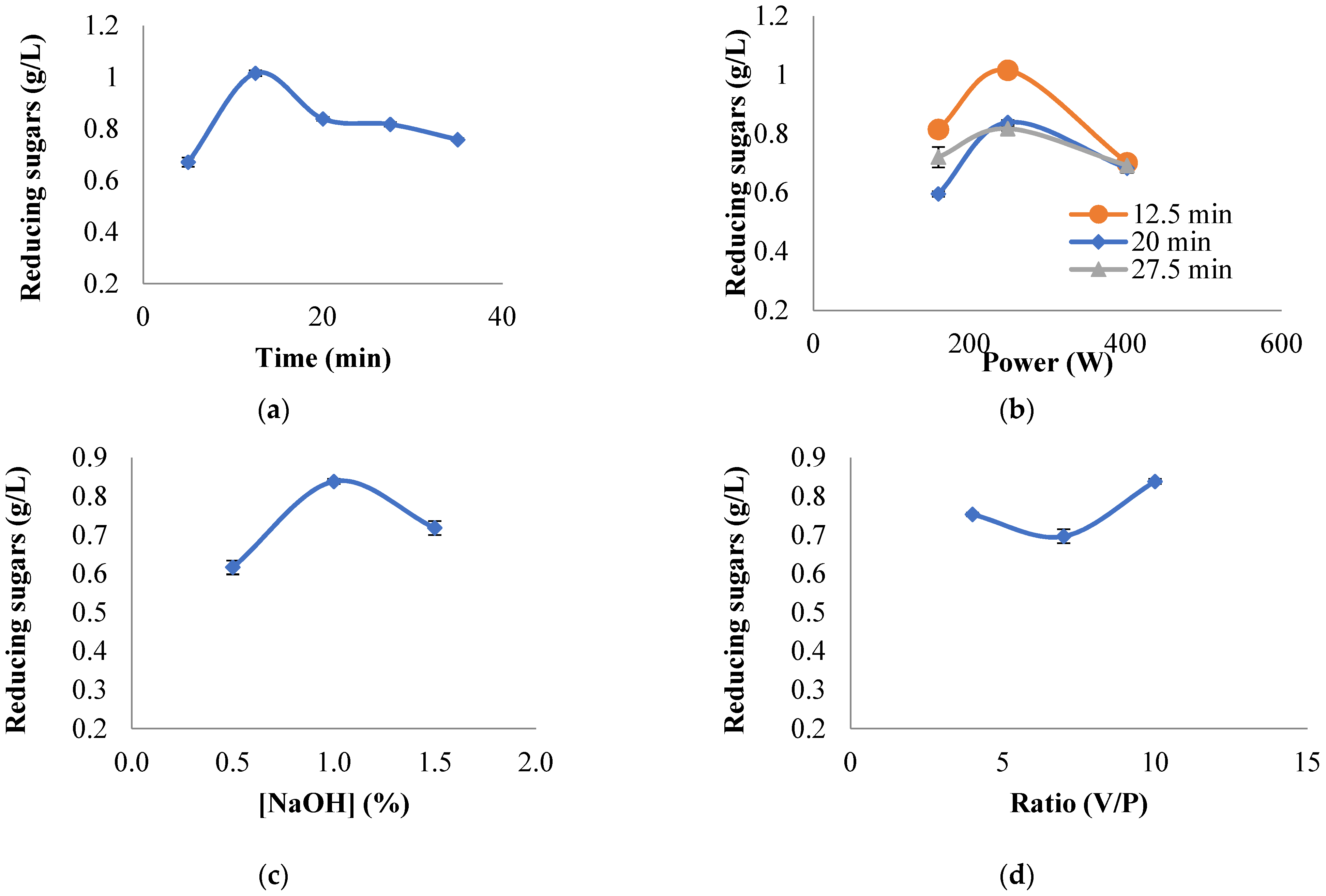
3.1. Efficiency of Microwave Pretreatment on Hydrolysis
3.2. Fermentation of Sugars to Bioethanol
3.3. Energy Consumption Calculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Lior, N. Energy resources and use: The present situation and possible paths to the future. Energy 2008, 33, 842–857. [Google Scholar] [CrossRef]
- Sánchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Alfonsín, V.; Maceiras, R.; Gutiérrez, C. Bioethanol production from industrial algae waste. Waste Manag. 2019, 87, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.A.; Inambao, F.L. Bioethanol production from different Matooke peels species: A surprising source for alternative fuel. Case Stud. Thermal Eng. 2019, 13, 100357. [Google Scholar] [CrossRef]
- Tye, Y.Y.; Lee, K.T.; Abdullah, W.N.W.; Leh, C.P. The world availability of non-wood lignocellulosic biomass for the production of cellulosic ethanol and potential pretreatments for the enhancement of enzymatic saccharification. Renew. Sustain. Energy Rev. 2016, 60, 155–172. [Google Scholar] [CrossRef]
- Zhang, C. Lignocellulosic Ethanol: Technology and Economics. In Alcohol Fuels—Current Technologies and Future Prospect; Yun, Y., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- MamathaKumari, M.; Praveen Kumar, D.; Haridoss, P.; DurgaKumari, V.; Shankar, M.V. Nanohybrid of titania/carbon nanotubes—nanohorns: A promising photocatalyst for enhanced hydrogen production under solar irradiation. Int. J. Hydrogen Energy 2015, 40, 1665–1674. [Google Scholar] [CrossRef]
- Bibi, R.; Imran, M.; Hussain, S.; Ditta, A. Algal bioethanol production technology: A trend towards sustainable. Renew. Sustain. Energy Rev. 2017, 71, 976–985. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Progr. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Kumar, M.N.; Ravikumar, R.; Thenmozhi, S.; Kumar, M.R.; Shankar, M.K. Choice of pretreatment technology for sustainable production of bioethanol from lignocellulosic biomass: Bottle necks and recommendations. Waste Biomass Valori. 2019, 10, 1693–1709. [Google Scholar] [CrossRef]
- Cardona, C.A.; Sánchez, O.J. Fuel ethanol production: Process design trends and integration opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef]
- Al-Haj Ibrahim, H. Pretreatment of straw for bioethanol production. Energy Procedia 2012, 14, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef] [PubMed]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Kapoor, K.; Garg, N.; Diwan, R.K.; Varshney, L.; Tyagi, A.K. Study the effect of gamma radiation pretreatment of sugarcane bagasse on its physcio-chemical morphological and structural properties. Radiat. Phys. Chem. 2017, 141, 190–195. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, X.; Chen, J.; Chen, L.; Su, X.; Liu, Y. Comparison of gamma irradiation and steam explosion pretreatment for ethanol production from agricultural residues. Biomass Bioenerg. 2012, 46, 301–308. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Kim, I.; Kim, K.H. Optimization of sono-assisted dilute sulfuric acid process for simultaneous pretreatment and saccharification of rice straw. Int. J. Environ. Sci. Technol. 2014, 11, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. Evaluation of ultrasound assisted potassium permanganate pre-treatment of spent coffee waste. Bioresour. Technol. 2017, 224, 680–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundar, S.; Bergey, N.S.; Cardona, L.S.; Stipanovic, A.; Driscoll, M. Electron beam pretreatment of switchgrass to enhance enzymatic hydrolysis to produce sugars for biofuels. Carbohydr. Polym. 2014, 100, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Gabhane, J.; Prince William, S.P.M.; Gadhe, A.; Rath, R.; Vaidya, A.N.; Wate, S. Pretreatment of banana agricultural waste for bio-ethanol production: Individual and interactive effects of acid and alkali pretreatments with autoclaving, microwave heating and ultrasonication. Waste Manag. 2014, 34, 498–503. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Comparison of microwave and conduction-convection heating autohydrolysis pretreatment for bioethanol production. Bioresour. Technol. 2017, 243, 273–283. [Google Scholar] [CrossRef]
- Kristiani, A.; Effendi, N.; Aristiawan, Y.; Aulia, F.; Sudiyani, Y. Effect of Combining Chemical and Irradiation Pretreatment Process to Characteristic of Oil Palm’s Empty Fruit Bunches as Raw Material for Second Generation Bioethanol. Energy Procedia 2015, 68, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Loow, Y.L.; Wu, T.Y.; Yang, G.H.; Jahim, J.M.; Teoh, W.H.; Mohammad, A.W. Role of energy irradiation in aiding pretreatment of lignocellulosic biomass for improving reducing sugar recovery. Cellulose 2016, 23, 2761–2789. [Google Scholar] [CrossRef]
- Maurya, D.P.; Vats, S.; Rai, S.; Negi, S. Optimization of enzymatic saccharification of microwave pretreated sugarcane tops through response surface methodology for biofuel. Indian J. Exp. Biol. 2013, 51, 992–996. [Google Scholar]
- Ha, G.-S.; El-Dalatony, M.M.; Kurade, M.B.; Salama, E.-S.; Basak, B.; Kang, D.; Roh, H.-S.; Lim, H.; Jeon, B.-H. Energy-efficient pretreatments for the enhanced conversion of microalgal biomass to biofuels. Bioresour. Technol. 2020, 309, 123333. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Wine, R.H. Use of detergents in the analysis of fibrous feed. IV. Determination of plant cell-wall constituents. J. Assoc. Anal. Chem. 1967, 50, 50–55. [Google Scholar]
- Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mekkawi, S.A.; Abdo, S.M.; Samhan, F.A.; Ali, G.H. Optimization of some fermentation conditions for bioethanol production from microalgae using response surface method. Bull. Natl. Res. Cent. 2019, 43, 164. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G.; Menka, A.; Koim-Puchowska, B. Microwave-assisted pretreatment of maize distillery stillage with the use of dilute sulfuric acid in the production of cellulosic ethanol. Bioresour. Technol. 2019, 278, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Griess, O.; Pulidindi, I.N.; Perkas, N.; Gedanken, A. Bioethanol production from Ficus religiosa leaves using microwave irradiation. J. Environ. Manag. 2016, 177, 20–25. [Google Scholar] [CrossRef]
- Pang, F.; Xue, S.; Yu, S.; Zhang, C.; Li, B.; Kang, Y. Effects of microwave power and microwave irradiation time on pretreatment efficiency and characteristics of corn stover using combination of steam explosion and microwave irradiation (SE-MI) pretreatment. Bioresour. Technol. 2012, 118, 111–119. [Google Scholar] [CrossRef]
- Vani, S.; Binod, P.; Kuttiraja, M.; Sindhu, R.; Sandhya, S.V.; Preeti, V.E.; Sukumaran, R.K.; Pandey, A. Energy requirement for alkali assisted microwave and high pressure reactor pretreatments of cotton plant residue and its hydrolysis for fermentable sugar production for biofuel application. Bioresour. Technol. 2012, 112, 300–307. [Google Scholar] [CrossRef]
- Chen, C.; Boldor, D.; Aita, G.; Walker, M. Ethanol production from sorghum by a microwave-assisted dilute ammonia pretreatment. Bioresour. Technol. 2012, 110, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Chen, X.; Wang, L.; Zhou, S.; Feng, H.; Chen, W.N.; Lau, R. Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour. Technol. 2013, 128, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Su, M.Y.; Tzeng, W.S.; Shyu, Y.T. An analysis of feasibility of bioethanol production from Taiwan sorghum liquor waste. Bioresour Technol. 2010, 101, 6669–6675. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Zieliński, M.; Dębowski, M. Microwave support of the alcoholic fermentation process of cyanobacteria Arthrospira platensis. Environ. Sci. Pollut. Res. 2020, 27, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Wen, Z. Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem. Eng. J. 2008, 38, 369–378. [Google Scholar] [CrossRef]
- Kappe, C.O. Microwaves in organic and medicinal chemistry. Weinheim 2005, 45, 1677–1678. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Chapple, C.; Ladisch, M.; Meilan, R. Loosening lignin’s grip on biofuel production. Nat. Biotechnol. 2007, 25, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xi, B.; Zhang, Y.; Angelidaki, I. Microwave pretreatment of rape straw for bioethanol production: Focus on energy efficiency. Bioresour. Technol. 2011, 102, 7937–7940. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G. Microwave-Mediated Biofuel Production; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Cellulose (%) | 37.3 ± 1.9 |
| Organic matter (%) | 13.2 ± 0.5 |
| Total Kjeldahl nitrogen (mg/kg) | 1180 ± 25 |
| Ammonium nitrogen (mg N/kg) | 11 ± 1.1 |
| Total organic carbon (%) | 2.9 ± 0.23 |
| Cd (µg/g) | 1.5 ± 0.02 |
| Ca (µg/g) | 70,100 ± 72 |
| Cr (µg/g) | 3.0 ± 0.15 |
| Cu (µg/g) | 3.3 ± 0.21 |
| Fe(µg/g) | 1210 ± 35 |
| Pb (µg/g) | 1.4 ± 0.09 |
| Mg (µg/g) | 5080 ± 33 |
| Test | Time (min) | [NaOH] (%) | Power (W) | Ratio (V/P) |
|---|---|---|---|---|
| 1 | 20.0 | 1 | 249 | 4:1 |
| 2 | 20.0 | 1 | 249 | 7:1 |
| 3 | 20.0 | 1 | 249 | 10:1 |
| 4 | 20.0 | 0.50 | 249 | 10:1 |
| 5 | 20.0 | 1.50 | 249 | 10:1 |
| 6 | 5.0 | 1 | 249 | 10:1 |
| 7 | 12.5 | 1 | 249 | 10:1 |
| 8 | 27.5 | 1 | 249 | 10:1 |
| 9 | 35.0 | 1 | 249 | 10:1 |
| 10 | 12.5 | 1 | 160 | 10:1 |
| 11 | 12.5 | 1 | 402 | 10:1 |
| 12 | 20.0 | 1 | 160 | 10:1 |
| 13 | 20.0 | 1 | 402 | 10:1 |
| 14 | 27.5 | 1 | 402 | 10:1 |
| 15 | 27.5 | 1 | 160 | 10:1 |
| Test | Energy Consumption (kJ/g Reduced Sugars) | Energy Consumption (kJ/g Bioethanol) |
|---|---|---|
| 3 | 1755.6 ± 35.4 | 3864.9 ± 34.9 |
| 6 | 438.9 ± 8.8 | 966.2 ± 8.7 |
| 7 | 1097.3 ± 22.1 | 2415.6 ± 21.8 |
| 8 | 2414.0 ± 48.7 | 5314.2 ± 47.9 |
| 9 | 3072.3 ± 62 | 6763.5 ± 61.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maceiras, R.; Alfonsín, V.; Seguí, L.; González, J.F. Microwave Assisted Alkaline Pretreatment of Algae Waste in the Production of Cellulosic Bioethanol. Energies 2021, 14, 5891. https://doi.org/10.3390/en14185891
Maceiras R, Alfonsín V, Seguí L, González JF. Microwave Assisted Alkaline Pretreatment of Algae Waste in the Production of Cellulosic Bioethanol. Energies. 2021; 14(18):5891. https://doi.org/10.3390/en14185891
Chicago/Turabian StyleMaceiras, Rocío, Víctor Alfonsín, Luis Seguí, and Juan F. González. 2021. "Microwave Assisted Alkaline Pretreatment of Algae Waste in the Production of Cellulosic Bioethanol" Energies 14, no. 18: 5891. https://doi.org/10.3390/en14185891
APA StyleMaceiras, R., Alfonsín, V., Seguí, L., & González, J. F. (2021). Microwave Assisted Alkaline Pretreatment of Algae Waste in the Production of Cellulosic Bioethanol. Energies, 14(18), 5891. https://doi.org/10.3390/en14185891