Cultivation of Autochthonous Microalgae for Biomass Feedstock: Growth Curves and Biomass Characterization for Their Use in Biorefinery Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Studied and Culture Conditions
2.2. Photobioreactors
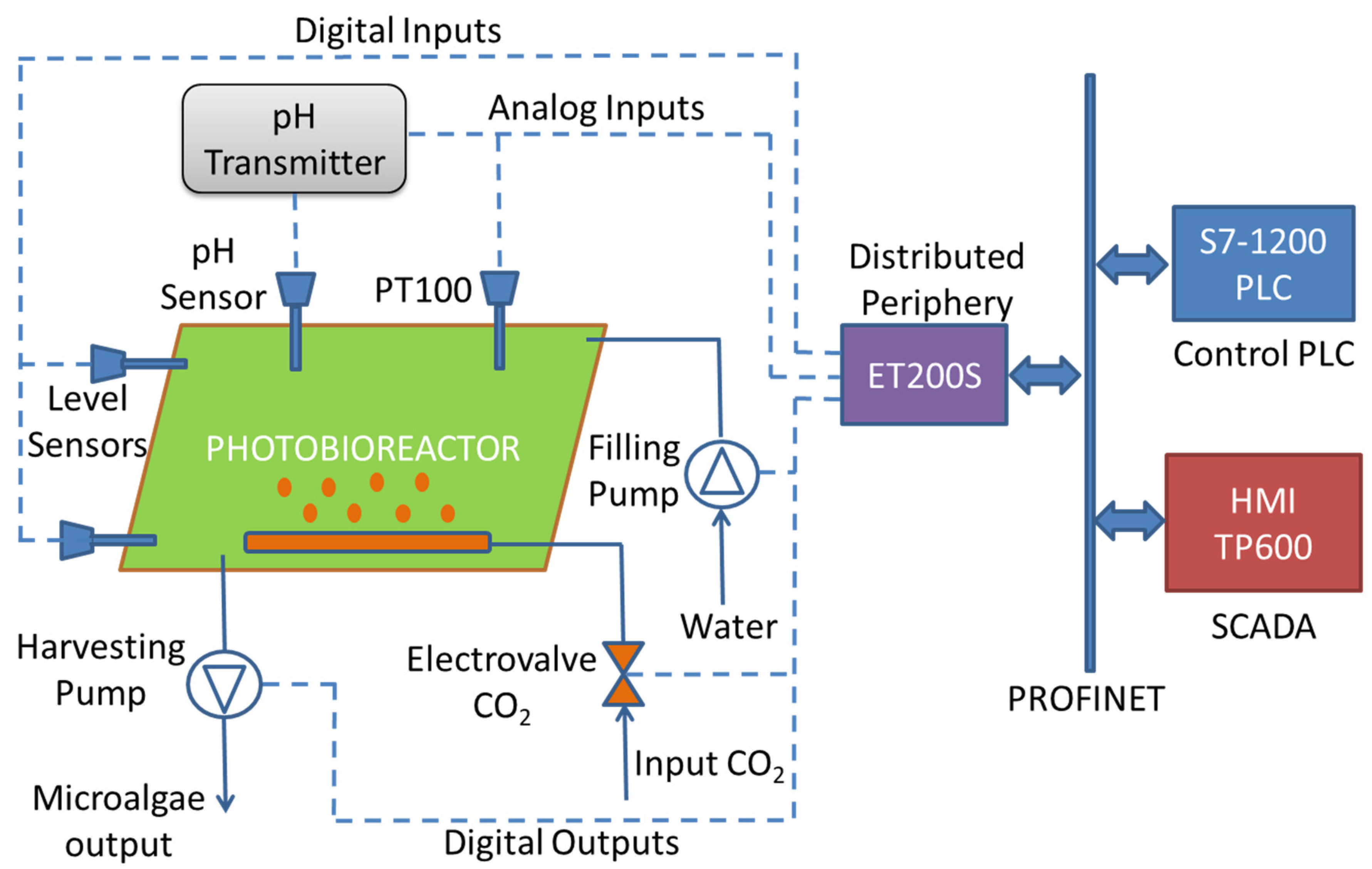
2.3. Automation and Monitoring System of the Pilot Plant
2.4. Analytical Methods
2.5. Growth Kinetic
3. Results and Discussion
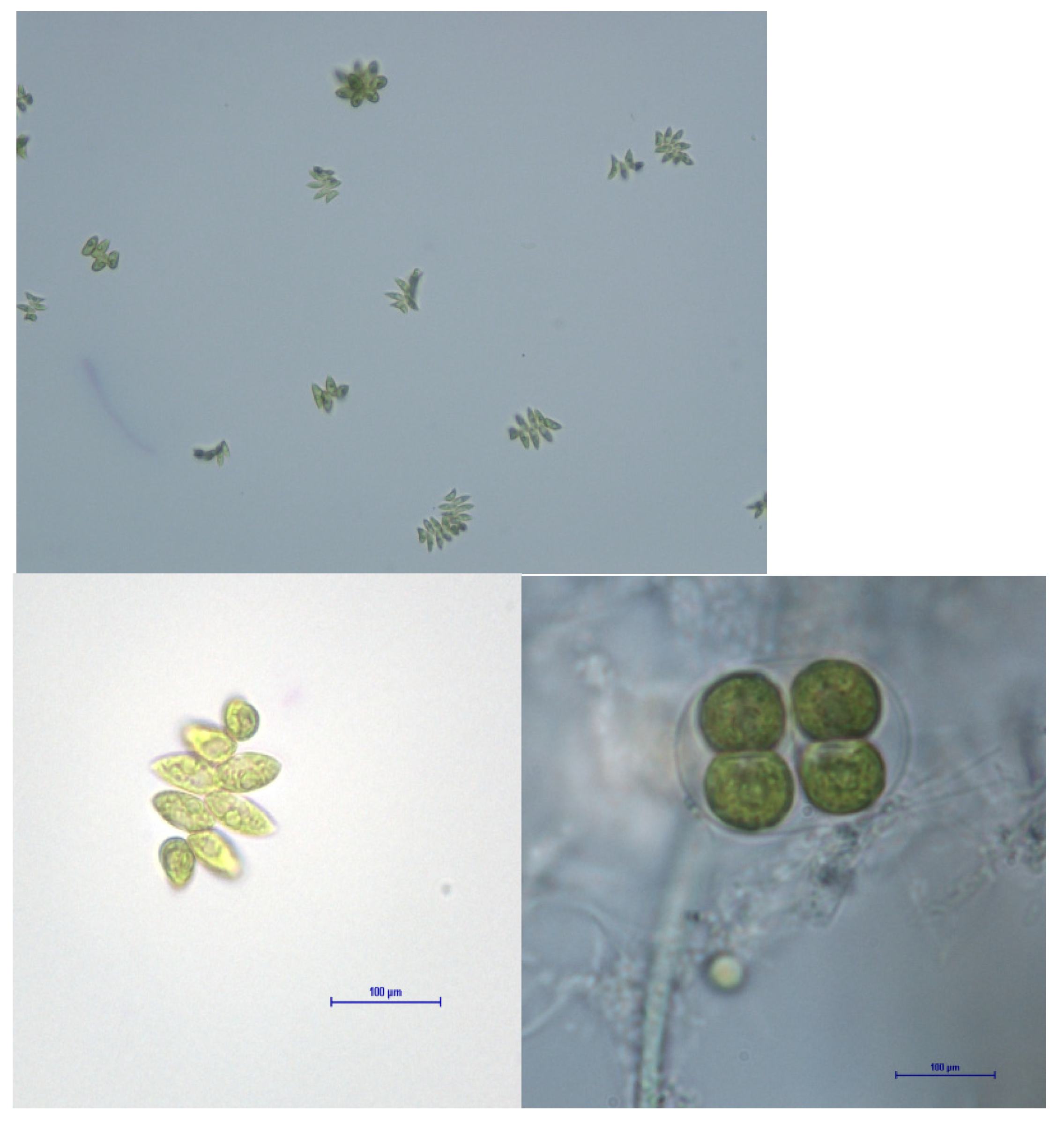
3.1. Microalgae Morphological Characterization
- −
- Scenedesmus sp.: it belongs to the Chlorophyta division, Chlorophyceae class, and to the Scenedesmus genus. It is characterized because it can form immobile colonies of lined cells forming flat sheets. The more frequent colonies have two or four cells but can have eight, sixteen, thirty-two and sometimes be unicellular. Normally, the end cells have two thorns up to 200 µm length that stand out. Each cell contains a unique parietal and one cellular wall (see Figure 3a).
- −
- Charca Brovales: At the beginning, this population belonged to a green-blue cyanobacteria group and different types of microalgae; among them, different cells were observed, with various shapes such as filamentous, round, oval and other microorganisms such as protozoa and bacteria. Therefore, it can be considered a consortium of different species. After a time period of 15–20 days of culturing and cooling in the laboratory photobioreactor with high CO2 concentrations, a proliferation of one specific microalga was observed. This was the mono-specific culture of a very small alga with a round form similar to Chlorella minutissima and was selected to form part of this study (see Figure 3b).
- −
- Arrocampo: The cells collected in this reservoir are green and round, and are very similar to Chlorellas sp. and Nannochloropsis sp. This microalga is unicellular and does not form colonies. Morphologically, it is very similar to the consortium named Charca Brovales (Figure 3c).
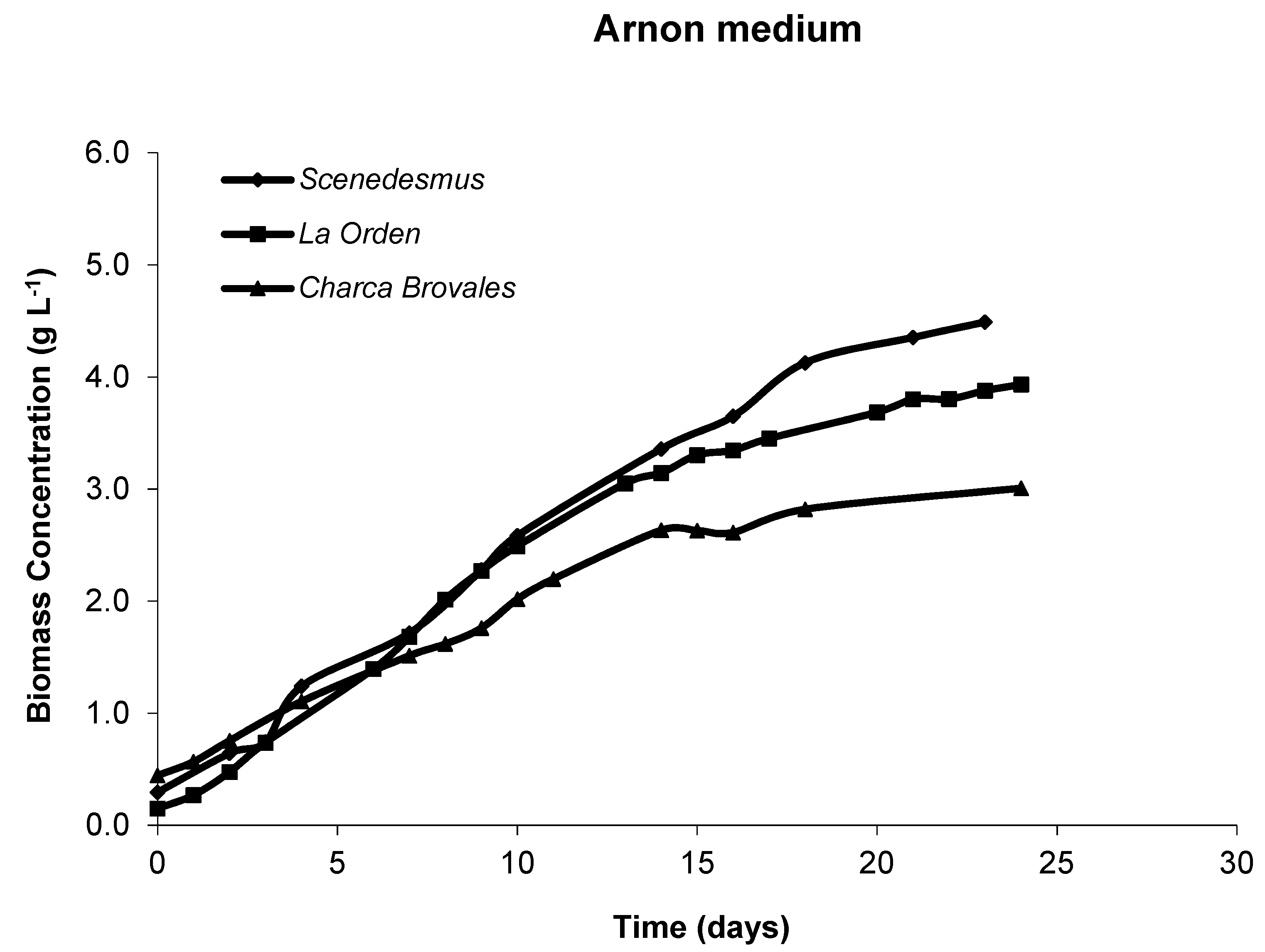
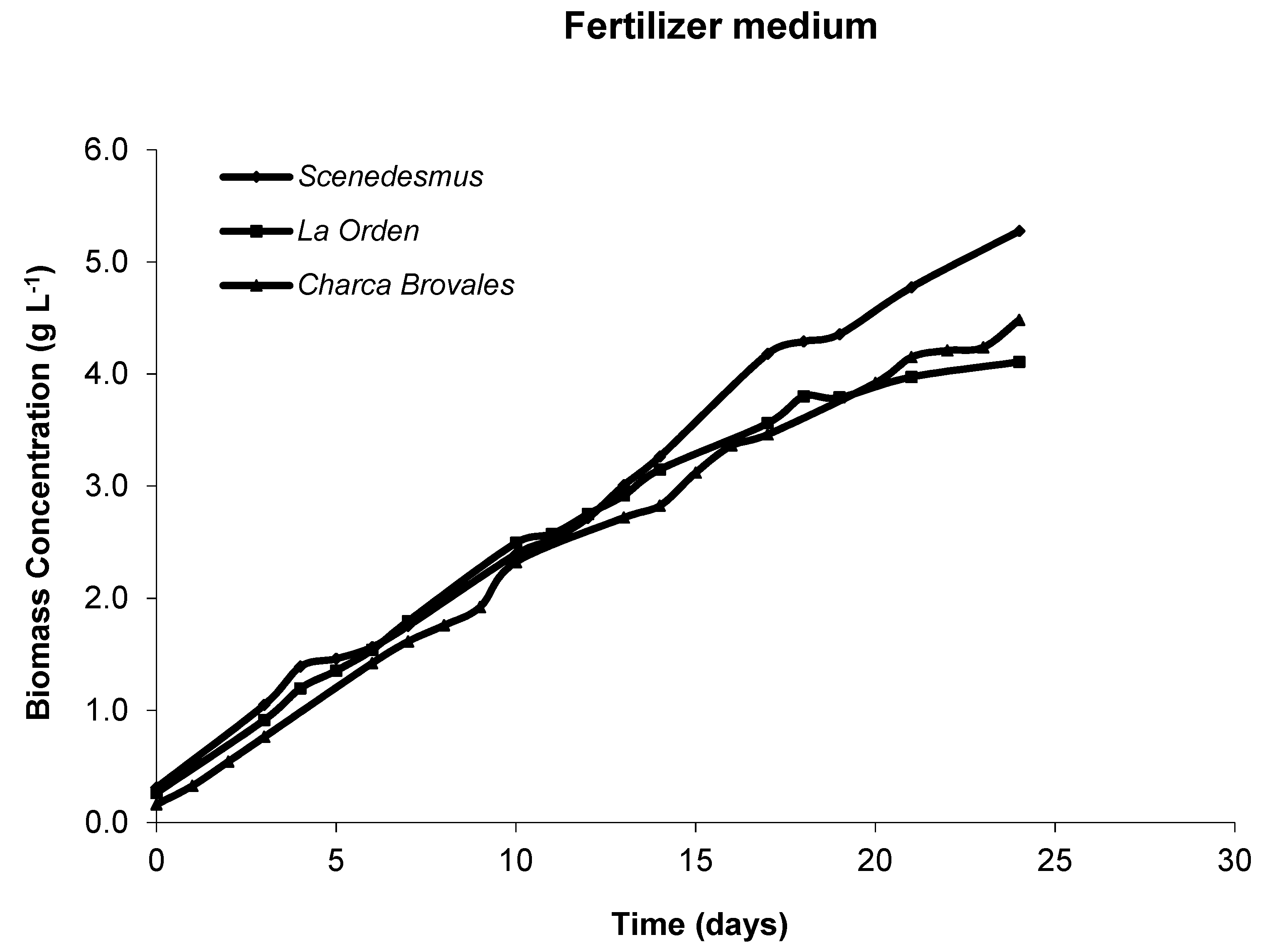
3.2. Microalgae Productivity and Growth Curves
3.2.1. Laboratory Photobioreactors
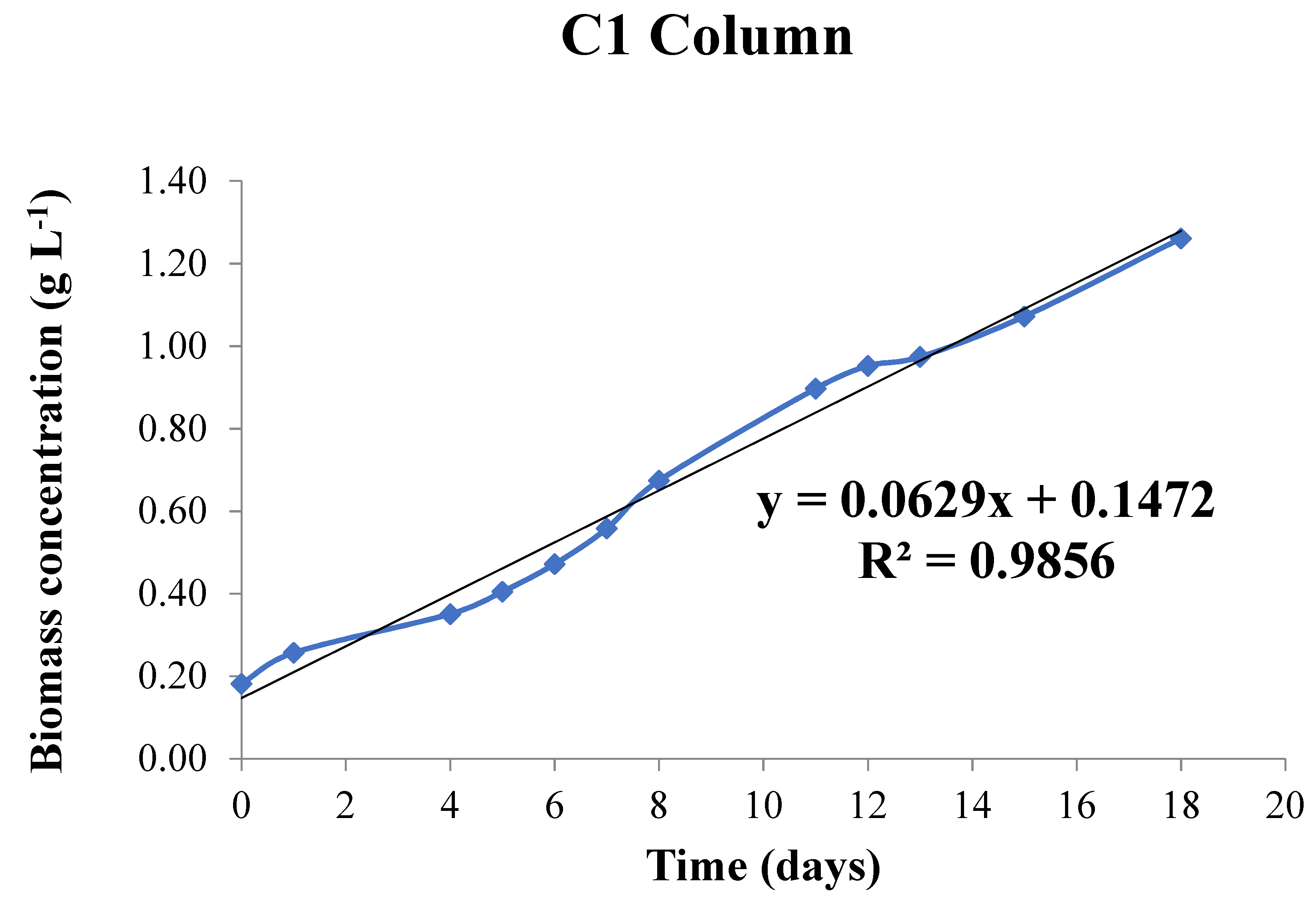
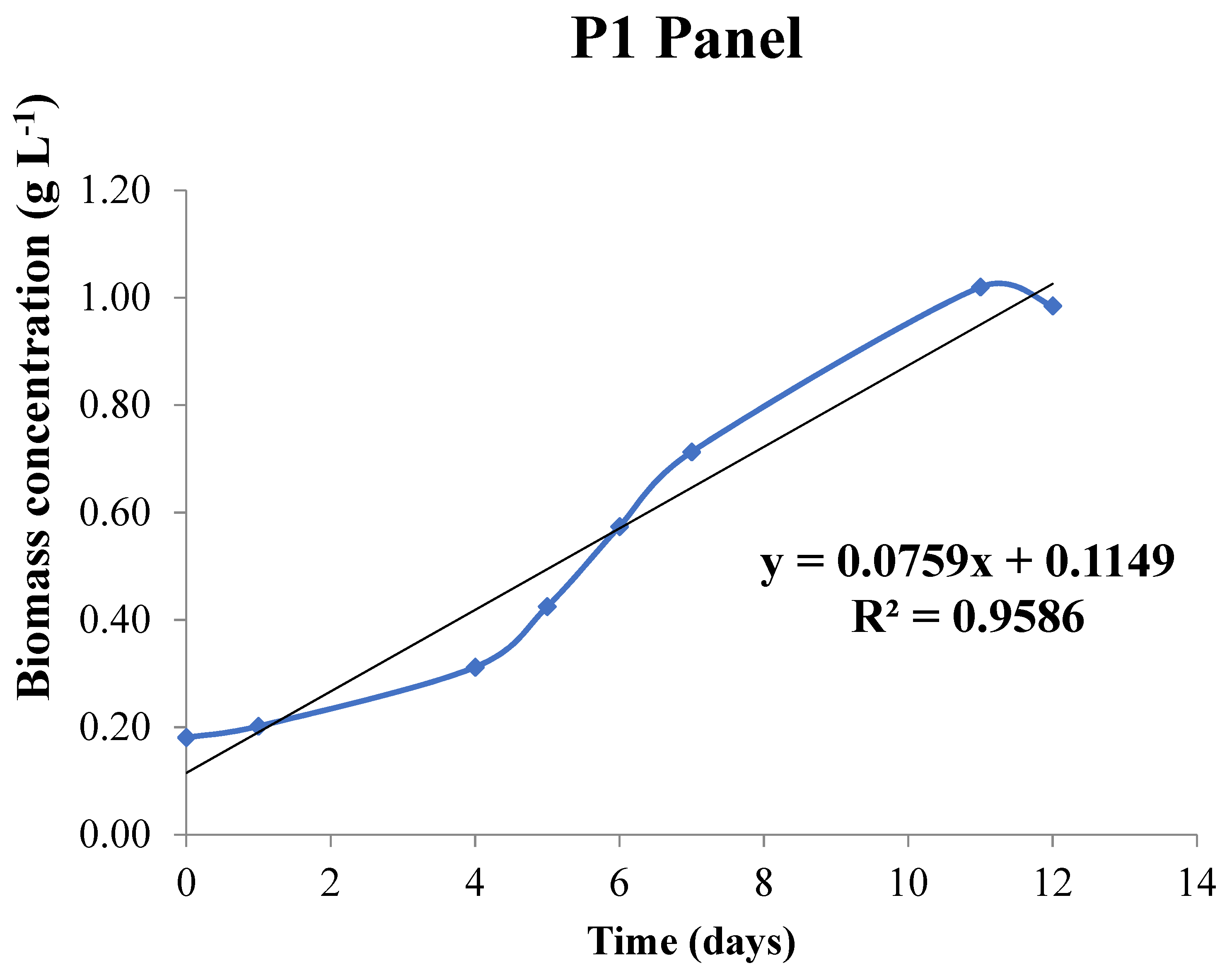
3.2.2. Pilot Plant
3.3. Characterization of Microalgae Biomass from Laboratory Photobioreactors
3.4. Microalgae Biomass Characterization from Exterior Photobioreactors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- European Commission. 2030 Objectives. Available online: http://ec.europa.eu/europe2020/index_es.htm (accessed on 20 February 2021).
- Suwelack, K.; Wüst, D. An approach to unify the appraisal framework for biomass conversion systems. Biomass Bioenergy 2015, 83, 354–365. [Google Scholar] [CrossRef]
- Okoro, V.O.; Sun, Z.; Birch, J. Meat processing waste as a potential feedstock for biochemicals and biofuels- A review of possible conversion technologies. J. Clean. Prod. 2017, 142, 1583–1608. [Google Scholar] [CrossRef]
- Dayananda, C.; Sarada, R.; Rani, M.U.; Shamala, T.R.; Ravishankar, G.A. Autotrophic cultivation of Botryococcus braunii for the production of hydrocarbons and exopolysaccharides in various media. Biomass Bioenergy 2007, 31, 87–93. [Google Scholar] [CrossRef]
- Deprá, M.C.; Dos Santos, A.M.; Severo, I.A.; Santos, A.B.; Zepka, L.Q.; Jacob-Lopes, E. Microalgal Biorefineries for Bioenergy Production: Can We Move from Concept to Industrial Reality? BioEnergy Res. 2018, 11, 727–747. [Google Scholar] [CrossRef]
- Pienkos, P.; Darzins, A. The promise and challenges of microalgal-derived biofuels. Biofuel Bioprod. Biorefining 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Ras, M.; Lardon, L.; Sialve, B.; Bernet, N.; Steyer, J.P. Experimental study on a coupled process of production and anaerobic digestion of Chorella vulgaris. Bioresour. Technol. 2011, 102, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Debowski, M.; Zielinski, M.; Grala, A.; Dudek, M. Algae biomass as an alternative substrate in biogas production technologies—Review. Renew. Sustain. Energy Rev. 2013, 27, 596–604. [Google Scholar] [CrossRef]
- Wang, M.; Park, C. Investigation of anaerobic digestion of Chlorella sp. and Micractinium sp. grown in high-nitrogen wastewater and their co-digestion with waste activated sludge. Biomass Bioenergy 2015, 80, 30–37. [Google Scholar] [CrossRef]
- Yun, H.S.; Ji, M.K.; Park, Y.T.; Salama, E.S.; Choi, J. Microalga, Acutodesmus obliquus KGE 30 as a potential candidate for CO2 mitigation and biodiesel production. Environ. Sci. Pollut. Res. 2016, 23, 17831–17839. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Gheda, S.F.; El-Sayed, A.E.B.; Shady, A.M.A.; El-Sheikh, M.E.; Schagerl, M. Outdoor cultivation of the green microalga Chlorella vulgaris under stress conditions as a feedstock for biofuel. Environ. Sci. Pollut. Res. 2019, 26, 18520–18532. [Google Scholar] [CrossRef]
- Batan, L.; Quinn, J.; Willson, B.; Bradley, T. Net energy and greenhouse gas emission evaluation of biodiesel derived from microalgae. Environ. Sci. Technol. 2010, 44, 7975–7980. [Google Scholar] [CrossRef]
- Liu, X.; Saydah, B.; Eranki, P.; Colosi, L.M.; Mitchell, B.G.; Rhodes, J.; Clarens, A.F. Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Bioresour. Technol. 2013, 148, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Moody, J.W.; McGinty, C.M.; Quinn, J.C. Global evaluation of biofuel potential from microalgae. Proc. Natl. Acad. Sci. USA 2014, 111, 8691–8696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scragg, A.H.; Illman, A.M.; Carden, A.; Shales, S.W. Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 2002, 23, 67–73. [Google Scholar] [CrossRef]
- Wijffels, R.; Barbosa, M. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [Green Version]
- Norsker, N.H.; Barbosa, M.J.; Vermue, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Murphy, C.F.; Allen, D.T. Energy-water nexus for mass cultivation of algae. Environ. Sci. Technol. 2011, 45, 5861–5868. [Google Scholar] [CrossRef]
- Alba, L.; Torri, C.; Samorì, C.; van der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. Hydrothermal treatment (HTT) of microalgae: Evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuels 2012, 26, 642–657. [Google Scholar] [CrossRef]
- Mu, D.; Min, M.; Krohn, B.; Mullins, K.A.; Ruan, R.; Hill, J. Life cycle environmental impacts of wastewater-based algal biofuels. Environ. Sci. Technol. 2014, 48, 11696–11704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, D.; Ruan, R.; Addy, M.; Mack, S.; Chen, P.; Zhou, Y. Life cycle assessment and nutrient analysis of various processing pathways in algal biofuel production. Bioresour. Technol. 2017, 230, 33–42. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Suali, E.; Sarbatly, R. Conversion of microalgae to biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- Kunjapur, A.M.; Bruce-Eldridge, R. Photobioreactor Design for Commercial Biofuel Production from Microalgae. Ind. Eng. Chem. Res. 2010, 49, 3516–3526. [Google Scholar] [CrossRef]
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshaka, A.; Cohen, Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 60, 497–503. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Sabio, E.; Ramiro, M.J. Preparation and properties of biodiesel from Cynara cardúnculus L. oil. Ind. Eng. Chem. Res. 1999, 38, 2927–2931. [Google Scholar] [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Sánchez, N.; Sánchez, R.; Encinar, J.M.; González, J.F.; Martínez, G. Complete analysis of castor oil methanolysis to obtain biodiesel. Fuel 2015, 147, 95–99. [Google Scholar] [CrossRef]
- Kim, N.J.; Li, H.; Jung, K.; Chang, C.H.; Lee, P.C. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour. Technol. 2011, 102, 7466–7469. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Matías, J.; Encinar, J.M.; González, J.; González, J.F. Optimisation of ethanol fermentation of Jerusalem artichoke tuber juice using simple technology for a decentralised and sustainable ethanol production. Energy Sustain. Dev. 2015, 25, 4–39. [Google Scholar] [CrossRef]
- Nahak, S.; Nahak, G.; Pradhan, I.; Sahu, R.K. Bioethanol from Marine Algae: A Solution to Global Warming Problem. J. Appl. Environ. Biol. Sci. 2011, 1, 74–80. [Google Scholar]
- Branyikova, I.; Marsalkova, B.; Doucha, J.; Branyik, T.; Bisova, K.; Zachleder, V. Microalgae-novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Wang, G.C.; Zhou, B.C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G.; Fernandes, B.D.; Abreu, A.P.; Vicente, A.A.; Teixeira, J.A. Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl. Energy 2011, 88, 3331–3335. [Google Scholar] [CrossRef] [Green Version]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Krzemieniewski, M. Anaerobic Co-digestion of the Energy Crop Sida hermaphrodita and Microalgae Biomass for Enhanced Biogas Production. Int. J. Environ. Res. 2017, 11, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Arnon Daniel, I.; McSwain, B.D.; Tsujimoto, H.Y.; Wada, K. Photochimical activity and components of membrane preparations from blue-green algae I. Coexistence of two photosystems in relation to cholorphyll and removal of phycoyanin. Biochim. Biophys. Acta 1974, 357, 231–245. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Chisti, Y. Production of microalgae for energy—An introduction. In Proceedings of the International Technical Conference on “Production and Use of Microalgae for Energy Purposes”, Madrid, Spain, 11 November 2008. [Google Scholar]
- Calderón, A.J.; González, I. Some hardware and instrumentation aspects of the development of an automation system for jar tests in drinking water treatment. Sensors 2017, 17, 2305. [Google Scholar] [CrossRef] [Green Version]
- Moxley, G.; Zhang, Y.H.P. More accurate determination of acid-labile carbohydrates in lignocellulose by modified quantitative saccharification. Energy Fuels 2007, 21, 684–3688. [Google Scholar] [CrossRef]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- Anderson, J.; Sorek, B. Microalgae: The Fuel of Tomorrow; University of Pittsburg: Pittsburgh, PA, USA, 2008. [Google Scholar]
- Encinar, J.M.; González, J.F.; Martínez, G.; Sánchez, N.; Pardal, A. Soybean oil transesterification by the use of a microwave flow system. Fuel 2012, 95, 386–393. [Google Scholar] [CrossRef]
- EPA 414.4. Methods for Chemical Analysis of Water and Wastes; U.S. Environmental Protection Agency, Environmental Monitoring and Support Laboratory: Washington, DC, USA, 1993. [Google Scholar]
- Bailey, J.E.; Ollis, D.F. Biochemical Engineering Fundamentals, 2nd ed.; McGraw-Hill: Singapore, 1986. [Google Scholar]
- Martínez-García, L. Elimination of CO2 with Native Microalgae. Ph.D. Thesis, University of León, Institute of Natural Resources, Area of Chemical Engineering, León, Spain, 2009; p. 213. [Google Scholar]
- Persico, M.M.; Moris, M.; Tranier, E.D.; Zanazzi, A.N.; Saubidet, A.A.; Beligni, M.V. Evaluación de un sistema exterior de cultivo masivo de la microalga marina Nannochloropsis occulata, en una zona templada oceánica de Argentina. Revista Latinoamericana de Biotecnología Ambiental y Algal 2011, 2, 30–48. [Google Scholar]
- Chang, E.H.; Yang, S.S. Some characteristics of microalgae isolated in Taiwan for biofixation of carbon dioxide. Bot. Bull. Acad. Sin. 2003, 44, 43–52. [Google Scholar]
- De Morais, M.G.; Costa, J.A. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.E.; Jiménez, J.M.; Farida, E.Y. Influence of phosphorus concentration on the growth kinetics and stoichiometry of the microalga Scenedesmus obliquus. Process. Biochem. 1997, 32, 657–664. [Google Scholar] [CrossRef]
- Hodaifa, G.; Martínez, M.; Sánchez, S. Use of industrial wastewater from olive-oil extraction for biomass production of Scenedesmus obliquus. Bioresour. Technol. 2008, 99, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.F.; Fernández-Sevilla, J.M.; Acién, F.G.; Cerón, M.C.; Pérez-Parra, J.; Molina-Grima, E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008, 79, 719–729. [Google Scholar] [CrossRef] [PubMed]
- San Pedro, A.; González-López, C.V.; Acién, F.G.; Molina-Grima, E. Marine microalgae selection and culture conditions optimization for biodiesel production. Bioresour. Technol. 2013, 134, 353–361. [Google Scholar] [CrossRef]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in chlorella strains calorific values when grown in low nitrogen medium. Enzym. Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Tsai, M.T.; Ong, S.C.; Chen, C.H.; Liu, C.S. Lipid accumulation and CO2 utilization of Nannochloropsis occulata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Chang, J.S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mallick, N. Scenedesmus obliquus as a potential source of biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Molina-Grima, E.; Fernández-Sevilla, J.M.; Sánchez-Pérez, J.A.; García-Camacho, F. A study on simultaneous photolimitation and photoinhibition in dense microalgal cultures taking into account incident and averaged irradiances. J. Biotechnol. 1996, 45, 59–69. [Google Scholar] [CrossRef]
- Richmond, A. Microalgaculture. Crit. Rev. Biotechnol. 1986, 4, 369–438. [Google Scholar] [CrossRef]
- Tredici, M.R.; Chini Zitelli, G. Efficiency of sunlight utilization: Tubular versus flat fotobioreactors. Biotechnol. Bioeng. 1998, 57, 187–197. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- Punín-Crespo, M.O.; Lage-Yusty, M.A. Determination of aliphatic hydrocarbons in the alga Himanthalia elongate. Ecotoxicol. Environ. Saf. 2004, 57, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Lee, S.; Oh, Y.; Kim, D.; Kwon, D.; Lee, C.; Lee, J. Converting carbohydrates extracted from marine algae into ethanol using various ethanolic Escherichia coli strains. Appl. Microbiol. Biotechnol. 2011, 164, 878–888. [Google Scholar] [CrossRef]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Characterization and optimization of carbohydrate production from an indigenous microalgae Chorella vulgaris FSP-E. Bioresour. Technol. 2013, 135, 157–165. [Google Scholar] [CrossRef]
- Encinar, J.M.; Sánchez, N.; Martínez, G.; García, L. Study of biodiesel production from animal fats with high free fatty acid content. Bioresour. Technol. 2011, 102, 10907–10914. [Google Scholar] [CrossRef]
- Sánchez, N. Obtención de Biodiésel Mediante Transesterificación de Aceite de Ricino y Grasas Animales. Aprovechamiento Energético de la Glicerina Como Subproducto Del Proceso. Ph.D. Thesis, Universidad de Extremadura, Badajoz, Spain, 18 May 2015. [Google Scholar]




| Dissolution 1 | Chemical Compound | Quantity(g L−1) | (mL L−1) * |
|---|---|---|---|
| solution stock 1 | Na2MoO4 2 H2O | 1.26 | 1 |
| solution stock D7-(Mo-V) | H3BO3 | 2.86 | 1 |
| MnCl2 4 H2O | 1.81 | ||
| ZnSO4 7 H2O | 0.222 | ||
| CuSO4 5 H2O | 0.79 | ||
| CoCl2 6 H2O | 0.403 | ||
| solution stock 2 | MgSO4 7 H2O | 124 | 1 |
| solution stock 3 | CaCl2 2 H2O | 15 | 1 |
| solution stock 4 | NaCl | 117 | 1 |
| solution stock Fe-EDTA | C10H16N2O8 | 16 g in 186 mL water | 1 |
| KOH | 10.4 g in 186 mL water | ||
| FeSO47H2O | 13.7 g in 384 mL water | ||
| solution stock 5 | NaNO3 2M | 170 | 10 |
| Dissolution 2 | |||
| solution stock 6 | K2HPO4 1M | 174 | 4 |
| Photobioreactor | Microalgae Population | µmax (d −1) | dt (Days) | Pmax (g L−1 d−1) |
|---|---|---|---|---|
| Laboratory | Scenedesmus sp (AM) | 0.504 | 1.35 | |
| Scenedesmus sp (FM) | 0.408 | 1.71 | ||
| La Orden (AM) | 0.864 | 0.81 | ||
| La Orden (FM) | 0.408 | 1.67 | ||
| Charca Brovales (AM) | 0.288 | 2.44 | ||
| Charca Brovales (FM) | 0.720 | 0.97 | ||
| Pilot Plant column | La Orden (FM) | 0.360 | 2 | 0.116 |
| Pilot plant panel | La Orden (FM) | 0.312 | 2.2 | 0266 |
| Microalgae Population | Elemental Analysis, % wt db | HHV, | ||||
|---|---|---|---|---|---|---|
| C/N | C | H | N | S | MJ kg−1 | |
| Laboratory photobioreactor Scenedesmus sp. (AM) | 17.9 | 43.0 | 6.65 | 2.79 | 0.356 | 21.2 |
| Scenedesmus sp. (FM) | 16.4 | 46.3 | 6.81 | 3.28 | 0.277 | 21.5 |
| La Orden (AM) | 16.5 | 48.9 | 7.28 | 3.46 | 0.245 | 21.8 |
| La Orden (FM) | 13.3 | 47.2 | 7.98 | 4.15 | 0.379 | 21.1 |
| Charca Brovales (AM) | 6.4 | 46.9 | 6.85 | 8.55 | 0.729 | 20.0 |
| Charca Brovales (FM) | 15.5 | 46.6 | 6.83 | 3.51 | 0.386 | 20.8 |
| Exterior photobioreactors La Orden column (FM) | 7.9 | 46.0 | 7.47 | 6.79 | 0.460 | 22.3 |
| La Orden panel (FM) | 6.7 | 48.3 | 6.76 | 8.41 | 0.592 | 20.7 |
| % wt db Lipids | |
|---|---|
| Microalgae Population | Hexane |
| Laboratory photobioreactor | |
| Scenedesmus sp. (AM) | 9.3 ± 0.3 |
| Scenedesmus sp. (FM) | 8.8 ± 0.2 |
| La Orden (AM) | 12.6 ± 0.5 |
| La Orden (FM) | 8.8 ± 0.3 |
| Charca Brovales (AM) | 4.5 ± 0.4 |
| Charca Brovales (FM) | 7.4 ± 0.15 |
| Exterior photobioreactors | |
| La Orden column (FM) | 6.07 ± 0.03 |
| La Orden panel (FM) | 3.21 ± 0.19 |
| Microalgae Population | Sugars Content % wt db | ||
|---|---|---|---|
| Fructose | Glucose | Sucrose | |
| Laboratory photobioreactor | |||
| Scenedesmus sp | 0.98 | 5.08 | 0.75 |
| La Orden | 0.71 | 1.45 | 0.51 |
| Charca Brovales | 0.81 | 2.01 | 0.31 |
| Microalgae population | Carbohydrates | ||
| (% wt db) | |||
| Exterior photobioreactors | |||
| La Orden column | 6.50 | ||
| La Orden panel | 9.22 | ||
| Origin | Animal Fats [67] | Rape Oil [27] | Soybean Oil [27] | Scenedesmus ob. Oil [57] | Parietochloris incisa Oil [25] | Chlorella vulgaris Oil [11] | Acutodesmus obliquus Oil [10] | “La Orden” Oil (this work) |
|---|---|---|---|---|---|---|---|---|
| Lauric acid C12:0 | 0.46 ± 0.05 | |||||||
| Myristic acid C14:0 | 1.38 | 0.07 | 0.09 | 1.33 ± 0.75 | ||||
| Palmitic acid C16:0 | 27.3 | 4.92 | 11.60 | 19.80 | 10.00 | 34.37 ± 1.56 | 34.0 | 31.50 ± 1.37 |
| Palmitoleic acid C16:1 | 4.01 | 0.24 | 0.11 | 4.06 | 2.00 | 2.41 ± 0.09 | ||
| Stearic acid C18:0 | 11.7 | 1.63 | 3.25 | 9.08 | 3.00 | 4.75 ± 1.2 | 3.17 ± 0.09 | |
| Oleic acid C18:1 | 44.4 | 66.59 | 25.09 | 16.41 | 16.00 | 44.91 ± 2.65 | 13.0 | 19.60 ± 1.56 |
| Linoleic acid C18:2 | 10.4 | 17.08 | 52.93 | 21.50 | 17.00 | 12.78 ± 1.87 | 7.8 | 9.32 ± 1.13 |
| Linolenic acid C18:3 | 0.62 | 7.75 | 5.95 | 12.3 | 3.00 | 1.40 ± 0.05 | 36.0 | 6.25 ± 1.05 |
| Nonadecanoic acid C19:0 | 4.71 ± 1.01 | |||||||
| Arachidonic acid C20:4 | 43.00 | |||||||
| Others | 0.19 | 1.71 | 0.99 | 16.60 | 3 | 9.2 | 23.10 ± 0.93 |
| Parameter | pH | E, mV | TS, % | VS, % | VST, % | CODT, mg O2 L−1 | N-ammonia, mg L−1 | C:N | Alkalinity, mg CaCO3 L−1 |
|---|---|---|---|---|---|---|---|---|---|
| “La Orden” consortium | 7.24 | 53 | 9.80 | 65.00 | 6.37 | 150,000 | 196 | 7.55 | 3754 |
| Inoculum | 7.50 | −414 | 2.86 | 51.29 | 1.47 | 47,000 | 1740 | 9.13 | 8568 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, J.F.; Cuello, T.B.; Calderón, A.J.; Calderón, M.; González, J.; Carmona, D. Cultivation of Autochthonous Microalgae for Biomass Feedstock: Growth Curves and Biomass Characterization for Their Use in Biorefinery Products. Energies 2021, 14, 4567. https://doi.org/10.3390/en14154567
González JF, Cuello TB, Calderón AJ, Calderón M, González J, Carmona D. Cultivation of Autochthonous Microalgae for Biomass Feedstock: Growth Curves and Biomass Characterization for Their Use in Biorefinery Products. Energies. 2021; 14(15):4567. https://doi.org/10.3390/en14154567
Chicago/Turabian StyleGonzález, Juan Félix, Teresa Belén Cuello, Antonio José Calderón, Manuel Calderón, Jerónimo González, and Diego Carmona. 2021. "Cultivation of Autochthonous Microalgae for Biomass Feedstock: Growth Curves and Biomass Characterization for Their Use in Biorefinery Products" Energies 14, no. 15: 4567. https://doi.org/10.3390/en14154567







