Influence of Fuel Type and Water Content Variation on Pollutant Emission Characteristics of a Biomass Circulating Fluidized Bed Boiler
Abstract
:1. Introduction
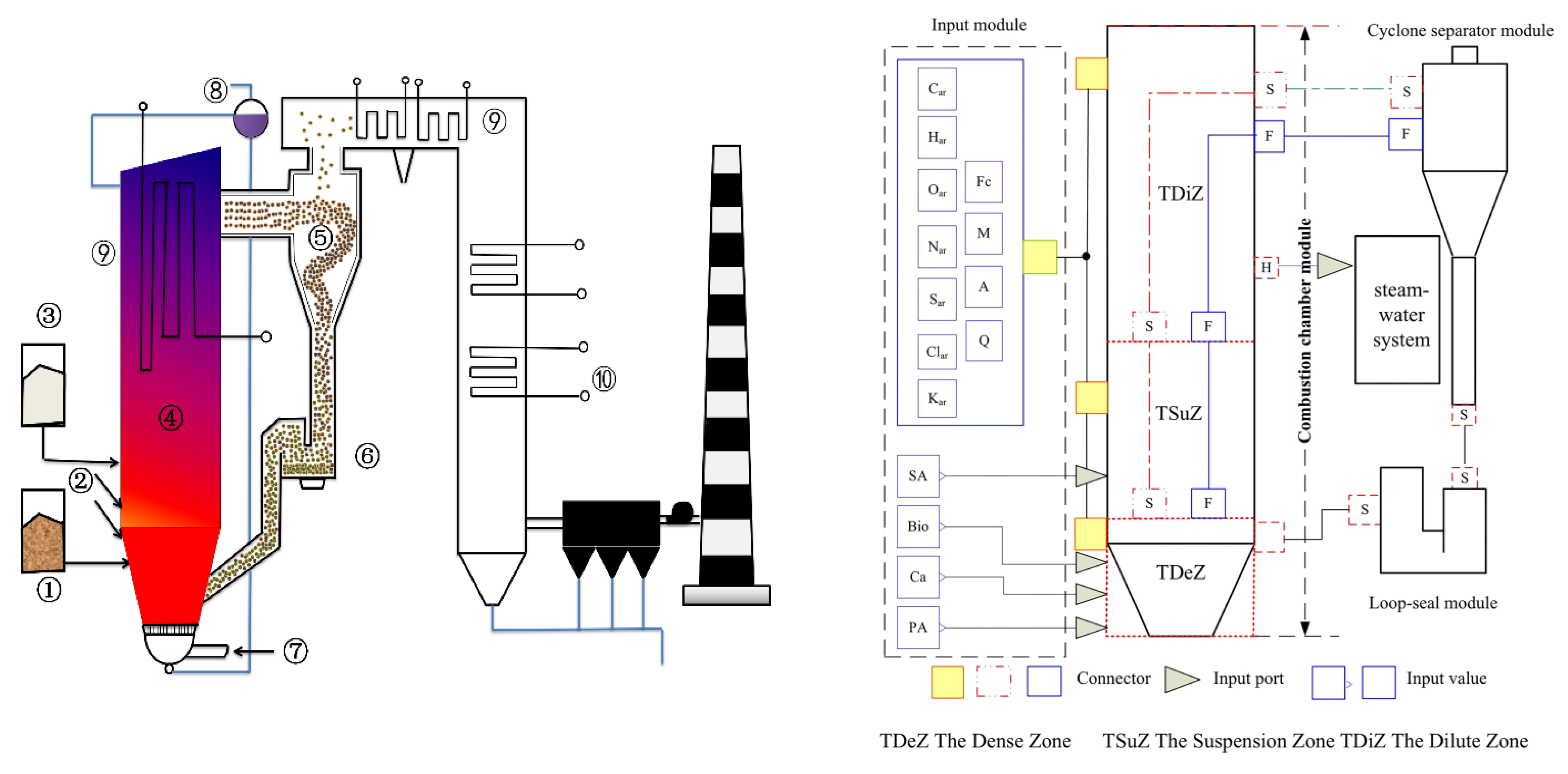
2. Modeling
2.1. Combustion System Model
2.1.1. Flow Model
2.1.2. Combustion Model
2.1.3. Heat Transfer Model
2.1.4. Mass and Energy Conservation Models
2.2. Steam-Water System
2.2.1. Economizer Model
2.2.2. Second-Order Model of Evaporation Zone
2.2.3. Calculation of Thermodynamic Properties of Water and Steam
2.3. Simulation Model
3. Results and Discussion
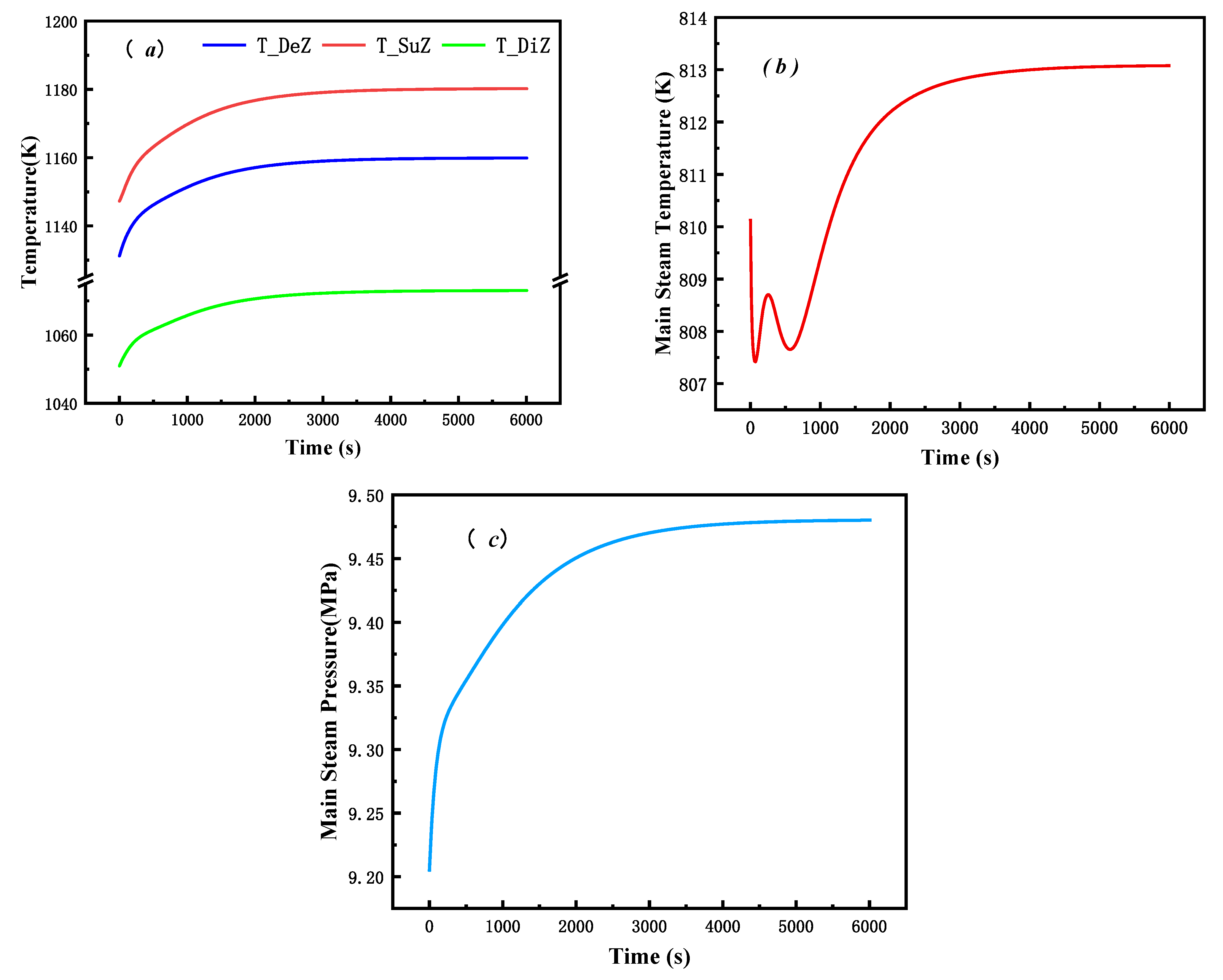
3.1. Steady-State Test
3.2. Dynamic Test
3.2.1. Verification of Dynamic Characteristics
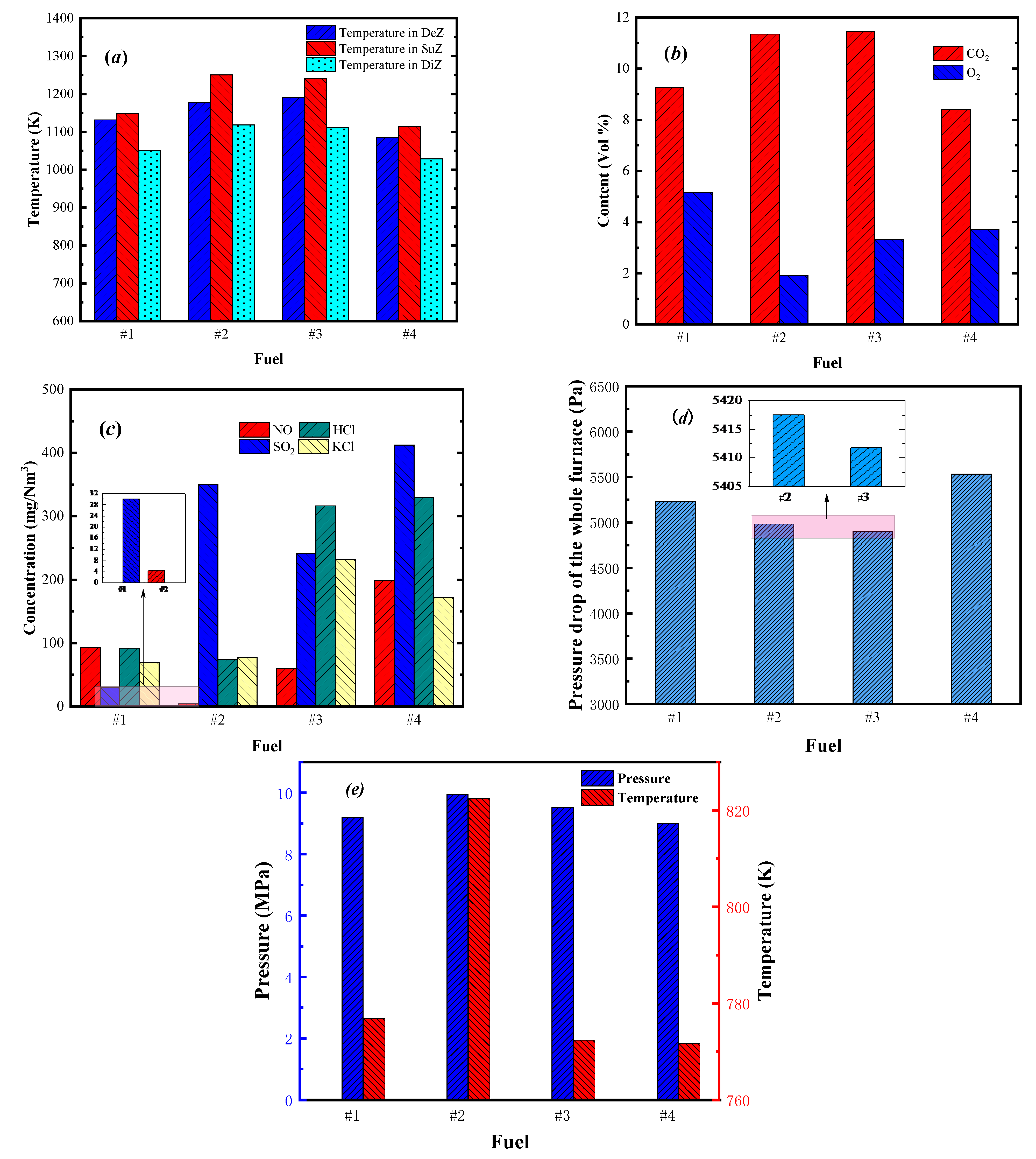
3.2.2. Dynamic Response of Fuel Variety Change
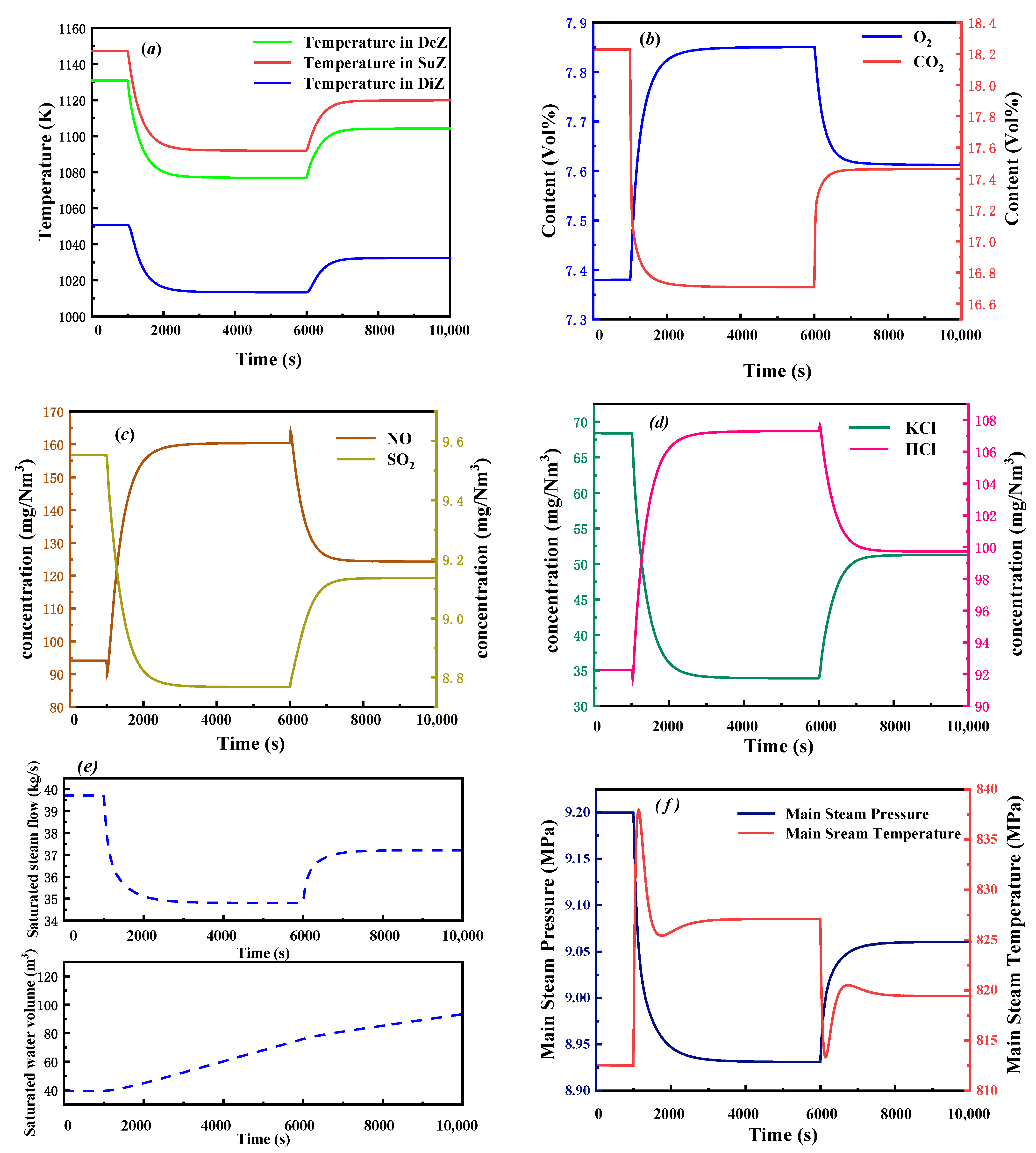
3.2.3. Dynamic Response of Fuel Moisture Content Change
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.G.; Feng, T.T.; Ma, Y.; Yan, Y.S.; Pan, X.F. Analysis on investment strategies in China: The case of biomass direct combustion power generation sector. Renew. Sust. Energy Rev. 2015, 42, 760–772. [Google Scholar]
- Saidur, R.; Atabani, A.; Demirbaş, A.; Hossain, M.S. A review on biomass as a fuel for boilers. Renew. Sust. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Nunes, L.; Loureiro, L.; Sá, L.; Silva, H. Evaluation of the potential for energy recovery from olive oil industry waste: Thermochemical conversion technologies as fuel improvement methods. Fuel 2020, 279, 118536. [Google Scholar] [CrossRef]
- Guo, F.; Zhong, Z. Co-combustion of anthracite coal and wood pellets: Thermodynamic snalysis, combustion efficiency, pollutant emissions and ash slagging. Environ. Pollut. 2018, 239, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, Z.; Motahari-Nezhad, M.; Banisaeed, M.; Shahraki, S.; Beheshti, M. A detailed study of oxy-fuel combustion of biomass in a circulating fluidized bed (CFB) combustor: Evaluation of catalytic performance of metal nanoparticles (Al, Ni) for combustion efficiency improvement. Energy 2016, 109, 1139–1147. [Google Scholar] [CrossRef]
- Doherty, W.; Reynolds, A.; Kennedy, D. The effect of air preheating in a biomass CFB gasifier using ASPEN Plus simulation. Biomass Bioenerg. 2009, 33, 1158–1167. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Tai, L.; Cheng, Z.; Chen, G.; Yan, B.; Hou, L. Biomass molded fuel in China: Current status, policies and suggestions. Sci. Total Environ. 2020, 724, 138345. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shen, J.; Li, Y.; Wang, M.; Lawal, A. Flexible operation of post-combustion solvent-based carbon capture for coalfired power plants using multi-model predictive control: A simulation study. Fuel 2018, 220, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, Z.; Xu, G.; Ren, J.; Li, J. Sustainability assessment of straw direct combustion power generation in China: From the environmental and economic perspectives of straw substitute to coal. J. Clean. Prod. 2020, 273, 122890. [Google Scholar] [CrossRef]
- Li, D.; Wu, D.S.; Xu, F.G.; Lai, J.H.; Shao, L. Literature overview of Chinese research in the field of better coal utilization-ScienceDirect. J. Clean. Prod. 2018, 185, 959–980. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Z.; Liu, Q.; Jianzhong, L.I.; Jun, H.E.; Neng, H.E.; Liao, Z. Design of Biomass-fired Circulating Fluidized Bed Boiler. Gas Heat 2014, 34, 6–8. [Google Scholar]
- Krzywanski, J.; Rajczyk, R.; Bednarek, M.; Wesolowska, M.; Nowak, W. Gas emissions from a large scale circulating fluidized bed boilers burning lignite and biomass. Fuel Process. Technol. 2013, 116, 27–34. [Google Scholar] [CrossRef]
- Nevalainen, H.; Jegoroff, M.; Saastamoinen, J.; Tourunen, A.; Jänttib, T.; Kettunen, A.; Johnsson, F.; Niklasson, F. Firing of coal and biomass and their mixtures in 50 kW and 12 MW circulating fluidized beds–phenomenon study and comparison of scales. Fuel 2007, 86, 2043–2051. [Google Scholar] [CrossRef]
- Gungor, A. Simulation of co-firing coal and biomass in circulating fluidized beds. Energy Convers. Manag. 2013, 65, 574–579. [Google Scholar] [CrossRef]
- Gungor, A. Two-dimensional biomass combustion modeling of CFB. Fuel 2008, 87, 1453–1468. [Google Scholar] [CrossRef]
- Sodja, A.; Škrjanc, I.; Zupančič, B. Cyber-physical modelling in Modelica with model-reduction techniques. J. Syst. Softw. 2020, 163, 110517. [Google Scholar] [CrossRef]
- Darton, R.C.; Lanauze, R.D.; Davidson, J.F.; Harrson, D. Bubble-growth due to coalescence in fluidized-beds. Trans. Inst. Chem. Eng. 1977, 55, 274–280. [Google Scholar]
- Wen, C.Y.; Yu, Y.H. A generalized method for predicting the minimum fluidization velocity. Aiche. J. 1966, 12, 610–612. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O. Entrainment of solids from fluidized beds I. Hold-up of solids in the freeboard II. Operation of fast fluidized beds. Powder Technol. 1990, 61, 193–206. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, W.; Shao, Y.; Liu, Q.; Liu, L.; Liu, G. Simulation of Combustion of Municipal Solid Waste and Coal in an Industrial-Scale Circulating Fluidized Bed Boiler. Energy Fuels 2017, 31, 14248–14261. [Google Scholar] [CrossRef]
- Zhong, W.; Xie, J.; Shao, Y.; Liu, X.; Jin, B. Three-dimensional modeling of olive cake combustion in CFB. Appl. Therm. Eng. 2015, 88, 322–333. [Google Scholar] [CrossRef]
- Hong, X.; Bai, J.; Wu, Y.; Wang, J.; Zhang, G. Dynamic simulation of a large scale 260 t/h CFB boiler based on SIMUCAD platform. In Proceedings of the 2009 ISECS International Colloquium on Computing, Communication, Control, and Management, Sanya, China, 8–9 August 2009; pp. 246–249. [Google Scholar]
- Svoboda, K.; Hartman, M.; Pohořelý, M.; Trnka, O. Modelling of effects of operating conditions and coal reactivity on temperature of burning particles in fluidized bed combustion. Acta Geodyn. Geomater. 2004, 1, 261–274. [Google Scholar]
- Thomson, T.M.; Khalid, H.; Lozano, J.J.; Sancho, E.; Delfosse, L. Modelling of gaseous pollutants emissions in circulating fluidized bed combustion of municipal refuse. Fuel 1998, 77, 1399–1410. [Google Scholar]
- Krzywanski, J.; Czakiert, T.; Blaszczuk, A.; Rajczyk, R.; Muskala, W.; Nowak, W. A generalized model of SO2 emissions from large- and small-scale CFB boilers by artificial neural network approach Part 2. SO2 emissions from large- and pilot-scale CFB boilers in O2/N2, O2/CO2 and O2/RFG combustion atmospheres. Fuel Process Technol. 2015, 139, 73–85. [Google Scholar] [CrossRef]
- Cao, W.; Martí-Rosselló, T.; Li, J.; Lue, L. Prediction of potassium compounds released from biomass during combustion. Appl. Energy 2019, 250, 1696–1705. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Adeosun, A.; Liu, B.; Ruan, R.; Li, S.; Tan, H. Particulate matter emission and K/S/Cl transformation during biomass combustion in an entrained flow reactor. J. Energy Ins. 2018, 91, 835–844. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Pyrolysis and Combustion of High-Chlorine Biomass. Energy Fuel 2011, 25, 4961–4971. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, J.; Han, K.; Wang, J.; Lu, C. Influence of BaCO3 on chlorine fixation, combustion characteristics and KCl conversion during biomass combustion. Fuel 2017, 208, 82–90. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Sommersacher, P.; Hochenauer, C.; Scharler, R. Multi-stage model for the release of potassium in single particle biomass combustion. Fuel 2020, 280, 118569. [Google Scholar] [CrossRef]
- Lith, S.; Alonso-Ramirez, V.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the Gas Phase of Inorganic Elements during Wood Combustion. Part 1: Development and Evaluation of Quantification Methods. Energy Fuel 2006, 20, 964–978. [Google Scholar] [CrossRef]
- Lith, S.; Alonso-Ramirez, V.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the Gas Phase of Inorganic Elements during Wood Combustion. Part 2: Influence of Fuel Composition. Energy Fuel 2008, 22, 1598–1609. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and Release to the Gas Phase of Cl, K, and S during Combustion of Annual Biomass. Energy Fuel 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Martin, H. Heat transfer between gas fluidized beds of solid particles and the surfaces of immersed heat exchanger elements, Part II. Chem. Eng. Process. 1984, 18, 157–169. [Google Scholar] [CrossRef]
- Badruddin, I.A.; Ahmed, N.; Al-Rashed, A.A.A.; Kanesan, J.; Kamangar, S.; Khaleed, H. Analysis of Heat and Mass Transfer in a Vertical Annular Porous Cylinder Using FEM. Transp. Porous Med. 2012, 91, 697–715. [Google Scholar] [CrossRef]
- Basu, P. Heat transfer in high temperature fast fluidized beds. Chem. Eng. Sci. 1990, 45, 3123–3136. [Google Scholar] [CrossRef]
- Ordys, A.W.; Pike, A.W.; Johnson, M.A.; Katebi, R.M.; Grimble, M.J. Modelling and Simulation of Power Generation Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Strm, K.J.; Bell, R.D. Drum-boiler dynamics. Automatica 2000, 36, 363–378. [Google Scholar]
- Aleksandrov, A.A.; Petrova, T.I.; Ochkov, V.F.; Smetanin, D.S. Annual scientific session of the International Association on the Properties of Water and Steam (IAPWS). Therm. Eng. 2008, 55, 621–622. [Google Scholar] [CrossRef]
- Eborn, J. On Model Libraries for Thermo-Hydraulic Applications. Ph.D. Thesis, Lund University, Lund, Sweden, 2001. [Google Scholar]
- Liu, Y.; Pan, L.; Liu, S.; Wu, X.; Wang, S. Modeling and Dynamic Characteristics of the Dense Phase Region of a Biomass-fired Circulating Fluidized Bed Combustion System Using Modelica. BioResources 2020, 15, 7443–7457. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, Y.; Li, X.; Yang, J. Modelling of 330 MW Circulating Fluidized Bed Boiler System Based on Deep Belief Network. J. Physics. Conf. Ser. 2020, 1486, 022035. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, H.; Zhu, H.; Su, Z.; Hao, Y. Multivariable Control Strategy for Combustion System of Circulating Fluidized Bed Boiler. In Proceedings of the 2012 Asia-Pacific Power and Energy Engineering Conference, Shanghai, China, 27–29 March 2012. [Google Scholar]
- Chen, Y.; Gou, X. Dynamic modeling and simulation of a 410 t/h Pyroflow CFB boiler. Comput. Chem. Eng. 2007, 31, 21–31. [Google Scholar] [CrossRef]

| Chemical Reaction | Reaction Rate | Reaction Rate Constant |
|---|---|---|
| Parameters | Model Output Values | Measured Value | Relative Error |
|---|---|---|---|
| Bed temperature of dense phase zone | 1132 K | 1058 K | 6.99% |
| Oxygen content of flue gas at furnace outlet | 5.19 % | 5.00% | 3.80% |
| NO concentration of flue gas at furnace outlet | 93.08 mg/Nm3 | 96.00 mg/Nm3 | 3.04% |
| SO2 concentration of flue gas at furnace outlet | 30.1 mg/Nm3 | 32.00 mg/Nm3 | 5.94% |
| Outlet temperature of economizer | 558.2 K | 560 K | 0.32% |
| Drum pressure | 10.49 MPa | 10.50 MPa | 0.10% |
| Outlet temperature of drum | 587.5 K | 588 K | 0.09% |
| Superheater export temperature | 812.5 K | 813 K | 0.06% |
| Outlet pressure of superheater | 9.19 MPa | 9.20 MPa | 0.11% |
| Main steam flow | 39.71 kg/s | 39.72 kg/s | 0.03% |
| Biomass Fuel | Elemental Compositions (ar, %) | Q (MJ/kg) | Proximate Analysis (ar, %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | K | Cl | M | FC | V | A | ||
| #1 | 31.32 | 3.45 | 23.72 | 0.17 | 0.01 | 0.104 | 0.080 | 10.96 | 40.01 | 13.48 | 45.19 | 1.32 |
| #2 | 45.07 | 6.75 | 30.05 | 0.06 | 0.12 | 0.160 | 0.073 | 12.45 | 37.14 | 20.13 | 41.46 | 1.27 |
| #3 | 42.19 | 4.40 | 34.6 | 0.52 | 0.08 | 0.253 | 0.273 | 12.21 | 30.88 | 18.03 | 47.19 | 3.90 |
| #4 | 44.01 | 5.93 | 36.14 | 0.29 | 0.14 | 0.468 | 0.270 | 11.32 | 40.01 | 12.76 | 42.74 | 4.49 |
| #5 | 31.32 | 3.45 | 23.72 | 0.17 | 0.01 | 0.104 | 0.080 | 10.23 | 45.02 | 12.65 | 41.05 | 1.27 |
| ar: as received basis. | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Liu, S.; Yao, D.; Kong, R.; Liu, Y. Influence of Fuel Type and Water Content Variation on Pollutant Emission Characteristics of a Biomass Circulating Fluidized Bed Boiler. Energies 2021, 14, 5962. https://doi.org/10.3390/en14185962
He J, Liu S, Yao D, Kong R, Liu Y. Influence of Fuel Type and Water Content Variation on Pollutant Emission Characteristics of a Biomass Circulating Fluidized Bed Boiler. Energies. 2021; 14(18):5962. https://doi.org/10.3390/en14185962
Chicago/Turabian StyleHe, Jianjie, Shanjian Liu, Di Yao, Ranran Kong, and Yaya Liu. 2021. "Influence of Fuel Type and Water Content Variation on Pollutant Emission Characteristics of a Biomass Circulating Fluidized Bed Boiler" Energies 14, no. 18: 5962. https://doi.org/10.3390/en14185962






