Ammonium Chloride (NH4Cl)—Ammonia (NH3): Sorption Characteristics for Heat Pump Applications
Abstract
:1. Introduction
1.1. Research Justification
1.2. Background
1.3. Summary
2. Methods and Materials
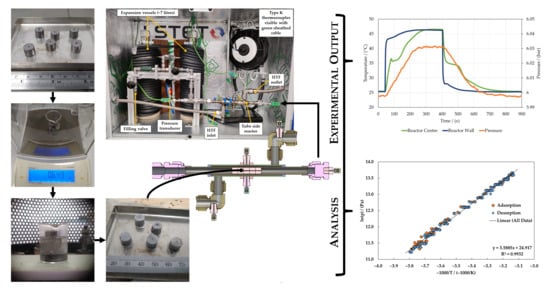
2.1. Composite Material Preparation
2.2. Analysis Methodology
2.3. Heat Transfer and Reaction Kinetics
3. Results and Discussion
3.1. Equilibrium Lines
3.2. Large Temperature Jump (LTJ) Modelling Results
3.2.1. Concentration Influence
3.2.2. MATLAB® Modelling Results
4. Further Work
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Salt | M.W./(g/mol) | Reaction Heat (ΔH)/(J/mol) | Reaction Entropy (ΔS)/(J/(mol∙K)) |
|---|---|---|---|
| NH3 L/V | 17.03 | 22,863 | 191.6 |
| NH4Cl (3-0) | 53.49 | 29,433 | 207.9 |
| NaBr (5.25-0) | 102.89 | 30,491 | 208.8 |
| PbCl2 (8-3.25) | 278.11 | 34,317 | 223.6 |
| BaCl2 (8-0) | 208.23 | 37,665 | 227.3 |
| CaCl2 (8-4) | 110.98 | 41,013 | 230.3 |
| SrCl2 (8-1) | 158.53 | 41,431 | 228.8 |
| CaCl2 (4-2) | 110.98 | 42,268 | 229.9 |
| MnCl2 (6-2) | 125.84 | 47,416 | 228.1 |
| FeCl2 (6-2) | 126.75 | 51,266 | 228.0 |
Appendix B

Appendix C


References
- Yang, Z.; Qu, M.; Gluesenkamp, K.R. Ammonia-based chemisorption heat pumps for cold-climate heating applications: A comprehensive review. Appl. Therm. Eng. 2020, 179, 115674. [Google Scholar] [CrossRef]
- Bao, H.S.; Wang, R.Z. A Review of Reactant Salts for Resorption Refrigeration Systems. Int. J. Air Cond. Refrig. 2010, 18, 165–180. [Google Scholar] [CrossRef]
- An, G.L.; Wang, L.W.; Gao, J. Two-stage cascading desorption cycle for sorption thermal energy storage. Energy 2019, 174, 1091–1099. [Google Scholar] [CrossRef]
- Bao, H.; Ma, Z.; Roskilly, A.P. A chemisorption power generation cycle with multi-stage expansion driven by low grade heat. Energy Convers. Manag. 2017, 150, 956–965. [Google Scholar] [CrossRef]
- Neveu, P.; Castaing, J. Solid-gas chemical heat pumps: Field of application and performance of the internal heat of reaction recovery process. Heat Recovery Syst. CHP 1993, 13, 233–251. [Google Scholar] [CrossRef]
- Climate Change Act. 2008. Available online: http://www.legislation.gov.uk/ukpga/2008/27/contents (accessed on 20 March 2021).
- Neveu, P.; Castaing-Lasvignottes, J. Development of a numerical sizing tool for a solid-gas thermochemical transformer—I. Impact of the microscopic process on the dynamic behaviour of a solid-gas reactor. Appl. Therm. Eng. 1997, 17, 501–518. [Google Scholar] [CrossRef]
- Bao, H.S.; Oliveira, R.G.; Wang, R.Z.; Wang, L.W.; Ma, Z.W. Working pairs for resorption refrigerator. Appl. Therm. Eng. 2011, 31, 3015–3021. [Google Scholar] [CrossRef]
- Li, T.X.; Wang, R.Z.; Kiplagat, J.K.; Chen, H. Experimental study and comparison of thermochemical resorption refrigeration cycle and adsorption refrigeration cycle. Chem. Eng. Sci. 2010, 65, 4222–4230. [Google Scholar] [CrossRef]
- Alefeld, G. Energiespeicherung durch heterogen-verdampfung. I. Physikalish-technische grundlagen (Energy storage by heterogeneous evaporation. I. Technical and physical principles). Wärme 1975, 81, 89–93. [Google Scholar]
- Rivero-Pacho, A.M.; Critoph, R.E.; Metcalf, S.J. Modelling and development of a generator for a domestic gas-fired carbon-ammonia adsorption heat pump. Renew. Energy 2017, 110, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Low Carbon Heating Technology Innovation: Grant Scheme. 2017. Available online: https://www.gov.uk/government/publications/low-carbon-heating-technology-innovation-grant-scheme (accessed on 7 September 2021).
- Wang, L.W.; Wang, R.Z.; Oliveira, R.G. A review on adsorption working pairs for refrigeration. Renew. Sustain. Energy Rev. 2009, 13, 518–534. [Google Scholar] [CrossRef]
- Worsøe-Schmidt, P. A solar-powered solid-absorption refrigeration system. Int. J. Refrig. 1979, 2, 75–84. [Google Scholar] [CrossRef]
- Worsøe-Schmidt, P. Solar refrigeration for developing countries using a solid-absorption cycle. Int. J. Ambient. Energy 1983, 4, 115–124. [Google Scholar] [CrossRef]
- Critoph, R.E. Activated carbon adsorption cycles for refrigeration and heat pumping. Carbon 1989, 27, 63–70. [Google Scholar] [CrossRef]
- Health and Safety Executive (HSE). Managing Legionella in Hot and Cold Water Systems. Available online: https://tinyurl.com/swsd2wz4 (accessed on 8 March 2021).
- UK Meteorological (MET) Office. Coventry (West Midlands Conurbation). MET Office. Available online: https://tinyurl.com/e55jb55r (accessed on 26 March 2021).
- Lepinasse, E.; Spinner, B. Production de froid par couplage de réacteurs solide-gaz I: Analyse des performances de tels systèmes (Cold production through coupling of solid-gas reactors I: Performance analysis). Int. J. Refrig. 1994, 17, 309–322. [Google Scholar] [CrossRef]
- Li, T.X.; Wang, R.Z.; Kiplagat, J.K.; Wang, L.W.; Oliveira, R.G. Thermodynamic study of a combined double-way solid–gas thermochemical sorption refrigeration cycle. Int. J. Refrig. 2009, 32, 1570–1578. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Jingyi, W. Adsorption Refrigeration Technology Theory and Application; John Wiley & Sons: Singapore, 2014. [Google Scholar]
- Bao, H.S.; Oliveira, R.G.; Wang, R.Z.; Wang, L.W. Choice of Low Temperature Salt for a Resorption Refrigerator. Ind. Eng. Chem. Res. 2010, 49, 4897–4903. [Google Scholar] [CrossRef]
- Goetz, V.; Spinner, B.; Lepinasse, E. A solid-gas thermochemical cooling system using BaCl2 and NiCl2. Energy 1997, 22, 49–58. [Google Scholar] [CrossRef]
- Van der Pal, M.; Critoph, R.E. Performance of CaCl2-reactor for application in ammonia-salt based thermal transformers. Appl. Therm. Eng. 2017, 126, 518–524. [Google Scholar] [CrossRef]
- Hinmers, S.; Critoph, R.E. Modelling the ammoniation of barium chloride for chemical heat transformations. Energies 2019, 12, 4404. [Google Scholar] [CrossRef] [Green Version]
- Aristov, Y.I.; Dawoud, B.; Glaznev, I.S. Elyas A new methodology of studying the dynamics of water sorption/desorption under real operating conditions of adsorption heat pumps: Experiment. Int. J. Heat Mass Transf. 2008, 51, 4966–4972. [Google Scholar] [CrossRef]
- Critoph, R.E.; Hinmers, S.; Atkinson, G.H. Ammonia-salt sorption: Testing and analysis, modelling and validation. Presented at the International Sorption Heat Pump Conference (Virtual). In Proceedings of the International Sorption Heat Pump Conference (Virtual), Berlin, Germany, 26 August 2021; p. 100. [Google Scholar]
- Mazet, N.; Amouroux, M.; Spinner, B. Analysis and experimental study of the transformation of a non-isothermal solid/gas reacting medium. Chem. Eng. Commun. 1991, 99, 155–174. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R. CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 4–46. [Google Scholar]
- Mazet, N.; Amouroux, M. Analysis of heat transfer in a non-isothermal solid-gas reacting medium. Chem. Eng. Commun. 1991, 99, 175–200. [Google Scholar] [CrossRef]
- Lebrun, M.; Spinner, B. Models of heat and mass transfers in solid-gas reactors used as chemical heat pumps. Chem. Eng. Sci. 1990, 45, 1743–1753. [Google Scholar] [CrossRef]
- Hinmers, S.; Atkinson, G.H.; Critoph, R.E.; van der Pal, M. Ammonia-Salt Reactions for Heat Pumping and Thermal Transforming Applications. Presented at the Heat Powered Cycles (HPC). In Proceedings of the Heat Powered Cycles (HPC), Bilbao, Spain, 16–19 September 2021. [Google Scholar]
- Atkinson, G.H.; Critoph, S.R.; Hinmers, E. Development of an ammonia-halide salt MATLAB® model. In Proceedings of the International Sorption Heat Pump Conference (Virtual), Berlin, Germany, 22–25 August 2021; Volume 47. [Google Scholar]
- Oliveira, R.G.; Xu, J.; Wang, C.Y.; Wang, R.Z. Resorption system for simultaneous heat and cold production. In Proceedings of the International Sorption Heat Pump Conference (ISHPC), Seoul, Korea, 23–26 September 2008. [Google Scholar]
- Touzain, P. Thermodynamic values of ammonia salt reactions for chemical sorption heat pumps. In Proceedings of the International Sorption Heat Pump Conference, Munich, Germany, 24–26 March 1999; pp. 225–238. [Google Scholar]
- Furrer, M. Thermoanalytical Study of Selected Complexes of Inorganic Chlorides with Ammonia and Ammonia Derivatives, Switzerland. 1980. Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:13652849 (accessed on 22 March 2020).













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkinson, G.H.; Hinmers, S.; Critoph, R.E.; van der Pal, M. Ammonium Chloride (NH4Cl)—Ammonia (NH3): Sorption Characteristics for Heat Pump Applications. Energies 2021, 14, 6002. https://doi.org/10.3390/en14186002
Atkinson GH, Hinmers S, Critoph RE, van der Pal M. Ammonium Chloride (NH4Cl)—Ammonia (NH3): Sorption Characteristics for Heat Pump Applications. Energies. 2021; 14(18):6002. https://doi.org/10.3390/en14186002
Chicago/Turabian StyleAtkinson, George H., Samuel Hinmers, Robert E. Critoph, and Michel van der Pal. 2021. "Ammonium Chloride (NH4Cl)—Ammonia (NH3): Sorption Characteristics for Heat Pump Applications" Energies 14, no. 18: 6002. https://doi.org/10.3390/en14186002
APA StyleAtkinson, G. H., Hinmers, S., Critoph, R. E., & van der Pal, M. (2021). Ammonium Chloride (NH4Cl)—Ammonia (NH3): Sorption Characteristics for Heat Pump Applications. Energies, 14(18), 6002. https://doi.org/10.3390/en14186002