Thermodynamics, Energy Dissipation, and Figures of Merit of Energy Storage Systems—A Critical Review
Abstract
:1. Introduction—The Need for Energy Storage
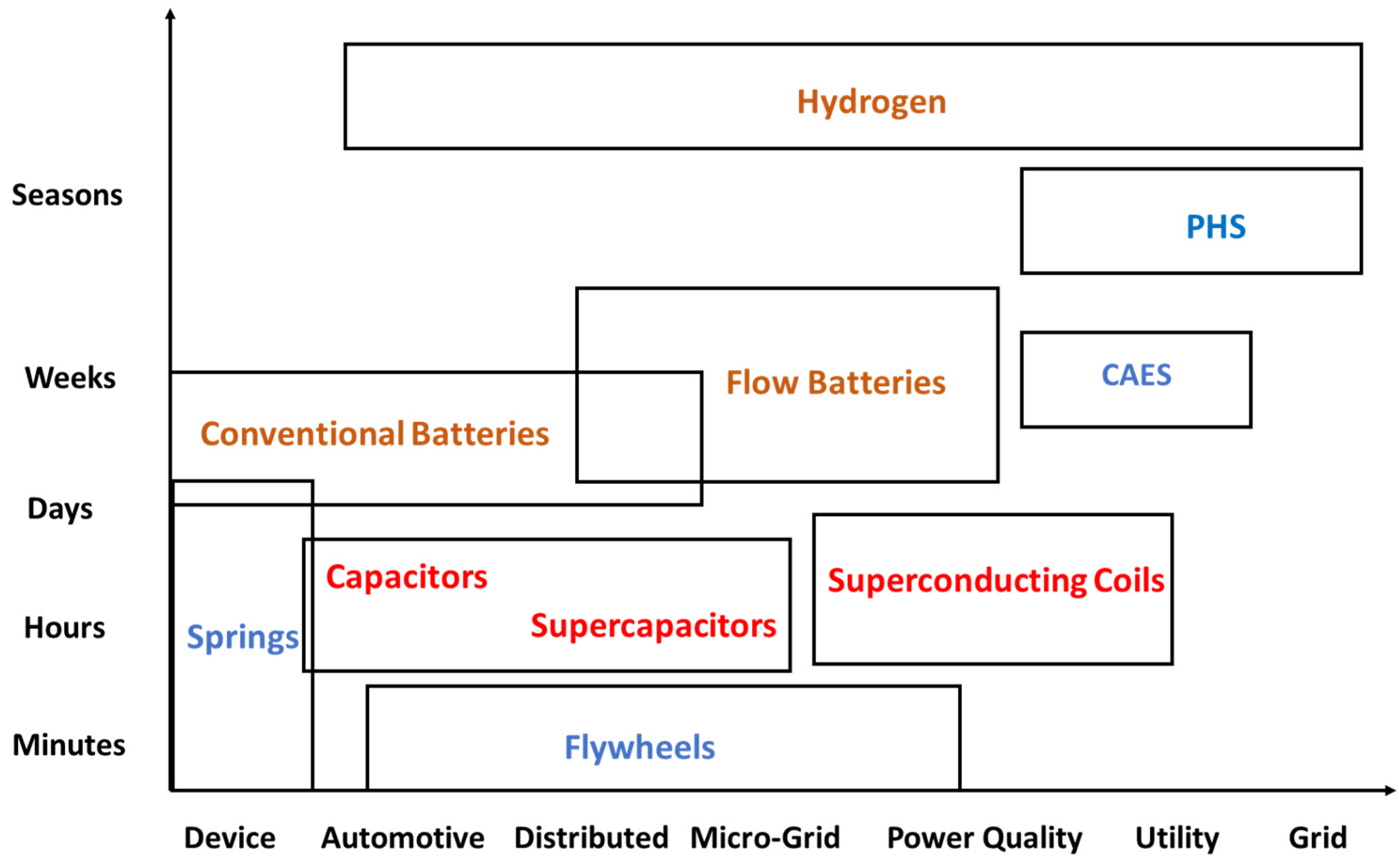
2. Classifications of Energy Storage Systems
- 1.
- Based on their final utilization, which is primarily determined by the quantity of energy stored and delivered. The following storage categories—from the highest to the lowest storage capacity—pertain to this classification.
- Grid-level: Storage that may supply an entire grid or large parts of a grid. A grid normally needs very large storage capacity to be used when the RESs do not generate sufficient power.
- Utility-level: Storage of an energy-generating corporation for sale at an optimum price, also of very large storage capacity. Due to the very high ES capacities, oftentimes, grid-level and utility-level systems are lumped as one category.
- Power quality (electric load correction): This is also at the utility level but entails lower storage capacity and faster response times. The primary purpose of this type of storage is to ensure uninterrupted electricity supply, for example, when, due to temporary cloudiness, the power generation of PV drops for short time periods.
- Microgrid: For the ES in a microgrid, which is composed of clusters of buildings or businesses, the storage is at the location of the consumers, who may optimize their storage systems and storage levels according to their energy demand. The microgrid systems have significantly lower capacity than utility-level storage systems.
- Distributed storage: e.g., to supply energy to a single commercial or business establishment or a household. These systems are also of relatively lower capacity.
- Automotive: This category includes the battery-operated vehicles, the fuel-cell vehicles and the hybrid vehicles. While the common automobile and marine batteries represent mature technologies, there is a great deal of research activity on the development of high-capacity and more efficient batteries for electric vehicles and hybrids.
- Device or appliance storage: e.g., energy for the operation of a cell phone, a grandfather clock or a mechanical watch.
- 2.
- Based on the type of energy in the storage systems.
- Mechanical: e.g., pumped hydro systems (PHSs), compressed air energy systems (CAESs), flywheels, springs, torsion bars, etc.
- Chemical: Includes all types of batteries, hydrogen-based systems, biofuels and synthetic fuels.
- Electrical/magnetic: e.g., capacitors, supercapacitors, superconducting coils, etc.
- Thermal: includes molten salts, steam, chilled water (for air conditioning) and phase-change materials.
3. Thermodynamic Properties and Figures of Merit
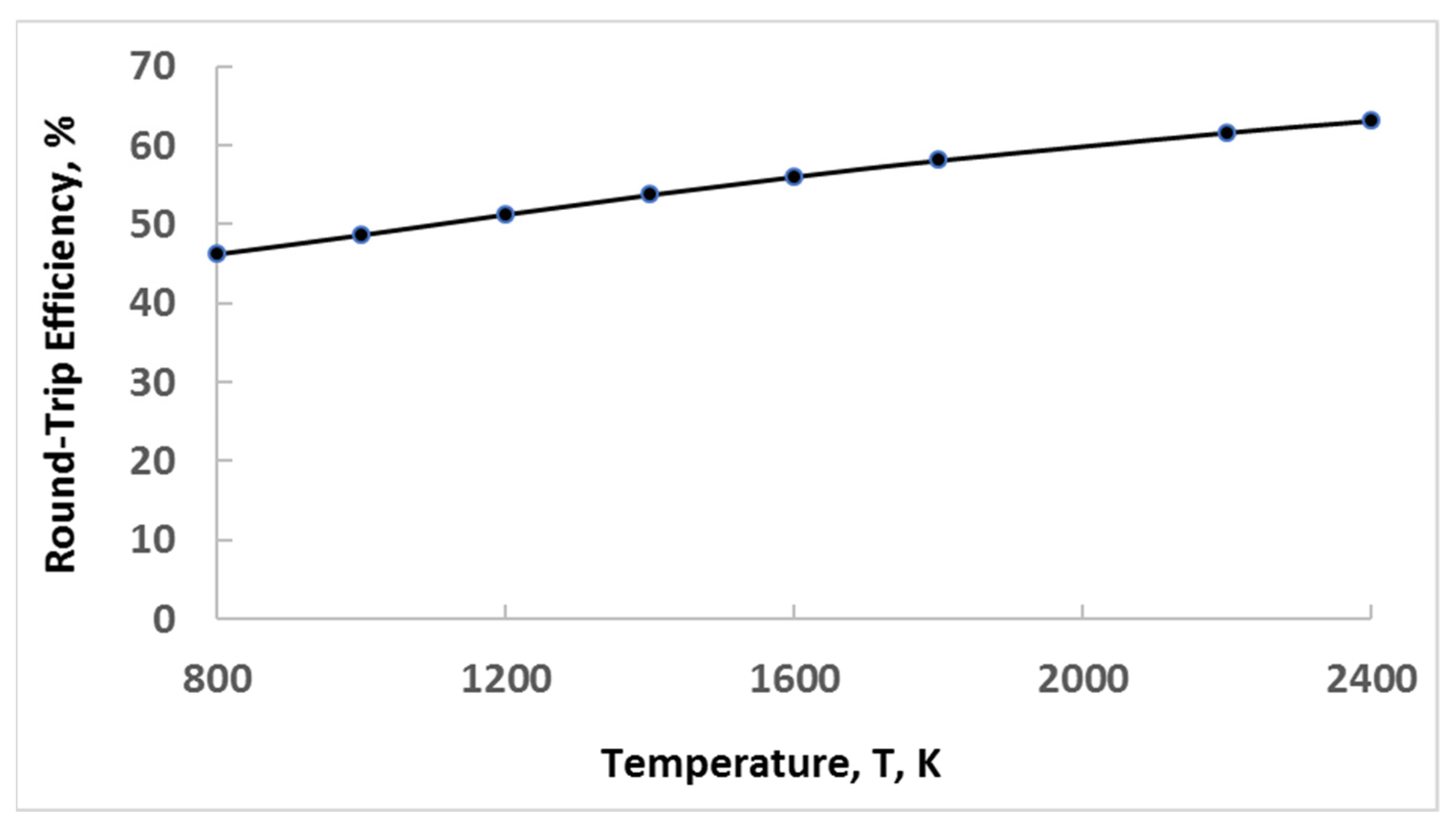
- Efficiency or, more precisely, round-trip efficiency is the ratio of the energy output of the storage system to the energy input. The input and output energies must be of the same type of energy, e.g., electricity or thermal, and must be expressed in the same units. Oftentimes the round-trip efficiency is expressed as the product of the charging efficiency and the generating efficiency [13,45].
- Specific energy is the energy stored per unit mass of the system. High specific energy is of importance in mobile systems (e.g., vehicles and aircraft) but insignificant for stationary systems, such as grid-level and utility-level systems (The specific energy (kJ/kg, or kWh/kg) appears in some publications as “energy density.” We follow here the international convention of thermodynamic properties and other quantities of interest, where per unit mass is defined as “specific” and per unit volume is defined as “density.”).
- Energy density is the energy stored per unit volume of the system. It measures how large the size of the storage system is and is important in distributed storage and mobile applications.
- Maximum depth of discharge is the fraction of energy stored that is practically usable. While most ES systems may discharge all of their energy, the manufacturers of batteries recommend that their equipment is not discharged below a certain state of charge, typically close to 20%. In such cases, the useful discharge capacity is 80% of the rated capacity of the system.
- Self-discharge time is the time for a fully charged system to discharge to a defined depth of discharge, when it does not supply any useful energy. For example, the self-discharge time for flywheels to 20% of their depth of discharge is on the order of minutes; the self-discharge time of solid-state batteries is on the order of months; and the self-discharge time of PHS storage systems is almost infinite.
- The cycle life is a number that indicates how many times the storage system may be charged and discharged, before its storage capacity and its round-trip efficiency deteriorate. At the end of the life cycle, the ES systems must be repaired, replaced or (in the case of batteries) regenerated.
4. Mechanical Energy Storage Systems
4.1. Pumped Hydroelectric Systems (PHSs)
- The pumping station due to the pump-motor efficiency.
- The ascending trip as friction in the pipeline.
- The descending trip as friction in the pipeline.
- The turbine station due to the turbine generator efficiency.
- Evaporation of water in the artificial lake.
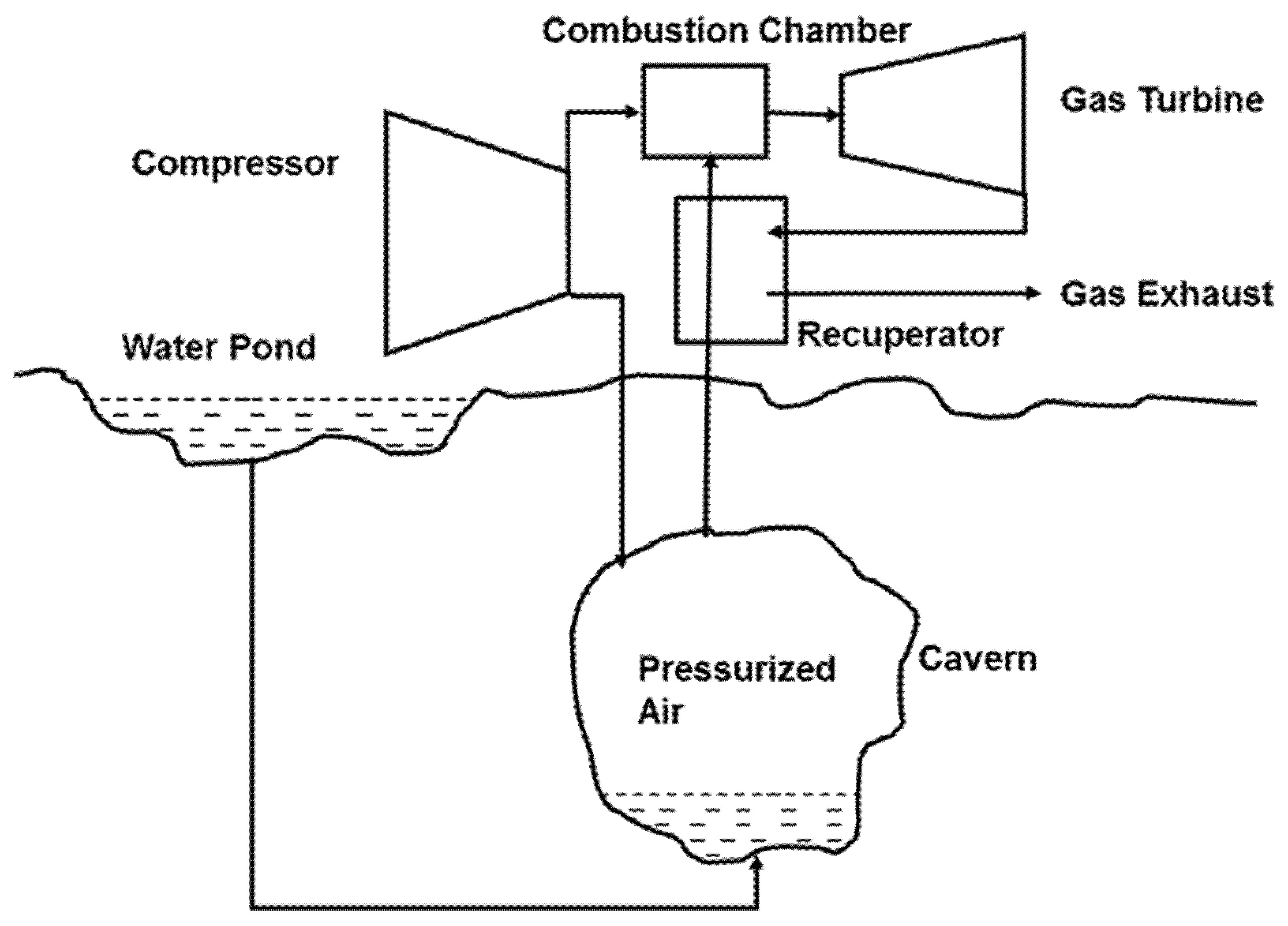
4.2. Compressed Air Energy Systems (CAESs)
- Adding the water column, as it is drawn in Figure 4, to maintain a constant pressure.
- Feeding the compressed air directly to a high-pressure turbine without further heating in the combustion chamber.
- Heat from the hot air at the end of the compression process is stored in another well-insulated medium to heat up the pressurized air before it is directed to the turbine. This is sometimes called an advanced adiabatic CAES (AA-CAES) [61]. Such systems have the advantage of being used in large (utility-scale) as well as smaller (microgrid-scale) versions with the heat stored as sensible or latent heat [62,63,64].
4.3. Flywheels
4.4. Springs and Torsion Bars
5. Electrical and Magnetic Energy Storage
5.1. Capacitors and Superacapacitors
5.2. Electric and Magnetic Coils—Superconductors
6. Electrochemical Energy Storage
6.1. Lead–Acid Batteries
6.2. Nickel-Based Batteries
6.3. Sodium-Based Batteries
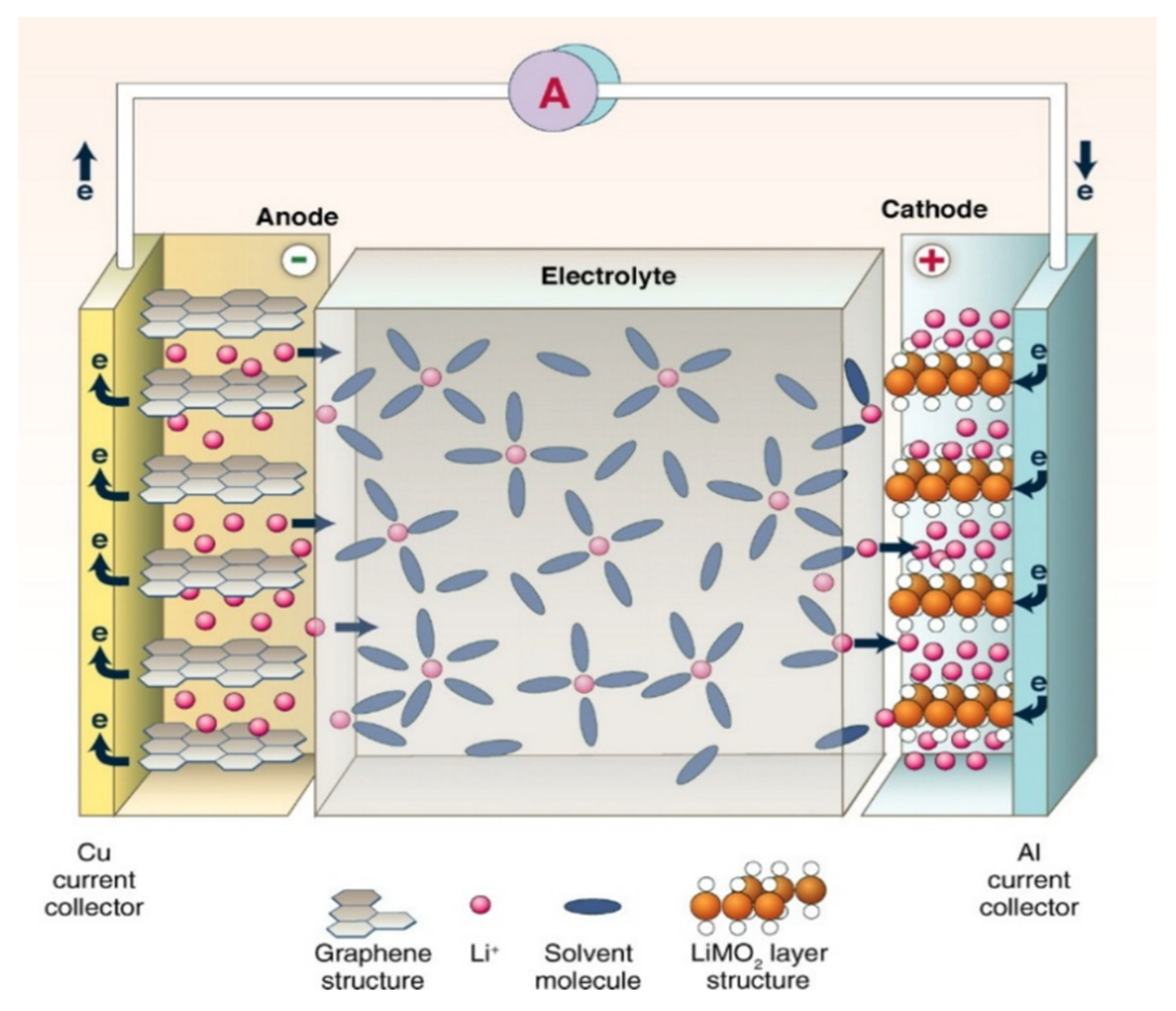
6.4. Lithium-Based Batteries
6.5. Metal-Air Batteries
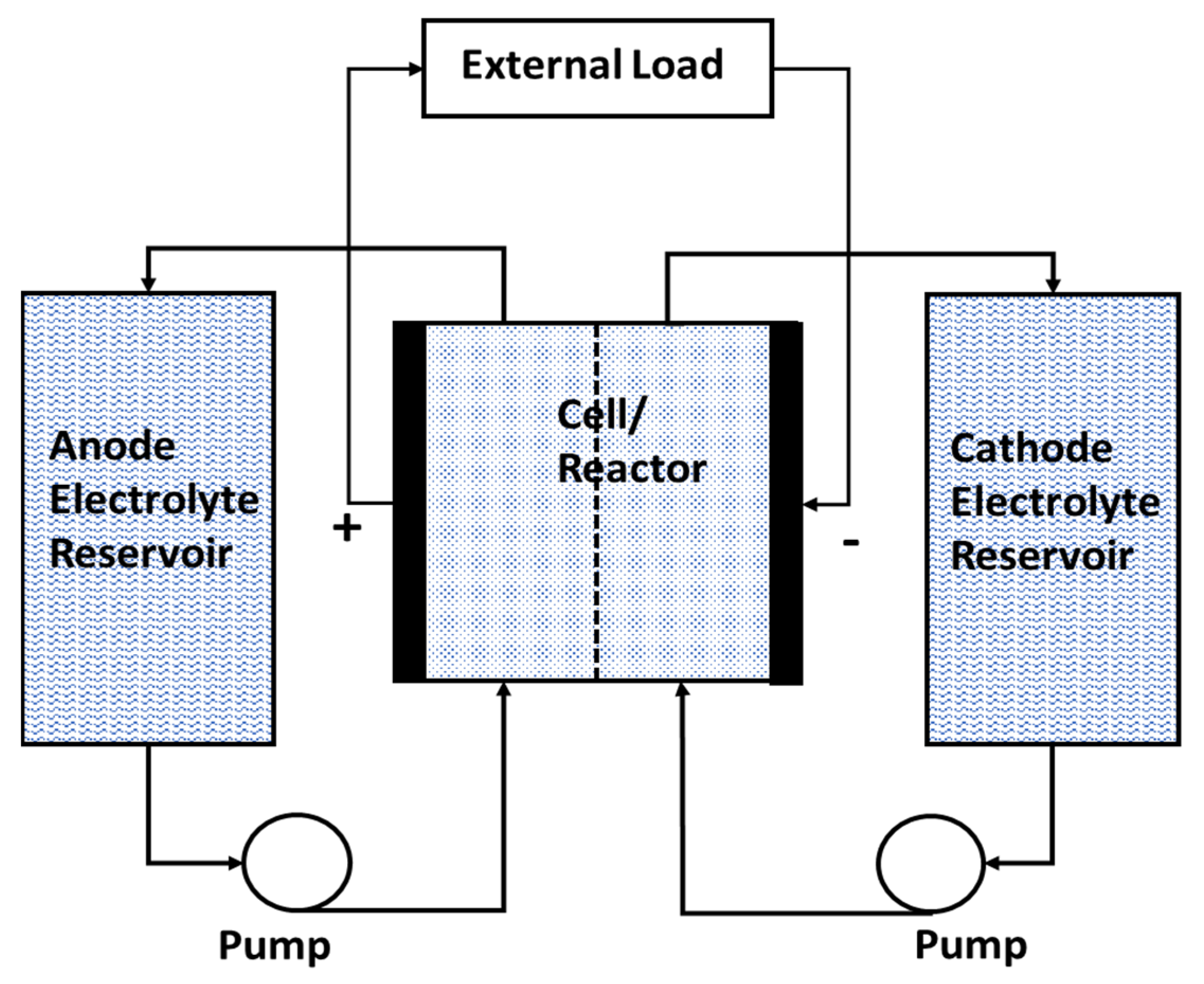
6.6. Flow Batteries
- Ability to discharge to 0% of charge (100% depth of discharge).
- Higher cycle life at 100% depth of discharge.
- No shelf-life limitations since flow batteries are non-perishable.
- Scalable ES capacity that depend only on the volume of the electrolyte reservoirs.
- The ability to store energy from any electricity-generating source.
- High rate of discharge over significant times.
- Fast recharge of the battery-operated systems by replenishing the electrolyte in the reservoirs.
- Low equivalent carbon emissions during their life cycle [134].
- Higher specific energy and energy density relative to the lead-acid batteries, but lower than that of lithium-based batteries.
- Significantly higher cost. However, the cost may improve with larger systems and longer hours of discharge.
- Relatively low round-trip efficiency.
- Limited operational temperature range, typically 10–35 °C.
6.7. Advantages and Disadvantages of Conventional Batteries
- Increase their specific energy and energy density.
- Reduce the recharge time and associated energy losses (dissipation).
- Eliminate or significantly diminish the internal current drift and self-discharge.
7. Hydrogen Energy Storage
- The energy density of hydrogen at ambient conditions is relatively high, but not as high as the energy density of liquid fuels. Compressed hydrogen and liquid hydrogen have significantly higher energy densities. As is shown in Table 5, the energy density is significantly higher at higher pressures with 1 m3 of hydrogen at 500 bar storing the equivalent of 1 MWh of electric energy. Liquid hydrogen at atmospheric pressure has extremely high specific energy and energy density but needs to be stored at about 20 K (−253 °C) [72,153].
- As hydrogen is a constituent of water in the hydrosphere, this element is abundant and almost inexhaustible.
- Hydrogen is a stable compound. It may be stored and used after a long time, e.g., for the seasonal storage of wind energy from the spring to the summer season.
- Hydrogen may be easily generated by the electrolysis or thermolysis of water and hydrocarbon splitting—all mature and well-known technologies [154,155,156,157]. Microbial electrolysis is also possible [158,159]. The efficiency of the several electrolysis processes is relatively high, in the range 70–90%, and hydrogen production does not appear to create an environmental pollution risk [160,161,162].
- The emissions from hydrogen conversion systems are water, a harmless and non-polluting compound.
- If released, hydrogen is not harmful to the environment and does not pose any health threats to humans.
- It may be used in IC engines and as combustion fuel in gas turbines, as well as in fuel cells.
- Hydrogen-operated automobiles and trucks do not emit pollutants and may be recharged in very short times. As such, hydrogen vehicles are ideal for inner city and long-distance transportation. There are already several operational “hydrogen routes” for hydrogen vehicles in Europe, Japan and several states in the USA [167,168].
- Since the hydrogen molecule is very small, hydrogen readily diffuses through the atomic matrices of metals, including steel, causing hydrogen embrittlement and decarburization. These processes weaken the hydrogen containers that need to be coated with special polymer films [170].
- It is flammable and explosive in air.
- As shown in Table 5, its energy density is very low under ambient conditions. This implies hydrogen needs to be transported and stored at high pressures.
8. Thermal Energy Storage
8.1. High-Temperature Storage
- To supply with heat a Rankine cycle for the generation of electricity. Gemasolar, the largest thermal solar power plant in the world (formerly known as Solar Tres), makes use of a large molten salt (primarily nitrate salts) storage tank. Heat is stored in a large tank containing the nitrates at 565 °C and is used during the dusk and night hours to raise steam for the turbines of the thermal solar power plant [198]. This enables Gemasolar to continuously generate electricity for fifteen hours after sunset, allowing the plant to run 24/7 during the summer months. The predecessor of this plant, Solar Two in California, also relied on molten salts to extend its hours of electricity generation. High-temperature TES systems enable the concentrated solar power plants to reach high-capacity factors and generate dispatchable power [199,200]. TES systems may also be used with nuclear power plants, both water and gas cooled; given that the thermal output of nuclear power plants must remain within narrow boundaries, the use of high-temperature TES systems in nuclear installations has been proposed to meet the electric power-demand fluctuations at the utility level [201].
- To supply high-temperature industrial processes. In such cases, steam (at both high and low pressure) is generated and stored in large, insulated tanks to supply industrial processes with heat at well-controlled temperatures for a plethora of applications [25,202,203,204]. A few types of district heating also use low-pressure steam [205,206].
- To supply heat to an absorption or adsorption cycle for cooling, a process commonly called solar cooling [207]. A temperature higher than 100 °C is considered to be of “high quality.” Cooling systems that are supplied with high-quality heat may operate chillers with two or three effects, the chillers are more efficient and their refrigeration capacity is significantly higher [206,208,209]. Figure 8 is a schematic diagram of a system that uses solar heat to supply a building with chilled water for air conditioning. When the heat from the collectors exceeds the heat required by the chiller, the hot water tank of the building is charged. Conversely, when the heat required by the chiller exceeds the supplied solar heat, the thermal storage is discharged to match the demand.
8.2. Lower-Temperature Storage
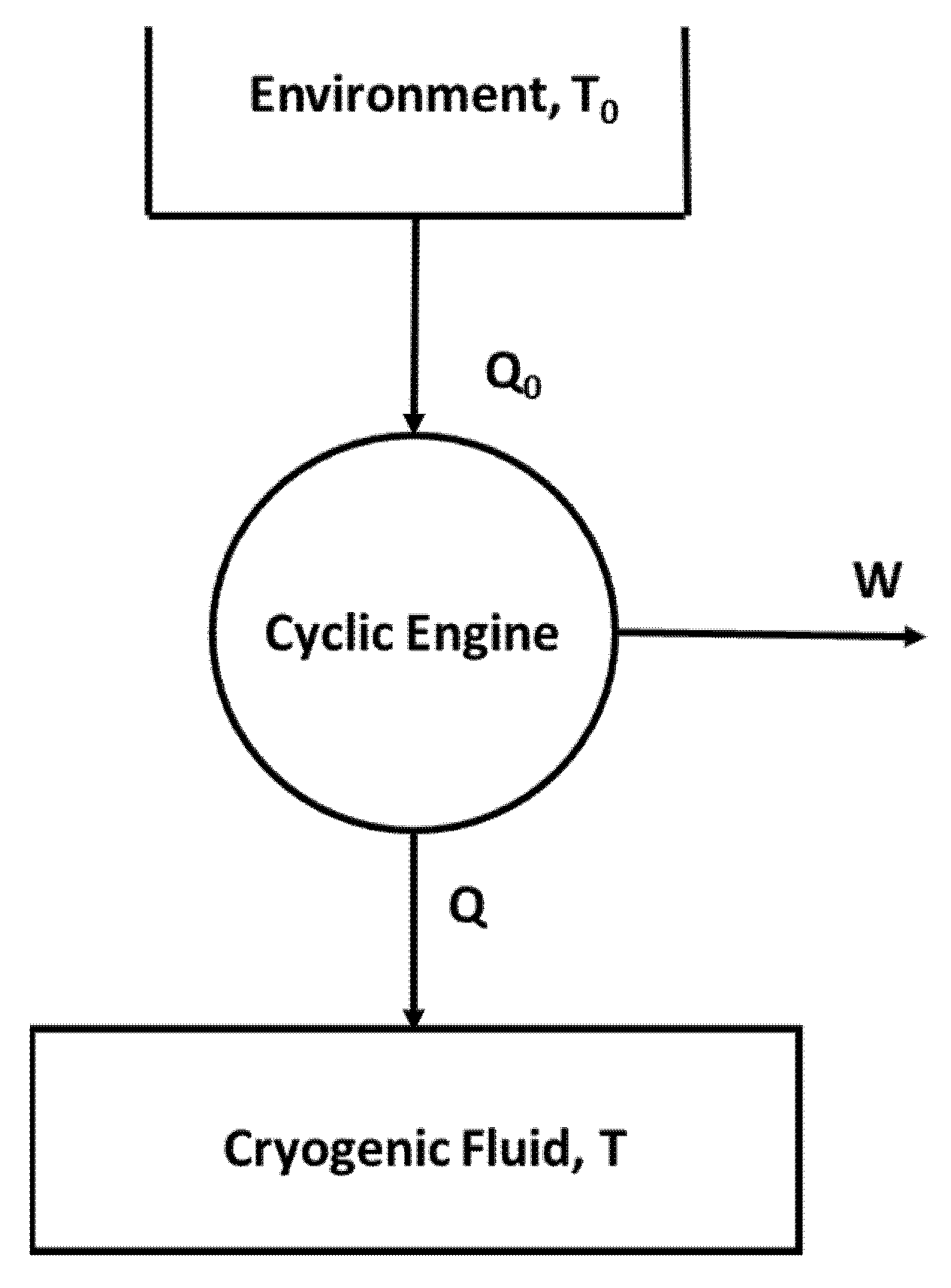
8.3. Cryogenic Temperature Storage
8.4. Thermo-Chemical Materials
8.5. Borehole Thermal Energy Storage
9. Cost and Price Considerations
10. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2007: Synthesis Report; Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Fisher, D.A.; Hales, C.H.; Wang, W.C.; Ko, M.K.W.; Sze, N.D. Model calculations of the relative effects of CFCs and their replacements on global warming. Nature 1990, 344, 513–516. [Google Scholar] [CrossRef]
- EIA. Kigali Amendment to the Montreal Protocol: A Crucial Step in the Fight against Catastrophic Climate Change. In Proceedings of the 22nd Conference of the Parties (CoP22) to the United Nations Framework Convention on Climate Change (UNFCCC), Marrakech, Morocco, 7–18 November 2016. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2014. 2016; EPA 430-R-16-002. Available online: https://www.osti.gov/biblio/1464240 (accessed on 20 September 2021).
- International Energy Agency. CO2 Emissions from Fuel Combustion—Overview; IEA-Chirat: Paris, France, 2019. [Google Scholar]
- International Energy Agency. Key World Statistics; IEA-Chirat: Paris, France, 2018. [Google Scholar]
- International Energy Agency. Key World Statistics; IEA-Chirat: Paris, France, 2020. [Google Scholar]
- Energy Information Agency. Coal Explained; US Department of Energy: Washington, DC, USA, 2020.
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emissions, Our World in Data. 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 10 April 2021).
- IPCC. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Miller, A.I.; Suppiah, S.; Duffey, R.B. Climate change gains more from nuclear substitution than from conservation. Nucl. Eng. Des. 2006, 236, 1657–1667. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, M.M.; Alameri, S.A. Emerging areas of nuclear power applications. Nucl. Eng. Des. 2019, 354, 110183. [Google Scholar] [CrossRef]
- Michaelides, E.E. Alternative Energy Systems; Springer: Heidelberg, Germany, 2012. [Google Scholar]
- Freeman, E.; Occello, D.; Barnes, F. ES for Electrical Systems in the USA. AIMS Energy 2016, 4, 856–875. [Google Scholar] [CrossRef]
- California ISO (CAISO). What the Duck Curve Tells Us about Managing a Green Grid. 2016. Available online: https://www.caiso.com/Documents/FlexibleResourcesHelpRenewables_FastFacts.pdf (accessed on 5 May 2021).
- Leonard, M.D.; Michaelides, E.E. Grid-Independent Residential Buildings with Renewable Energy Sources. Energy 2018, 148C, 448–460. [Google Scholar] [CrossRef]
- Available online: http://www.ercot.com/gridinfo/generation (accessed on 15 April 2021).
- Michaelides, E.E. ES and Installed Wind Capacity Requirements for the Substitution of Fossil Fuels in the Electricity Generation Sector. J. Energy Power Technol. 2021, 3, 18. [Google Scholar] [CrossRef]
- Denholm, P.; O’Connell, M.; Brinkman, G.; Jorgenson, J. Over-Generation from Solar Energy in California: A Field Guide to the Duck Chart; National Renewable Energy Laboratory: Golden, CO, USA, 2015. [Google Scholar]
- Schoenung, S.M.; Keller, J.O. Commercial potential for renewable hydrogen in California. Int. J. Hydrogen Energy 2017, 42, 13321–13328. [Google Scholar] [CrossRef]
- Zhang, N.; Lu, X.; McElroy, M.B.; Nielsen, C.P.; Chen, X.; Deng, Y. Reducing curtailment of wind electricity in China by employing electric boilers for heat and pumped hydro for ES. Appl. Energy 2016, 184, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Denholm, P.; Margolis, R.M. Evaluating the limits of solar photovoltaics (PV) in electric power systems utilizing ES and other enabling Technologies. Energy Policy 2007, 35, 4424–4433. [Google Scholar] [CrossRef]
- Chaudhary, P.; Rizwan, M. Energy management supporting high penetration of solar photovoltaic generation for smart grid using solar forecasts and pumped hydro storage system. Renew. Energy 2018, 118, 928–946. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, N.; Du, E.; Mia, M.; Peng, F.; Kang, C. Probabilistic duck curve in high PV penetration power system: Concept, modeling, and empirical analysis in China. Appl. Energy 2019, 242, 205–215. [Google Scholar] [CrossRef]
- Michaelides, E.E. Energy, the Environment, and Sustainability; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Lazar, J. Teaching the “Duck” to Fly; RAP Publications: Paris, France, 2014; Available online: www.raponline.org (accessed on 10 April 2021).
- Sanandaji, B.M.; Vincent, T.L.; Poolla, K. Ramping rate flexibility of residential HVAC loads. IEEE Trans. Sustain. Energy 2016, 7, 865–874. [Google Scholar] [CrossRef]
- Hassan, A.S.; Cipcigan, L.; Jenkins, N. Optimal battery storage operation for PV systems with tariff incentives. Appl. Energy 2017, 20, 422–441. [Google Scholar] [CrossRef]
- Sevilla, F.R.S.; Parra, D.; Wyrsch, N.; Patel, M.; Kienzle, F.; Korba, P. Techno-economic analysis of battery storage and curtailment in a distribution grid with high PV penetration. J. Energy Storage 2018, 18, 73–83. [Google Scholar] [CrossRef]
- Ding, Y.; Shao, C.; Yan, J.; Song, Y.; Zhang, C.; Guo, C. Economical flexibility options for integrating fluctuating wind energy in power systems: The case of China. Appl. Energy 2018, 228, 426–436. [Google Scholar] [CrossRef]
- Kumar, N.; Venkataraman, S.; Lew, D.; Brinkman, G.; Palchak, D.; Cochran, J. Retrofitting Fossil Power Plants for Increased Flexibility. In Proceedings of the ASME Power Conference, Baltimore, MD, USA, 28–31 July 2014. [Google Scholar]
- Janko, S.A.; Arnold, M.R.; Johnson, N.G. Implications of high-penetration renewables for ratepayers and utilities in the residential solar photovoltaic (PV) market. Appl. Energy 2016, 180, 37–51. [Google Scholar] [CrossRef]
- Michaelides, E.E. Fossil Fuel Substitution with Renewables for Electricity Generation—Effects on Sustainability Goals. Eur. J. Sustain. Dev. Res. 2020, 4, em0111. [Google Scholar] [CrossRef] [Green Version]
- IRENA. Electricity Storage and Renewables: Costs and Markets to 2030; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2017. [Google Scholar]
- Schmidt, O.; Hawkes, A.; Gambhir, A.; Staffell, I. The future cost of electrical ES based on experience rates. Nat. Energy 2017, 2, 17110. [Google Scholar] [CrossRef]
- Darling, R.M.; Gallagher, K.G.; Kowalski, J.A.; Ha, S.; Brushett, F.R. Pathways to low-cost electrochemical ES: A comparison of aqueous and nonaqueous flow batteries. Energy Environ. Sci. 2014, 7, 3459–3477. [Google Scholar] [CrossRef] [Green Version]
- Janoschka, T.; Martin, N.; Martin, U.; Friebe, C.; Morgenstern, S.; Hiller, H.; Hager, M.D.; Schubert, U.S. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 2015, 527, 78–81. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Saktisahdan, T.J.; Jannifar, A.; Hasan, M.H.; Matseelar, H.S.C. A review of available methods and development on ES.; Technology update. Renew. Sustain. Energy Rev. 2014, 33, 532–545. [Google Scholar] [CrossRef]
- Argyrou, M.C.; Christodoulides, P.; Kalogirou, S.A. ES for electricity generation and related processes: Technologies appraisal and grid scale applications. Renew. Sustain. Energy Rev. 2018, 94, 804–821. [Google Scholar] [CrossRef]
- Zakeri, B.; Syri, S. Electrical ES systems: A comparative life cycle cost analysis. Renew. Sustain. Energy Rev. 2015, 42, 569–596. [Google Scholar] [CrossRef]
- Hoppmann, J.; Volland, J.; Schmidt, T.S.; Hoffmann, T.H. The economic viability of battery storage for residential solar photovoltaic systems—A review and a simulation model. Renew. Sustain. Energy Rev. 2014, 39, 1101–1118. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A review on the role, cost and value of hydrogen energy systems for deep decarbonization. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shahd, N.; Warda, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Parra, D.; Swierczynski, M.; Stroeb, D.I.; Norman, S.A.; Abdond, A.; Worlitschek, J.; O’Doherty, T.; Rodrigues, L.; Gillotte, M.; Zhang, X.; et al. An interdisciplinary review of ES for communities: Challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 79, 730–749. [Google Scholar] [CrossRef]
- Katsivelakis, M.; Bargiotas, D.; Daskalopulu, A.; Panapakidis, I.P.; Tsoukalas, L. Techno-Economic Analysis of a Stand-Alone Hybrid System: Application in Donoussa Island, , Greece. Energies 2021, 14, 1868. [Google Scholar] [CrossRef]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future ES technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Morabito, A.; Steimes, J.; Bontems, O.; Al Zohbi, G.; Hendrick, P. Set-up of a pump as turbine use in micro-pumped hydro ES: A case of study in Froyennes Belgium. J. Phys. Conf. Ser. 2017, 813, 012033. [Google Scholar] [CrossRef]
- de Oliveira e Silva, G.D.; Hendrick, P. Pumped hydro ES in buildings. Appl. Energy 2016, 179, 1242–1250. [Google Scholar] [CrossRef]
- United States Department of Energy. DOE Global ES Database. 2021. Available online: https://www.sandia.gov/ess-ssl/global-energy-storage-database-home (accessed on 20 May 2021).
- Yang, C.; Jackson, R.B. Opportunities and barriers to pumped-hydro ES in the United States. Renew. Sustain. Energy Rev. 2011, 15, 839–844. [Google Scholar] [CrossRef]
- Poullikkas, A. Optimization analysis for pumped ES systems in small isolated power systems. J. Power Technol. 2013, 93, 78–89. [Google Scholar]
- Beaudin, M.; Zareipour, H.; Schellenberglabe, A.; Rosehart, W. ES for mitigating the variability of renewable electricity sources: An updated review. Energy Sustain. Dev. 2010, 14, 302–314. [Google Scholar] [CrossRef]
- Pump Efficiency—What Is Efficiency? 2012. Available online: https://www.pumpsandsystems.com/pump-efficiency-what-efficiency (accessed on 20 May 2021).
- Hydraulic Efficiency. Mobil Service. 2018. Available online: https://www.mobil.com/industrial (accessed on 20 May 2021).
- Liu, X.; Luo, Y.; Karney, B.; Wang, W. A selected literature review of efficiency improvements in hydraulic turbines. Renew. Sustain. Energy Rev. 2015, 51, 18–28. [Google Scholar] [CrossRef]
- Efficiency in Electrcity Generation, Report by Euro Electric in Collaboration with VGB. July 2003. Available online: https://wecanfigurethisout.org/ENERGY/Web_notes/Bigger_Picture/Where_do_we_go_Supporting_Files/Efficiency%20in%20Electricity%20Generation%20-%20EURELECTRIC.pdf (accessed on 20 September 2021).
- Paik, J.; Sotiropoulos, F.; Sale, M.J. Numerical simulation of swirling flow in complex hydroturbine draft tube using unsteady statistical turbulence models. J. Hydraul. Eng. 2005, 131, 441–456. [Google Scholar] [CrossRef]
- Munson, B.R.; Young, D.F.; Okiishi, T.H.; Huwbsch, W.W. Fundamentals of Fluid Mechanics; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- American Concrete Pipe Association. Design data—22, Flotation of Circular Concrete Pipe. 2012. Available online: https://www.concretepipe.org/pipe-box-resources/design/design-data/ (accessed on 20 September 2021).
- American Concrete Pipe Association. Flow Friction Characteristics of Concrete Pressure Pipe. ACPA Technical Series. 2021. Available online: https://acppa.org/wp-content/uploads/2011/05/ACPPA-TechSeries-11-FlowCharacteristics-1.pdf (accessed on 20 September 2021).
- Wolf, D.; Kanngießer, A.; Budt, M.; Doetsch, C. Adiabatic compressed air ES co-located with wind energy-multifunctional storage commitment optimization for the German market using GOMES. Energy Syst. 2012, 3, 181–208. [Google Scholar] [CrossRef]
- Madlener, R.; Latz, J. Economics of centralized and decentralized compressed air ES for enhanced grid integration of wind power. Appl. Energy 2013, 101, 299–309. [Google Scholar] [CrossRef]
- Geissbühler, L.; Becattini, V.; Zanganeh, G.; Zavattoni, S.; Barbato, M.; Haselbacher, A.; Steinfeld, A. Pilot-scale demonstration of advanced adiabatic compressed air energy storage, Part 1: Plant description and tests with sensible thermal-energy storage. J. Energy Storage 2018, 17, 129–139. [Google Scholar] [CrossRef]
- Becattini, V.; Geissbühler, L.; Zanganeh, G.; Haselbacher, A.; Steinfeld, A. Pilot-scale demonstration of advanced adiabatic compressed air energy storage, Part 2: Tests with combined sensible/latent thermal-energy storage. J. Energy Storage 2018, 17, 140–152. [Google Scholar] [CrossRef]
- Salgi, G.; Lund, H. System behavior of compressed-air energy-storage in Denmark with a high penetration of renewable energy sources. Appl. Energy 2008, 85, 182–189. [Google Scholar] [CrossRef]
- Lund, H.; Salgi, G. The role of compressed air ES (CAES) in future sustainable energy systems. Energy Convers. Manag. 2009, 50, 1172–1179. [Google Scholar] [CrossRef]
- Lund, H.; Salgi, G.; Elmegaard, B.; Andersen, A.N. Optimal operation strategies of compressed air ES (CAES) on electricity spot markets with fluctuating prices. Appl. Therm. Eng. 2009, 29, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Drury, E.; Denholm, P.; Sioshansi, R. The value of compressed air ES in energy and reserve markets. Energy 2011, 36, 4959–4973. [Google Scholar] [CrossRef]
- Gu, Y.; McCalley, J.; Ni, M.; Bo, R. Economic modeling of compressed air ES. Energies 2013, 6, 2221–2241. [Google Scholar] [CrossRef]
- Swiss Federal Office of Energy (SFOE). Demonstration of the Ability of Caverns for Compressed Air Storage with Thermal En-ergy Recuperation; Swiss Federal Office of Energy (SFOE): Bern, Switzerland, 2016. [Google Scholar]
- Michaelides, E.E. Exergy and the Conversion of Energy; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- REFPROP. Reference Fluid Thermodynamic and Transport. Properties, Version 9.1; NIST Standard Reference Data Basis: Washington DC, USA, 2012. [Google Scholar]
- Koshizuka, N.; Ishikawa, F.; Nasu, H. Progress of superconducting bearing technologies for flywheel ES systems. Phys. C 2003, 386, 444–450. [Google Scholar] [CrossRef]
- Mousavi, G.S.M.; Faraji, F.; Majazi, A.; Al-Haddad, K. A comprehensive review of flywheel ES system technology. Renew. Sustain. Energy Rev. 2017, 67, 477–490. [Google Scholar] [CrossRef]
- Sebastián, R.; Peña, D.; Alzola, R. Flywheel ES systems: Review and simulation for an isolated wind power system. Renew. Sustain. Energy Rev. 2012, 16, 6803–6813. [Google Scholar] [CrossRef]
- Rendell, D.; Shaw, S.R.; Pool, P.J.; Oberlin-Harris, C. Thirty Year Operational Experience of the JET Flywheel Generators. Proc. 28th Symp. On Fusion Technology (SOFT-28). Fusion Eng. Des. 2015, 98–99, 1140–1143. [Google Scholar] [CrossRef]
- Intrator, J.; Elkind, E.; Abele, A.; Weissman, S.; Sawchuk, M.; Bartlett, E. 2020 Strategic Analysis of ES in California; California Energy Commission: Sacramento, CA, USA, 2011; CEC-500-2011-047.
- Prodromidis, G.N.; Coutelieris, F.A. Simulations of economical and technical feasibility of battery and flywheel hybrid ES systems in autonomous projects. Renew. Energy 2012, 39, 149–153. [Google Scholar] [CrossRef]
- Wang, D.; Ren, C.; Sivasubramaniam, A.; Urgaonkar, B.; Fathy, H. ES in datacenters: What, where, and how much? Perform. Eval. Rev. 2012, 40, 187–198. [Google Scholar] [CrossRef]
- Probstein, R.F. Physicochemical Hydrodynamics, 2nd ed.; Elsevier: New York, NY, USA, 1994. [Google Scholar]
- Carrasco, J.M.; Franquelo, L.G.; Bialasiewicz, J.T.; Galván, E.; Guisado, R.C.P.; Prats, Á.M.; Leon, J.I.; Moreno-Alfonso, N. Power-electronic systems for the grid integration of renewable energy sources: A survey. IEEE Trans. Ind. Electron. 2006, 53, 1002–1016. [Google Scholar] [CrossRef]
- Hall, P.J.; Mirzaeian, M.; Fletcher, S.I.; Sillars, F.B.; Rennie, A.J.R.; Shitta-Bey, G.O.; Wilson, G.; Cruden, A.; Carter, R. ES in electrochemical capacitors: Designing functional materials to improve performance. Energy Env. Sci. 2011, 3, 1238–1251. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical ES system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Rani, J.R.; Thangavel, R.; Oh, S.I.; Lee, Y.S.; Jang, J.H. An Ultra-High-Energy Density Supercapacitor; Fabrication Based on Thiol-functionalized Graphene Oxide Scrolls. Nanomaterials 2019, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Rani, J.R.; Thangavel, R.; Oh, S.I.; Woo, J.M.; Chandra Das, N.; Kim, S.Y.; Lee, Y.S.; Jang, J.H. High Volumetric Energy Density Hybrid Supercapacitors Based on Reduced Graphene Oxide Scrolls. ACS Appl. Mater. Interfaces 2017, 9, 22398–22407. [Google Scholar] [CrossRef]
- Patil, A.M.; Kitiphatpiboon, N.; An, X.; Hao, X.; Li, S.; Hao, X.; Abudula, A.; Guan, G. Fabrication of a High-Energy Flexible All-Solid-State Supercapacitor Using Pseudocapacitive 2D-Ti3C2Tx-MXene and Battery-Type Reduced Graphene Oxide/Nickel-Cobalt Bimetal Oxide Electrode Materials. ACS Appl. Mater. Interfaces 2020, 12, 52749–52762. [Google Scholar] [CrossRef]
- Michaelides, E.E. Nanofluidics: Thermodynamic and Transport Properties; Springer: New York, NY, USA, 2014. [Google Scholar]
- Liu, S.; Sun, S.; You, X.Z. Inorganic nanostructured materials for high performance electrochemical supercapacitors. Nanoscale 2014, 6, 2037–2045. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, H.; Yuan, W.; Zhang, M.; Lai, X. Design and Cost Estimation of Superconducting Magnetic ES (SMES) Systems for Power Grids. In Proceedings of the IEEE Power and Energy Society General Meeting, Vancouver, BC, Canada, 1–5 July 2013. [Google Scholar]
- Drozdov, A.P.; Eremets, M.I.; Troyan, I.A.; Ksenofontov, V.; Shylin, S.I. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 2015, 525, 73–76. [Google Scholar] [CrossRef]
- Drozdov, A.P.; Kong, P.P.; Minkov, V.S.; Besedin, S.P.; Kuzovnikov, M.A.; Mozaffari, S.; Balicas, L.; Balakirev, F.; Graf, D.E.; Prakapenka, V.B.; et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nat. Cell Biol. 2019, 569, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Snider, E.; Dasenbrock-Gammon, N.; McBride, R.; Debessai, M.; Vindana, H.; Vencatasamy, K.; Lawler, K.V.; Salamat, A.; Dias, R.P. Room-temperature superconductivity in a carbonaceous sulfur hydride. Nat. Cell Biol. 2020, 586, 373–377. [Google Scholar] [CrossRef]
- Xue, X.; Cheng, K.; Sutanto, D.A. Study of the status and future of superconducting magnetic ES in power systems. Supercond. Sci. Technol. 2006, 19, R31–R39. [Google Scholar] [CrossRef]
- Cole, J.F. Battery ES systems—An emerging market for lead/acid batteries. J. Power Sources 1995, 53, 239–243. [Google Scholar] [CrossRef]
- Parker, C.D. Lead–acid battery ES systems for electricity supply networks. J. Power Sources 2001, 100, 18–28. [Google Scholar] [CrossRef]
- Lam, L.T.; Louey, R. Development of ultra-battery for hybrid-electric vehicle applications. J. Power Sources 2006, 158, 1140–1148. [Google Scholar] [CrossRef]
- Cho, J.; Jeong, S.; Kim, Y. Commercial and research battery technologies for electrical ES applications. Prog. Energy Combust. Sci. 2015, 48, 84–101. [Google Scholar] [CrossRef]
- Poullikkas, A. A comparative overview of large-scale battery systems for electricity storage. Renew. Sustain. Energy Rev. 2013, 27, 778–788. [Google Scholar] [CrossRef]
- Goncalves-Lacerda, V.; Barbosa-Mageste, A.; Boggione-Santos, I.J.; Henrique-Mendes, L. Separation of Cd and Ni from NiCd batteries by an environmentally safe methodology employing aqueous two-phase systems. J. Power Sources 2009, 193, 908–913. [Google Scholar] [CrossRef]
- Avril, S.; Arnaud, G.; Florentin, A.; Vinard, M. Multi-objective optimization of batteries and hydrogen storage technologies for remote photovoltaic systems. Energy 2010, 35, 5300–5308. [Google Scholar] [CrossRef]
- Connolly, D.; Lund, H.; Mathiesen, B.V.; Leahy, M. The first step towards a 100% renewable energy system for Ireland. Appl. Energy 2011, 88, 502–507. [Google Scholar] [CrossRef]
- Kawakami, N.; Iijima, Y.; Sakanaka, Y.; Fukuhara, M.; Ogawa, K.; Bando, M. Development and Field Experiences of Stabilization System Using 34 MW NAS Batteries for a 51 MW Wind Farm. In Proceedings of the 2010 IEEE International Symposium on Industrial Electronics, Bari, Italy, 4–7 July 2010. [Google Scholar]
- Yuan, Y.; Zhang, X.; Ju, P.; Qian, K.; Fu, Z. Applications of battery ES system for wind power dispatchability purpose. Electr. Power Syst. Res. 2012, 93, 54–60. [Google Scholar] [CrossRef]
- Sebastián, R.; Peña-Alzola, R. Simulation of an isolated wind diesel system with battery ES. Electr. Power Syst. Res. 2001, 81, 677–686. [Google Scholar] [CrossRef]
- Wen, Z.; Cao, J.; Gu, Z.; Xu, X.; Zhang, F.; Lin, Z. Research on sodium sulfur battery for ES. Solid State Ionics 2008, 179, 1697–1701. [Google Scholar] [CrossRef]
- Doughty, D.H.; Butler, P.C.; Akhil, A.A.; Clark, N.H.; Boyes, J.D. Batteries for large-scale stationary electrical ES. Electrochem. Soc. Interface 2010, 19, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical ES for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Nazri, G.A.; Pistoia, G.F. (Eds.) Lithium Batteries: Science and Technology; Science and Business Media: New York, NY, USA, 2009. [Google Scholar]
- Cai, X.; Lai, L.; Shen, Z.; Lin, J. Graphene and graphene-based composites as Li-ion battery electrode materials and their application in full cells. J. Mater. Chem. A 2017, 5, 15423–15446. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Z.; Sun, Z.; Wu, T.; Gao, Y.; Li, H.; Li, F.; Niu, L. An advanced lithium ion battery based on a high quality graphitic graphene anode and a Li [Ni0.6 Co0.2 Mn0.2]O2 cathode. Electrochim. Acta 2018, 259, 48–55. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4468. [Google Scholar] [CrossRef]
- Smith, K.; Wang, C.Y. Solid-state diffusion limitations on pulse operation of a lithium ion cell for hybrid electric vehicles. J. Power Sources 2006, 161, 628–639. [Google Scholar] [CrossRef]
- Hall, C. Future Batteries, Coming Soon: Charge in Seconds, Last Months and Power over the Air, Pocket, 22 March 2021. Available online: https://www.pocket-lint.com/gadgets/news/130380-future-batteries-coming-soon-charge-in-seconds-last-months-and-power-over-the-air (accessed on 6 June 2021).
- Michaelides, E.E. Thermodynamics and Energy Consumption of Electric Vehicles. Energy Convers. Manag. 2019, 203, 112246. [Google Scholar] [CrossRef]
- Lowy, J. Feds Urge Airlines to Ban Laptops in Checked Bags, Citing Fire, Explosion Risk; Associate Press: New York, NY, USA, 2017. [Google Scholar]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical ES technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef] [Green Version]
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene balls for lithium rechargeable batteries with fast charging and high volumetric energy densities. Nat. Commun. 2017, 8, 1516. [Google Scholar]
- International Electrotechnical Commission (IEC); Electrical ES: Geneva, Switzerland, 2011.
- Leadbetter, J.; Swan, L.G. Selection of battery technology to support grid- integrated renewable electricity. J. Power Sources 2012, 216, 376–386. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Pelham, E.; Lindfield, R.; Bae, C.; Saleem, M. Ruthenium based redox flow battery for solar ES. Energy Conv. Manag. 2001, 52, 2501–2508. [Google Scholar] [CrossRef]
- Huang, K.-L.; Li, X.-G.; Liu, S.-Q.; Tan, N.; Chen, L.-Q. Research progress of vanadium redox flow battery for energys torage in China. Renew. Energy 2008, 33, 186–192. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.Y.; Yang, J.H.; Kim, H.T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Akinyele, D.; Belikov, J.; Levron, Y. Battery storage technologies for electrical applications: Impact in stand-alone photovoltaic systems. Energies 2017, 10, 1760. [Google Scholar] [CrossRef] [Green Version]
- Ponce-de-León, C.; Frías-Ferrer, A.; González-García, J.; Szánto, D.A.; Walsh, F.C. Redox flow cells for energy conversion. J. Power Sources 2006, 160, 716–732. [Google Scholar] [CrossRef] [Green Version]
- Lex, P.; Jonshagen, B. The zinc bromine battery system for utility and remote area applications. Power Eng. J. 1999, 13, 142–148. [Google Scholar] [CrossRef]
- Arenas, L.F.; Loh, A.; Trudgeon, D.P.; Li, X.; Ponce de León, C.; Walsh, F.C. The characteristics and performance of hybrid redox flow batteries with zinc negative electrodes for ES. Renew. Sustain. Energy Rev. 2018, 90, 992–1016. [Google Scholar] [CrossRef] [Green Version]
- Scamman, D.P.; Gavin, W.R.; Roberts, E.P.L. Numerical modelling of a bromide—polysulphide redox flow battery. Part 1: Modelling approach and validation for a pilot-scale system. J. Power Sources 2009, 189, 1220–1230. [Google Scholar] [CrossRef]
- Rydh, C.J. Environmental assessment of vanadium redox and lead—acid bat-teries for stationary ES. J. Power Sources 1999, 80, 21–29. [Google Scholar] [CrossRef]
- Aditya, S.K.; Das, D. Battery ES for load frequency control of an interconnected power system. Electr. Power Syst. Res. 2001, 58, 179–185. [Google Scholar] [CrossRef]
- Koohi-Kamali, S.; Tyagi, V.V.; Rahim, N.A.; Panwar, N.L.; Mokhlis, H. Emergence of ES technologies as the solution for reliable operation of smart power systems: A review. Renew. Sustain. Energy Rev. 2013, 25, 135–165. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent progress in redox flow battery research and development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Hartikainen, T.; Mikkonen, R.; Lehtonen, J. Environmental advantages of super- conducting devices in distributed electricity-generation. Appl. Energy 2007, 84, 29–38. [Google Scholar] [CrossRef]
- Leung, P.; Li, X.; Ponce De León, C.; Berlouis, L.; Low, C.T.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in ES. RSC Adv. 2012, 2, 10125–10256. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in flow battery research and development. J. Electrochem. Soc. 2011, 158, R55–R79. [Google Scholar] [CrossRef]
- Uhrig, M.; Koenig, S.; Suriyah, M.R.; Leibfried, T. Lithium-based vs. vanadium redox flow batteries—A comparison for home storage systems. Energy Procedia 2016, 99, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.renogy.com/deep-cycle-agm-battery-12-volt-200ah/ (accessed on 11 August 2021).
- Trócoli, R.; Morata, A.; Erinmwingbovo, C.; La Mantia, F.; Tarancó, A. Self-discharge in Li-ion aqueous batteries: A case study on LiMn2O4. Electrochim. Acta 2021, 373, 137847. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. Lihium–Sulfur Batteries with the Lowest Self-Discharge and the Longest Shelf life. ACS Energy Lett. 2017, 2, 1056–1061. [Google Scholar] [CrossRef]
- Battery University. What Does Elevated Self-Discharge Do? 2021. Available online: https://batteryuniversity.com/learn/article/elevating_self_discharge (accessed on 21 June 2021).
- Bajpai, P.; Dash, V. Hybrid renewable energy systems for power generation in stand-alone applications: A review. Renew. Sustain. Energy Rev. 2012, 16, 2926–2939. [Google Scholar] [CrossRef]
- Li, N.; Uckun, C.; Constantinescu, E.M.; Birge, J.R.; Hedman, K.W.; Botterud, A. Flexible Operation of Batteries in Power System Scheduling with Renewable Energy. IEEE Trans. Sustain. Energy 2016, 7, 685–696. [Google Scholar] [CrossRef]
- Seong, W.M.; Park, K.Y.; Lee, M.W.; Moon, S.; Oh, K.; Park, H.; Lee, S.; Kang, K. Abnormal self-discharge in lithium-ion batteries. Energy Environ. Sci. 2018, 11, 970–978. [Google Scholar]
- DeValeria, M.K.; Michaelides, E.E.; Michaelides, D.N. Energy and Thermal Storage in Clusters of Grid-Independent Buildings. Energy 2019, 190, 116440. [Google Scholar] [CrossRef]
- Cusenza, M.A.; Guarino, F.; Longo, S.; Mistretta, M.; Cellura, M. Reuse of Electric Vehicle Batteries in Buildings: An Integrated Load Match Analysis and Life Cycle Assessment Approach. Energy Build. 2019, 186, 339–354. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-Ion Batteries towards Circular Economy: A., Literature Review of Opportunities and Issues of Recycling Treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Faessler, B. Stationary, Second Use Battery ES Systems and Their Applications: A Research Review. Energies 2021, 14, 2335. [Google Scholar] [CrossRef]
- Bockris, J.O. The Origin of Ideas on a Hydrogen Economy and Its Solution to the Decay of the Environment. Intern. J. Hydrog. Energy 2002, 27, 731–740. [Google Scholar] [CrossRef]
- Rifkin, J. The Hydrogen Economy: The Creation of the Worldwide Energy Web and the Redistribution of Power on Earth; Tarcher/Penguin: New York, NY, USA, 2003. [Google Scholar]
- Moran, M.J.; Shapiro, H.N. Fundamentals of Engineering Thermodynamics, 6th ed.; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Anderson, J.; Greonkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Mazloomi, K.; Sulaiman, N.; Moayedi, H. Review—Electrical Efficiency of Electrolytic Hydrogen Production. Int. J. Electrochem. Sci. 2012, 7, 3314–3326. [Google Scholar]
- Mao, Y.; Gao, Y.; Dong, W.; Wu, H.; Song, Z.; Zhao, X.; Sun, J.; Wang, W. Hydrogen production via a two-step water splitting thermochemical cycle based on metal oxide—A review. Appl. Energy 2020, 267, 114860. [Google Scholar] [CrossRef]
- Wang, M.; Zuo, Y.; Wang, J.; Wang, Y.; Shen, X.; Qiu, B.; Cai, L.; Zhou, F.; Lau, S.P.; Chai, Y. Remarkably Enhanced Hydrogen Generation of Organolead Halide Perovskites via Piezocatalysis and Photocatalysis. Adv. Energy Mater. 2019, 9, 1901801. [Google Scholar] [CrossRef]
- Salam, M.K.; Ahmed, K.; Akter, N.; Hossain, T.; Abdullah, B. A review of hydrogen production via biomass gasification and its prospect in Bangladesh. Int. J. Hydrogen Energy 2018, 43, 14944–14973. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Farhana-Azman, N.; Chandrasekhar, K.; Sahaid-Kalil, M. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, V.; Chen, G.; Zhang, Y. Study of IrxRu1-xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochim. Acta 2009, 54, 6250–6256. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Jua, H.K.; Badwalb, S.; Giddey, S. A comprehensive review of carbon and hydrocarbon assisted water electrolysis for hydrogen production. Appl. Energy 2018, 231, 502–533. [Google Scholar] [CrossRef]
- Gillette, J.; Kolpa, R. Overview of Interstate Hydrogen Pipeline Systems. Argonne National Laboratory Report 2007; ANL: Chicago, IL, USA, 2007. [Google Scholar]
- Ball, M.; Weeda, M. The hydrogen economy—Vision or reality? Int. J. Hydrogen Energy 2015, 40, 7903–7919. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.; Thornton, A.; Hill, M.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- BEUC. Low Carbon Cars in the 2020s: Consumer Impacts and EU Policy Implications; Bureau Européen des Unions de Consommateurs: Brussells, Belgium, 2016. [Google Scholar]
- Fuel Cell Electric Vehicles, Alternative Fuels Data Center; US Department of Energy, EERE, 2021. Available online: https://afdc.energy.gov/vehicles/fuel_cell.html (accessed on 24 September 2011).
- Shiva-Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Fekete, J.R.; Sowards, J.W.; Amaro, R.L. Economic impact of applying high strength steels in hydrogen gas pipelines. Int. J. Hydrogen Energy 2015, 40, 10547–10558. [Google Scholar] [CrossRef] [Green Version]
- Cardella, U.; Decker, L.; Klein, H. Roadmap to economically viable hydrogen liquefaction. Int. J. Hydrogen Energy 2017, 42, 13329–13338. [Google Scholar] [CrossRef]
- Krasae-in, S.; Stang, J.H.; Neksa, P. Development of large-scale hydrogen liquefaction processes from 1898 to 2009. Int. J. Hydrogen Energy 2010, 35, 4524–4533. [Google Scholar] [CrossRef]
- Wilhelmsen, O.; Berstad, D.; Aasen, A.; Neksa, P.; Skaugen, G. Reducing the exergy destruction in the cryogenic heat exchangers of hydrogen liquefaction processes. Int. J. Hydrogen Energy 2018, 43, 5033–5047. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.D.; Yang, Z.X.; Zhu, Y.Q. Porous carbon-based materials for hydrogen storage: Advancement and challenges. J. Mater. Chem. 2013, 1, 9365–9381. [Google Scholar] [CrossRef]
- Blankenship, I.I.T.S.; Balahmar, N.; Mokaya, R. Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nat. Commun. 2017, 8, 1545. [Google Scholar] [CrossRef] [Green Version]
- Crivello, J.C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. Mater. Sci Process. 2016, 122, 97. [Google Scholar] [CrossRef] [Green Version]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids. 2015, 84 (Suppl. C), 96–106. [Google Scholar] [CrossRef]
- Shao, H.; He, L.; Lin, H.; Li, H.W. Progress and trends in magnesium-based materials for energy-storage research: A review. Energy Technol. 2018, 6, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Graetz, J.; Reilly, J.J.; Yartys, V.A.; Maehlen, J.P.; Bulychev, B.M.; Antonov, V.E.; Tarasov, B.P.; Gabis, I.E. Aluminum hydride as a hydrogen and ES material: Past, present and future. J. Alloys Compd. 2011, 509S, S517–S528. [Google Scholar] [CrossRef]
- Graetz, J.; Hauback, B.C. Recent developments in aluminum-based hydrides for hydrogen storage. MRS Bull. 2013, 38, 473–479. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.-W.; Jensen, T.R. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ren, Z.; Zhang, X.; Jian, N.; Yang, Y.; Gao, M.; Pan, H. Development of catalyst-enhanced sodium alanate as an advanced hydrogen-storage material for mobile applications. Energy Technol. 2018, 6, 487–500. [Google Scholar] [CrossRef]
- Ley, M.B.; Meggouh, M.; Moury, R.; Peinecke, K.; Felderhoff, M. Development of hydrogen storage tank systems based on complex metal hydrides. Materials 2015, 8, 5891–5921. [Google Scholar] [CrossRef]
- Gregory DH, Lithium nitrides, imides and amides as lightweight, reversible hydrogen stores. J. Mater. Chem. 2008, 18, 2321–2330. [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Pfromm, P.H. Towards sustainable agriculture: Fossil-free ammonia. J. Renew. Sustain. Energy 2017, 9, 034702. [Google Scholar] [CrossRef] [Green Version]
- Muller, K.; Brooks, K.; Autrey, T. Hydrogen storage in formic acid: A comparison of process options. Energy Fuels 2017, 31, 12603–12611. [Google Scholar] [CrossRef]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source-recent developments and future trends. Energy Env. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.P.; Surya Prakash, G.K.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products-closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef]
- Pontzen, F.; Liebner, W.; Gronemann, V.; Rothaemel, M.; Ahlers, B. CO2-based methanol and DME-Efficient technologies for industrial scale production. Catal. Today 2011, 171, 242–250. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Mahkamov, K. Solar ES using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Sharma, S.D.; Sagara, K. Latent heat storage materials and systems: A review. Int. J. Green Energy 2003, 2, 1–56. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal ES with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Keenan, J.H. A Steam Chart for Second Law Analysis. A Study of Thermodynamics Availability in a Steam Power Plant. Mech. Eng. 1932, 54, 195–204. [Google Scholar]
- Kestin, J. Availability—The Concept and Associated Terminology. Energy-Int. J. 1980, 5, 679–692. [Google Scholar] [CrossRef] [Green Version]
- Ahrendts, J. Reference States. Energy-Int. J. 1980, 5, 667–677. [Google Scholar] [CrossRef]
- Burgaleta, J.I.; Arias, S.; Ramirez, D. Gemasolar, the First Tower Thermosolar Commercial Plant with Molten Salt Storage. In Proceedings of the SolarPACES Congress, Granada, Spain, 20–23 September 2011. [Google Scholar]
- Relloso, S.; Gutierrez, Y. Real Application of Molten Salt Thermal Storage to Obtain High Capacity Factors in Parabolic Trough Plants. In Proceedings of the SolarPACES Congress, Las Vegas, NV, USA, 4–7 March 2008. [Google Scholar]
- Olaso, J.; Ortega, J.I. Solar Power Dispatchability through Thermal storage—Solar TRES. In Proceedings of the SolarPACES Congress, Las Vegas, NV, USA, 4–7 March 2008. [Google Scholar]
- Denholm, P.; King, J.C.; Kutcher, C.F.; Wilson, P.P.H. Decarbonizingtheelectric sector: Combining renewable and nuclear energy using thermal storage. Energy Policy 2012, 44, 301–311. [Google Scholar] [CrossRef]
- Deng, T.; Tian, L.; Liu, J. Spatial and temporal multiscale analysis on ES in heat supply units’ boiler and heat supply nets. Proc. CSEE 2017, 37, 599–606. [Google Scholar]
- Wang, L.; Yu, S.; Kong, F.; Sun, X.; Zhou, Y.; Zhong, W.; Lin, X. A study on ES characteristics of industrial steam heating system based on dynamic modeling. Energy Rep. 2020, 6, 190–198. [Google Scholar] [CrossRef]
- Deng, T.; Tian, L.; Liu, J. A control method of heat supply units for improving frequency control and peak load regulation ability with thermal storage in heat supply net. Proc. CSEE 2015, 35, 3626–3633. [Google Scholar]
- Lund, H.; Werner, S.; Wiltshire, R.; Svendsen, S.; Thorsen, J.E.; Hvelplund, F.; Mathiesen, B.V. 4th generation district heating (4GDH). Integrating smart thermal grids into future sustainable energy systems. Energy 2014, 68, 1–11. [Google Scholar] [CrossRef]
- Wang, H.; Meng, H. Improved thermal transient modeling with new 3-order numerical solution for a district heating network with consideration of the pipe wall’s thermal inertia. Energy 2018, 160, 171–183. [Google Scholar] [CrossRef]
- Pintaldi, S.; Perfumo, C.; Sethuvenkatraman, S.; White, S.; Rosengarten, G. A review of thermal ES technologies and control approaches for solar cooling. Renew. Sustain. Energy Rev. 2015, 41, 975–995. [Google Scholar] [CrossRef]
- Eicker, U.; Pietruschka, D. Design and performance of solar powered absorption cooling systems in office buildings. Energy Build. 2009, 41, 81–91. [Google Scholar] [CrossRef]
- Yabase, H.; Makita, K. Steam Driven Triple Effect Absorption Solar Cooling System. In Proceedings of the International Refrigeration and Air Conditioning Conference, Paper 1272; Purdue: Lafayette, IN, USA, 2012. [Google Scholar]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal ES technologies and systems for concentrating solar power plants. Prog. Energy Combustion Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Lu, Z.S.; Wang, R.Z.; Xia, Z.Z.; Lu, X.R.; Yang, C.B.; Ma, Y.C. Study of a novel solar adsorption cooling system and a solar absorption cooling system with new CPC collectors. Renew. Energy 2013, 50, 299–306. [Google Scholar] [CrossRef]
- Li, Z.; Sumathy, K. Experimental studies on a solar powered air conditioning system with partitioned hot water storage tank. Sol. Energy 2001, 71, 285–297. [Google Scholar] [CrossRef]
- Calise, F. High temperature solar heating and cooling systems for different Mediterranean climates: Dynamic simulation and economic assessment. Appl. Therm. Eng. 2012, 32, 108–124. [Google Scholar] [CrossRef]
- Kousksou, T.; Bruel, P.; Jamil, A.; El Rhafiki, T.; Zeraouli, Y. ES: Applications and challenges. Sol. Energy Mater. Sol. Cells 2014, 120, 59–80. [Google Scholar] [CrossRef]
- Pinel, P.; Cruickshank, C.A.; Beausoleil-Morrison, I.; Wills, A. A review of available methods for seasonal storage of solar thermal energy in residential applications. Renew. Sustain. Energy Rev. 2011, 15, 3341–3359. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal ES with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Gil, A.; Oró, E.; Peiró, G.; Álvarez, S.; Cabeza, L.F. Material selection and testing for thermal ES in solar cooling. Renew. Energy 2013, 57, 366–371. [Google Scholar] [CrossRef]
- Hodapp, R.T. Dallas/Fort Worth International Airport District Energy Plant Upgrades Project—Making More with Less. In Proceedings of the Sustainable Communities Conference, Dallas, TX, USA, 18–20 March 2009. [Google Scholar]
- IEA. 2021. Available online: https://www.iea.org/topics/buildings (accessed on 21 July 2021).
- Gottschall, T.; Gràcia-Condal, A.; Fries, M.; Taubel, A.; Pfeuffer, L.; Mañosa, L.; Planes, A.; Skokov, K.P.; Gutfleisch, O. A multicaloric cooling cycle that exploits thermal hysteresis. Nat. Mater. 2018, 17, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Hess, T.; Maier, L.M.; Bachmann, N.; Corhan, P.; Schäfer-Welsen, O.; Wöllenstein, J.; Bartholomé, K. Thermal hysteresis and its impact on the efficiency of first-order caloric materials. J. Appl. Phys. 2020, 127, 075103. [Google Scholar] [CrossRef]
- Rodionov, V.; Amirov, A.; Annaorazov, M.; Lähderanta, E.; Granovsky, A.; Aliev, A.; Rodionova, V. Thermal Hysteresis Control in Fe49Rh51 Alloy through Annealing Process. Processes 2021, 9, 772. [Google Scholar] [CrossRef]
- Wen, D.S.; Chen, H.S.; Ding, Y.L.; Dearman, P. Liquid nitrogen injection into water: Pressure build-up and heat transfer. Cryogenics 2006, 46, 740–748. [Google Scholar] [CrossRef]
- Knowlen, C.; Williams, J.; Mattick, A.T.; Deparis, H.; Hertzberg, A. Quasi-Isothermal Expansion Engines for Liquid Nitrogen Automotive Propulsion. SAE Tech. Pap. Ser. 1997. [Google Scholar] [CrossRef] [Green Version]
- Knowlen, C.; Mattick, A.T.; Bruckner, A.P.; Hertzberg, A. High Efficiency Energy Conversion Systems for Liquid Nitrogen Automobiles. SAE Tech. Pap. Ser. 1998. [Google Scholar] [CrossRef] [Green Version]
- Tatsidjodoung, P.; le Pierrès, N.; Luo, L. A review of potential materials for thermal ES in building applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Pan, Z.H.; Zhao, C.Y. Gasesolid thermochemical heat storage reactors for high-temperature applications. Energy 2017, 130, 155–173. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Recent Advances in Thermochemical ES via Solid–Gas Reversible Reactions at High Temperature. Energies 2020, 13, 5859. [Google Scholar] [CrossRef]
- Zhou, X.; Mahmood, M.; Chen, J.; Yang, T.; Xiao, G.; Ferrari, M.L. Validated model of thermochemical energy storage based on cobalt oxides. Appl. Therm. Eng. 2019, 159, 113965. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. A critical review of thermochemical ES systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical ES and conversion: A-state-of-the-art review of the experimental research under practical conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Zsembinszki, G.; Solé, A.; Barreneche, C.; Prieto, C.; Fernández, A.; Cabeza, L. Review of Reactors with Potential Use in Thermochemical ES in Concentrated Solar Power Plants. Energies 2018, 11, 2358. [Google Scholar] [CrossRef] [Green Version]
- Hartlieb, P.; Toifl, M.; Kuchar, F.; Meisels, R.; Antretter, T. Thermo-physical properties of selected hard rocks and their relation to microwave-assisted comminution. Miner. Eng. 2016, 91, 34–41. [Google Scholar] [CrossRef]
- Ochsner, K. Geothermal Heat Pumps—A Guide for Planning and Installing; Earthscan: London, UK, 2008. [Google Scholar]
- Banks, D. An Introduction to Thermogeology: Ground Source Heating and Cooling, 2nd ed.; Wiley: Chichester, UK, 2012. [Google Scholar]
- Sibbitt, B.; Onno, T.; McClenahan, D. The Drake Landing solar community project—Early results. In Proceedings of the 32. annual conference of the Solar Energy Society of Canada and the 2. Canadian Solar Research Network conference, Calgary, AB, Canada, 10–14 June 2007. [Google Scholar]
- Sibbitt, B.; McClenahan, D.; Djebbar, R.; Thornton, J.; Wong, B.; Carriere, J.; Kokko, J. The performance of a high solar fraction seasonal storage district heating system–five years of operation. Energy Procedia 2012, 30, 856–865. [Google Scholar] [CrossRef] [Green Version]
- Lundh, M.; Dalenbäck, J.O. Swedish solar heated residential area with seasonal storage in rock: Initial evaluation. Renew. Energy 2008, 33, 703–711. [Google Scholar] [CrossRef]
- Kjellsson, E.; Hellström, G.; Perers, B. Optimization of systems with the combination of ground-source heat pump and solar collectors in dwellings. Energy 2010, 35, 2667–2673. [Google Scholar] [CrossRef]
- Gustafsson, A.-M.; Westerlund, L.; Hellström, G. CFD modelling of natural convection in a groundwater-filled borehole heat exchanger. Appl. Therm. Eng. 2010, 30, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Skarphagen, H.; Banks, D.; Frengstad, B.S.; Gether, H. Design Considerations for Borehole Thermal Energy Storage (BTES): A Review with Emphasis on Convective Heat Transfer. Geofluids 2019, 2019, 1–26. [Google Scholar] [CrossRef]
- Michaelides, E.E. A New Model for the Lifetime of Fossil Fuel Resources. Nat. Resour. Res. 2017, 26, 161–175. [Google Scholar] [CrossRef]
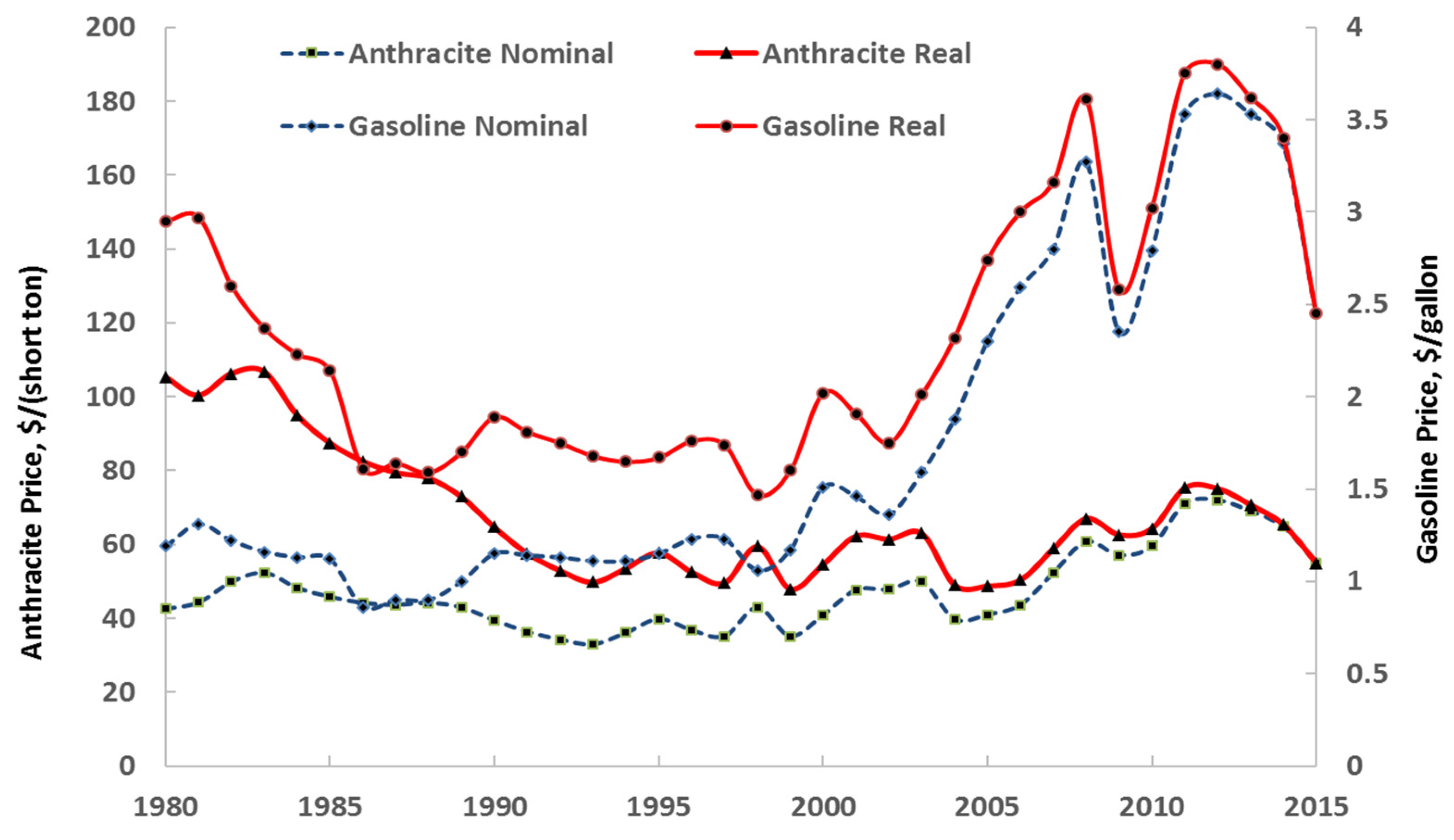
- Available online: https://www.energy.gov/eere/vehicles/fact-915-march-7-2016-average-historical-annual-gasoline-pump-price-1929-2015 (accessed on 20 November 2019).
- Available online: https://www.eia.gov/totalenergy/data/annual/index.php (accessed on 31 January 2021).
- Michaelides, E.E. Thermodynamic Analysis and Power Requirements of CO2 Capture, Transportation, and Storage in the Ocean. Energy 2021, 230, 120804. [Google Scholar] [CrossRef]
- Mongird, K.; Viswanathan, V.; Alam, J.; Vartanian, C.; Sprenkle, V.; Baxter, R. 2020 Grid Energy Storage Technology Cost and Performance Assessment; DOE/PA-0204; Pacific Northwest National Laboratory: Seattle WA, USA, 2020. [Google Scholar]
- Sander, M.; Gehring, R. LIQHYSMES—A novel ES concept for variable renewable energy sources using hydrogen and SMES. IEEE Trans. Appl. Supercond. 2011, 21, 1362–1366. [Google Scholar] [CrossRef]
- Hamajima, T.; Amata, H.; Iwasaki, T.; Atomura, N.; Tsuda, M.; Miyagi, D.; Shintomi, T.; Makida, Y.; Takao, T.; Munakata, K.; et al. Application of SMES and fuel cell system combined with liquid hydrogen vehicle station to renewable energy control. IEEE Trans. Appl. Supercond. 2012, 22, 5701704. [Google Scholar] [CrossRef]
- Thounthong, P.; Raël, S.; Davat, B. Control strategy of fuel cell/supercapacitors hybrid power sources for electric vehicle. J. Power Sources 2006, 158, 806–814. [Google Scholar] [CrossRef]
- Uzunoglu, M.; Alam, M.S. Dynamic modeling, design, and simulation of a combined PEM fuel cell and ultracapacitor system for stand-alone residential applications. IEEE Trans. Energy Convers 2006, 21, 767–775. [Google Scholar] [CrossRef]
- Martinez, M.; Molina, M.G.; Frack, F.; Mercado, P.E. Dynamic modeling, simulation and control of hybrid ES system based on compressed air and supercapacitors. Lat. Am. Trans. IEEE 2013, 11, 466–472. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Bonucci, F.; Castellini, L.; Castellini, S.; Ottaviano, A.; Pelosi, D.; Zuccari, A. Dynamic analysis of a hybrid ES system (H-ESS) coupled to a photovoltaic (PV) plant. Energies 2018, 11, 396. [Google Scholar] [CrossRef] [Green Version]
- Beaman, B.G.; Rao, G.M. Hybrid Battery and Flywheel ES System for LEO Spacecraft. In Proceedings of the Annual Battery Conference on Applications and Devices, Proceeding of the IEEE 13th Annual Battery Conference on Applications and Advances, Long Beach, CA, USA, 16 January 1998; pp. 113–116. [Google Scholar]
- Lemofouet, S.; Rufer, A. A Hybrid ES System Based on Compressed Air and Supercapacitors with Maximum Efficiency Point Tracking (MEPT). IEEE Trans. Ind. Electron. 2006, 53, 1105–1115. [Google Scholar] [CrossRef]





| Height, H | 300 | 500 | 1000 |
|---|---|---|---|
| Specific energy, kJ/kg | 2.9 | 4.9 | 9.8 |
| Energy density, kJ/m3 | 2943 | 4905 | 9810 |
| Mass flow rate, kg/s | 39,741 | 23,845 | 11,922 |
| Volume flow rate, m3/s | 39.7 | 23.8 | 11.9 |
| Volume for 4 h, m3 | 572,276,767 | 343,366,060 | 171,683,030 |
| Mass for 4 h, kg | 572,276,767,352 | 343,366,060,411 | 71,683,030,205 |
| Nominal OD, Inches | ID, m | Number of Pipelines | Velocity, m/s | Friction Factor | Frictional Loss, MW/km | Efficiency, % per km | Efficiency, 5 km, % |
|---|---|---|---|---|---|---|---|
| 36 | 0.762 | 11 | 4.75 | 0.032 | 1.05 | 90.12 | 59.46 |
| 48 | 1.016 | 6 | 4.90 | 0.025 | 1.22 | 93.75 | 72.40 |
| 72 | 1.524 | 3 | 4.35 | 0.019 | 1.01 | 97.40 | 87.66 |
| 96 | 2.032 | 2 | 3.67 | 0.012 | 0.55 | 99.05 | 95.35 |
| T, K | P, bar | Specific Energy, kJ/kg | Energy Density, kJ/m3 | ηex |
|---|---|---|---|---|
| 300 | 60 | 351.4 | 24,722 | 53.6 |
| 400 | 60 | 366.0 | 18,838 | 55.8 |
| 500 | 60 | 401.0 | 16,402 | 61.1 |
| 300 | 80 | 376.0 | 35,251 | 52.5 |
| 400 | 80 | 390.8 | 26,642 | 54.6 |
| 500 | 80 | 426.1 | 23,055 | 59.5 |
| Cathode | Voltage, V | Charge Capacity, A-hr/kg | Specific Energy, kJ/kg (kWh/kg) |
|---|---|---|---|
| LiCoO2 | 3.7 | 140 | 1865, (0.518) |
| LiMnO2 | 4.0 | 100 | 1440, (0.400) |
| LiFePO4 | 3.3 | 120 | 1426, (0.396) |
| Li2FePO4F | 3.6 | 115 | 1490, (0.414) |
| T, K | P, bar | ρ, kg/m3 | Specific Energy | Energy Density | ||
|---|---|---|---|---|---|---|
| Gaseous | MJ/kg | kWh/kg | MJ/m3 | kWh/m3 | ||
| 300 | 1 | 0.1 | 118.6 | 32.9 | 9.6 | 2.7 |
| 300 | 10 | 0.8 | 118.6 | 32.9 | 95.3 | 26.5 |
| 300 | 100 | 7.6 | 118.6 | 32.9 | 904.4 | 251.2 |
| 300 | 200 | 14.4 | 118.6 | 32.9 | 1707.8 | 474.4 |
| 300 | 500 | 30.7 | 118.6 | 32.9 | 3636.5 | 1010.1 |
| 300 | 700 | 39.1 | 118.6 | 32.9 | 4631.7 | 1286.6 |
| 300 | 1000 | 49.2 | 118.6 | 32.9 | 5839.2 | 1622.0 |
| Liquid | ||||||
| 20.324 | 1 | 70.9 | 118.6 | 32.9 | 8408.6 | 2335.7 |
| Specific Energy, kJ/kg | Energy Density, MJ/m3 | Round-Trip Efficiency, % | |
|---|---|---|---|
| PHS | 3–10 | 3–10 | 55–75 |
| CAES | 300–400 | 16–36 | 45–60 |
| Flywheels | 5–100 | 100–5000 | 70–95 |
| Supercapacitors | 100–750 | 100–220 | 80–95 |
| Superconducting Coils | 50,000–200,000 | 80,000–240,000 | 90–97 |
| Conventional Batteries | 70–2000 | 120–1600 | 75–90 |
| Flow Batteries | 500–1000 | 500–1000 | 60–85 |
| Hydrogen | 118,600 | 10–8400 | 60–75 |
| Thermal Storage | 100–1200 | 100–2000 | 50–90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaelides, E.E. Thermodynamics, Energy Dissipation, and Figures of Merit of Energy Storage Systems—A Critical Review. Energies 2021, 14, 6121. https://doi.org/10.3390/en14196121
Michaelides EE. Thermodynamics, Energy Dissipation, and Figures of Merit of Energy Storage Systems—A Critical Review. Energies. 2021; 14(19):6121. https://doi.org/10.3390/en14196121
Chicago/Turabian StyleMichaelides, Efstathios E. 2021. "Thermodynamics, Energy Dissipation, and Figures of Merit of Energy Storage Systems—A Critical Review" Energies 14, no. 19: 6121. https://doi.org/10.3390/en14196121
APA StyleMichaelides, E. E. (2021). Thermodynamics, Energy Dissipation, and Figures of Merit of Energy Storage Systems—A Critical Review. Energies, 14(19), 6121. https://doi.org/10.3390/en14196121





