A Review of Hydrogen as a Fuel in Internal Combustion Engines
Abstract
:1. Introduction
2. Production Methods of Hydrogen
2.1. Steam Methane Reforming
2.2. Electrolysis
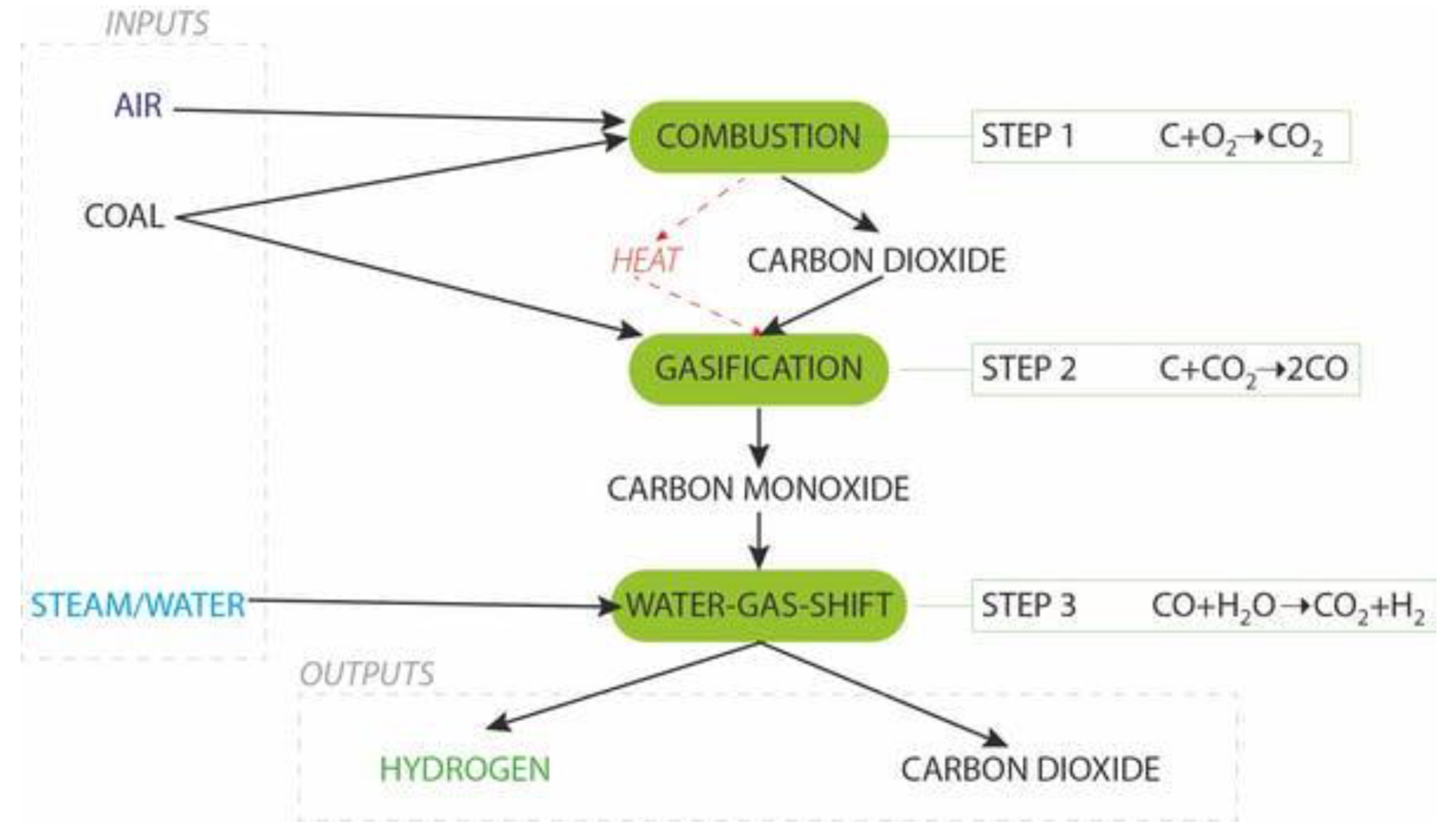
2.3. Coal Gasification
2.4. Biomass Gasification
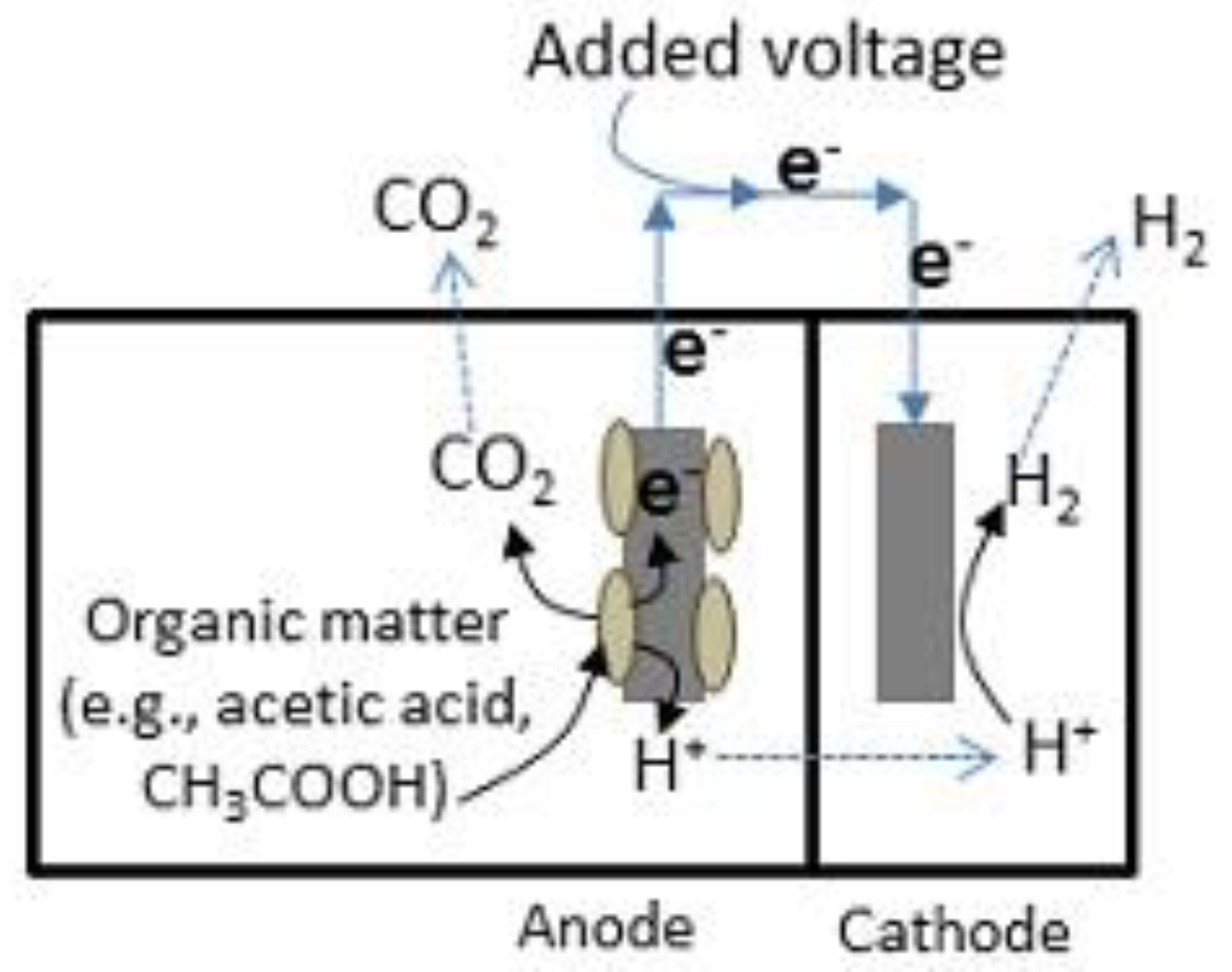
2.5. Microbial Biomass Conversion
2.6. Thermochemical Water Spliting Cycles
3. Methods of Using Hydrogen in Engines
3.1. Use of Hydrogen as a Direct Fuel
3.2. Use of Hydrogen as a Secondary Fuel
4. Methods to Provide Hydrogen to Internal Combustion Engines
4.1. Fuel Carburetion Technique
4.2. Inlet Manifold and Inlet Port Injection Technique
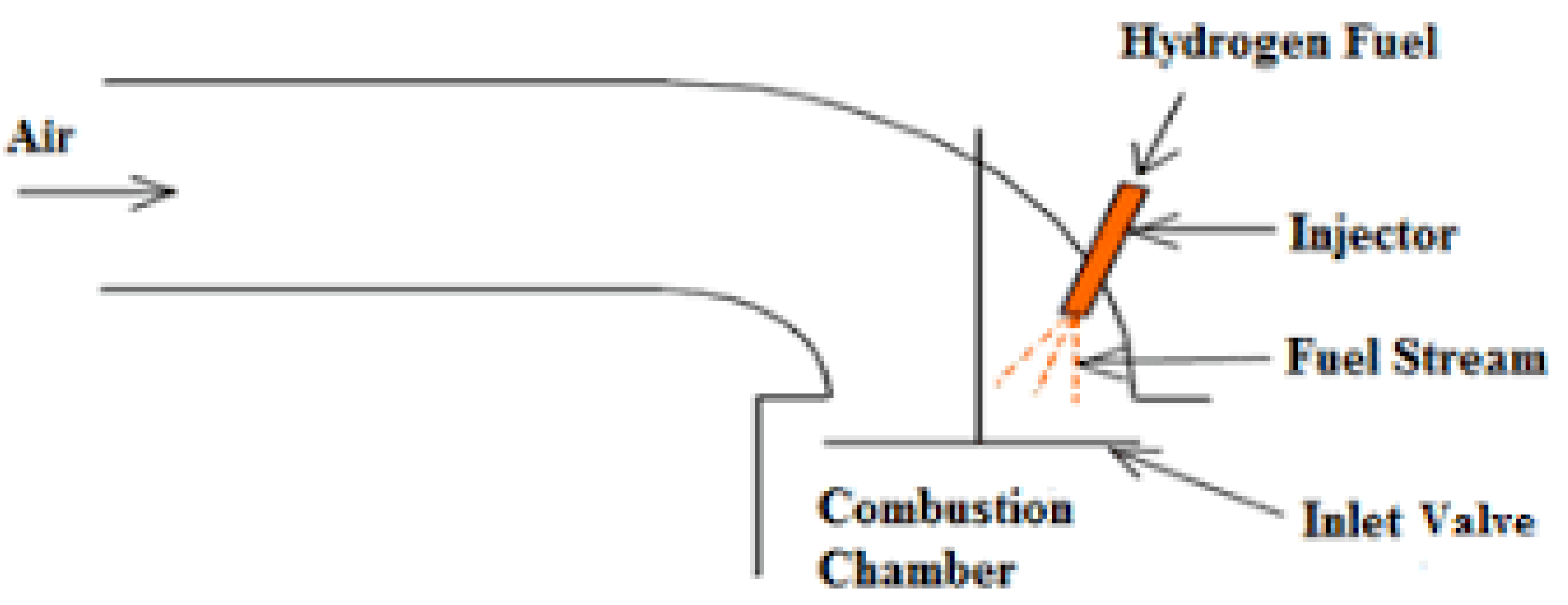
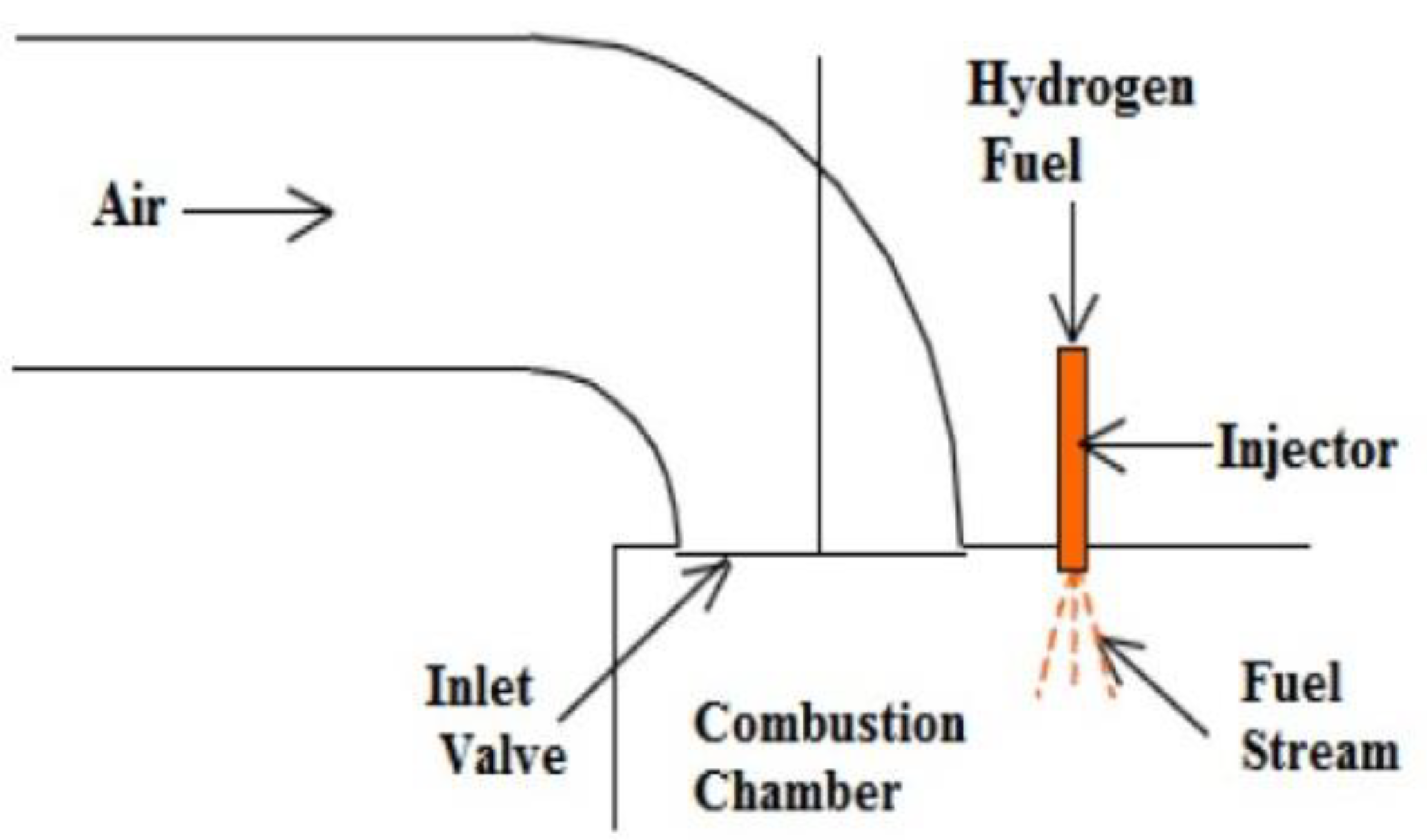
4.3. Direct Injection Systems
5. Impact of Hydrogen Fuels on the Performance of Internal Combustion (IC) Engines
5.1. Power and Torque
5.2. Brake Thermal Efficiency
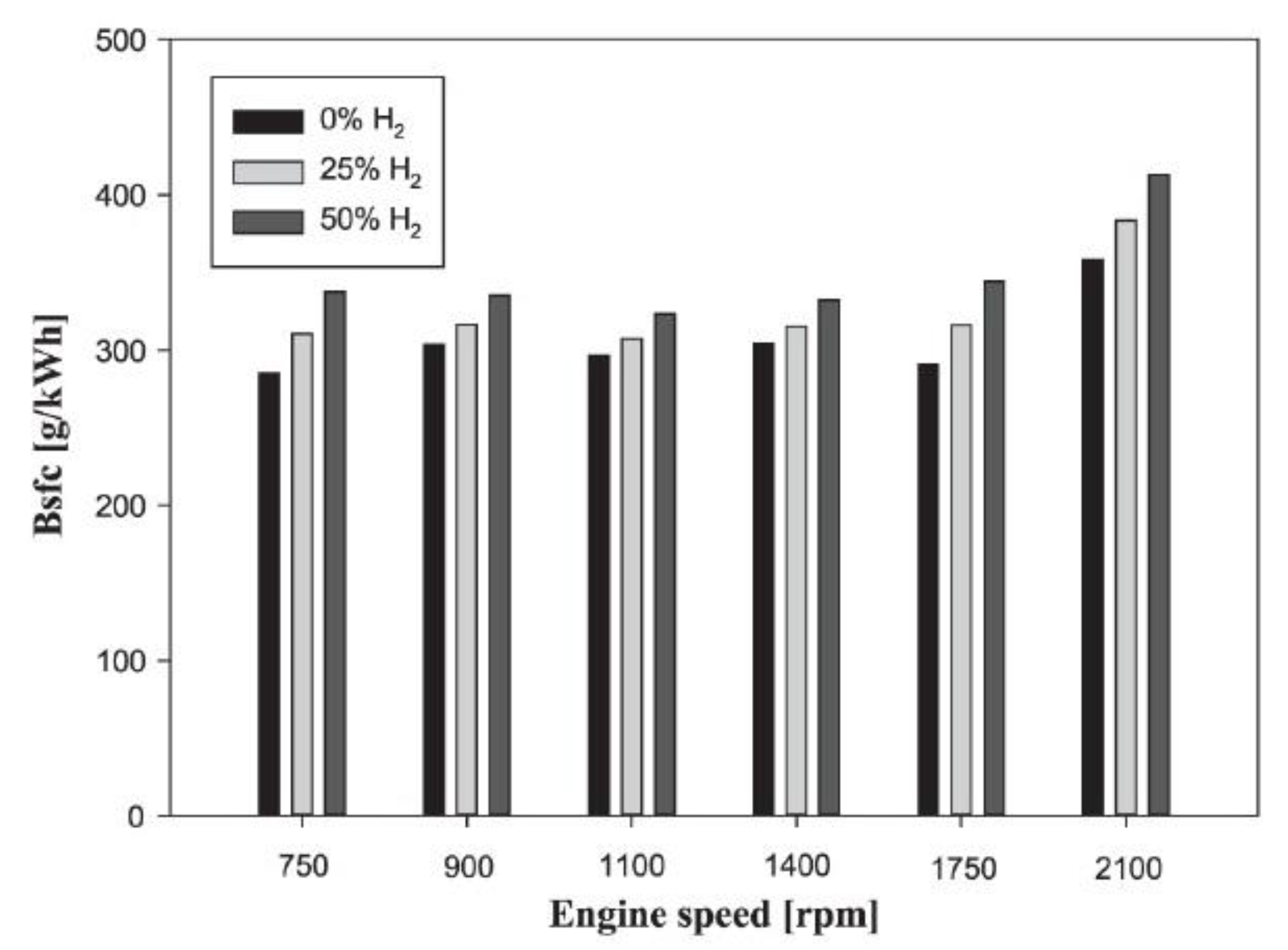
5.3. Brake-Specific Fuel Consumption
6. Impact of Hydrogen Fuels on Emissions of Internal Combustion (IC) Engines
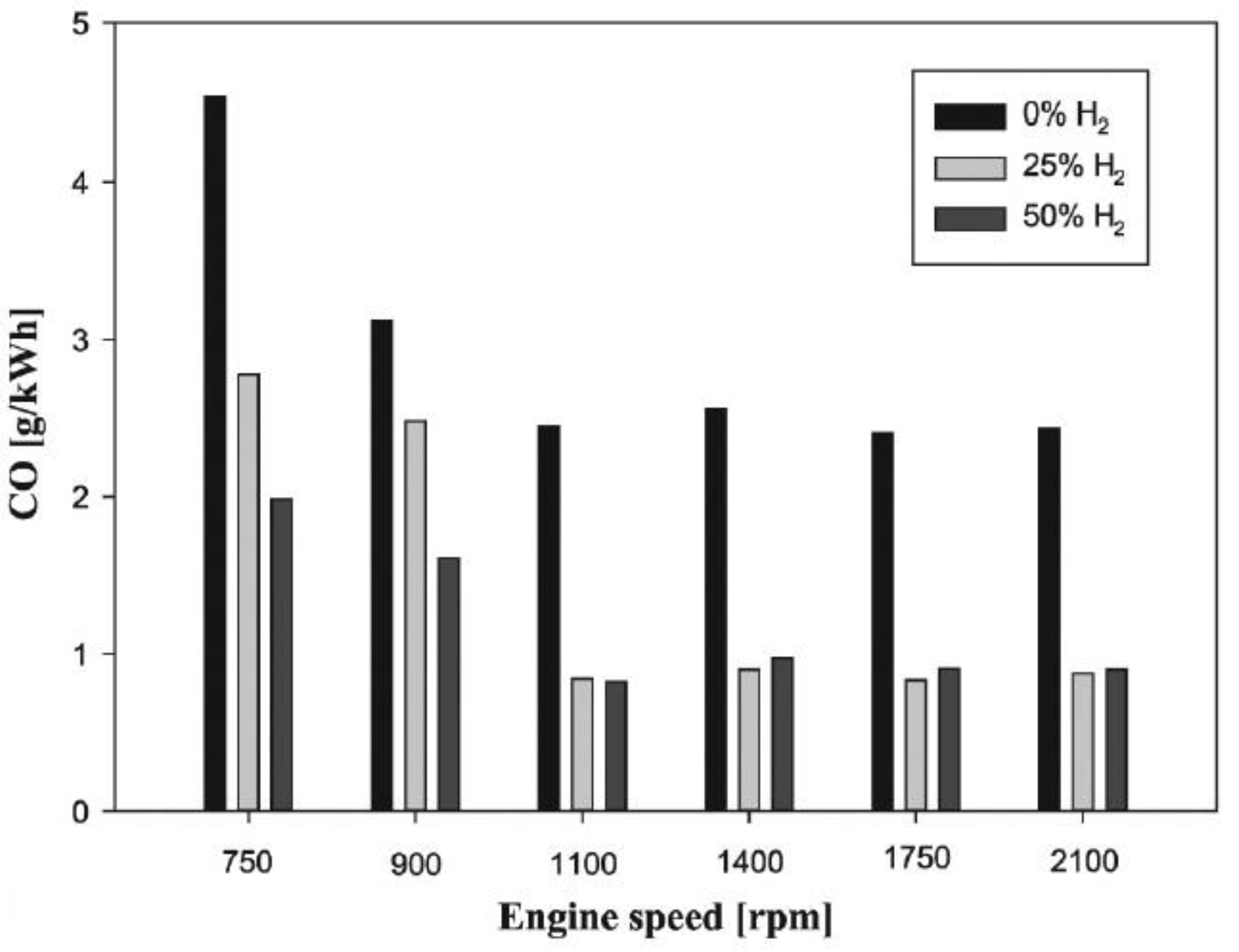
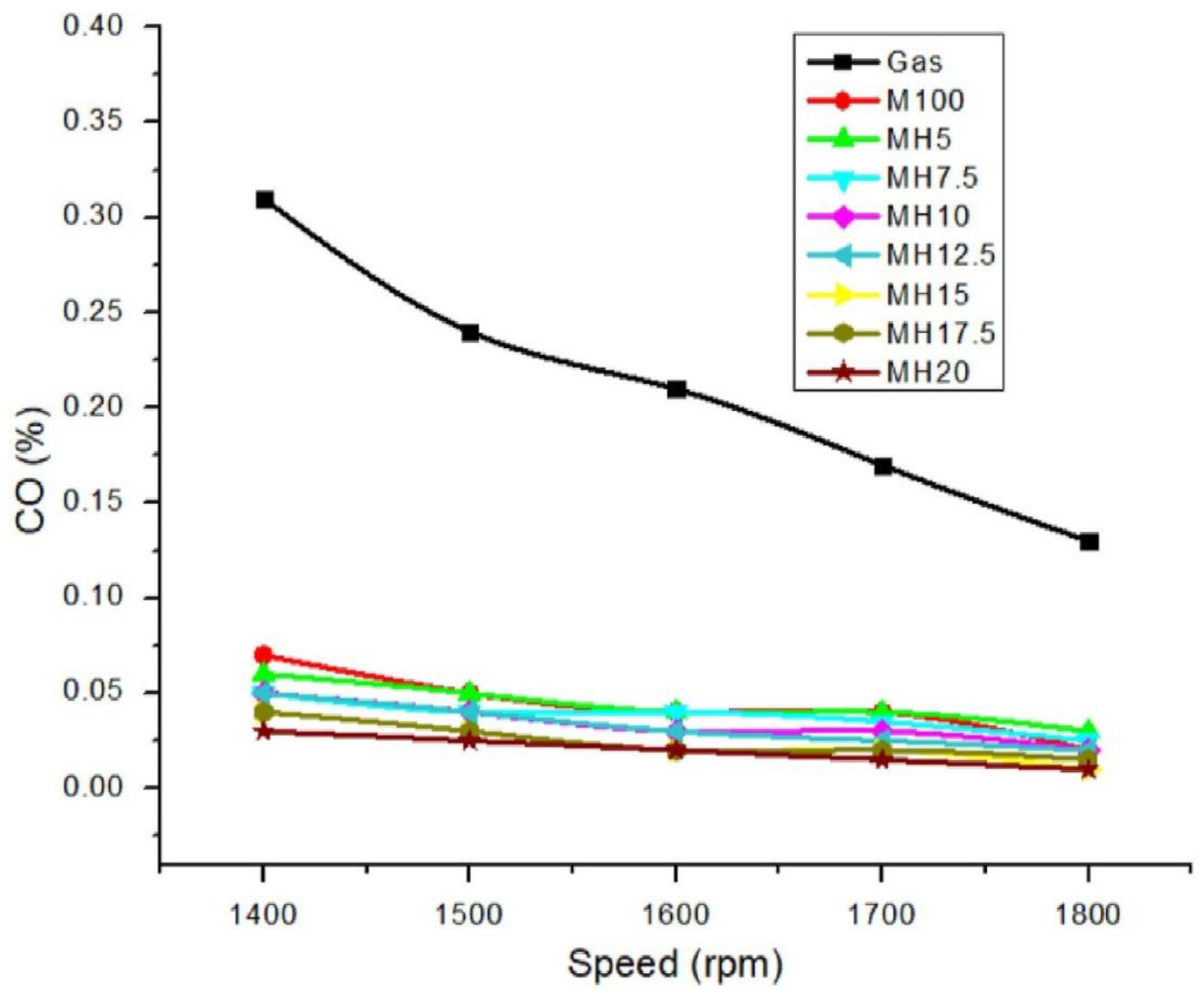
6.1. CO Emissions
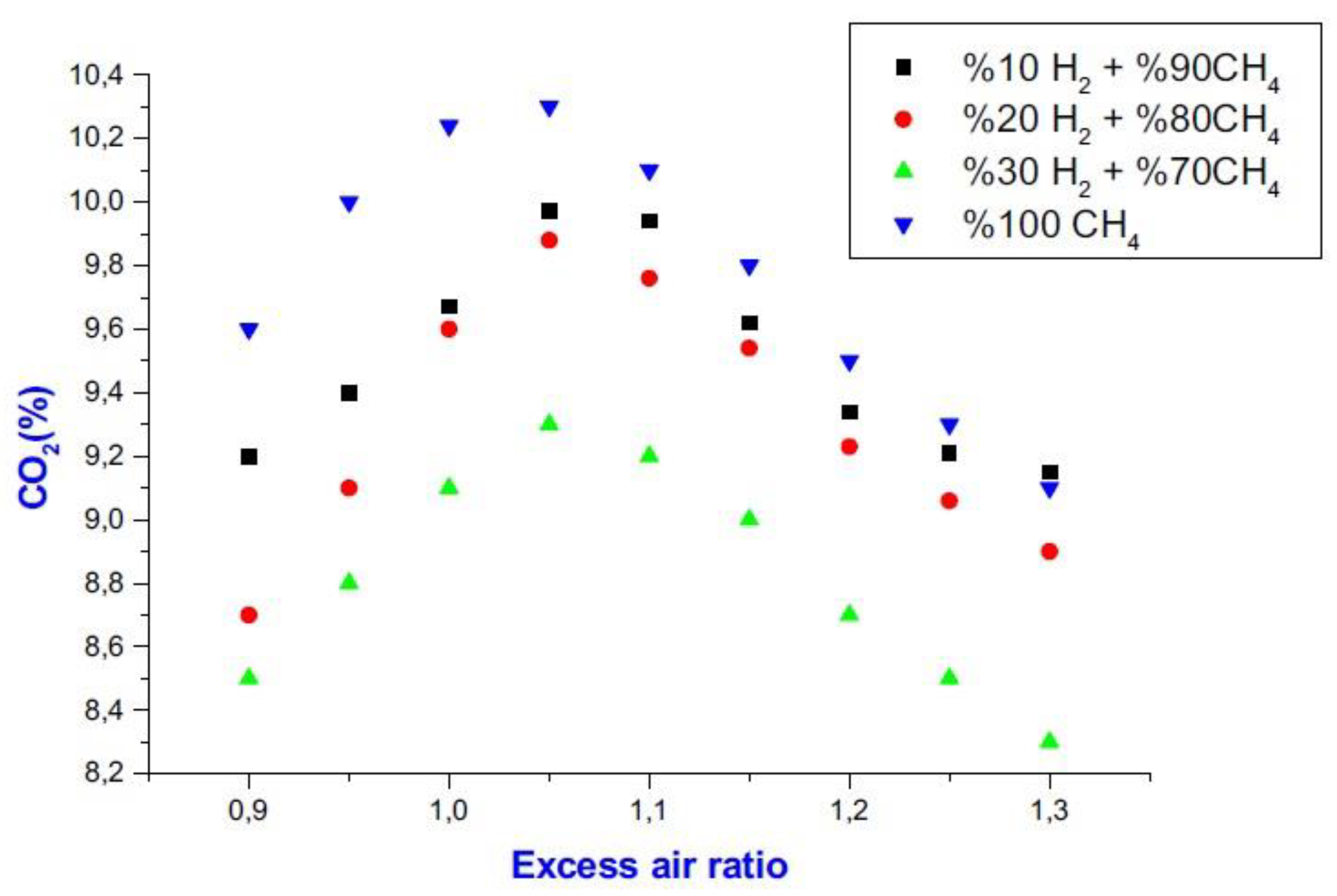
6.2. CO2 Emissions
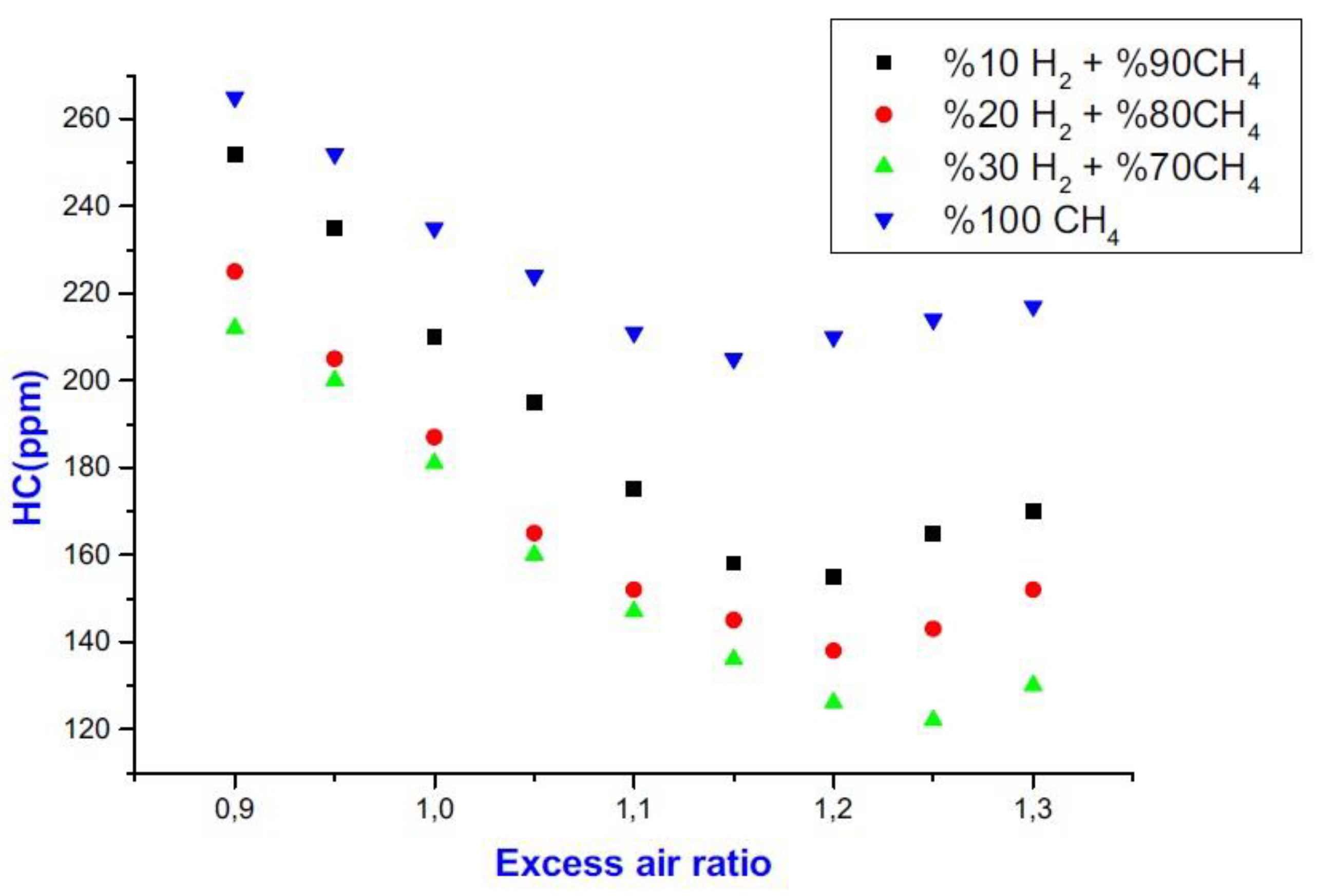
6.3. UHC Emissions
6.4. NOx Emissions
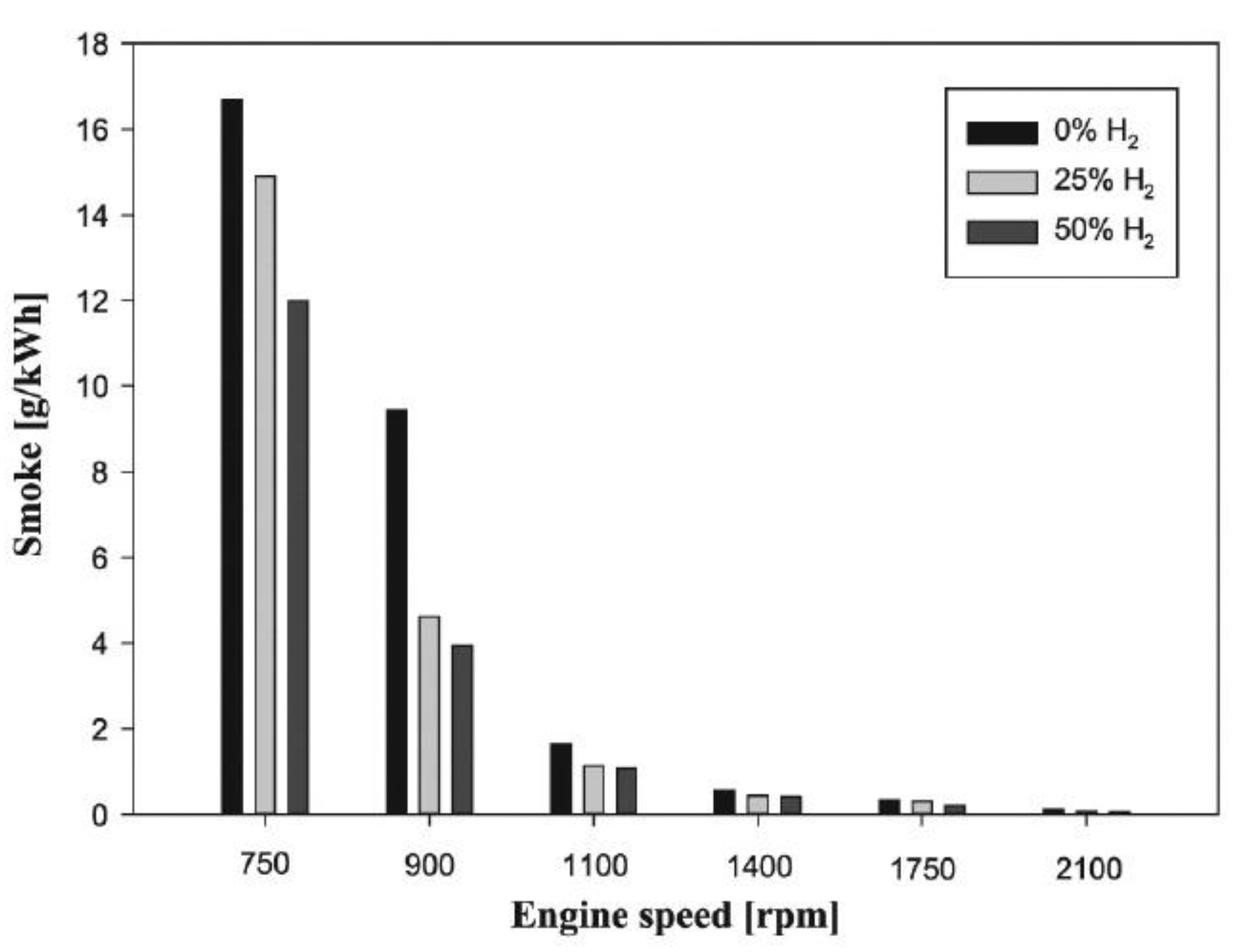
6.5. Soot Emissions
7. Conclusions
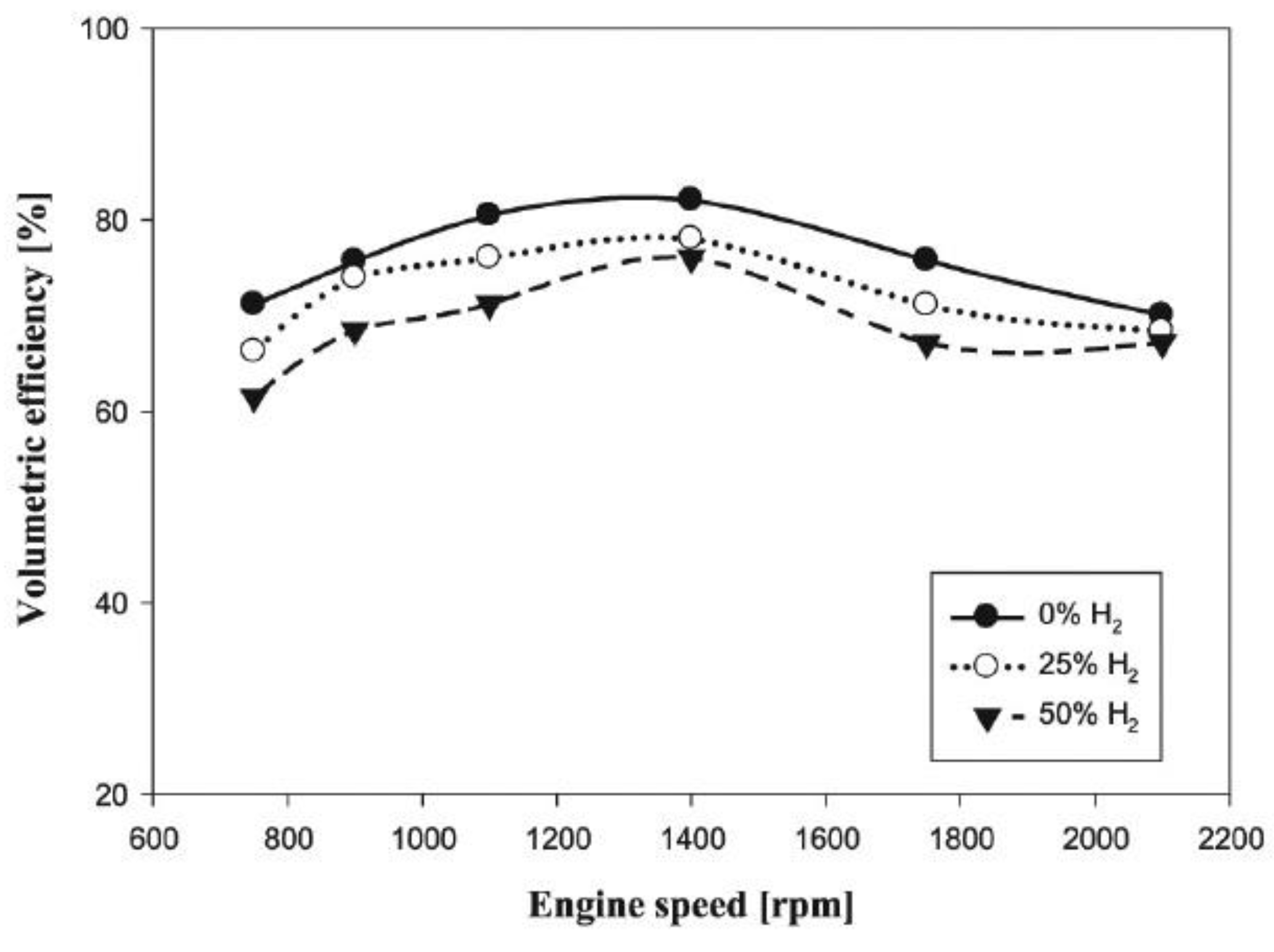
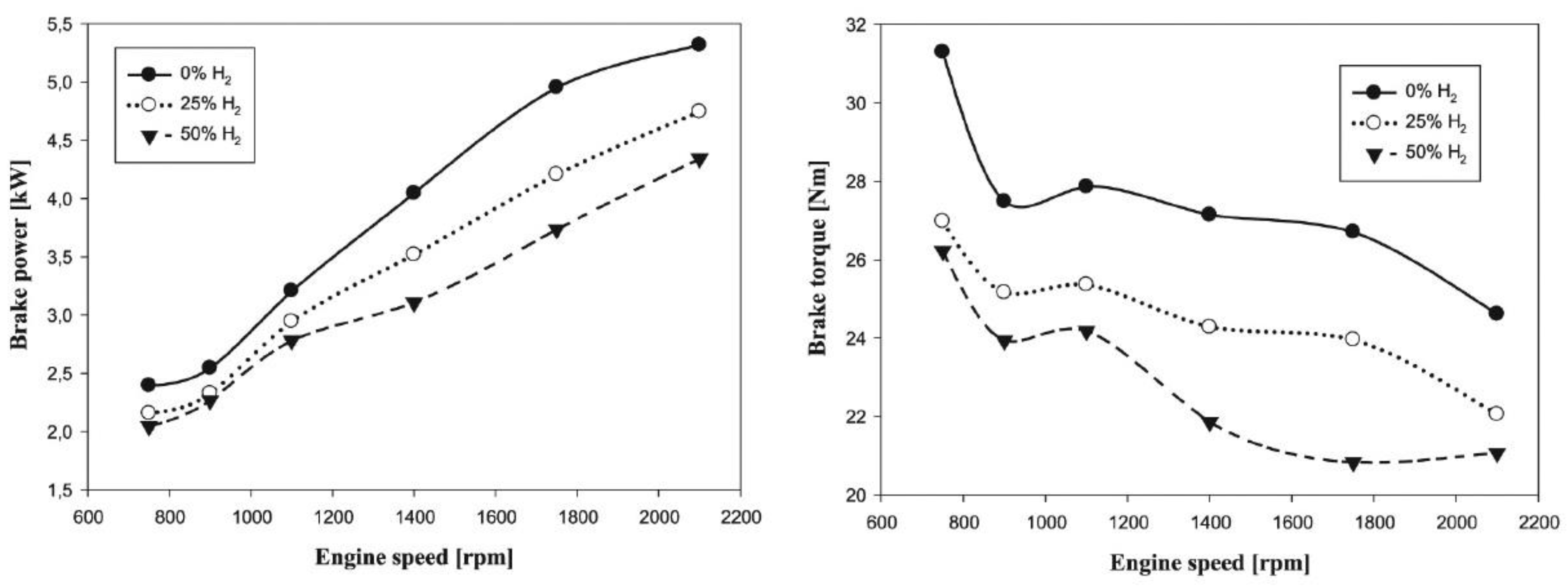
- When hydrogen is used as an additive in spark ignition (SI) and compression ignition (CI) engines, it will reduce the volumetric efficiency of the engine because the lower heat value (LHV) of hydrogen (120 MJ/kg) is higher than those of diesel (43.6 MJ/kg) and gasoline (43.4 MJ/kg). This reduction in volumetric efficiency will reduce engine power and torque.
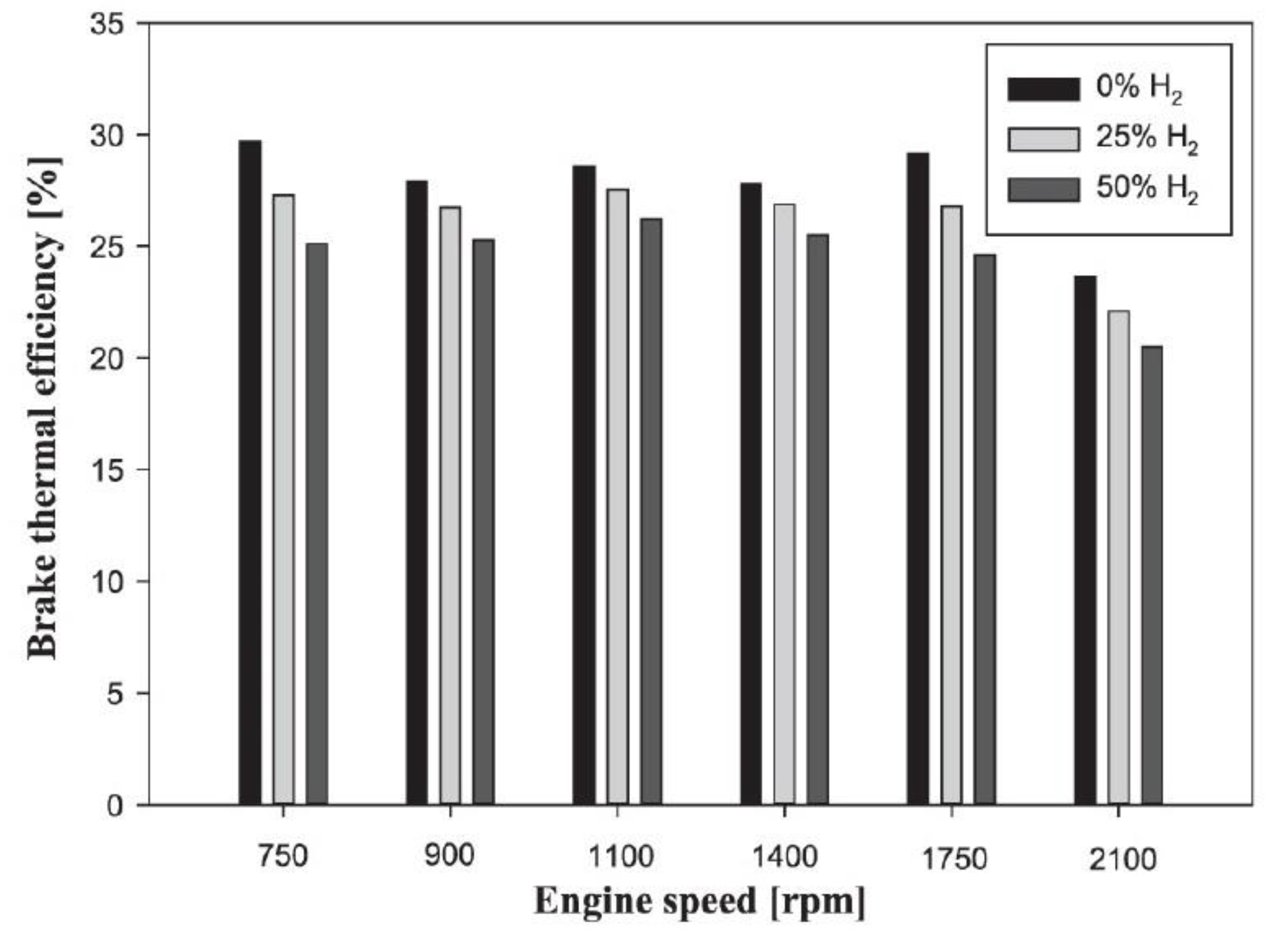
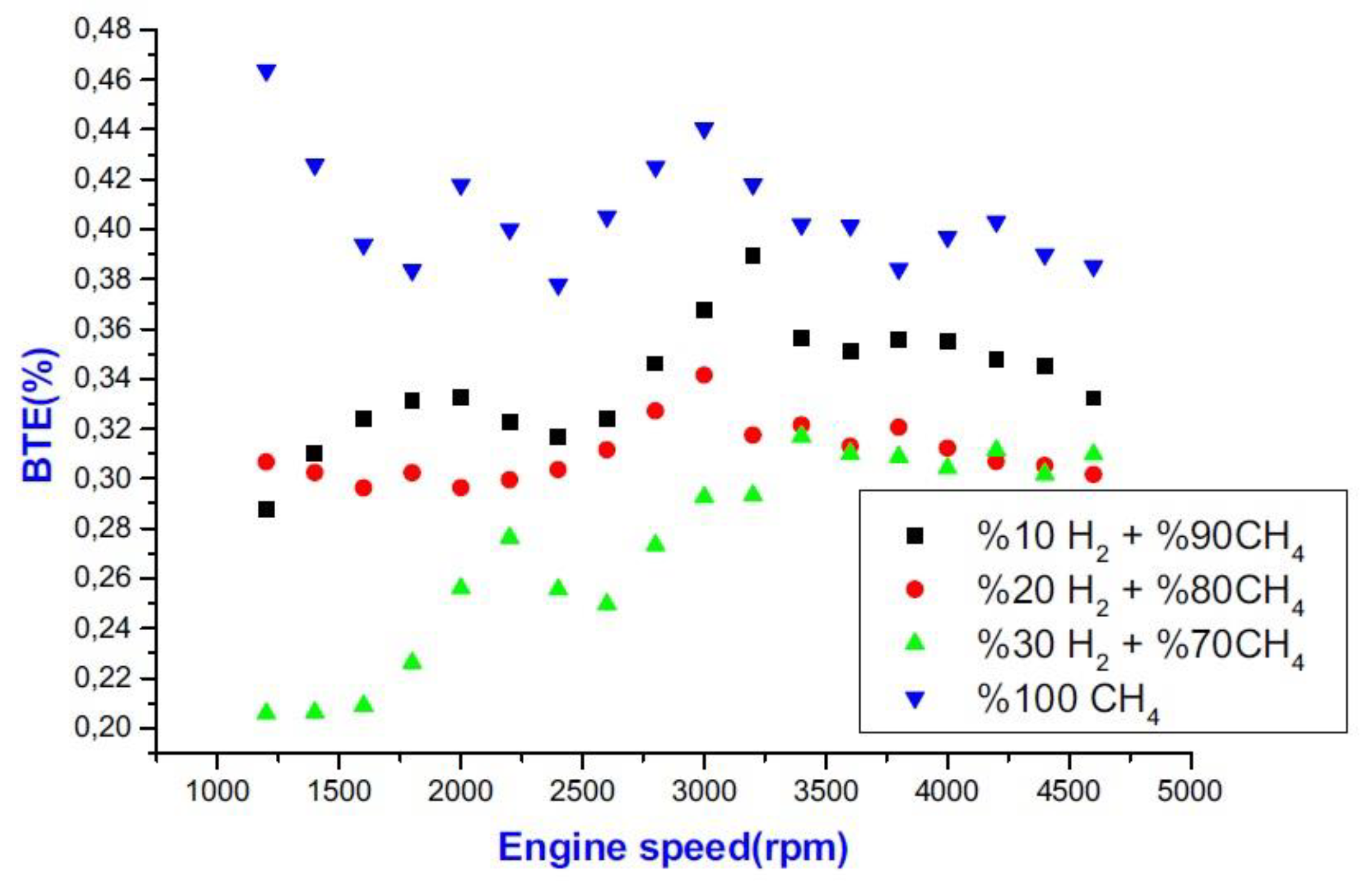
- Due to the high molecular thermal capacity of hydrogen and to the fact that with the addition of hydrogen in internal combustion engines, the progress of the combustion phase changes and reduces the combustion efficiency, in SI and CI engines the addition of hydrogen as a fuel decreases brake thermal efficiency (BTE).
- Hydrogen burns quickly and has a nine-fold faster flame speed in a diesel dual-fuel engine. The heat release rate rises as the load rises and hydrogen substitution rises. For this reason, adding hydrogen to diesel fuel in CI engines increases brake-specific fuel consumption (BSFC). By injecting hydrogen at varied ratios, the equivalent brake-specific fuel consumption (BSFC) increases compared to what observed when using gasoline due to the reduction in engine power (when hydrogen injection methods that lower engine power are utilized).
- Adding hydrogen to internal combustion engines reduces the amount of carbon monoxide emissions from CI and SI engines. Because hydrogen is not a hydrocarbon fuel since its molecule lacks carbon, increasing the hydrogen mass fraction in the fuel will lower the rate of hydrocarbon synthesis. Furthermore, a high hydrogen flame raises the cylinder’s pressure and improves combustion efficiency. Because of its high diffusion coefficient, pre-combustion hydrogen produces a more homogenous flammable mixture and improves oxygen availability. As a result of these factors, the amounts of carbon monoxide (CO) and unburned hydrocarbon (UHC) produced by internal combustion engines are lowered.
- When hydrogen is used as a fuel in internal combustion engines, the H/C rate rises, resulting in a decrease in combustion time and an improvement in combustion efficiency. On the other hand, because hydrogen is a clean fuel, it does not emit CO2, and its usage reduces CO2 emissions.
- Because of its features such as fast flame speed, low ignition energy required, and high adiabatic temperature, hydrogen is regarded as a suitable fuel for combustion. These features contribute to a rise in the temperature of the working fluid in the cylinder as well as to an increase in NOx.
- The high coefficient of hydrogen emission and the greater access of fuel to oxygen will increase the homogeneity of the flammable mixture and the amount of H/C in the total fuel, which will reduce the soot in diesel engines.
- With the usage of hydrogen in the majority of internal combustion engines, hazardous exhaust pollutants are reduced, and engines’ overall performance improves. When considering its environmental and economic benefits, hydrogen is a clean and sustainable energy source.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini, S.E.; Butler, B. An overview of development and challenges in hydrogen powered vehicles. Int. J. Green Energy 2020, 17, 13–37. [Google Scholar] [CrossRef] [Green Version]
- Shadidi, B.; Yusaf, T.; Alizadeh, H.H.A.; Ghobadian, B. Experimental investigation of the tractor engine performance using diesohol fuel. Appl. Energy 2014, 114, 874–879. [Google Scholar] [CrossRef]
- Short-Term Energy Outlook. Available online: https://www.eia.gov/outlooks/steo/report/global_oil.php (accessed on 7 July 2021).
- Demirbas, A. Biohydrogen; Springer Publishing Co: London, UK, 2009. [Google Scholar]
- Chang, X.; Ma, T.; Wu, R. Impact of urban development on residents’ public transportation travel energy consumption in China: An analysis of hydrogen fuel cell vehicles alternatives. Int. J. Hydrogen Energy 2019, 44, 16015–16027. [Google Scholar] [CrossRef]
- How Many Cars Are There in the World Today? Available online: https://www.rfidtires.com/how-many-cars-world.html (accessed on 24 October 2018).
- World Business Council for Sustainable Development (WBCSD). Mobility 2030: Meeting the Challenges to Sustainability. The Sustainable Mobility Project; World Business Council for Sustainable Development (WBCSD): Geneva, Switzerland, 2004. [Google Scholar]
- Ettefaghi, E.; Ghobadian, B.; Rashidi, A.; Najafi, G.; Khoshtaghaza, M.H.; Rashtchi, M. A novel bio-nano emulsion fuel based on biodegradable nanoparticles to improve diesel engines performance and reduce exhaust emissions. Renew. Energy 2018, 125, 64–72. [Google Scholar] [CrossRef]
- Najafi, G.; Ghobadian, B.; Yusaf, T.F.; Rahimi, H. Combustion analysis of a CI engine performance using waste cooking biodiesel fuel with an artificial neural network aid. Am. J. Appl. Sci. 2007, 4, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Yusaf, T.; Hamawand, I.; Baker, P.; Najafi, G. The effect of methanol-diesel blended ratio on ci engine performance. Int. J. Automot. Mech. Eng. 2013, 8, 1385–1395. [Google Scholar] [CrossRef]
- Das, L.M. 7—Hydrogen-fueled internal combustion engines. In Woodhead Publishing Series in Energy. Compendium of Hydrogen Energy; Barbir, F., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 177–217. [Google Scholar]
- Ali, O.M.; Mamat, R.; Najafi, G.; Yusaf, T.; Ardebili, S.M.S. Optimization of biodiesel-diesel blended fuel properties and engine performance with ether additive using statistical analysis and response surface methods. Energies 2015, 8, 14136–14150. [Google Scholar] [CrossRef] [Green Version]
- Yasin, M.H.M.; Mamat, R.; Najafi, G.; Ali, O.M.; Yusop, A.F.; Ali, M.H.J.R. Potentials of palm oil as new feedstock oil for a global alternative fuel: A review. Renew. Sustain. Energy Rev. 2017, 79, 1034–1049. [Google Scholar] [CrossRef] [Green Version]
- Awad, O.I.; Ali, O.M.; Mamat, R.; Abdullah, A.; Najafi, G.; Kamarulzaman, M. Using fusel oil as a blend in gasoline to improve SI engine efficiencies: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 69, 1232–1242. [Google Scholar] [CrossRef] [Green Version]
- Yazid, M.N.A.W.M.; Sidik, N.A.C.; Mamat, R.; Najafi, G.; Transfer, M. A review of the impact of preparation on stability of carbon nanotube nanofluids. Int. Comun. Heat Mass Transf. 2016, 78, 253–263. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A.; Ganjehkaviri, A. An overview of renewable hydrogen production from thermochemical process of oil palm solid waste in Malaysia. Energy Convers. Manag. 2015, 94, 415–429. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Shivaprasad, K.; Kumar, G.; Guruprasad, K. Performance, emission and fuel induction system of hydrogen fuel operated spark ignition engine—A review. Int. J. Mod. Eng. Res. 2012, 2, 565–571. [Google Scholar]
- Fayaz, H.; Saidur, R.; Razali, N.; Anuar, F.S.; Saleman, A.R.; Islam, M.R. An overview of hydrogen as a vehicle fuel. Int. J. Hydrogen Energy 2012, 16, 5511–5528. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Pagliaro, M.; Konstandopoulos, A.G. Solar Hydrogen: Fuel of the Future; Royal Society of Chemistry: Cambridge, UK, 2012. [Google Scholar]
- Balat, M.; Balat, M. Political, economic and environmental impacts of biomass-based hydrogen. Int. J. Hydrogen Energy 2010, 34, 3589–3603. [Google Scholar] [CrossRef]
- Wallington, T.J.; Lambert, C.K.; Ruona, W.C. Diesel vehicles and sustainable mobility in the U.S. Energy Pol. 2013, 54, 47–53. [Google Scholar] [CrossRef]
- Mwangi, J.K.; Lee, W.-J.; Chang, Y.-C.; Chen, C.-Y.; Wang, L.-C. An overview: Energy saving and pollution reduction by using green fuel blends in diesel engines. Appl. Energy 2015, 159, 214–236. [Google Scholar] [CrossRef]
- Gill, S.S.; Tsolakis, A.; Herreros, J.M.; York, A.P.E. Diesel emissions improvements through the use of biodiesel or oxygenated blending components. Fuel 2012, 95, 578–586. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shahd, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Reitz, R.D. Directions in internal combustion engine research. Combust Flame 2013, 160, 1–8. [Google Scholar] [CrossRef]
- White, C.M.; Steeper, R.R.; Lutz, A.E. The hydrogen-fueled internal combustion engine: A technical review. Int. J. Hydrogen Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- Kahraman, E.; Ozcanlı, S.C.; Ozerdem, B. An experimental study on performance and emission characteristics of a hydrogen fuelled spark ignition engine. Int. J. Hydrogen Energy 2007, 32, 2066–2072. [Google Scholar] [CrossRef] [Green Version]
- Jingding, L.; Linsong, G.; Tianshen, D. Formation and restraint of toxic emissions in hydrogen-gasoline mixture fueled engines. Int. J. Hydrogen Energy 1998, 23, 971–975. [Google Scholar] [CrossRef]
- King, R.; Rand, M. The hydrogen engine. Can. J. Technol. 1955, 33, 44569. [Google Scholar] [CrossRef]
- Ji, C.; Wang, S. Effect of hydrogen addition on combustion and emissions performance of a spark ignition gasoline engine at lean conditions. Int. J. Hydrogen Energy 2009, 34, 7823–7834. [Google Scholar] [CrossRef]
- Das, L.M. Hydrogeneoxygen reaction mechanism and its implication to hydrogen engine combustion. Int. J. Hydrogen Energy 1996, 21, 703–715. [Google Scholar] [CrossRef]
- Andrea, T.D.; Henshaw, P.F.; Ting, D.S.K. The addition of hydrogen to a gasoline-fuelled SI engine. Int. J. Hydrogen Energy 2004, 29, 1541–1552. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill Book Co: New York, NY, USA, 1988. [Google Scholar]
- Padiyar, S. Properties of hydrogen. In Proceedings of Summer School of Hydrogen Energy; IIT Madras: Chennai, India, 1985. [Google Scholar]
- Li, H.; Karim, G.A. An Experimental Investigation of S.I. Engine operation on gaseous fuels lean mixtures. SAE Trans. 2005, 114, 1600–1608. [Google Scholar]
- Duan, J.; Liu, F.; Sun, B. Backfire control and power enhancement of a hydrogen internal combustion engine. Int. J. Hydrogen Energy 2014, 39, 4581–4589. [Google Scholar] [CrossRef]
- Ji, C.; Wang, S. Effect of hydrogen addition on the idle performance of a spark ignited gasoline engine at stoichiometric condition. Int. J. Hydrogen Energy 2009, 34, 3546–3556. [Google Scholar] [CrossRef]
- Salek, F.; Zamen, M.; Hosseini, S.V. Experimental study, energy assessment and improvement of hydroxyl generator coupled with a gasoline engine. Energy Rep. 2020, 6, 146–156. [Google Scholar] [CrossRef]
- Salek, F.; Zamen, M.; Hosseini, S.V.; Babaie, M. Novel hybrid system of pulsed HHO generator/TEG waste heat recovery for CO reduction of a gasoline engine. Int. J. Hydrogen Energy 2020, 45, 23576–23586. [Google Scholar] [CrossRef]
- Faizal, M.; Chuah, L.; Lee, C.; Hameed, A.; Lee, J.; Shankar, M. Review of hydrogen fuel for internal combustion engines. J. Mech. Eng. Res. Dev. 2019, 42, 35–46. [Google Scholar]
- Kojima, Y.; Kawai, Y.; Kimbara, M.; Nakanishi, H.; Matsumoto, S. Hydrogen Generation by Hydrolysis Reaction of Lithium Borohydride. Int. J. Hydrogen Energy 2004, 29, 1213–1217. [Google Scholar] [CrossRef]
- Estimating the Carbon Footprint of Hydrogen Production. Available online: https://www.forbes.com/sites/scottmendelson (accessed on 21 August 2020).
- Hydrogen Production: Natural Gas Reforming. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming (accessed on 3 August 2021).
- Hydrogen Production: Electrolysis. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis (accessed on 8 August 2021).
- Ozturk, M.; Dincer, I. A comprehensive review on power-to-gas with hydrogen options for cleaner applications. Int. J. Hydrogen Energy 2021, 46, 31511–31522. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. Assessment and optimization of an integrated wind power system for hydrogen and methane production. Energy Convers. Manag. 2018, 177, 693–703. [Google Scholar] [CrossRef]
- Explainer: How do We Make Hydrogen from Coal, and Is It Really a Clean Fuel? Available online: https://theconversation.com/explainer-how-do-we-make-hydrogen-from-coal-and-is-it-really-a-clean-fuel94911#:~:text=To%20produce%20hydrogen%20from%20coal,heat%20for%20the%20gasification%0reaction (accessed on 13 April 2018).
- Hydrogen Production: Biomass Gasification. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-biomass-gasification (accessed on 5 August 2021).
- Hydrogen Production: Biomass-Derived Liquid Reforming. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-biomass-derived-liquid-reforming (accessed on 22 August 2021).
- Safari, F.; Norouzi, O.; Tavasoli, A. Hydrothermal gasification of Cladophora glomerata macroalgae over its hydrochar as a catalyst for hydrogen-rich gas production. Bioresour. Technol. 2016, 222, 232–241. [Google Scholar] [CrossRef]
- Safari, F.; Javani, N.; Yumurtaci, Z. Hydrogen production via supercritical water gasification of almond shell over algal and agricultural hydrochars as catalysts. Int. J. Hydrogen Energy 2018, 43, 1071–1080. [Google Scholar] [CrossRef]
- Hydrogen Production: Microbial Biomass Conversion. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-microbial-biomass-conversion (accessed on 17 August 2021).
- Dincer, I.; Zamfirescu, C. Sustainable Hydrogen Production; Elsevier: Amesterdam, NL, USA, 2017. [Google Scholar]
- Hoskins, A.L.; Millican, S.L.; Czernik, C.E. Continuous on-sun solar thermochemical hydrogen production via an isothermal redox cycle. Appl. Energy 2019, 249, 368–376. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A study on the Fe–Cl thermochemical water splitting cycle for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 18867–18875. [Google Scholar] [CrossRef]
- Tsujimura, T.; Mikami, S.; Achicha, N.; Tokunaga, Y.; Senda, J.; Fujimoto, H. A study of direct injection diesel engine fueled with hydrogen. SAE Trans. 2003, 112, 390–405. [Google Scholar]
- Dipioglu, I. Investigation of Production of Hydrogen on Vehicle and Usage of Internal Combustion Engines with Petroleum Origin Fuels. Master’s Thesis, G.U., Turkey, 1998. [Google Scholar]
- Murcak, A. Experimental Analysis of the Effect of Hydrogen Fuel on Diesel Engine Performance and Exhaust Emissions. Master’s Thesis, Automotive Department, S.U., Turkey, 2003. [Google Scholar]
- Birsen, E.B. Use of Hydrogen Fuel in Internal Combustion Engines. Master’s Thesis, Natural Sciences Institute, Erciyes University, Kayseri, Turkey, 2008. [Google Scholar]
- Akal, D.; Öztuna, S.; Büyükakın, M.K. A review of hydrogen usage in internal combustion engines (gasoline-Lpg-diesel) from combustion performance aspect. Int. J. Hydrogen Energy 2020, 45, 35257–35268. [Google Scholar] [CrossRef]
- Overend, E. Hydrogen Combustion Engines. Master’s Thesis, The University of Edinburgh, Scotland, UK, 1999. [Google Scholar]
- Gadallah, H.A.; Elshenawy, A.E.; Elzahaby, M.A.; El-Salmawy, A.H.; Bawady, H.A. Effect of direct water injection on performance and emissions of a hydrogen fuelled direct injection engine. In Proceedings of the Ecologic Vehicles and Renewable Energies Monaco 2009, Monaco, France, 26–29 March 2009; pp. 178–191. [Google Scholar]
- Shudo, T.; Omori, K.; Hiyama, O. NOx reduction and NO2 emission characteristics in rich-lean combustion of hydrogen. Int. J. Hydrogen Energy 2008, 33, 4689–4693. [Google Scholar] [CrossRef] [Green Version]
- Kose, H. Experimental Investigation of the Effect of Hydrogen Addition in Dual Fuel Mode on Engine Performance and Emission. Master’s Thesis, Natural Sciences Institute, Selçuk University, Konya, Turkey, 2012. [Google Scholar]
- Saravanan, N.; Nagarajan, G. Performance and emission studies on port injection of hydrogen with varied flow rates with Diesel as an ignition source. Appl. Energy 2010, 87, 2218–2229. [Google Scholar] [CrossRef]
- Chitragar, P.R.; Shivaprasad, K.V.; Kumar, G.N. Use of Hydrogen in Internal Combustion Engines: A Comprehensive Study. J. Mech. Eng. Biomech. 2016, 1, 84–96. [Google Scholar]
- Das, M.L. Hydrogen engine: Research and development (R&D) programmers in Indian Institute of Technology (IIT), Delhi. Int. J. Hydrogen Energy 2002, 27, 953–965. [Google Scholar]
- Ciniviz, M.; Hüseyin, K. Hydrogen use in internal combustion engine: A review. Int. J. Automot. Eng. Technol. 2012, 1, 1–15. [Google Scholar]
- Elsemary, I.M.M.; Attia, A.A.A.; Elnagar, K.H.; Elaraqy, A.A.M. Experimental investigation on performance of single cylinder spark ignition engine fueled with hydrogen-gasoline mixture. Appl. Therm. Eng. 2016, 106, 850–854. [Google Scholar] [CrossRef]
- Sohret, Y.; Gurbuz, H.; Akcay, I.H. Energy and exergy analyses of a hydrogen fueled SI engine: Effect of ignition timing and compression ratio. Energy 2019, 175, 410–422. [Google Scholar] [CrossRef]
- Wang, S.; Ji, C.; Zhang, B.; Liu, X. Lean burn performance of a hydrogen-blended gasoline engine at the wide open throttle condition. Appl. Energy 2014, 136, 43–50. [Google Scholar] [CrossRef]
- Yu, X.; Li, G.; Du, Y.; Guo, Z.; Shang, Z.; He, F.; Shen, Q.; Li, D.; Li, Y. A comparative study on effects of homogeneous or stratified hydrogen on combustion and emissions of a gasoline/hydrogen SI engine. Int. J. Hydrogen Energy 2019, 44, 25974–25984. [Google Scholar] [CrossRef]
- Acikgoz, B.; Celik, C. An experimental study on performance and emission characteristics of a methane–hydrogen fuelled gasoline engine. Int. J. Hydrogen Energy 2012, 37, 18492–18497. [Google Scholar] [CrossRef]
- Gong, C.; Li, Z.; Yi, L.; Liu, F. Comparative study on combustion and emissions between methanol port-injection engine and methanol direct-injection engine with H2-enriched portinjection under lean-burn conditions. Energy Convers. Manag. 2019, 200, 112096. [Google Scholar] [CrossRef]
- Kak, A.; Kumar, N.; Singh, B.; Singh, S.; Gupta, D. Comparative study of emissions and performance of hydrogen boosted SI engine powered by gasoline methanol blend and gasoline ethanol blend. SAE Technol. Pap. 2015, 10, 1677–1776. [Google Scholar]
- Karagöz, Y.; Sandalcı, T.; Yüksek, L.; Dalkılıç, A.S.; Wongwises, S. Effect of hydrogen–diesel dual-fuel usage on performance, emissions and diesel combustion in diesel engines. Adv. Mech. Eng. 2016, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nuthan Prasad, B.S.; Pandey, J.K.; Kumar, G.N. Effect of hydrogen enrichment on performance, combustion, and emission of a methanol fueled SI engine. Int. J. Hydrogen Energy 2021, 46, 25294–25307. [Google Scholar] [CrossRef]
- Yamin, J.A.; Hamdan, M.A. The performance of hydrogen-powered 4-stroke SI engine using locally designed fuel regulator. J. Braz. Soc. Mech. Sci. Eng. 2010, 32, 195–199. [Google Scholar] [CrossRef]
- Christodoulou, F.; Megaritis, A. Experimental investigation of the effects of simultaneous hydrogen and nitrogen addition on the emissions and combustion of a diesel engine. Int. J. Hydrogen Energy 2014, 39, 2692–2702. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Pournazeri, S.; Princevac, M.; Miller, J.W.; Mahalingam, S.; Khan, M.Y.; Jayaram, V.; Welch, A.W. Effect of hydrogen addition on criteria and greenhouse gas emissions for a marine diesel engine. Int. J. Hydrogen Energy 2014, 39, 11336–11345. [Google Scholar] [CrossRef] [Green Version]
- Salvi, B.L.; Subramanian, K.A. Experimental investigation on effects of compression ratio and exhaust gas recirculation on backfire, performance and emission characteristics in a hydrogen fuelled spark ignition engine. Int. J. Hydrogen Energy 2016, 41, 5842–5855. [Google Scholar] [CrossRef]
- Vorst, W.; Finegold, J.G. Automotive hydrogen engines, and Onboard storage Methods. In Hydrogen Energy Fundamentals; Sun-Sentinel: Miami Beach, FL, USA, 1975. [Google Scholar]
- Koten, H. Hydrogen effects on the diesel engine performance and emissions. Int. J. Hydrogen Energy 2018, 43, 10511–10519. [Google Scholar] [CrossRef]
- Masood, M.; Ishrat, M.M.; Reddy, A.S. Computational combustion and emission analysis of hydrogen–diesel blends with experimental verification. Int. J. Hydrogen Energy 2007, 32, 2539–2547. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, L.; Han, J.; Chen, X. Investigating the effect of increasing specific heat and the influence of charge cooling of water injection in a TGDI engine. Appl. Therm. Eng. 2019, 149, 1105–1113. [Google Scholar] [CrossRef]
- Elsemary, I.M.; Attia, A.A.; Elnagar, K.H.; Elsaleh, M.S. Spark timing effect on performance of gasoline engine fueled with mixture of hydrogen gasoline. Int. J. Hydrogen Energy 2017, 42, 30813–30820. [Google Scholar] [CrossRef]
- Wang, J.; Duan, X.; Liu, Y.; Wang, W.; Liu, J.; Lai, M.-C. Numerical investigation of water injection quantity and water injection timing on the thermodynamics, combustion and emissions in a hydrogen enriched lean burn natural gas SI engine. Int. J. Hydrogen Energy 2020, 45, 17935–17952. [Google Scholar] [CrossRef]
- Xu, P.; Ji, C.; Wang, S.; Cong, X.; Ma, Z.; Tang, C. Effects of direct water injection on engine performance in engine fueled with hydrogen at varied excess air ratios and spark timing. Fuel 2020, 269, 117209. [Google Scholar] [CrossRef]
- Li, A.; Zheng, Z.; Peng, T. Effect of water injection on the knock, combustion, and emissions of a direct injection gasoline engine. Fuel 2020, 268, 117376. [Google Scholar] [CrossRef]
- Vinod, S.Y.; Soni, S.L.; Sharma, D. Performance and emission studies of direct injection C.I. engine in duel fuel mode (hydrogen-diesel) with EGR. Int. J. Hydrogen Energy 2012, 36, 3807–3817. [Google Scholar]
- Akar, M.A.; Kekilli, E.; Bas, O.; Yildizhan, S.; Serin, H.; Ozcanli, M. Hydrogen enriched waste oil biodiesel usage in compression ignition engine. Int. J. Hydrogen Energy 2018, 43, 18046–18052. [Google Scholar] [CrossRef]
- Rocha, H.M.Z.; Pereira, R.S.; Nogueira, M.F.M.; Belchior, C.R.P.; Tostes, M.E.L. Experimental investigation of hydrogen addition in the intake air of compressed ignition engines running on biodiesel blend. Int. J. Hydrogen Energy 2017, 42, 4530–4539. [Google Scholar] [CrossRef]
- Deheri, C.; Acharya, S.K.; Thatoi, D.N.; Mohanty, A.P. A review on performance of biogas and hydrogen on diesel engine in dual fuel mode. Fuel 2020, 260, 116337. [Google Scholar] [CrossRef]
- Salek, F.; Babaie, M.; Hosseini, S.V.; Anwar Bég, O. Multi-objective optimization of the engine performance and emissions for a hydrogen/gasoline dual-fuel engine equipped with the port water injection system. Int. J. Hydrogen Energy 2021, 46, 10535–10547. [Google Scholar] [CrossRef]
- Bari, S.; Esmaeil, M.M. Effect of H2/O2 addition in increasing the thermal efficiency of a diesel engine. Fuel 2010, 89, 378–383. [Google Scholar] [CrossRef]
- Jamrozik, A.; Grab-Rogaliński, K.; Tutak, W. Hydrogen effects on combustion stability, performance and emission of diesel engine. Int. J. Hydrogen Energy 2020, 45, 19936–19947. [Google Scholar] [CrossRef]
- Ghazal, O.H. Performance and combustion characteristic of CI engine fueled with hydrogen enriched diesel. Int. J. Hydrogen Energy 2013, 38, 15469–15476. [Google Scholar] [CrossRef]
- Yilmaz, I.; Tastan, M. Investigation of hydrogen addition to methanol-gasoline blends in an SI engine. Int. J. Hydrogen Energy 2018, 43, 20252–20261. [Google Scholar] [CrossRef]
- Lanz, A.; Heffel, J.; Messer, C. Hydrogen Fuel Cell Engines and Related Technologies; Energy Technology Training Center, College of the Desert: Palm Desert, CA, USA, 2001. [Google Scholar]
- Shivaprasad, K.V.; Chitragar, P.R.; Kumar, G.N. Effect of Hydrogen Addition on Combustion and Emission Characteristics of High Speed Spark Ignition Engine—An Experimental Study. SAE Technol. Pap. 2015, 14, 1684–1693. [Google Scholar]
- Shirk Matthew, G.; McGuire Thomas, P.; Neal Gary, L.; Haworth Daniel, C. Investigation of a H2-assisted combustion system for a light-duty diesel vehicle. Int. J. Hydrogen Energy 2008, 33, 7237–7244. [Google Scholar] [CrossRef]
- Szwaja, S.; Grab-Rogalinski, K. Hydrogen combustion in a compression ignition diesel engine. Int. J. Hydrogen Energy 2009, 34, 4413–4421. [Google Scholar] [CrossRef]






| Properties | Hydrogen | Gasoline | Diesel |
|---|---|---|---|
| Carbohydrate content (mass percent) | 0 | 84 | 86 |
| Molecular mass | 2.015 | 110 | 170 |
| A/F stoichiometric ratio | 34.3 | 14.6 | 17 |
| Temperature of ignition (K) | 858 | 530 | - |
| Temperature of adiabatic flame (K) | 2384 | 2270 | 2300 |
| 293 K (cm/s) flame speed | 237 | 41.5 | - |
| Flammability limits (vol percent in air) | 4.1–75 | 1.5–7.6 | 0.6–5.5 |
| Quenching the gap (cm) | 0.06 | 0.2 | - |
| Lower heating value per kilogram (MJ/kg) | 120 | 44 | - |
| Diffusion coefficient (cm2/s) under stoichiometric conditions | 0.61 | 0.05 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shadidi, B.; Najafi, G.; Yusaf, T. A Review of Hydrogen as a Fuel in Internal Combustion Engines. Energies 2021, 14, 6209. https://doi.org/10.3390/en14196209
Shadidi B, Najafi G, Yusaf T. A Review of Hydrogen as a Fuel in Internal Combustion Engines. Energies. 2021; 14(19):6209. https://doi.org/10.3390/en14196209
Chicago/Turabian StyleShadidi, Behdad, Gholamhassan Najafi, and Talal Yusaf. 2021. "A Review of Hydrogen as a Fuel in Internal Combustion Engines" Energies 14, no. 19: 6209. https://doi.org/10.3390/en14196209






