Modeling and Optimization of Microwave-Based Bio-Jet Fuel from Coconut Oil: Investigation of Response Surface Methodology (RSM) and Artificial Neural Network Methodology (ANN)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
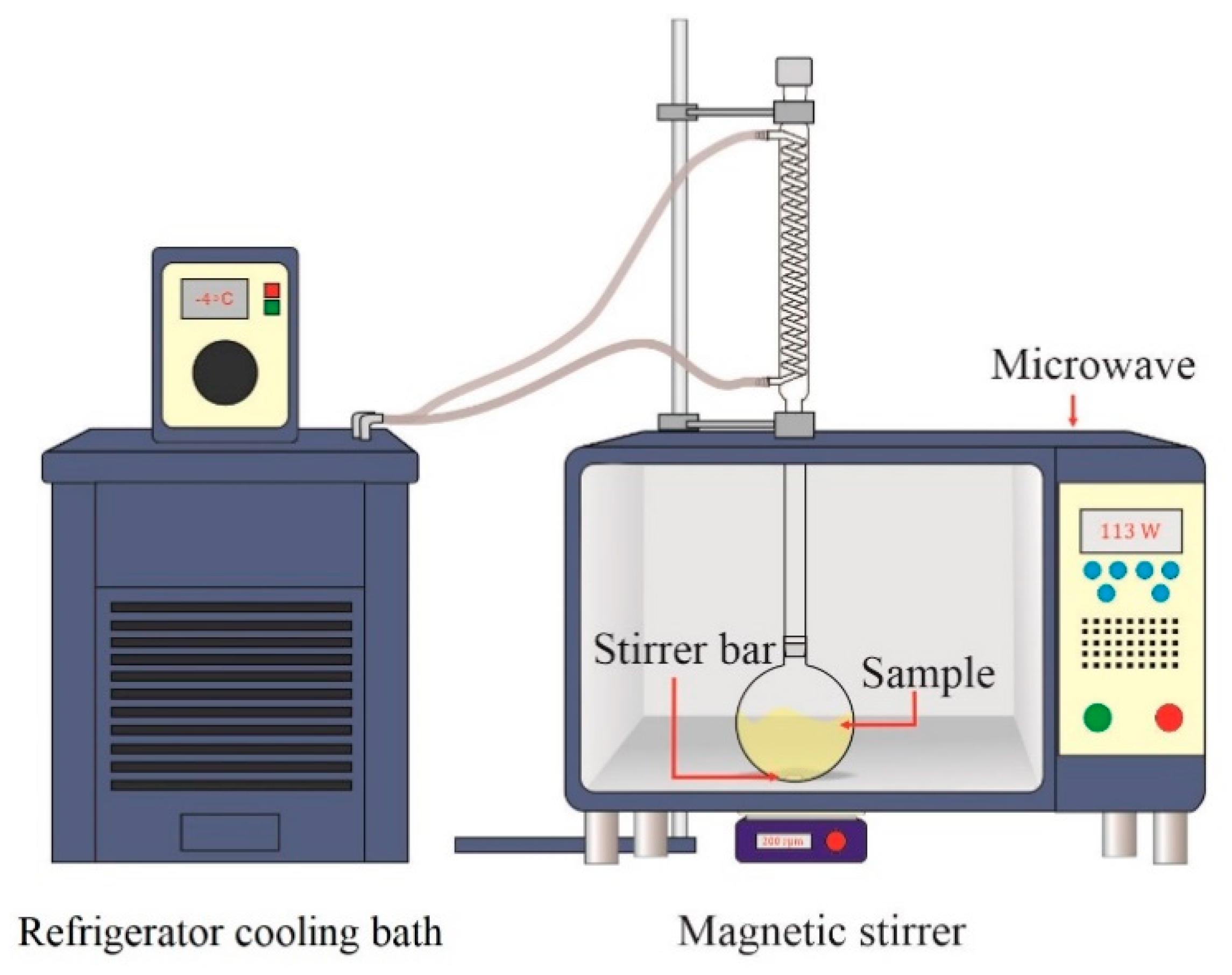
2.2. Microwave-Assisted Catalytic Transesterification
2.3. Distillation
2.4. Response Surface Methodology (RSM)
2.5. Artificial Neural Network (ANN)
2.6. Ant Colony Optimization (ACO)
2.7. Statistical Evaluation of the Developed Models
2.8. Sensitivity Analysis
2.9. Optimization of Transesterification Process Variables
2.10. Bio-Jet Fuel Properties
3. Results
3.1. RSM
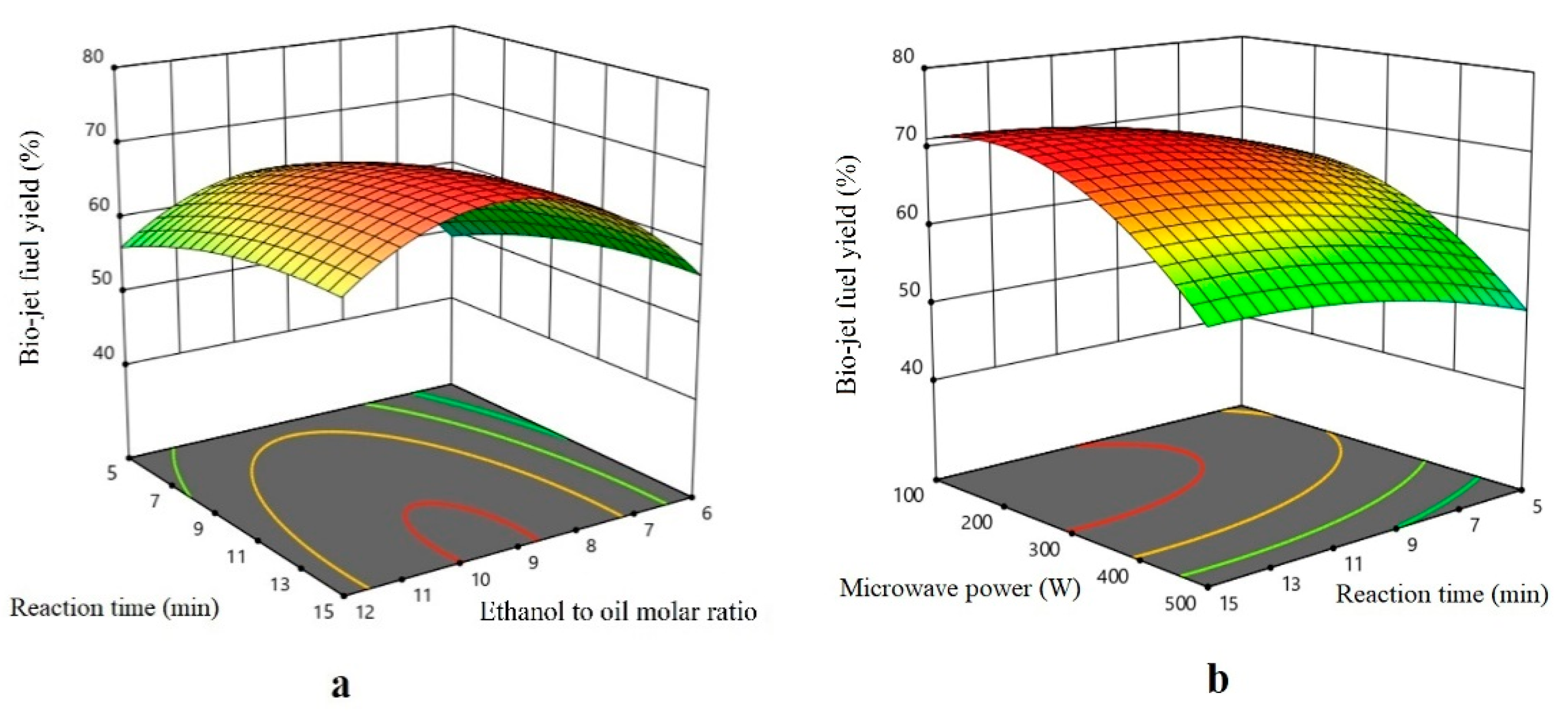
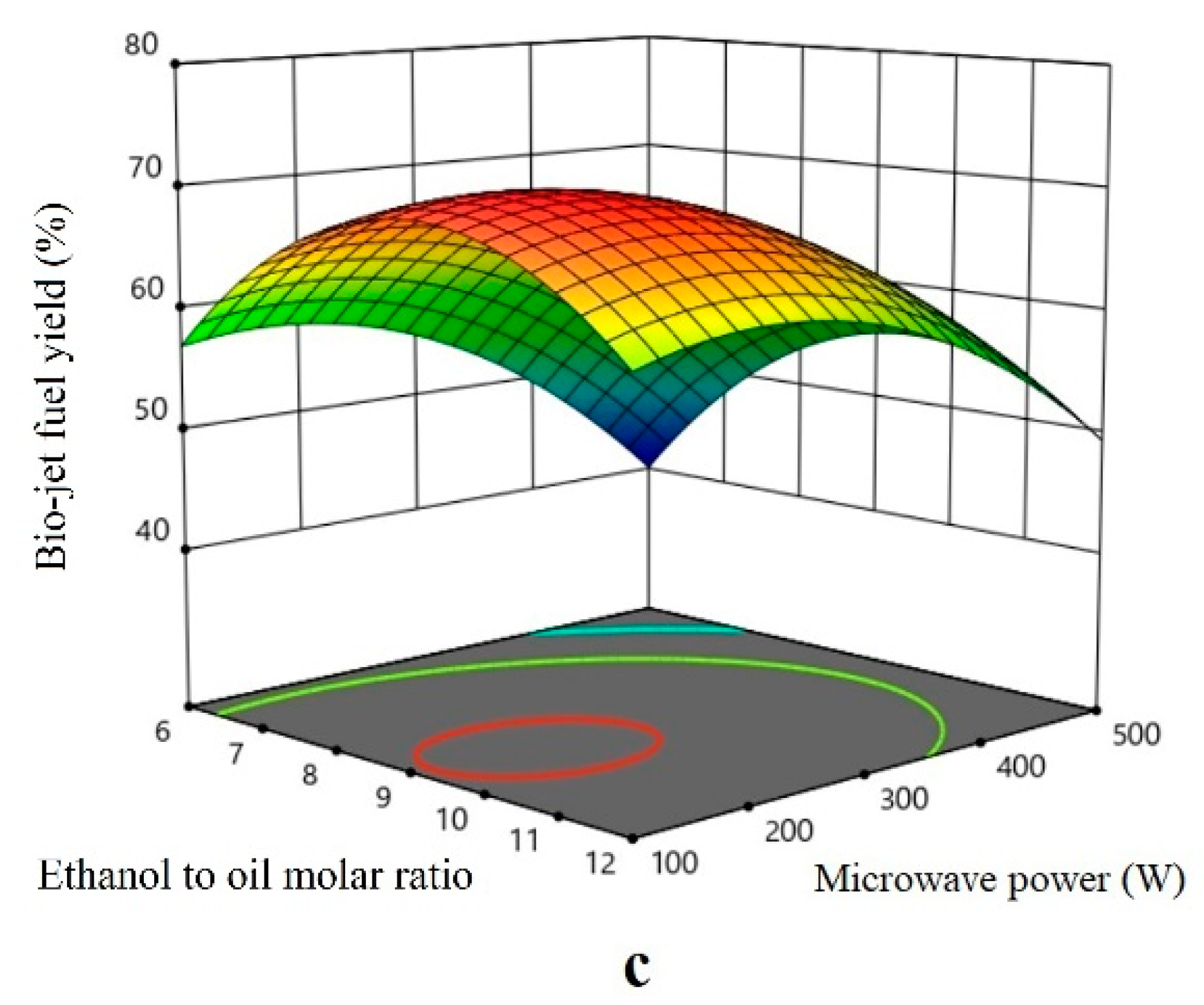
Effect of the Parameter
Effect of the Ethanol to Oil Molar Ratio
Effect of the Reaction Time
Effect of the Microwave Power
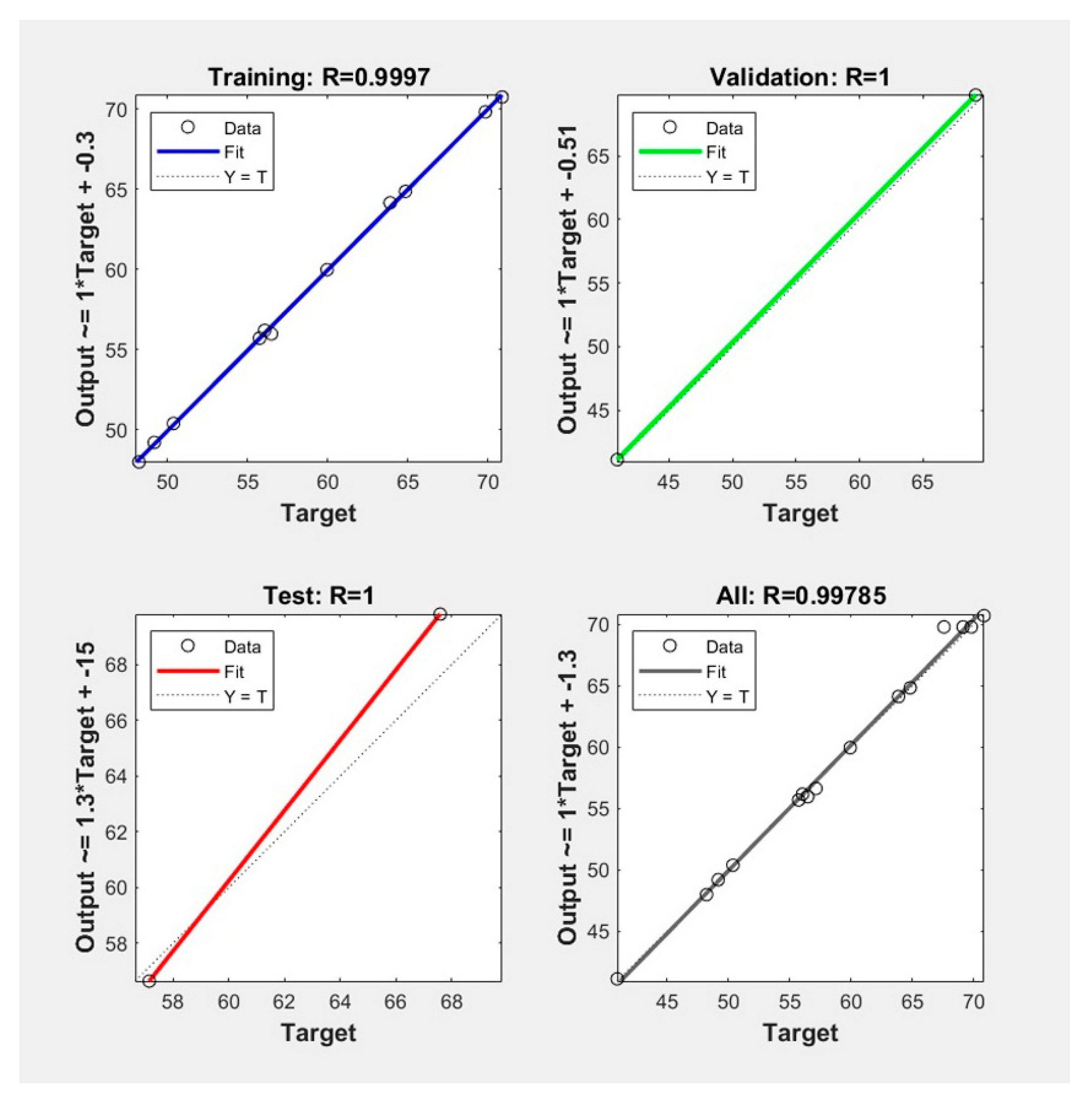
3.2. Analysis of the Developed ANN Model
3.3. Comparison of the Predictive Capability of Models
3.4. Sensitivity Analysis Results of the Input Variables on the Developed Models
3.5. Optimization of the Process Variables for Bio-Jet Fuel
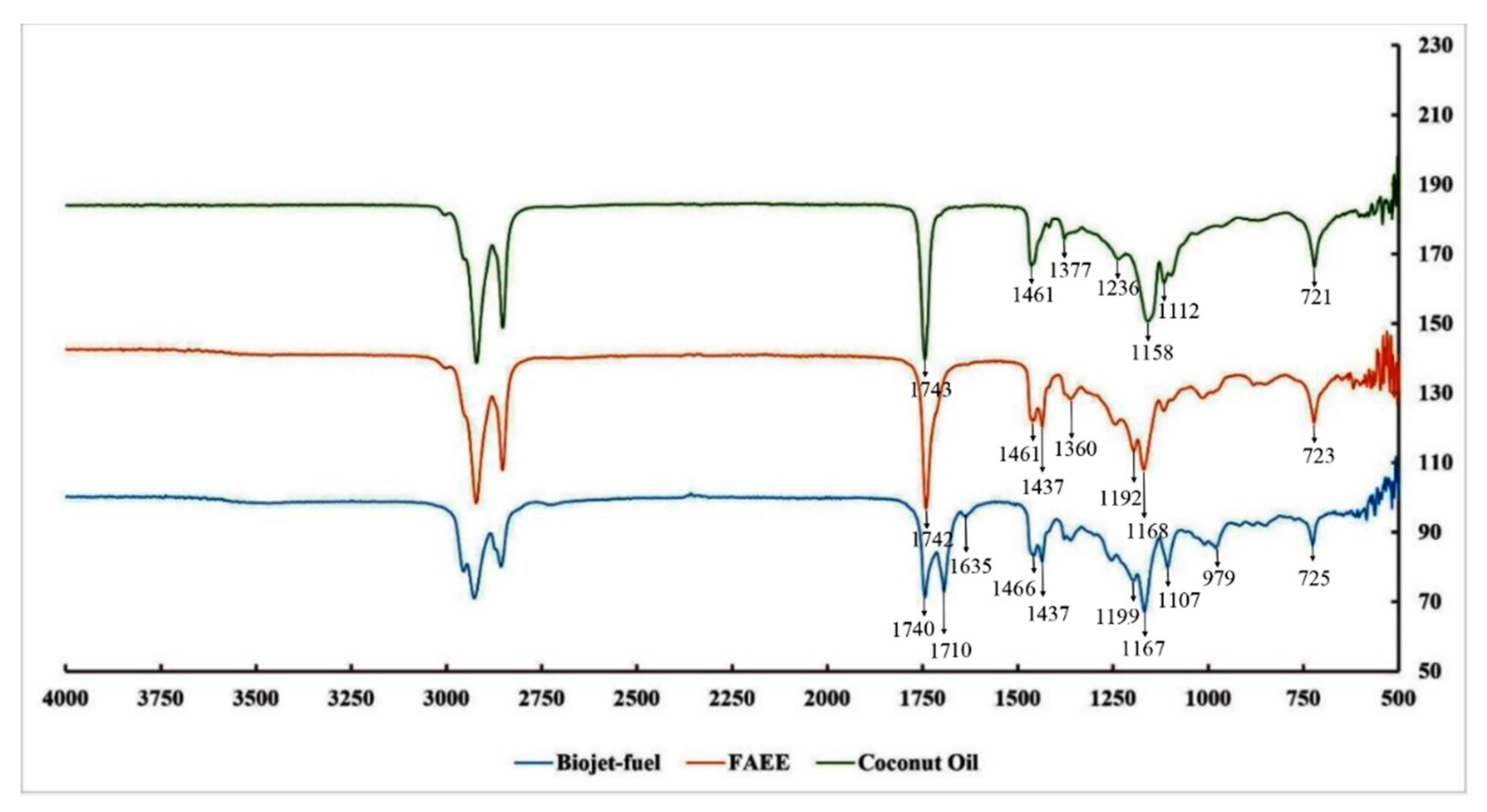
3.6. FTIR
3.7. Bio-Jet Fuel Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malaysiakini Ten Years Before Known Oil, Gas Reserves Run Dry 2019. Available online: https://www.malaysiakini.com/news/467781 (accessed on 24 June 2020).
- Silitonga, A.S.S.; Masjuki, H.H.H.; Ong, H.C.; Sebayang, A.H.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.M.I.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Holtsmark, N.; Agheb, E.; Molinas, M.; Høidalen, H.K. High frequency wind energy conversion system for offshore DC collection grid—Part II: Efficiency improvements. Sustain. Energy Grids Netw. 2016, 5, 177–185. [Google Scholar] [CrossRef]
- Heriche, H.; Rouabah, Z.; Bouarissa, N. High-efficiency CIGS solar cells with optimization of layers thickness and doping. Opt. Int. J. Light Electron. Opt. 2016, 127, 11751–11757. [Google Scholar] [CrossRef]
- Saifuddin, N.; Ong, M.Y.; Priatharsini, P. Optimization of photosynthetic hydrogen gas production by green alga in sulfur deprived condition. Indian J. Sci. Technol. 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Abdul Latif, N.I.S.; Ong, M.Y.; Nomanbhay, S. Hydrothermal liquefaction of Malaysia’s algal biomass for high-quality bio-oil production. Eng. Life Sci. 2019, 19, 246–269. [Google Scholar] [CrossRef] [Green Version]
- Mazaheri, H.; Ong, H.C.; Masjuki, H.H.; Amini, Z.; Harrison, M.D.; Wang, C.-T.; Kusumo, F.; Alwi, A. Rice bran oil based biodiesel production using calcium oxide catalyst derived from Chicoreus brunneus shell. Energy 2018, 144, 10–19. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Salman, B.; Ong, M.Y.; Nomanbhay, S.; Salema, A.A.; Sankaran, R.; Show, P.L. Thermal analysis of nigerian oil palm biomass with sachet-water plasticwastes for sustainable production of biofuel. Processes 2019, 7, 475. [Google Scholar] [CrossRef] [Green Version]
- Mohd Mokhta, Z.; Ong, M.Y.; Salman, B.; Nomanbhay, S.; Salleh, S.F.; Chew, K.W.; Show, P.L.; Chen, W.H. Simulation studies on microwave-assisted pyrolysis of biomass for bioenergy production with special attention on waveguide number and location. Energy 2020, 190, 116474. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Fattah, I.M.R.; Maitra, S.; Mahlia, T.M.I. A ranking scheme for biodiesel underpinned by critical physicochemical properties. Energy Convers. Manag. 2021, 229, 113742. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Leong, K.Y. Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers. Manag. 2014, 81, 30–40. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Hassan, M.H.; Ong, H.C.; Kusumo, F. Analysis of the performance, emission and combustion characteristics of a turbocharged diesel engine fuelled with Jatropha curcas biodiesel-diesel blends using kernel-based extreme learning machine. Environ. Sci. Pollut. Res. 2017, 24, 25383–25405. [Google Scholar] [CrossRef] [PubMed]
- IEA. Electricity Information 2019; OECD: Paris, France, 2019. [Google Scholar]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Shamsuddin, A.H.; Wang, C.T.; Indra Mahlia, T.M.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Gholami, A.; Pourfayaz, F.; Maleki, A. Recent Advances of Biodiesel Production Using Ionic Liquids Supported on Nanoporous Materials as Catalysts: A Review. Front. Energy Res. 2020, 8, 144. [Google Scholar] [CrossRef]
- ATAG. Aviation Benefits beyond Borders; Air Transport Action Group: Geneva, Switzerland, 2018. [Google Scholar]
- Jeff, O. Fact Sheet: The Growth in Greenhouse Gas Emissions from Commercial Aviation; Environmental and Energy Study Institute: Washington, DC, USA, 2019. [Google Scholar]
- Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; de Lira-Flores, J.A.; Hernández, S. A review on the production processes of renewable jet fuel. Renew. Sustain. Energy Rev. 2017, 79, 709–729. [Google Scholar] [CrossRef]
- Llamas, A.; García-Martínez, M.J.; Al-Lal, A.M.; Canoira, L.; Lapuerta, M. Biokerosene from coconut and palm kernel oils: Production and properties of their blends with fossil kerosene. Fuel 2012, 102, 483–490. [Google Scholar] [CrossRef]
- Wang, W.C.; Tao, L. Bio-jet fuel conversion technologies. Renew. Sustain. Energy Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Chen, M.; Fang, Y.; Tan, T. Highly efficient conversion of plant oil to bio-aviation fuel and valuable chemicals by combination of enzymatic transesterification, olefin cross-metathesis, and hydrotreating. Biotechnol. Biofuels 2018, 11, 30. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y. A Review of Microwave-Assisted Reactions for Biodiesel Production. Bioengineering 2017, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Yusoff, M.F.M.; Xu, X.; Guo, Z. Comparison of fatty acid methyl and ethyl esters as biodiesel base stock: A review on processing and production requirements. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 525–531. [Google Scholar] [CrossRef]
- Mendow, G.; Veizaga, N.S.; Sánchez, B.S.; Querini, C.A. Biodiesel production by two-stage transesterification with ethanol. Bioresour. Technol. 2011, 102, 10407–10413. [Google Scholar] [CrossRef]
- Kishi, A.; Shironita, S.; Umeda, M. H2O2 detection analysis of oxygen reduction reaction on cathode and anode catalysts for polymer electrolyte fuel cells. J. Power Sources 2012, 197, 88–92. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Rodríguez, J.J.; Tejedor, A. Biodiesel fuels from vegetable oils: Transesterification of Cynara cardunculus L. Oils with ethanol. Energy Fuels 2002, 16, 443–450. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimisation. Fuel 2016, 180, 164–174. [Google Scholar] [CrossRef]
- Dev, S.R.S.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. FDTD modeling and simulation of microwave heating of in-shell eggs. Prog. Electromagn. Res. M 2010, 13, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Ong, M.Y.; Nomanbhay, S. Design and Modeling of an Enhanced Microwave Reactor for Biodiesel Production. Int. J. Sci. Res. Publ. 2018, 8, 527–534. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Patil, P.D.; Reddy, H.; Muppaneni, T.; Ponnusamy, S.; Cooke, P.; Schuab, T.; Deng, S. Microwave-mediated non-catalytic transesterification of algal biomass under supercritical ethanol conditions. J. Supercrit. Fluids 2013, 79, 67–72. [Google Scholar] [CrossRef]
- Silitonga, A.; Mahlia, T.; Shamsuddin, A.; Ong, H.; Milano, J.; Kusumo, F.; Sebayang, A.; Dharma, S.; Ibrahim, H.; Husin, H.; et al. Optimization of Cerbera manghas Biodiesel Production Using Artificial Neural Networks Integrated with Ant Colony Optimization. Energies 2019, 12, 3811. [Google Scholar] [CrossRef] [Green Version]
- Oloko-Oba, M.I.; Taiwo, A.E.; Ajala, S.O.; Solomon, B.O.; Betiku, E. Performance evaluation of three different-shaped bio-digesters for biogas production and optimization by artificial neural network integrated with genetic algorithm. Sustain. Energy Technol. Assess. 2018, 26, 116–124. [Google Scholar] [CrossRef]
- Ishola, N.B.; Okeleye, A.A.; Osunleke, A.S.; Betiku, E. Process modeling and optimization of sorrel biodiesel synthesis using barium hydroxide as a base heterogeneous catalyst: Appraisal of response surface methodology, neural network and neuro-fuzzy system. Neural Comput. Appl. 2019, 31, 4929–4943. [Google Scholar] [CrossRef]
- Dharma, S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Silitonga, A.S.; Kusumo, F.; Mahlia, T.M.I. Optimization of biodiesel production process for mixed Jatropha curcas-Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Kusumo, F.; Mahlia, T.M.I.; Shamsuddin, A.H.; Ong, H.C.; Ahmad, A.R.; Ismail, Z.; Ong, Z.C.; Silitonga, A.S. The effect of multi-walled carbon nanotubes-additive in physicochemical property of rice brand methyl ester: Optimization analysis. Energies 2019, 12, 3291. [Google Scholar] [CrossRef] [Green Version]
- Sebayang, A.H.; Masjuki, H.H.; Ong, H.C.; Dharma, S.; Silitonga, A.S.; Kusumo, F.; Milano, J. Optimization of bioethanol production from sorghum grains using artificial neural networks integrated with ant colony. Ind. Crops Prod. 2017, 97, 146–155. [Google Scholar] [CrossRef]
- Chandra Mohan, B.; Baskaran, R. A survey: Ant Colony Optimization based recent research and implementation on several engineering domain. Expert Syst. Appl. 2012, 39, 4618–4627. [Google Scholar] [CrossRef]
- Truong, N.T.T.; Boontawan, A. Development of Bio-Jet Fuel Production Using Palm Kernel Oil and Ethanol. Int. J. Chem. Eng. Appl. 2017, 8, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Kusumo, F.; Dharma, S.; Sebayang, A.H.; Cheah, M.Y.; Wang, C.T. Physicochemical property enhancement of biodiesel synthesis from hybrid feedstocks of waste cooking vegetable oil and Beauty leaf oil through optimized alkaline-catalysed transesterification. Waste Manag. 2018, 80, 435–449. [Google Scholar] [CrossRef]
- Ong, M.Y.; Chew, K.W.; Show, P.L.; Nomanbhay, S. Optimization and kinetic study of non-catalytic transesterification of palm oil under subcritical condition using microwave technology. Energy Convers. Manag. 2019, 196, 1126–1137. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Mahlia, T.M.I.; Kusumo, F.; Dharma, S.; Sebayang, A.H.; Sembiring, R.W.; Shamsuddin, A.H. Intensification of Reutealis trisperma biodiesel production using infrared radiation: Simulation, optimisation and validation. Renew. Energy 2019, 133, 520–527. [Google Scholar] [CrossRef]
- Binnal, P.; Amruth, A.; Basawaraj, M.P.; Chethan, T.S.; Murthy, K.R.S.; Rajashekhara, S. Microwave-assisted esterification and transesterification of dairy scum oil for biodiesel production: Kinetics and optimisation studies. Indian Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Sabio, E.; Ramiro, M.J. Preparation and properties of biodiesel from Cynara cardunculus L. oil. Ind. Eng. Chem. Res. 1999, 38, 2927–2931. [Google Scholar] [CrossRef]
- Lee, J.S.; Saka, S. Biodiesel production by heterogeneous catalysts and supercritical technologies. Bioresour. Technol. 2010, 101, 7191–7200. [Google Scholar] [CrossRef]
- Saifuddin, A.; Chua, K.H. Production of Ethyl Ester (Biodiesel) from Used Frying Oil: Optimization of Transesterification Process Using Microwave Irradiation. MJ. Chem. 2004, 6, 77–82. [Google Scholar]
- Oyerinde, A.; Bello, E. Use of Fourier Transformation Infrared (FTIR) Spectroscopy for Analysis of Functional Groups in Peanut Oil Biodiesel and Its Blends. Br. J. Appl. Sci. Technol. 2016, 13, 1–14. [Google Scholar] [CrossRef]
- Jimoh, A.; Abdulkareem, A.S.; Afolabi, A.S.; Odigure, J.O.; Odili, U.C. Production and Characterization of Biofuel from Refined Groundnut Oil. In Energy Conservation; InTech: London, UK, 2012; pp. 10–12. [Google Scholar]
- Saifuddin, N.; Refal, H. Spectroscopic Analysis of Structural Transformation in Biodiesel Degradation. Res. J. Appl. Sci. Eng. Technol. 2014, 8, 1149–1159. [Google Scholar] [CrossRef]
- Rabaev, M.; Landau, M.V.; Vidruk-Nehemya, R.; Koukouliev, V.; Zarchin, R.; Herskowitz, M. Conversion of vegetable oils on Pt/Al2O3/SAPO-11 to diesel and jet fuels containing aromatics. Fuel 2015, 161, 287–294. [Google Scholar] [CrossRef]
- Amanda, B. The Future of Advanced Bio-Jet Fuel; Linköping University: Linköping, Sweden, 2017. [Google Scholar]
- Cheng, F.; Brewer, C.E. Producing jet fuel from biomass lignin: Potential pathways to alkyl-benzenes and cycloalkanes. Renew. Sustain. Energy Rev. 2017, 72, 673–722. [Google Scholar] [CrossRef]



| Fatty Acid | Composition (wt%) |
|---|---|
| Caprylic acid, C8:0 | 6.0 |
| Capric acid, C10:0 | 5.1 |
| Lauric acid, C12:0 | 48.5 |
| Myristic acid, C14:0 | 16.9 |
| Palmitic acid, C16:0 | 9.6 |
| Stearic acid, C18:0 | 2.3 |
| Oleic acid, C18:1 | 8.2 |
| Linoleic acid, C18:2 | 3.4 |
| Experimental Level | Coconut Oil to Ethanol Molar Ratio, F1 | Reaction Time, F2 (min) | Microwave Power, F3 (W) |
|---|---|---|---|
| Low level, L (−1) | 1:6 | 5 | 100 |
| Medium level, M (0) | 1:9 | 10 | 300 |
| High level, H (+1) | 1:12 | 15 | 500 |
| Source | Sequential p-Value | Lack of Fit p-Value | Adjusted R-Squared | Predicted R-Squared | Remarks |
|---|---|---|---|---|---|
| Linear | 0.0418 | 0.0211 | 0.3787 | 0.2289 | |
| 2 Factors Interaction | 0.9898 | 0.0143 | 0.1574 | −0.4111 | |
| Quadratic | <0.0001 | 0.4277 | 0.9792 | 0.9130 | Suggested |
| Cubic | 0.4277 | 0.9839 | Aliased |
| Experimental Run | Coconut Oil to Ethanol Molar Ratio, F1 | Reaction Time, F2 (min) | Microwave Power, F3 (W) | Experimental Bio-Jet Fuel Yield (%) | Predicted Bio-Jet Fuel Yield (%) RSM | Predicted Bio-Jet Fuel Yield (%) ANN |
|---|---|---|---|---|---|---|
| 1 | 1:6 | 10 | 500 | 40.92 | 40.07 | 41.10 |
| 2 | 1:9 | 15 | 100 | 70.86 | 71.12 | 70.73 |
| 3 | 1:12 | 5 | 300 | 56.05 | 56.11 | 56.18 |
| 4 | 1:6 | 5 | 300 | 48.21 | 49.32 | 47.97 |
| 5 | 1:12 | 15 | 300 | 63.88 | 62.77 | 64.13 |
| 6 | 1:9 | 15 | 500 | 55.73 | 56.64 | 55.69 |
| 7 | 1:9 | 5 | 100 | 64.83 | 63.92 | 64.84 |
| 8 | 1:9 | 5 | 500 | 50.36 | 50.10 | 50.38 |
| 9 | 1:6 | 10 | 100 | 57.14 | 56.94 | 56.64 |
| 10 | 1:12 | 10 | 500 | 49.16 | 49.36 | 49.19 |
| 11 | 1:12 | 10 | 100 | 59.94 | 60.79 | 59.96 |
| 12 * | 1:9 | 10 | 300 | 69.82 | 68.85 | 69.81 |
| 13 | 1:6 | 15 | 300 | 56.47 | 56.41 | 55.96 |
| 14 * | 1:9 | 10 | 300 | 69.15 | 68.85 | 69.81 |
| 15 * | 1:9 | 10 | 300 | 67.59 | 68.85 | 69.81 |
| Source | Sum of Squares | df | Mean Square | F-Value * | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 1127.25 | 9 | 125.25 | 74.3 | <0.0001 | significant |
| F1-Ethanol | 86.4 | 1 | 86.4 | 51.25 | 0.0008 | |
| F2-Time | 94.46 | 1 | 94.46 | 56.04 | 0.0007 | |
| F3-Power | 400.45 | 1 | 400.45 | 237.55 | <0.0001 | |
| F1F2 | 0.0462 | 1 | 0.0462 | 0.0274 | 0.875 | |
| F1F3 | 7.4 | 1 | 7.4 | 4.39 | 0.0903 | |
| F2F3 | 0.1089 | 1 | 0.1089 | 0.0646 | 0.8095 | |
| 420.99 | 1 | 420.99 | 249.74 | <0.0001 | ||
| 15.11 | 1 | 15.11 | 8.96 | 0.0303 | ||
| 150.55 | 1 | 150.55 | 89.31 | 0.0002 | ||
| Residual | 8.43 | 5 | 1.69 | |||
| Lack of Fit | 5.81 | 3 | 1.94 | 1.48 | 0.4277 | not significant |
| Pure Error | 2.62 | 2 | 1.31 | |||
| Cor Total | 1135.68 | 14 |
| Statistical Analysis | RSM | ANN |
|---|---|---|
| R | 0.9963 | 0.9979 |
| R2 | 0.9926 | 0.9957 |
| RSME | 0.5619 | 0.4048 |
| SEP (%) | 0.9563 | 0.6889 |
| MAE | 0.0414 | 0.0220 |
| Chi-square | 0.0096 | 0.0061 |
| Modeling Method | Ethanol | Time | Power | Predicted | Observed |
|---|---|---|---|---|---|
| RSM | 9.35 | 14.34 | 189.53 | 72.49 | 73.02 |
| ANN | 9.25 | 12.66 | 163.69 | 74.8 | 74.45 |
| Properties | Unit | ASTM D1655 | This Work | Soybean [53] | Palm [42] |
|---|---|---|---|---|---|
| Density at 15 °C | kg/m3 | 775–840 | 788 | 776 | 866.3 |
| Kinetic viscosity at −20 °C | cSt | <8 | 6.52 | 3.30 | - |
| Flash point | °C | >38 | 55 | 48.5 | 105 |
| Freezing point | °C | <−47 | −16 | - | −50 |
| Lower heating value | MJ/kg | >42.8 | 43.5 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, M.Y.; Nomanbhay, S.; Kusumo, F.; Raja Shahruzzaman, R.M.H.; Shamsuddin, A.H. Modeling and Optimization of Microwave-Based Bio-Jet Fuel from Coconut Oil: Investigation of Response Surface Methodology (RSM) and Artificial Neural Network Methodology (ANN). Energies 2021, 14, 295. https://doi.org/10.3390/en14020295
Ong MY, Nomanbhay S, Kusumo F, Raja Shahruzzaman RMH, Shamsuddin AH. Modeling and Optimization of Microwave-Based Bio-Jet Fuel from Coconut Oil: Investigation of Response Surface Methodology (RSM) and Artificial Neural Network Methodology (ANN). Energies. 2021; 14(2):295. https://doi.org/10.3390/en14020295
Chicago/Turabian StyleOng, Mei Yin, Saifuddin Nomanbhay, Fitranto Kusumo, Raja Mohamad Hafriz Raja Shahruzzaman, and Abd Halim Shamsuddin. 2021. "Modeling and Optimization of Microwave-Based Bio-Jet Fuel from Coconut Oil: Investigation of Response Surface Methodology (RSM) and Artificial Neural Network Methodology (ANN)" Energies 14, no. 2: 295. https://doi.org/10.3390/en14020295
APA StyleOng, M. Y., Nomanbhay, S., Kusumo, F., Raja Shahruzzaman, R. M. H., & Shamsuddin, A. H. (2021). Modeling and Optimization of Microwave-Based Bio-Jet Fuel from Coconut Oil: Investigation of Response Surface Methodology (RSM) and Artificial Neural Network Methodology (ANN). Energies, 14(2), 295. https://doi.org/10.3390/en14020295






