Numerical Simulation Based on the Canister Test for Shale Gas Content Calculation
Abstract
:1. Introduction
2. Principles and Experiments
2.1. Gas Flow Model and Numerical Simulation
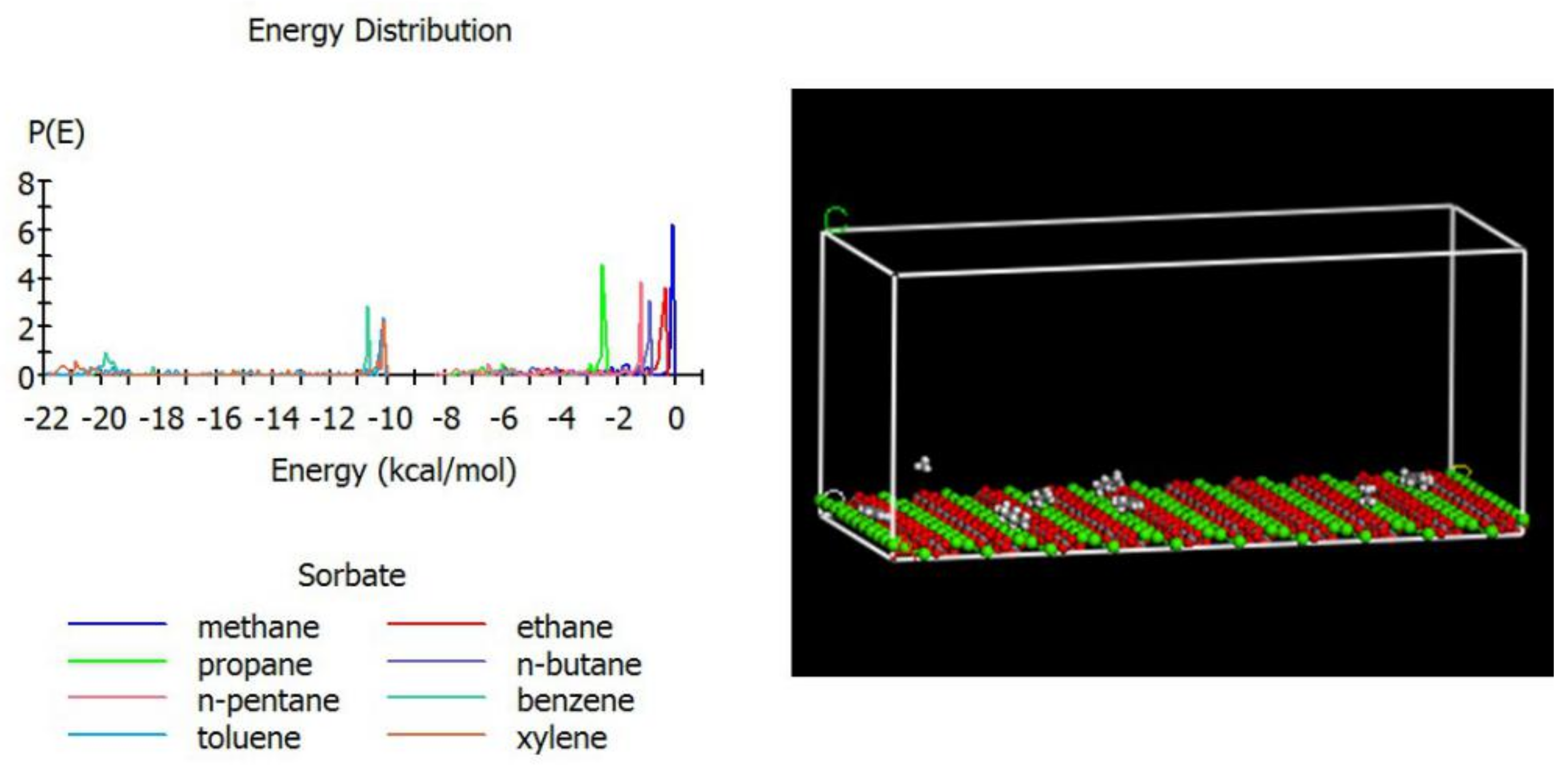
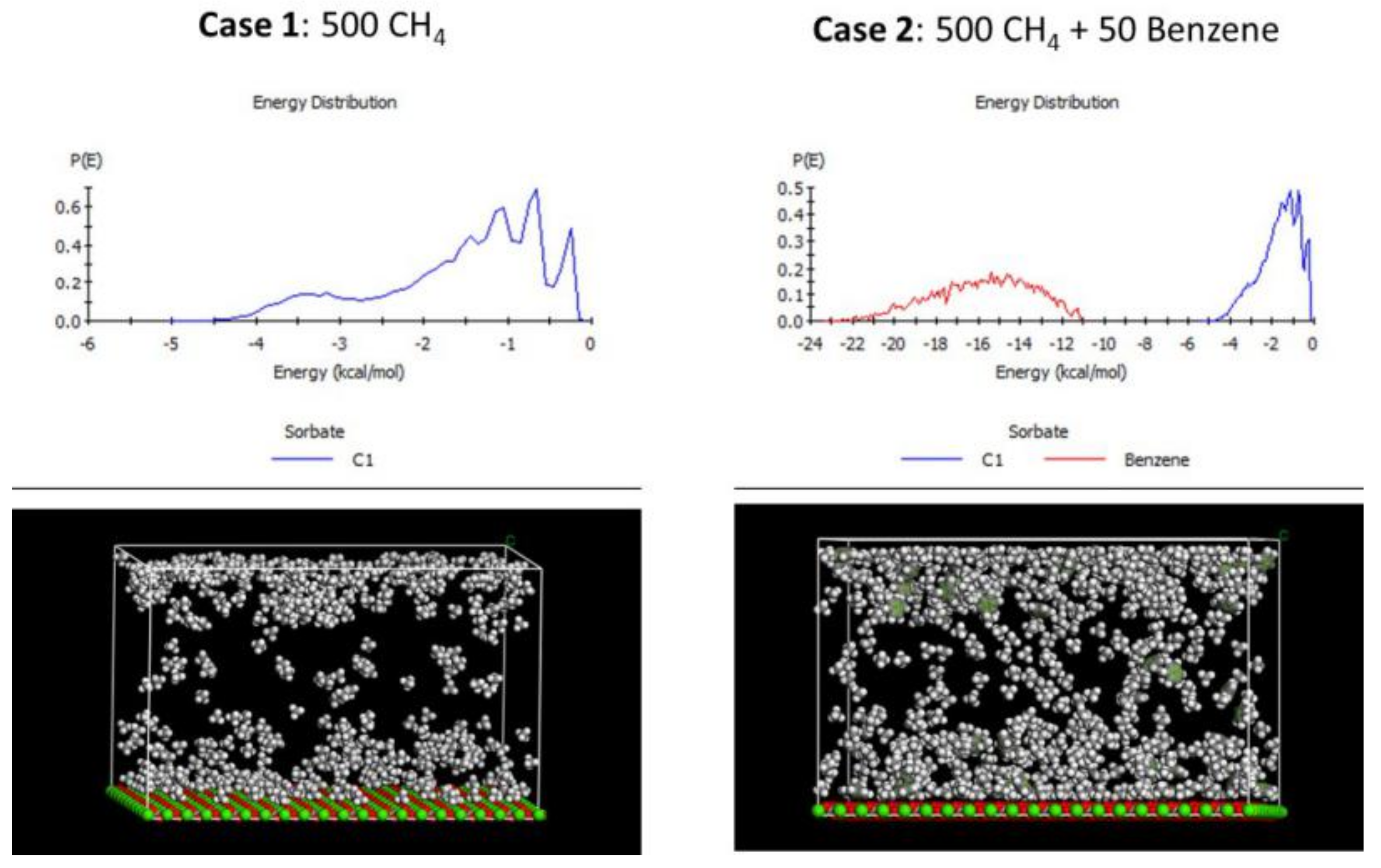
2.2. Molecular Simulation
- (1)
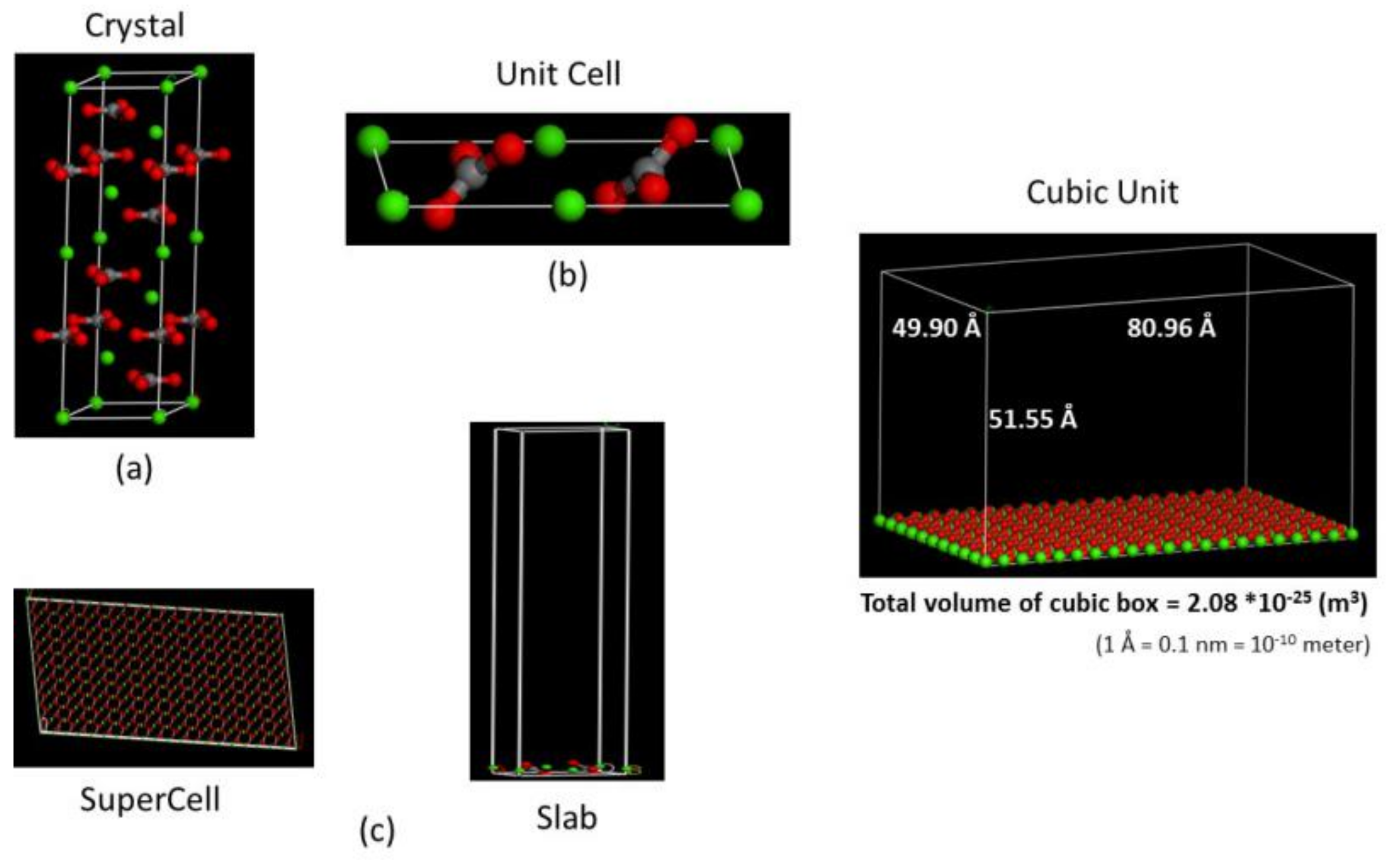
- The crystal structure of calcite is taken as a = 4.99 Å, b = 4.99 Å, and c = 17.61 Å, α = 90°, β = 90°, and γ = 120° with space group R-3C (167) (Figure 1a).
- (2)
- The {10–14} surface of calcite is cleaved to generate a 2D unit cell (Figure 1b): a corner Ca atom is shared by four cells, so the net charge is + 0.5; a bridged Ca atom is shared by two cells, so the net charge is +1.0; the carbonate CO32- species has a net charge of −2; thus, the total net charge of the unit cell is + 0.5 × 4 + 1.0 × 2 − 2 × 2 = 0.
- (3)
- A 10 × 10 supercell is created from the unit cell and expand to the slab high of 50 Å. Because the single calcite layer has a height of 1.55 Å, the total height of the cubic cell is 51.55 Å (Figure 1c).
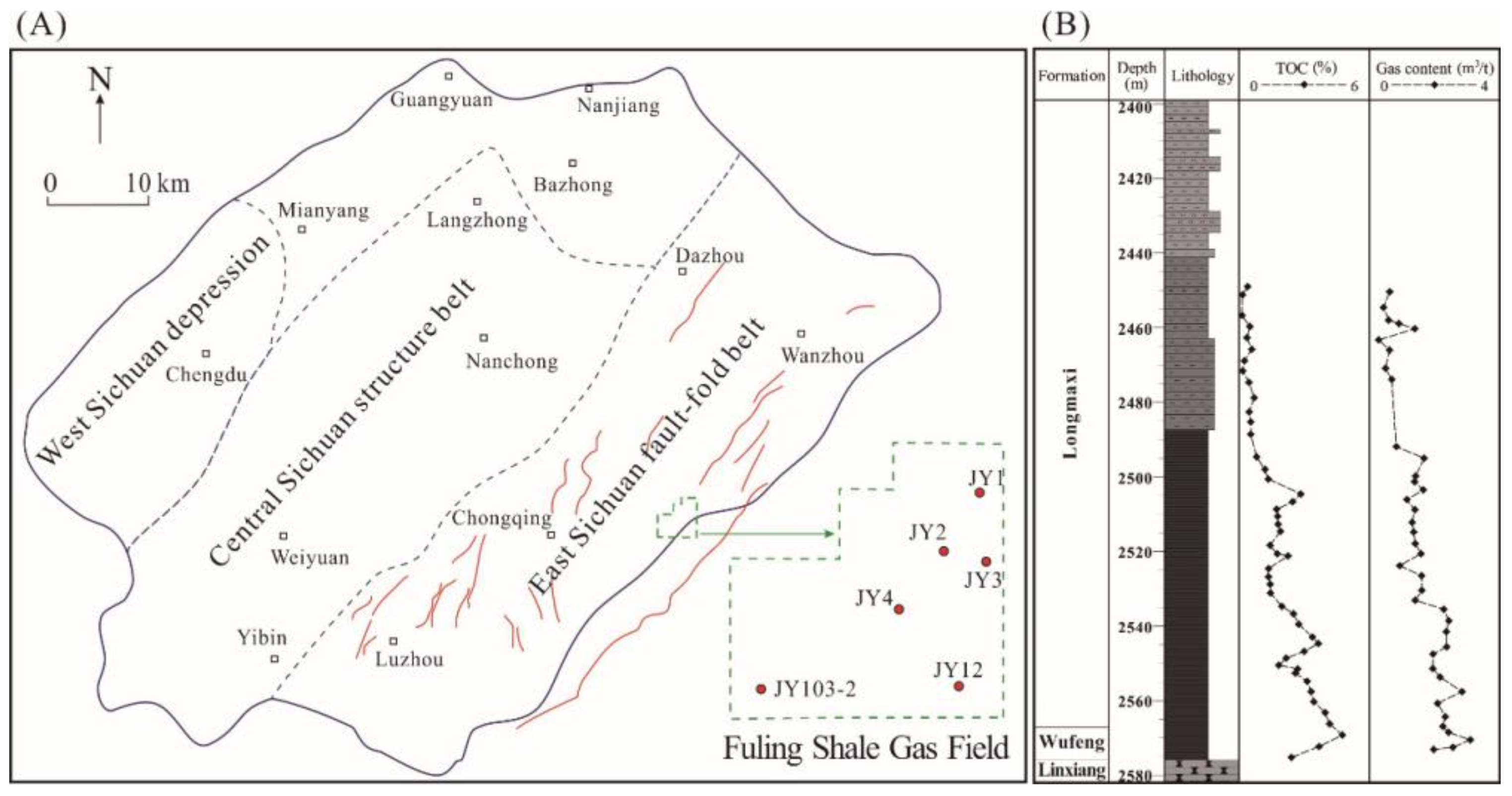
2.3. Geological Settings
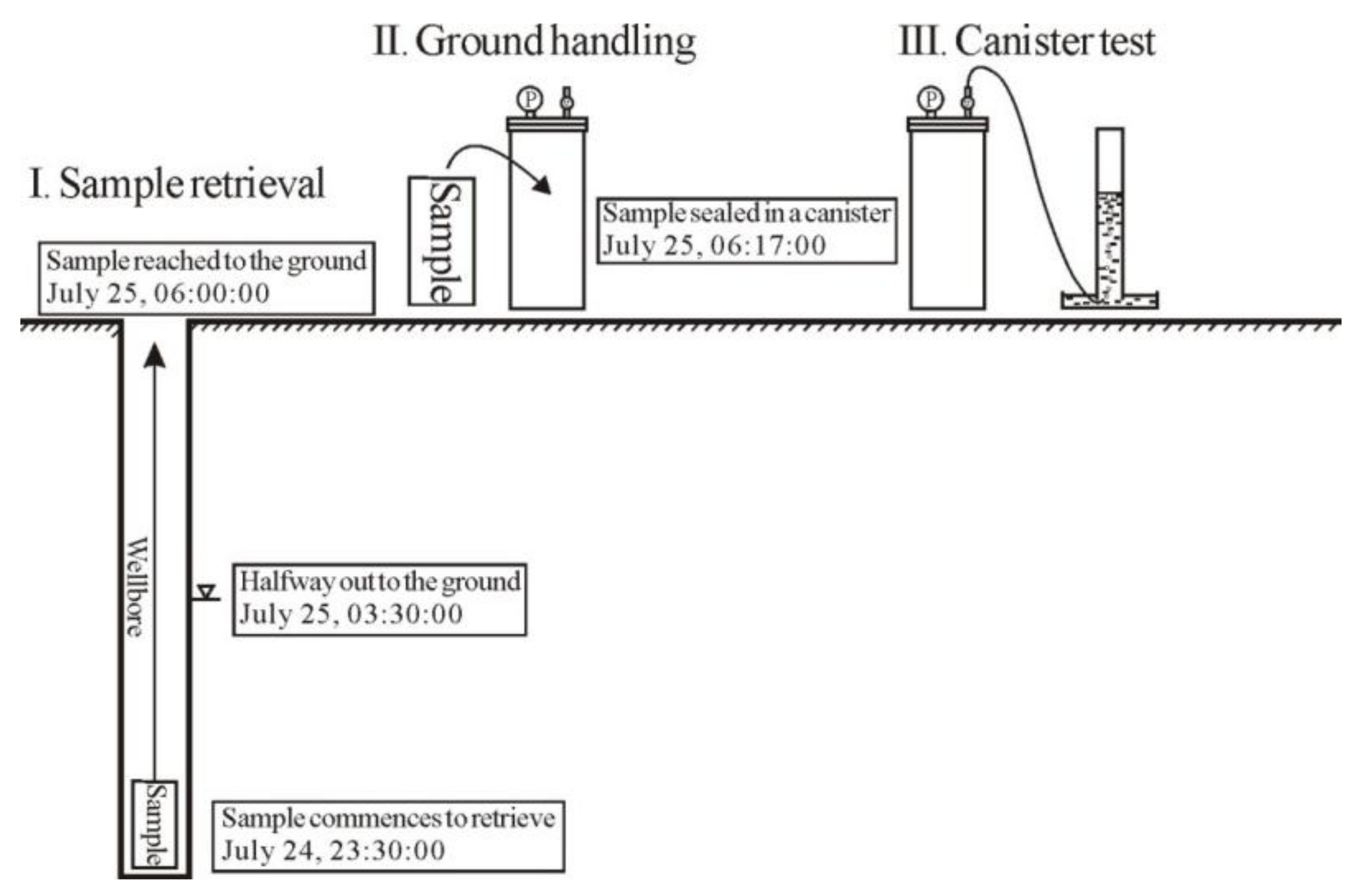
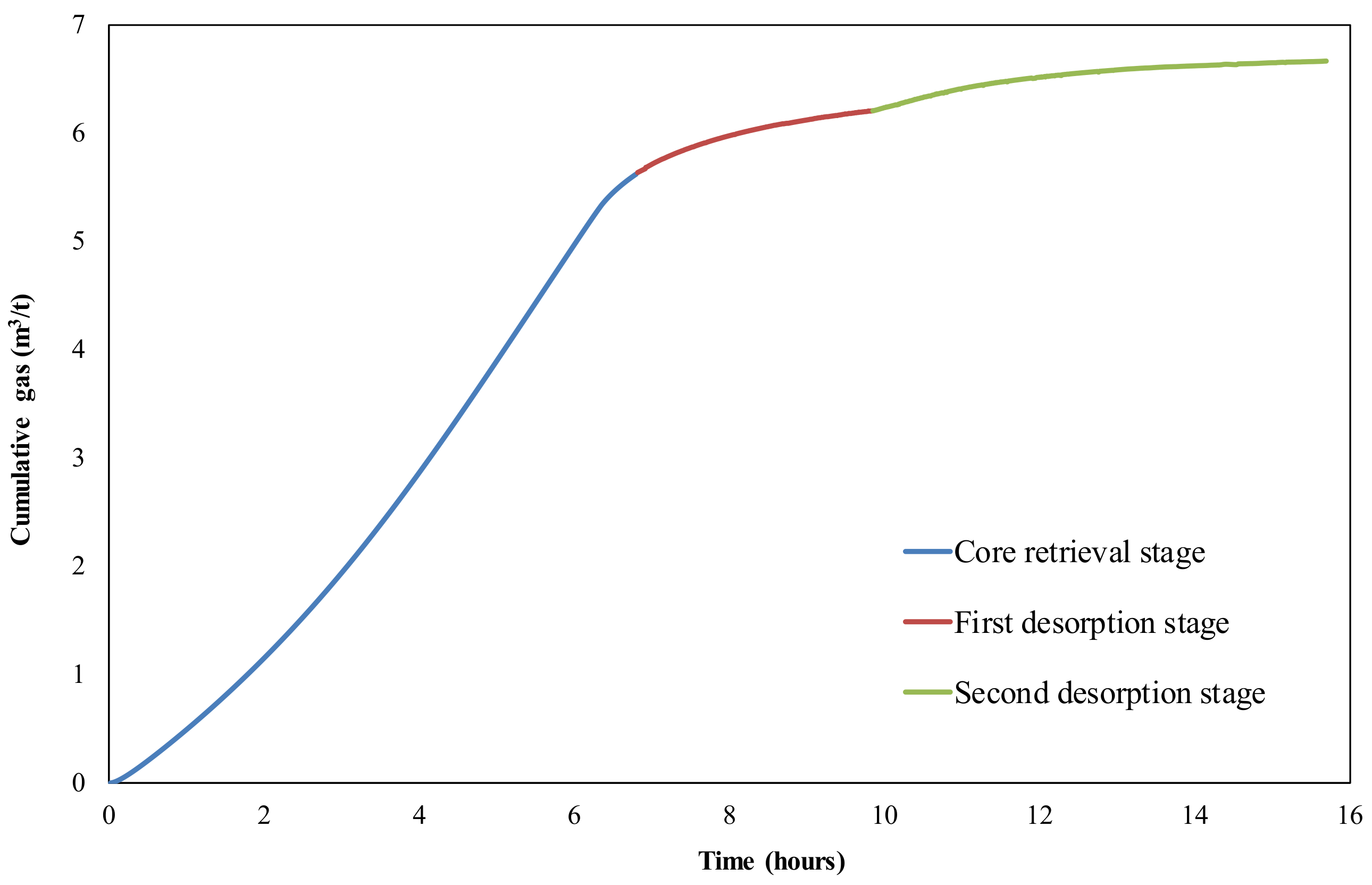
2.4. Sample and Canister Desorption
3. Results and Discussion
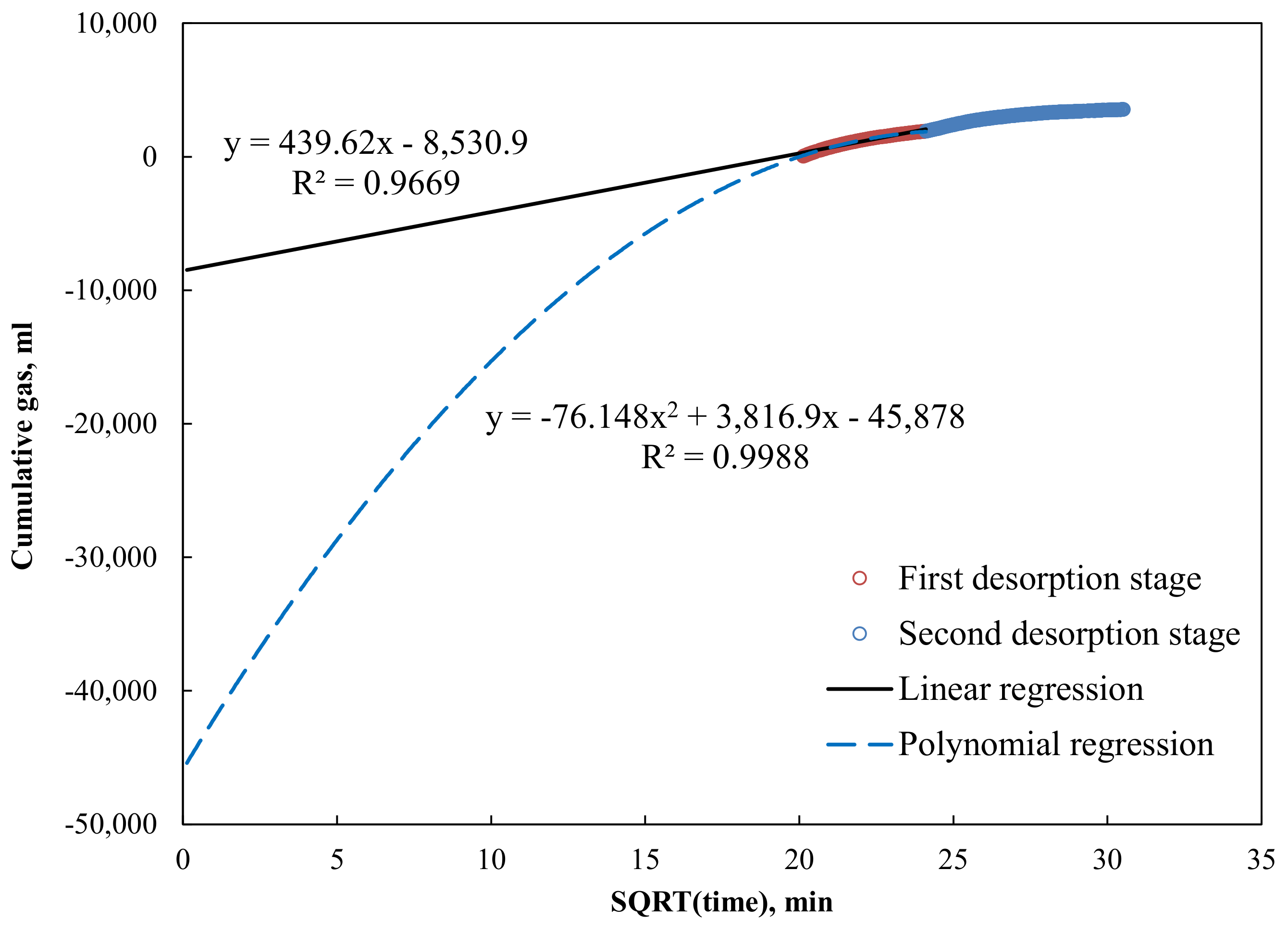
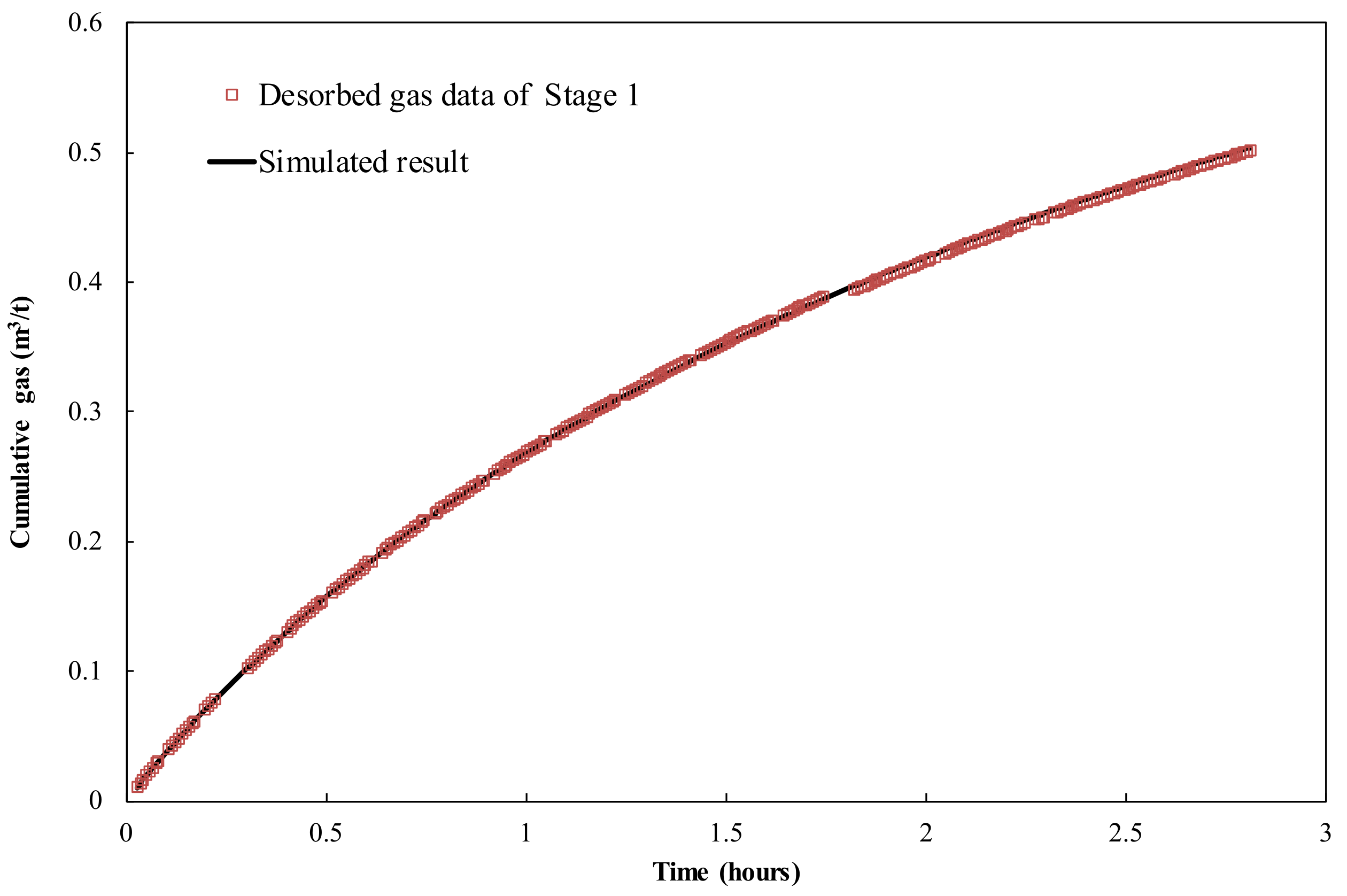
3.1. Canister Desorbed Gas and Lost Gas Calculations
3.2. Total Gas in Place (GIP) Based on Volumetric Approaches
3.3. Numerical Models
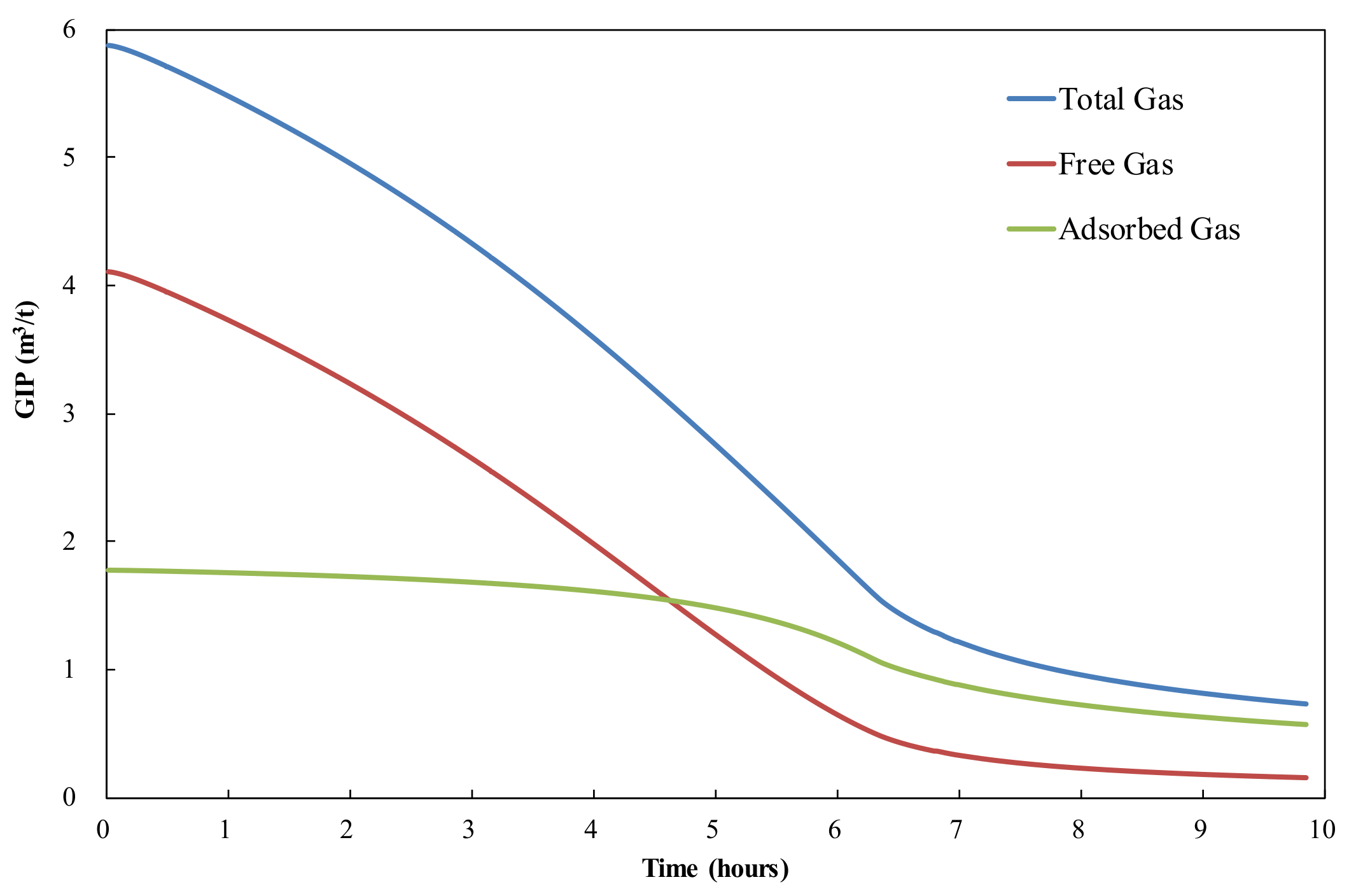
3.4. Free Gas vs. Adsorbed Gas
3.5. Hydrocarbon Storage in Pore Space
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Energy. Energy Information Administration. Annual Energy Outlook 2014: With Projections to 2040; Report No.: DOE/EIA-0383(2014); United States Department of Energy: Washington, DC, USA, 2014. [Google Scholar]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Curtis, M.E. Structural characterization of gas shales on the micro-and nano-scales. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar] [CrossRef]
- Slatt, R.M.; O’Brien, N.R. Pore types in the Barnett and Woodford gas shales: Contribution to understanding gas storage and migration pathways in fine-grained rocks. AAPG Bull. 2011, 95, 2017–2030. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M.; Power, I.M. Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analysis: Examples from the Barnnet, Woodford, Haynesville, Marcellus, and Doig units. AAPG Bull. 2012, 96, 1099–1119. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Solano, N.; Bustin, R.M.; Bustin, A.M.M.; Chalmers, G.R.L.; He, L.; Melnichenko, Y.B.; Radlinski, A.P.; Blach, T.P. Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 2013, 103, 606–616. [Google Scholar] [CrossRef]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Law, B.E.; Spencer, C.W. Gas in tight reservoirs–An emerging major source of energy. In The Future of Energy Gases; U.S. Geological Survey Professional Paper 1570; United States Government Printing Office: Washington, DC, USA, 1993; p. 233. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar] [CrossRef]
- Javadpour, F.; Fisher, D.; Unsworth, M. Nanoscale gas flow in shale gas sediments. J. Can. Pet. Technol. 2007, 46, 55–61. [Google Scholar] [CrossRef]
- Etminan, S.R.; Javadpour, F.; Maini, B.B.; Chen, Z.X. Measurement of gas storage processes in shale and of the molecular diffusion coefficient in kerogen. Int. J. Coal Geol. 2014, 123, 10–19. [Google Scholar] [CrossRef]
- Tugan, M.F.; Sinayuc, C. A new fully probabilistic methodology and a software for assessing uncertainties and managing risks in shale gas projects at any maturity stage. J. Pet. Sci. Eng. 2018, 168, 107–118. [Google Scholar] [CrossRef]
- Bertard, C.; Bruyet, B.; Gunther, J. Determination of desorbable gas concentration of coal (Direct Method). Int. J. Rock Mech. Min. Sci. 1970, 7, 43–65. [Google Scholar] [CrossRef]
- Kissell, F.N.; McCulloch, C.M.; Elder, C.H. The Direct Method of Determining Methane Content of Coalbeds for Ventilation Design; Report of Investigations 7767; University of Michigan Library: Ann Arbor, MI, USA, 1973; pp. 1–17. [Google Scholar]
- McLennan, J.D.; Schafer, P.S.; Pratt, T.J. A Guide to Determining Coalbed Gas Content; Topical Report GRI-94/0396; Gas Research Institute, 1995; Available online: https://sales.gastechnology.org/Guide-to-Determing-Coalbed-Gas-Content.html (accessed on 28 September 2021).
- Diamond, W.P.; Schatzel, S.J. Measuring the gas content of coal: A review. Int. J. Coal Geol. 1998, 35, 311–331. [Google Scholar] [CrossRef]
- Waechter, N.B.; Hampton, G.L., III; Shipps, J.C. Overview of coal and shale gas measurement: Field and laboratory procedures. In Proceedings of the International Coalbed Methane Symposium, Tuscaloosa, AL, USA, 3–7 May 2004. [Google Scholar]
- Yee, D.; Seidle, J.P.; Hanson, W.B. Gas sorption on coal and measurement of gas content. In Hydrocarbons from Coal; Law, B.E., Rice, D.D., Eds.; The American Association of Petroleum Geologists: Tulsa, OK, USA, 1993; pp. 203–218. [Google Scholar] [CrossRef]
- Diamond, W.P.; Schatzel, S.J.; Garcia, F.; Ulery, J.P. The Modified Direct Method: A Solution for Obtaining Accurate Coal Desorption Measurements; International Coalbed Methane Symposium: Tuscaloosa, AL, USA, 2001; pp. 331–342. [Google Scholar]
- Shtepani, E.; Noll, L.A.; Elrod, L.W.; Jacobs, P.M. A new regression-based method for accurate measurement of coal and shale gas content. SPE Reserv. Eval. Eng. 2012, 13, 359–364. [Google Scholar] [CrossRef]
- Javadpour, F. Nanopores and gas permeability of gas flow in mudrocks (Shales and Siltstone). J. Can. Pet. Technol. 2009, 48, 16–21. [Google Scholar] [CrossRef]
- Shabro, V.; Torres-Verdín, C.; Javadpour, F. Numerical simulation of shale-gas production: From pore-scale modeling of slip-flow, Knudsen diffusion, and Langmuir desorption to reservoir modeling of compressible fluid. In Proceedings of the SPE North American Unconventional Gas Conference and Exhibition, The Woodlands, TX, USA, 14 June 2011. [Google Scholar] [CrossRef] [Green Version]
- Shabro, V.; Torres-Verdín, C.; Sepehrnoori, K. Forecasting gas production in organic shale with the combined numerical simulation of gas diffusion in kerogen, Langmuir desorption from kerogen surfaces, and advection in nanopores. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Javadpour, F.; Michael, G.E. Novel analytical core-sample analysis indicates higher gas content in shale-gas reservoirs. SPE J. 2015, 20, 1–12. [Google Scholar] [CrossRef]
- Metwally, Y.M.; Sondergeld, C.H. Measuring low permeabilities of gas-sands and shales using a pressure transmission technique. Int. J. Rock Mech. Min. Sci. 2011, 48, 1135–1144. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Gasparik, M.; Amann-Hildenbrand, A.; Gensterblum, Y.; Krooss, B.M. Experimental study of fluid transport processes in the matrix system of the European organic-rich shales: I. Scandinavian Alum Shale. Mar. Pet. Geol. 2014, 51, 79–99. [Google Scholar] [CrossRef]
- Bhandari, A.R.; Flemings, P.B.; Polito, P.J.; Cronin, M.B.; Bryant, S.L. Anisotropy and stress dependence of permeability in the Barnett Shale. Transp. Porous Media 2015, 108, 393–411. [Google Scholar] [CrossRef]
- Ma, Y.; Pan, Z.; Zhong, N.; Connell, L.D.; Down, D.I.; Lin, W.; Zhang, Y. Experimental study of anisotropic gas permeability and its relationship with fracture structure of Longmaxi Shales, Sichuan Basin, China. Fuel 2016, 180, 106–115. [Google Scholar] [CrossRef]
- Kunda, P.K.; Cohen, I.M.; Dowling, D.R. Fluid Mechanics, 5th ed.; Elsevier Academic Press: Cambridge, MA, USA, 2012; pp. 96–99. [Google Scholar]
- Cui, X.; Bustin, A.M.M.; Bustin, R.M. Measurements of gas permeability and diffusivity of tight reservoir rocks: Different approaches and their applications. Geofluids 2009, 9, 208–223. [Google Scholar] [CrossRef]
- Mattar, L.; Brar, G.S.; Aziz, K. Compressibility of natural gases. J. Can. Pet. Technol. 1975, 14, 77–80. [Google Scholar] [CrossRef]
- Ghedan, S.G.; Aljawad, M.S.; Poettmann, F.H. Compressibility of natural gases. J. Pet. Sci. Eng. 1993, 10, 157–162. [Google Scholar] [CrossRef]
- Lohrenz, J.; Bray, B.G.; Clark, C.R. Calculating viscosities of reservoir fluids from their compositions. J. Pet. Technol. 1964, 16, 1171–1176. [Google Scholar] [CrossRef]
- Freeman, C.M.; Moridis, G.J.; Blasingame, T.A. A numerical study of microscale flow behavior in tight gas and shale gas reservoir systems. Transp. Porous Media 2009, 90, 253–268. [Google Scholar] [CrossRef]
- Darabi, H.; Ettehad, A.; Javadpour, F.; Sepehrnoori, K. Gas flow in ultra-tight shale strata. J. Fluid Mech. 2012, 710, 641–658. [Google Scholar] [CrossRef]
- Firouzi, M.; Alnoaimi, K.; Kovscek, A.; Wilcox, J. Klinkenberg effect on predicting and measuring helium permeability in gas shales. Int. J. Coal Geol. 2014, 123, 62–68. [Google Scholar] [CrossRef]
- Kazemi, M.; Takbiri-Borujeni, A. An analytical model for shale gas permeability. Int. J. Coal Geol. 2015, 146, 188–197. [Google Scholar] [CrossRef]
- Tang, G.H.; Tao, W.Q.; He, Y.L. Gas slippage effect on microscale porous flow using the lattice Boltzmann method. Phys. Rev. E 2005, 72, 056301. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.A.; Chalaturnyk, R. Expansion of the Klinkenberg’s slippage equation to low permeability porous media. Int. J. Coal Geol. 2014, 123, 2–9. [Google Scholar] [CrossRef]
- Moghaddam, R.N.; Jamiolahmady, M. Slip flow in porous media. Fuel 2016, 173, 298–310. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, F. Characteristics of shale nanopore system and its internal gas flow: A case study of the lower Silurian Longmaxi Formation shale from Sichuan Basin, China. J. Nat. Gas Geosci. 2017, 2, 303–311. [Google Scholar] [CrossRef]
- Maurer, J.; Tabeling, P.; Joseph, P.; Willaime, H. Second-order slip laws in microchannels for helium and nitrogen. Phys. Fluids 2003, 15, 2613–2621. [Google Scholar] [CrossRef]
- Sakhaee-Pour, A.; Bryant, S.L. Gas permeability of shale. SPE Reserv. Eval. Eng. 2012, 15, 401–409. [Google Scholar] [CrossRef]
- Dongari, N.; Agrawal, A.; Agrawal, A. Analytical solution of gaseous slip flow in long microchannels. Int. J. Heat Mass Transf. 2007, 50, 3411–3421. [Google Scholar] [CrossRef]
- Kirkpatrick, S.; Gelatt, C.D.; Vecchi, M.P. Optimization by Simulated Annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulation. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Rexer, T.F.T.; Benham, M.J.; Aplin, A.C.; Thomas, K.M. Methane adsorption on shale under simulated geological temperature and pressure conditions. Energy Fuels 2013, 27, 3099–3109. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Tian, H.; Xiao, X.; Cheng, P.; Zhou, Q.; Wei, Q. Geochemical characterization and methane adsorption capacity of overmature organic-rich Lower Cambrian shales in northeast Guizhou region, southwest China. Mar. Pet. Geol. 2017, 86, 858–873. [Google Scholar] [CrossRef]
- Guo, T. The Fuling Shale Gas Field—a highly productive Silurian gas shale with high thermal maturity and complex evolution history, southeastern Sichuan Basin, China. Interpretation 2015, 3, SJ25–SJ34. [Google Scholar] [CrossRef]
- Ambrose, R.J.; Hartman, R.C.; Diaz-Campos, M.; Akkutlu, I.Y.; Sondergeld, C.H. Shale gas-in-place calculations Part I: New pore-scale considerations. SPE J. 2012, 17, 219–229. [Google Scholar] [CrossRef]
- Beskok, A.; Karniadakis, G.E. Report: A model for flows in channels, pipes, and ducts at micro and nano scales. Microscale Thermophys. Eng. 1999, 3, 43–77. [Google Scholar] [CrossRef]
- Gou, Q.; Xu, S. Quantitative evaluation of free gas and adsorbed gas content of Wufeng-Longmaxi shales in the Jiaoshiba area, Sichuan Basin, China. Adv. Geo-Energy Res. 2019, 3, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Chen, G.; Xu, C.; Tong, M.; Ni, B.; Bao, H. Quantitative evaluation of adsorbed and free gas and their mutual conversion in Wufeng-Longmaxi shale, Fuling area. Oil Gas Geol. 2019, 40, 1248–1258. (In Chinese) [Google Scholar]
- Montgomery, S.L.; Jarvie, D.M.; Bowker, K.A.; Pollastro, R.M. Mississippian Barnett Shale, Fort Worth basin, north-central Texas: Gas-shale play with multi-trillion cubic foot potential. AAPG Bull. 2005, 89, 155–175. [Google Scholar] [CrossRef]
- Tian, H.; Li, T.; Zhang, T.; Xiao, X. Characterization of methane adsorption on overmature Lower Silurian-Upper Ordovician shales in Sichuan Basin, southwest China: Experimental results and geological implications. Int. J. Coal Geol. 2016, 156, 36–49. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Zhang, R.; Zhao, H.; Krooss, B.M. Investigation on the methane sorption capacity of marine shales from Sichuan Basin, China. Int. J. Coal Geol. 2015, 146, 104–117. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, X.; Tian, H.; Zhou, Q.; Cheng, P. Geological models of gas in place of the Longmaxi shale in Southeast Chongqing, South China. Mar. Pet. Geol. 2016, 73, 433–444. [Google Scholar] [CrossRef]

| Approach | Calculation Rule | Gas Content (m3/t rock) |
|---|---|---|
| Direct Method | Linear extrapolation to “time zero” | 2.11 |
| Polynomial extrapolation to “time zero” | 4.66 | |
| Linear extrapolation to true “time zero” | 3.40 | |
| Polynomial extrapolation to true “time zero” | 13.91 | |
| Volumetric Approach | Conventional equation | 6.56 |
| Ambrose’s new equation | 5.47 | |
| Numerical Simulation | Ignoring adsorbed phase volume | 6.62 |
| Considering adsorbed phase volume | 5.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Lu, L.; Tian, H.; Yang, Y.; Jiang, C.; Ma, Q. Numerical Simulation Based on the Canister Test for Shale Gas Content Calculation. Energies 2021, 14, 6518. https://doi.org/10.3390/en14206518
Kang S, Lu L, Tian H, Yang Y, Jiang C, Ma Q. Numerical Simulation Based on the Canister Test for Shale Gas Content Calculation. Energies. 2021; 14(20):6518. https://doi.org/10.3390/en14206518
Chicago/Turabian StyleKang, Shujuan, Le Lu, Hui Tian, Yunfeng Yang, Chengyang Jiang, and Qisheng Ma. 2021. "Numerical Simulation Based on the Canister Test for Shale Gas Content Calculation" Energies 14, no. 20: 6518. https://doi.org/10.3390/en14206518






