Characteristics of Water Contaminants from Underground Coal Gasification (UCG) Process—Effect of Coal Properties and Gasification Pressure
Abstract
:1. Introduction
2. Materials and Methods
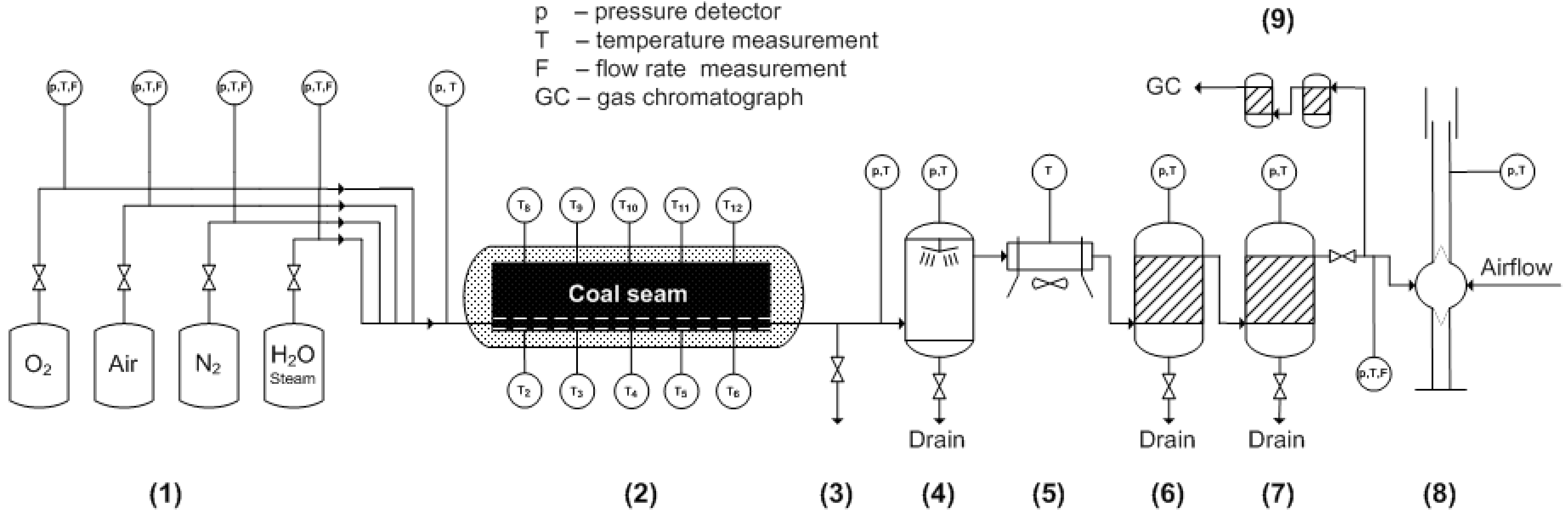
2.1. Coal Samples and UCG Experiments
2.2. Post-Processing Water Sampling
2.3. Chemical Analyses
2.4. Linear Correlation Analysis
3. Results and Discussion
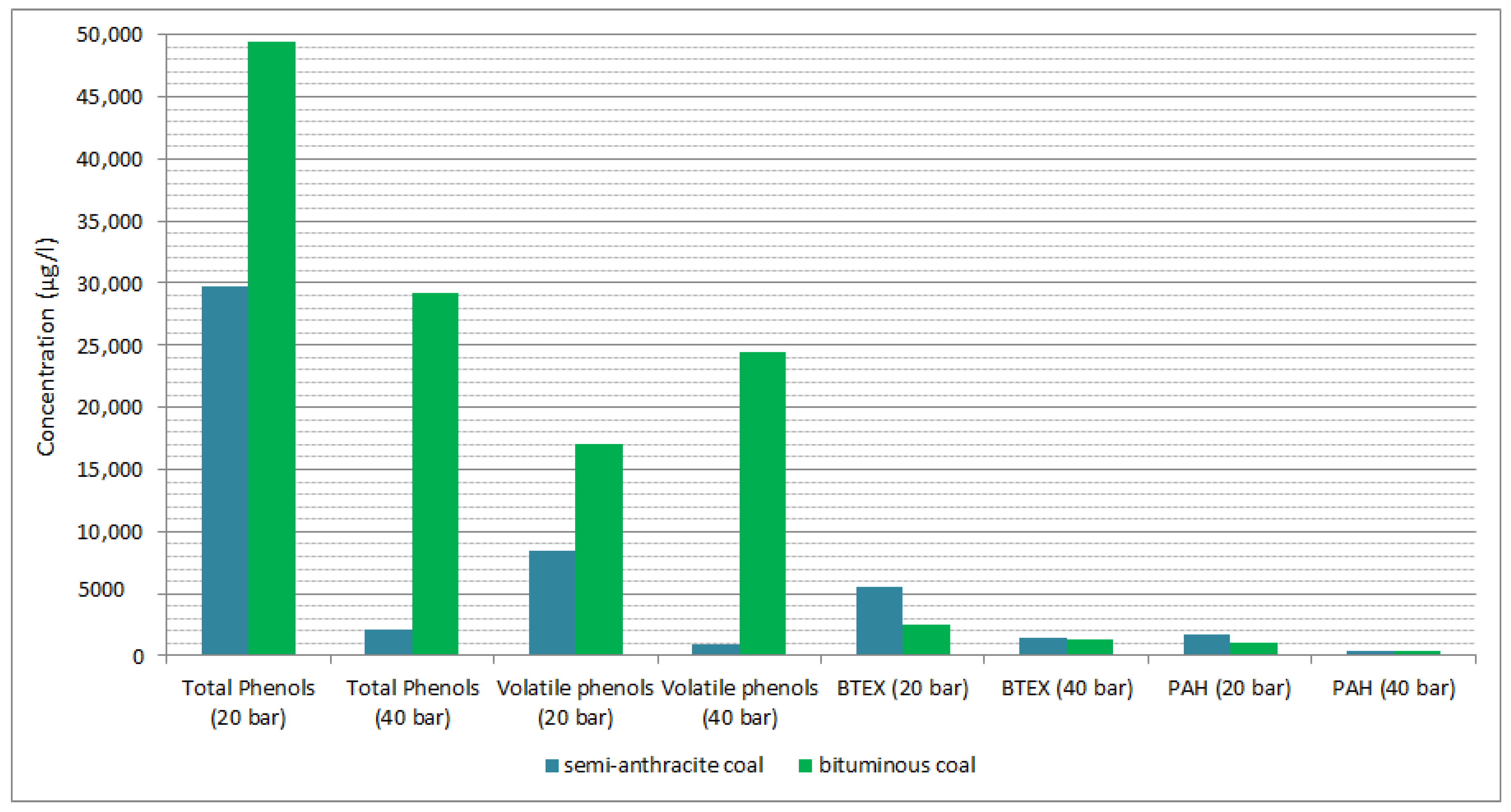
3.1. Coal Type Effect
3.2. Effect of Gasification Pressure
3.3. Pearson’s Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapusta, K.; Wiatowski, M.; Stańczyk, K.; Zagorscak, R.; Thomas, H.R. Large-scale experimental investigations to evaluate the feasibility of producing methane-rich gas (SNG) through under-ground coal gasification process. Effect of coal rank and gasification pressure. Energies 2020, 13, 1334. [Google Scholar] [CrossRef] [Green Version]
- Knapp, R. Environmental challenges for the global coal industry. World Coal Institute, Coal/ Power Focus. Asian J. Min. 2000, 208, 15–18. [Google Scholar]
- Chen, L.; Li, C.; Xu, B.; Xing, B.; Yi, G.; Huang, G.; Zhang, C.; Liu, J. Microbial degradation of organic pollutants in groundwater related to underground coal gasification. Energy Sci. Eng. 2019, 7, 2098–2111. [Google Scholar] [CrossRef] [Green Version]
- Balat, M. Status of Fossil Energy Resources: A Global Perspective. Energy Sources Part B Econ. Plan. Policy 2007, 2, 31–47. [Google Scholar] [CrossRef]
- Kapusta, K.; Stańczyk, K. Pollution of water during underground coal gasification of hard coal and lignite. Fuel 2011, 90, 1927–1934. [Google Scholar] [CrossRef]
- Xu, M.; Xin, L.; Liu, W.; Hu, X.; Cheng, W.; Li, C.; Wang, Z. Study on the physical properties of coal pyrolysis in underground coal gasification channel. Powder Technol. 2020, 376, 573–592. [Google Scholar] [CrossRef]
- Wiatowski, M.; Kapusta, K. Evolution of tar compounds in raw gas from a pilot-scale underground coal gasification (UCG) trial at Wieczorek mine in Poland. Fuel 2020, 276, 118070. [Google Scholar] [CrossRef]
- Bhutto, A.; Bazmi, A.; Zahedi, G. Underground coal gasification: From fundamentals to applications. Prog. Energy Combust. Sci. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Khadse, A.; Qayyumi, M.; Mahajani, S.; Aghalayam, P. Underground coal gasification: A new clean coal utilization technique for India. Energy 2007, 32, 2061–2071. [Google Scholar] [CrossRef]
- Pankiewicz-Sperka, M.; Stańczyk, K.; Płaza, G.; Kwaśniewska, J.; Nałęcz-Jawecki, G. Assessment of the chemical, microbiological and toxicological aspects of post-processing water from under-ground coal gasification. Ecotoxicol. Environ. Saf. 2014, 108, 294–301. [Google Scholar] [CrossRef]
- Yang, D.; Koukouzas, N.; Green, M.; Sheng, Y. Recent development on underground coal gasifica-tion and subsequent CO2 storage. J. Energy Inst. 2016, 89, 469–484. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification. Part I: Field demonstrations and proces performance. Prog. Energy Combust. Sci. 2018, 67, 158–187. [Google Scholar] [CrossRef]
- Wiatowski, M.; Kapusta, K.; Świądrowski, J.; Cybulski, K.; Ludwik-Pardała, M.; Grabowski, J.; Stańczyk, K. Technological aspects of underground coal gasification in the Experimental “Barbara” Mine. Fuel 2015, 159, 454–462. [Google Scholar] [CrossRef]
- Shafirovich, E.; Varma, A. Underground Coal Gasification: A Brief Review of Current Status. Ind. Eng. Chem. Res. 2009, 48, 7865–7875. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Production of synthetic natural gas (SNG) from coal and dry biomass–A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- van der Meijden, C.M.; Veringa, H.J.; Rabou, L.P. The production of synthetic natural gas (SNG): A comparison of three wood gasification systems for energy balance and overall efficiency. Biomass-Bioenergy 2010, 34, 302–311. [Google Scholar] [CrossRef]
- Kapusta, K.; Stańczyk, K. Chemical and toxicological evaluation of underground coal gasification (UCG) effluents. The coal rank effect. Ecotoxicol. Environ. Saf. 2015, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Strugała –Wilczek, A.; Basa, W.; Kapusta, K.; Soukup, K. In situ sorption phenomena can mitigate potential negative environmental effects of underground coal gasification (UCG)—An experimental study of phenol removal on UCG-derived residues in the aspect of contaminant retardation. Eco-Toxicol. Environ. Saf. 2021, 208, 111710. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, J.; Korczak, K.; Tokarz, A. Aquatic risk assessment based on the results of research on mine waters as a part of a pilot underground coal gasification process. Process. Saf. Environ. Prot. 2020, 148, 548–558. [Google Scholar] [CrossRef]
- Smoliński, A.; Stańczyk, K.; Kapusta, K.; Howaniec, N. Chemometric study of the ex situ under-ground coal gasification wastewater experimental data. Water Air Soil Pollut. 2012, 223, 5745–5758. [Google Scholar] [CrossRef] [Green Version]
- Bryant, C.W.; Cawein, C.C.; King, P.H. Biological Treatability of In Situ Coal Gasification Wastewater. J. Environ. Eng. 1988, 114, 400–414. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Yang, S.; Zhang, T.; Li, Y. Pretreatment of Coal Gasification Wastewater by Acidification Demulsion. Chin. J. Chem. Eng. 2006, 14, 398–401. [Google Scholar] [CrossRef]
- Shi, J.; Han, Y.; Xu, C.; Han, H. Biological coupling process for treatment of toxic and refractory compounds in coal gasification wastewater. Rev. Environ. Sci. Bio/Technol. 2018, 17, 765–790. [Google Scholar] [CrossRef]
- Thomas, M.; Zdebik, D.; Niewiara, E. Removing Phenols from Post-Processing Wastewater Originating from Underground Coal Gasification Using Coagulation-Flocculation and the H2O2/UV Process. Pol. J. Environ. Stud. 2018, 27, 2757–2763. [Google Scholar] [CrossRef]
- Zhang, G.; Wan, X.; Li, W.; Yang, Y.; Wang, L. Advanced treatment of coal gasification wastewater by catalytic oxidation with trace ozone. Chin. J. Environ. Eng. 2013, 7, 263–267. [Google Scholar]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and bio-logical treatments for wastewater decontamination—A review. Sci. Total. Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Li, P.; Ailijiang, N.; Cao, X.; Lei, T.; Liang, P.; Zhang, X.; Huang, X.; Teng, J. Pretreatment of coal gasification wastewater by adsorption using activated carbons and activated coke. Colloids Surf. A: Physicochem. Eng. Asp. 2015, 482, 177–183. [Google Scholar] [CrossRef]
- Priyadharshini, S.; Bakthavatsalam, A. A comparative study on growth and degradation behavior of C. pyrenoidosa on synthetic phenol and phenolic wastewater of a coal gasification plant. J. Environ. Chem. Eng. 2019, 7, 103079. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Philip, L. Performance evaluation of attached biofilm reactors for the treatment of wastewater contaminated with aromatic hydrocarbons and phenolic compounds. J. Environ. Chem. Eng. 2017, 5, 3852–3864. [Google Scholar] [CrossRef]
- Wang, W.; Han, H.; Yuan, M.; Li, H.; Fang, F.; Wang, K. Treatment of coal gasification wastewater by a two-continuous UASB system with step-feed for COD and phenols removal. Bioresour. Technol. 2011, 102, 5454–5460. [Google Scholar] [CrossRef] [PubMed]


| Coal | ||
|---|---|---|
| Parameter | “Six-Feet” Semi-Anthracite | “Wesoła” Bituminous |
| As received | ||
| Total Moisture Wtr, % | 1.15 ± 0.40 | 3.60 ± 0.40 |
| Ash Atr, % | 4.61 ± 0.30 | 8.74 ± 40 |
| Volatiles Vr, % | 9.92 ± 0.12 | 27.67 ± 0.50 |
| Total Sulphur Str, % | 1.55 ± 0.04 | 0.31 ± 0.02 |
| Calorific value Qir, kJ/kg | 33,416 ± 220 | 28,798 ± 200 |
| Analytical | ||
| Moisture Wa, % | 0.84 ± 0.30 | 2.18 ± 0.27 |
| Ash Aa, % | 4.62 ± 0.30 | 8.87 ± 0.63 |
| Volatiles Va, % | 9.95 ± 0.13 | 28.08 ± 0.92 |
| Combustion Heat Qsa, kJ/kg | 34,414 ± 228 | 30,317 ± 161 |
| Calorific value Qia, kJ/kg | 33,527 ± 221 | 29,258 ± 201 |
| Total Sulphur Sa, % | 1.55 ±0.04 | 0.31 ± 0.08 |
| Carbon Cta, % | 87.31 ± 0.66 | 75.35 ± 1.13 |
| Hydrogen Hta, % | 3.97 ± 0.28 | 4.61 ±0.40 |
| Nitrogen Na, % | 1.29 ± 0.12 | 1.20 ± 0.22 |
| Oxygen Oda, % | 0.50 ± 0.05 | 7.65 ± 0.1 |
| Specific Gravity, g/cm3 | 1.35 ± 0.028 | 1.40 ±0.018 |
| Vitrinite reflectance, Ro, % | 1.67 ± 0.03 | 0.91 ± 0.03 |
| Vitrinite, V, vol.% | 72 ± 6 | 59 ± 6 |
| Liplinite, L, vol.% | 0 ± 1 | 6 ± 4 |
| Inertinite, I, vol.% | 28 ± 3 | 35 ± 7 |
| Mineral matter, MM, vol.% | 2 ± 1 | 4 ± 3 |
| Element | “Six-Feet” Semi-Anthracite | “Wesoła” Bituminous |
|---|---|---|
| mg/kg (ppm) | ||
| As | 10 | 0 |
| B | 14 | 18 |
| Cd | 0 | 1 |
| Co | 10 | 0.5 |
| Cr | 73 | 0.3 |
| Cu | 25 | 13 |
| Hg | 0.22 | 0.02 |
| Mn | 218 | 357 |
| Mo | 4 | 0.1 |
| Ni | 52 | 2.6 |
| Pb | 27 | 0.8 |
| Sb | 17 | 0.4 |
| Se | 0 | 2.2 |
| Zn | 14 | 8.1 |
| % mass | ||
| Al | 1.05 | 0.07 |
| Fe | 1.04 | 1.43 |
| K | 0.09 | 0.002 |
| Ti | 0.04 | 0.001 |
| Coal Type | Semi-Anthracite “Six Feet” (South Wales, UK) | Semi-Anthracite “Six Feet” (South Wales, UK) | Bituminous “Wesoła” Coal (Upper Silesia, Poland) | Bituminous “Wesoła” Coal (Upper Silesia, Poland) |
|---|---|---|---|---|
| Gasification Reagent | O2/H2O | O2/H2O | O2/H2O | O2/H2O |
| Gasification Pressure, bar | 20 | 40 | 20 | 40 |
| Experiment duration | 96 | 96 | 96 | 96 |
| Average Gas Production Rate, Nm3/h | 9.0 | 9.4 | 9.3 | 9.4 |
| Gas Yield, Nm3/kg of coal consumed | 1.98 | 1.98 | 1.77 | 1.70 |
| Gas calorific value, Q, MJ/Nm3 | 11.7 | 12.1 | 9.2 | 10.4 |
| Coal gasified, kg | 436.1 | 455.5 | 504.0 | 530.2 |
| Total wastewater production, kg | 46.5 | 38.6 | 67.3 | 55.2 |
| Parameters | Unit | Semi-Anthracite Coal | Bituminous Coal | ||
|---|---|---|---|---|---|
| 20 Bar | 40 Bar | 20 Bar | 40 Bar | ||
| pH | pH | 6.4 | 5.2 | 5.3 | 4.9 |
| Conductivity | µS/cm | 1228.38 | 253.38 | 942 | 1006.71 |
| CODCr | mg/L O2 | 151.63 | 48.63 | 322.71 | 185.91 |
| Ammonia nitrogen | mg/L N | 160.11 | 11.68 | 96.41 | 95.74 |
| Chlorides | mg/L | 11.15 | 11.68 | 29.18 | 45.94 |
| Cyanides | mg/L | 1.11 | 1.43 | 1.7 | 0.87 |
| Total phenols volatile | mg/L | 8.45 | 0.87 | 17.04 | 24.46 |
| Sulphates | mg/L | 33.51 | 47.66 | 42.86 | 52.97 |
| Sulphides | mg/L | 1.04 | 0.04 | 0.97 | 0.02 |
| Mn | mg/L | 0.017 | 0.021 | 0.018 | 0.012 |
| Fe | mg/L | 0.823 | 0.284 | 0.131 | 0.245 |
| Sb | mg/L | 0.036 | 0.121 | 0.064 | 0.013 |
| As | mg/L | 0.036 | <0.02 | <0.01 | <0.01 |
| B | mg/L | 0.072 | 0.056 | 0.130 | 0.252 |
| Cr | mg/L | 0.013 | 0.012 | 0.010 | 0.006 |
| Zn | mg/L | 0.021 | 0.499 | 0.320 | 0.200 |
| Al | mg/L | 0.031 | 0.046 | 0.029 | 0.023 |
| Cd | mg/L | <0.0005 | 0.001 | <0.0005 | <0.0005 |
| Co | mg/L | 0.004 | 0.003 | <0.003 | <0.003 |
| Cu | mg/L | 0.005 | 0.010 | 0.009 | 0.002 |
| Mo | mg/L | 0.005 | <0.005 | 0.026 | <0.005 |
| Ni | mg/L | 0.098 | 0.312 | 0.051 | 0.027 |
| Pb | mg/L | <0.005 | 0.064 | 0.046 | 0.060 |
| Hg | mg/L | <0.0005 | <0.0005 | <0.0005 | <0.0005 |
| Se | mg/L | 0.016 | 0.017 | 0.036 | 0.027 |
| Ti | mg/L | <0.0005 | 0.001 | 0.001 | <0.0005 |
| Total BTEX | µg/L | 5483.13 | 1496.73 | 2514.32 | 1354.37 |
| Including benzene | µg/L | 4156.08 | 1341.43 | 2196.75 | 1059.07 |
| Total PAH | µg/L | 1657.98 | 361.99 | 1090.34 | 407.2 |
| Including Naphthalene | µg/L | 1321.25 | 320.88 | 905 | 305.74 |
| Total Phenols | mg/L | 29.73 | 2.14 | 49.46 | 29.25 |
| pH | Cond. | CODCr | NH4+ | Cl− | CN− | Volatile Phenols | SO42− | S2− | Fe | B | Zn | Al | Ni | Pb | Se | BTEX | PAH | Phenols | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | ||||||||||||||||||
| Cond. | 0.55 | 1.00 | |||||||||||||||||
| CODCr | 0.20 | 0.56 | 1.00 | ||||||||||||||||
| NH4+ | 0.63 | 0.99 | 0.53 | 1.00 | |||||||||||||||
| Cl− | −0.57 | −0.07 | 0.23 | −0.16 | 1.00 | ||||||||||||||
| CN− | 0.19 | 0.00 | 0.19 | 0.02 | −0.11 | 1.00 | |||||||||||||
| Volatile phenols | 0.39 | 0.77 | 0.87 | 0.75 | 0.07 | 0.18 | 1.00 | ||||||||||||
| SO42− | −0.64 | 0.05 | 0.10 | −0.02 | 0.33 | −0.14 | 0.04 | 1.00 | |||||||||||
| S2− | 0.31 | 0.44 | 0.13 | 0.46 | −0.14 | 0.02 | 0.15 | 0.13 | 1.00 | ||||||||||
| Fe | −0.10 | −0.15 | −0.14 | −0.16 | −0.03 | 0.04 | −0.15 | 0.04 | 0.06 | 1.00 | |||||||||
| B | −0.16 | 0.59 | 0.57 | 0.52 | 0.41 | −0.25 | 0.65 | 0.40 | −0.07 | −0.13 | 1.00 | ||||||||
| Zn | −0.57 | −0.55 | −0.44 | −0.56 | −0.02 | −0.02 | −0.46 | 0.30 | −0.34 | −0.20 | −0.19 | 1.00 | |||||||
| Al | −0.49 | −0.18 | −0.13 | −0.23 | 0.28 | 0.24 | −0.10 | 0.59 | −0.21 | −0.06 | 0.02 | 0.53 | 1.00 | ||||||
| Ni | −0.26 | −0.28 | −0.35 | −0.27 | −0.17 | 0.23 | −0.28 | 0.34 | −0.07 | 0.45 | −0.24 | 0.50 | 0.66 | 1.00 | |||||
| Pb | −0.71 | −0.30 | −0.15 | −0.35 | 0.36 | −0.33 | −0.22 | 0.60 | −0.21 | −0.04 | 0.22 | 0.58 | 0.58 | 0.38 | 1.00 | ||||
| Se | 0.35 | 0.87 | 0.61 | 0.83 | 0.09 | 0.03 | 0.83 | 0.14 | 0.25 | −0.10 | 0.66 | −0.41 | −0.11 | −0.25 | −0.14 | 1.00 | |||
| BTEX | 0.35 | 0.21 | 0.18 | 0.22 | −0.16 | 0.35 | 0.21 | −0.20 | 0.45 | 0.66 | −0.21 | −0.50 | −0.21 | 0.16 | −0.44 | 0.16 | 1.00 | ||
| PAH | 0.37 | 0.63 | 0.31 | 0.64 | −0.07 | −0.12 | 0.32 | 0.15 | 0.89 | −0.09 | 0.16 | −0.50 | −0.25 | −0.28 | −0.24 | 0.37 | 0.35 | 1.00 | |
| Phenols | 0.43 | 0.67 | 0.75 | 0.66 | −0.09 | 0.31 | 0.83 | −0.06 | 0.34 | −0.08 | 0.38 | −0.53 | −0.18 | −0.32 | −0.34 | 0.63 | 0.39 | 0.50 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pankiewicz-Sperka, M.; Kapusta, K.; Basa, W.; Stolecka, K. Characteristics of Water Contaminants from Underground Coal Gasification (UCG) Process—Effect of Coal Properties and Gasification Pressure. Energies 2021, 14, 6533. https://doi.org/10.3390/en14206533
Pankiewicz-Sperka M, Kapusta K, Basa W, Stolecka K. Characteristics of Water Contaminants from Underground Coal Gasification (UCG) Process—Effect of Coal Properties and Gasification Pressure. Energies. 2021; 14(20):6533. https://doi.org/10.3390/en14206533
Chicago/Turabian StylePankiewicz-Sperka, Magdalena, Krzysztof Kapusta, Wioleta Basa, and Katarzyna Stolecka. 2021. "Characteristics of Water Contaminants from Underground Coal Gasification (UCG) Process—Effect of Coal Properties and Gasification Pressure" Energies 14, no. 20: 6533. https://doi.org/10.3390/en14206533
APA StylePankiewicz-Sperka, M., Kapusta, K., Basa, W., & Stolecka, K. (2021). Characteristics of Water Contaminants from Underground Coal Gasification (UCG) Process—Effect of Coal Properties and Gasification Pressure. Energies, 14(20), 6533. https://doi.org/10.3390/en14206533







