Mill Scale Addition to Reduce Hydrogen Sulfide Production in Anaerobic Digestion
Abstract
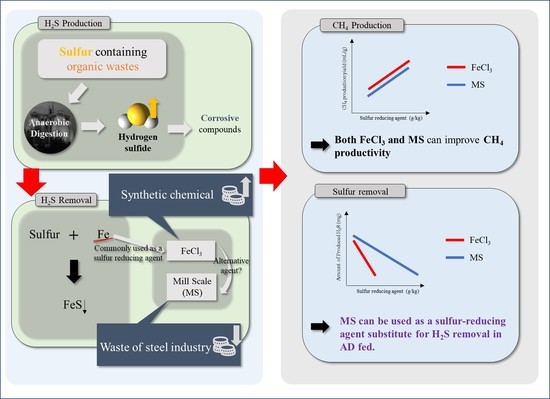
:1. Introduction
2. Materials and Methods
2.1. Preparation of Feedstock and Inoculum
2.2. Batch Test
2.3. Analytical Method
3. Results and Discussion
3.1. Effect of Iron Chloride Addition on Anaerobic Digestion
3.2. Effect of Mill Scale Addition on Anaerobic Digestion
3.3. Performance Comparison between Iron Chloride and Mill Scale Addition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abubakar, B.S.U.I.; Ismail, N. Anaerobic digestion of cow dung for biogas production. ARPN J. Eng. Appl. Sci. 2014, 7, 169–172. [Google Scholar]
- Ho, S.H.; Yang, Z.K.; Nagarajan, D.; Chang, J.S.; Ren, N.Q. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, C.; Wang, S.; Ren, N.Q.; Ho, S.H. Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chem. Eng. J. 2020, 384, 123244. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A review of biogas purification processes. Biofuels Bioprod. Biorefining 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Sposob, M.; Nam, J.Y.; Park, J.G.; Kim, T.H.; Hwang, Y.; Jeong, S.M.; Yun, Y.M. Starvation pretreatment enhances sulfidogenic operation of two-stage anaerobic digestion system for biogas production with low H2S content. J. Clean. Prod. 2021, 290, 125166. [Google Scholar] [CrossRef]
- Persson, M.; Jönsson, O.; Wellinger, A. Biogas Upgrading to Vehicle Fuel Standards and Grid Injection. IEA Bioenergy Task 2006, 37, 1–34. [Google Scholar]
- Wu, W.M.; Hickey, R.F.; Zeikus, J.G. Characterization of metabolic performance of methanogenic granules treating brewery wastewater: Role of sulfate-reducing bacteria. Appl. Environ. Microbiol. 1991, 57, 3438–3449. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Qu, Q.; Li, J.; Zhuo, Y.; Zhong, C.; Peng, D. Performance of ammonium chloride dosage on hydrogen sulfide in-situ prevention during waste activated sludge anaerobic digestion. Bioresour. Technol. 2019, 276, 91–96. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z. Desulphurisation of biogas: A systematic qualitative and economic-based quantitative review of alternative strategies. ChemEngineering 2019, 3, 76. [Google Scholar] [CrossRef] [Green Version]
- Kadam, R.; Panwar, N.L. Recent advancement in biogas enrichment and its applications. Renew. Sustain. Energy Rev. 2017, 73, 892–903. [Google Scholar] [CrossRef]
- Ahmad, W.; Sethupathi, S.; Kanadasan, G.; Lau, L.C.; Kanthasamy, R. A review on the removal of hydrogen sulfide from biogas by adsorption using sorbents derived from waste. Rev. Chem. Eng. 2019, 37, 407–431. [Google Scholar] [CrossRef]
- Regenspurg, S.; Iannotta, J.; Feldbusch, E.; Zimmermann, F.J.; Eichinger, F. Hydrogen sulfide removal from geothermal fluids by Fe (III)-based additives. Geotherm. Energy 2020, 8, 1–22. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Shirai, T.; Yamashiro, T.; Yasui, S.; Iwasaki, M.; Ihara, I.; Nishida, T.; Tangtaweewipat, S.; Umetsu, K. Valorizing waste iron powder in biogas production: Hydrogen sulfide control and process performances. J. Environ. Manag. 2018, 208, 134–141. [Google Scholar] [CrossRef]
- Ma, N.; Houser, J.B.; Wood, L.A. Production of cleaner mill scale by dynamic separation of the mill scale from the fast-moving flume water at a hot rolling mill. J. Clean. Prod. 2018, 176, 889–894. [Google Scholar] [CrossRef]
- World Steel Association. Available online: https://www.worldsteel.org/media-centre/press-releases/2021/world-steel-in-figures-2021.html (accessed on 3 June 2021).
- Martín, M.I.; López, F.A.; Torralba, J.M. Production of sponge iron powder by reduction of rolling mill scale. Ironmak. Steelmak. 2012, 39, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Lay, J.J.; Lee, Y.J.; Noike, T. Feasibility of biological hydrogen production from organic fraction of municipal solid waste. Water Res. 1999, 33, 2579–2586. [Google Scholar] [CrossRef]
- Lay, J.J.; Li, Y.Y.; Noike, T. Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res. 1997, 31, 1518–1524. [Google Scholar] [CrossRef]
- APH Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Park, C.M.; Novak, J.T. The effect of direct addition of iron (III) on anaerobic digestion efficiency and odor causing compounds. Water Sci. Technol. 2013, 68, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, S.; Yun, Y.M.; Kang, S. Stimulation of Biomethane Productivity in Anaerobic Digestion Using Electro-Conductive Carbon-Nanotube Hollow-Fiber Media. Minerals 2021, 11, 179. [Google Scholar] [CrossRef]
- Hwang, Y.; Sivagurunathan, P.; Lee, M.K.; Yun, Y.M.; Song, Y.C.; Kim, D.H. Enhanced hydrogen fermentation by zero valent iron addition. Int. J. Hydrog. Energy 2019, 44, 3387–3394. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.N.; Hwang, O.; Jung, M.W.; Ahn, B.K.; Kim, H.; Jo, G.; Yun, Y.M. Comprehensive analysis of microbial dynamics linked with the reduction of odorous compounds in a full-scale swine manure pit recharge system with recirculation of aerobically treated liquid fertilizer. Sci. Total Environ. 2021, 777, 146122. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.M.; Sung, S.; Shin, H.S.; Han, J.I.; Kim, H.W.; Kim, D.H. Producing desulfurized biogas through removal of sulfate in the first-stage of a two-stage anaerobic digestion. Biotechnol. Bioeng. 2017, 114, 970–979. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Food Waste I | Food Waste II | Inoculum |
|---|---|---|---|
| TCOD (g/L) | 102.5 ± 1.1 | 130.0 ± 0.9 | 26 ± 6 |
| SCOD (g/L) | 71.5 ± 1.0 | 75.0 ± 0.4 | 14 ± 4 |
| SCOD/TCOD (%) | 70 | 58 | 54 |
| TS (g/L) | 98.1 ± 0.6 | 113.1 ± 1.2 | 21 ± 1 |
| VS (g/L) | 85.2 ± 0.2 | 96.5 ± 0.4 | 19 ± 3 |
| Sulfur content (%) | 0.45 ± 0.00 | 0.19 ± 0.02 | 0.27 ± 0.00 |
| pH | 4.1 | 4.1 | 7.2 |
| FeCl3 Dosage (g FeCl3/kg TCOD) | CH4 Yield (mL CH4/g TCOD) | CH4 Production Rate (mL/d) | λ (d) | H2S Content (ppmv) | Total Amount of Sulfur Reduced to H2S (mg) | Theoretical Value of Sulfur Reacted with FeCl3 (mg H2S/g FeCl3) | Final pH |
|---|---|---|---|---|---|---|---|
| 0 | 211 ± 3 | 18.9 ± 0.3 | 9.0 ± 0.2 | 2,520 ± 426 | 2.37 ± 0.327 | - | 7.4 ± 0.1 |
| 11 | 258 ± 4 | 23.1 ± 1.1 | 8.0 ± 0.0 | 947 ± 267 | 0.95 ± 0.062 | 129 ± 33 | 7.4 ± 0.1 |
| 22 | 289 ± 14 | 22.9 ± 2.1 | 8.4 ± 0.2 | 271 ± 62 | 0.24 ± 0.038 | 97 ± 18 | 7.4 ± 0.2 |
| 33 | 269 ± 11 | 16.4 ± 4.3 | 7.6 ± 0.1 | 88 ± 24 | 0.08 ± 0.020 | 70 ± 13 | 7.4 ± 0.0 |
| 44 | 332 ± 2 | 31.8 ± 0.4 | 9.0 ± 0.3 | 46 ± 22 | 0.05 ± 0.037 | 53 ± 10 | 7.4 ± 0.0 |
| 67 | 283 ± 18 | 19.3 ± 5.6 | 7.7 ± 0.1 | 37 ± 20 | 0.03 ± 0.009 | 35 ± 7 | 7.4 ± 0.0 |
| 89 | 328 ± 16 | 28.2 ± 0.8 | 9.5 ± 0.0 | 19 ± 20 | 0.023 ± 0.004 | 26 ± 5 | 7.4 ± 0.1 |
| Mill Scale Dosage (g MS/kg TCOD) | CH4 Yield (mL CH4/g TCOD) | CH4 Production Rate (mL/d) | λ (d) | H2S Content (ppmv) | Total Amount of Sulfur Reduced to H2S (mg sulfur) | Theoretical Value of Sulfur Reacted with Mill Scale (mg H2S/mg MS) | Final pH |
|---|---|---|---|---|---|---|---|
| 0 | 260 ± 20 | 36.9 ± 2.0 | 9.0 ± 0.1 | 801 ± 74 | 1.88 ± 0.010 | - | 7.2 ± 0.0 |
| 5 | 251 ± 47 | 39.4 ± 1.6 | 8.0 ± 0.2 | 783 ± 120 | 1.64 ± 0.003 | 48 ± 2 | 7.2 ± 0.0 |
| 10 | 265 ± 26 | 37.1 ± 0.3 | 8.4 ± 0.0 | 703 ± 80 | 1.60 ± 0.045 | 28 ± 5 | 7.2 ± 0.1 |
| 20 | 277 ± 13 | 40.3 ± 2.2 | 7.6 ± 0.3 | 510 ± 81 | 1.20 ± 0.007 | 34 ± 0 | 7.2 ± 0.0 |
| 40 | 250 ± 51 | 46.5 ± 1.2 | 9.0 ± 0.3 | 262 ± 61 | 0.57 ± 0.092 | 33 ± 3 | 7.2 ± 0.2 |
| 160 | 287 ± 8 | 56.4 ± 0.1 | 9.5 ± 0.5 | 29 ± 29 | 0.08 ± 0.017 | 11 ± 0 | 7.2 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, B.-K.; Kim, T.-H.; Seon, J.; Park, S.-K.; Yun, Y.-M. Mill Scale Addition to Reduce Hydrogen Sulfide Production in Anaerobic Digestion. Energies 2021, 14, 6542. https://doi.org/10.3390/en14206542
Ahn B-K, Kim T-H, Seon J, Park S-K, Yun Y-M. Mill Scale Addition to Reduce Hydrogen Sulfide Production in Anaerobic Digestion. Energies. 2021; 14(20):6542. https://doi.org/10.3390/en14206542
Chicago/Turabian StyleAhn, Byung-Kyu, Tae-Hoon Kim, Jiyun Seon, Seung-Kyun Park, and Yeo-Myeong Yun. 2021. "Mill Scale Addition to Reduce Hydrogen Sulfide Production in Anaerobic Digestion" Energies 14, no. 20: 6542. https://doi.org/10.3390/en14206542