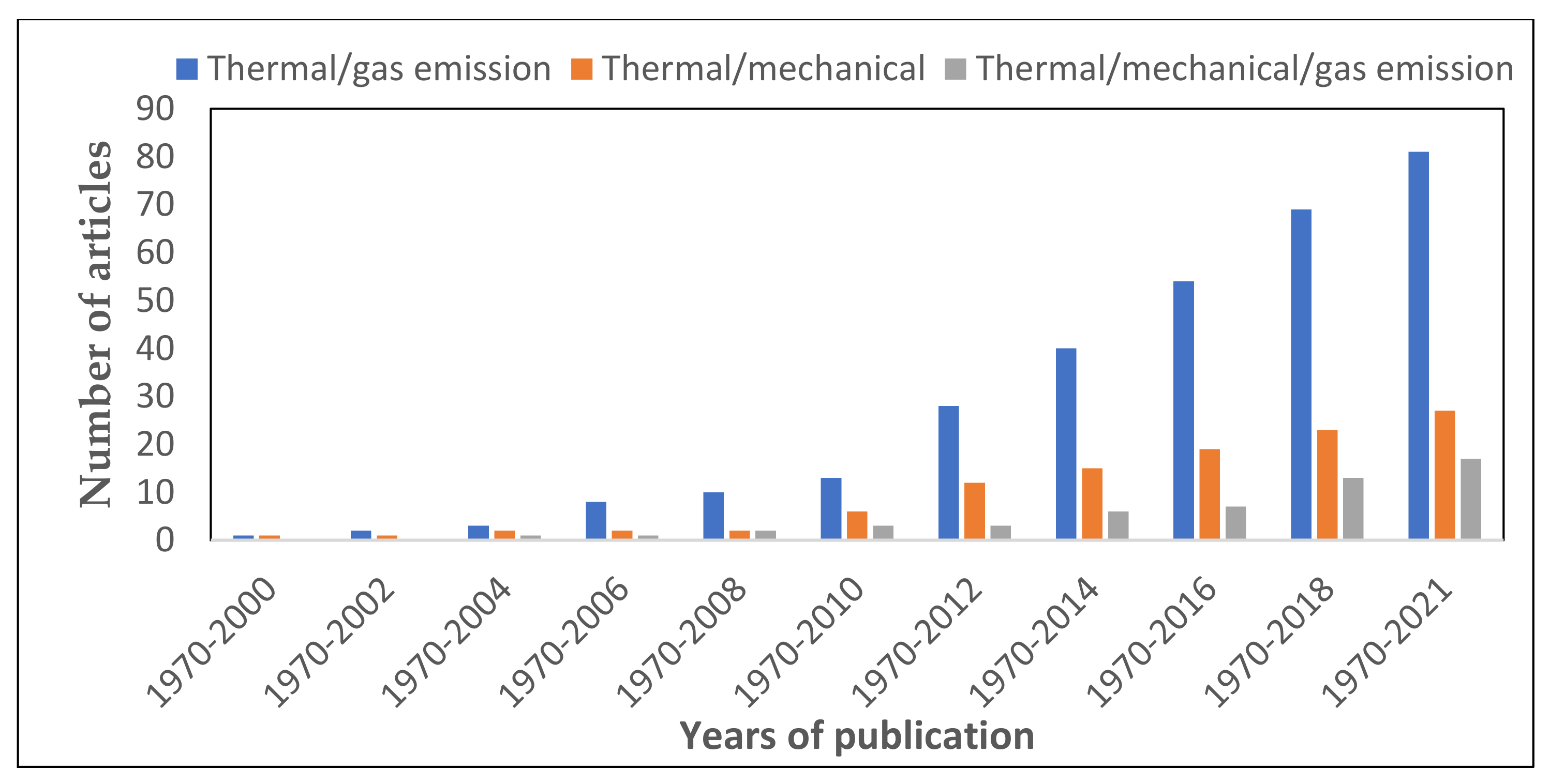
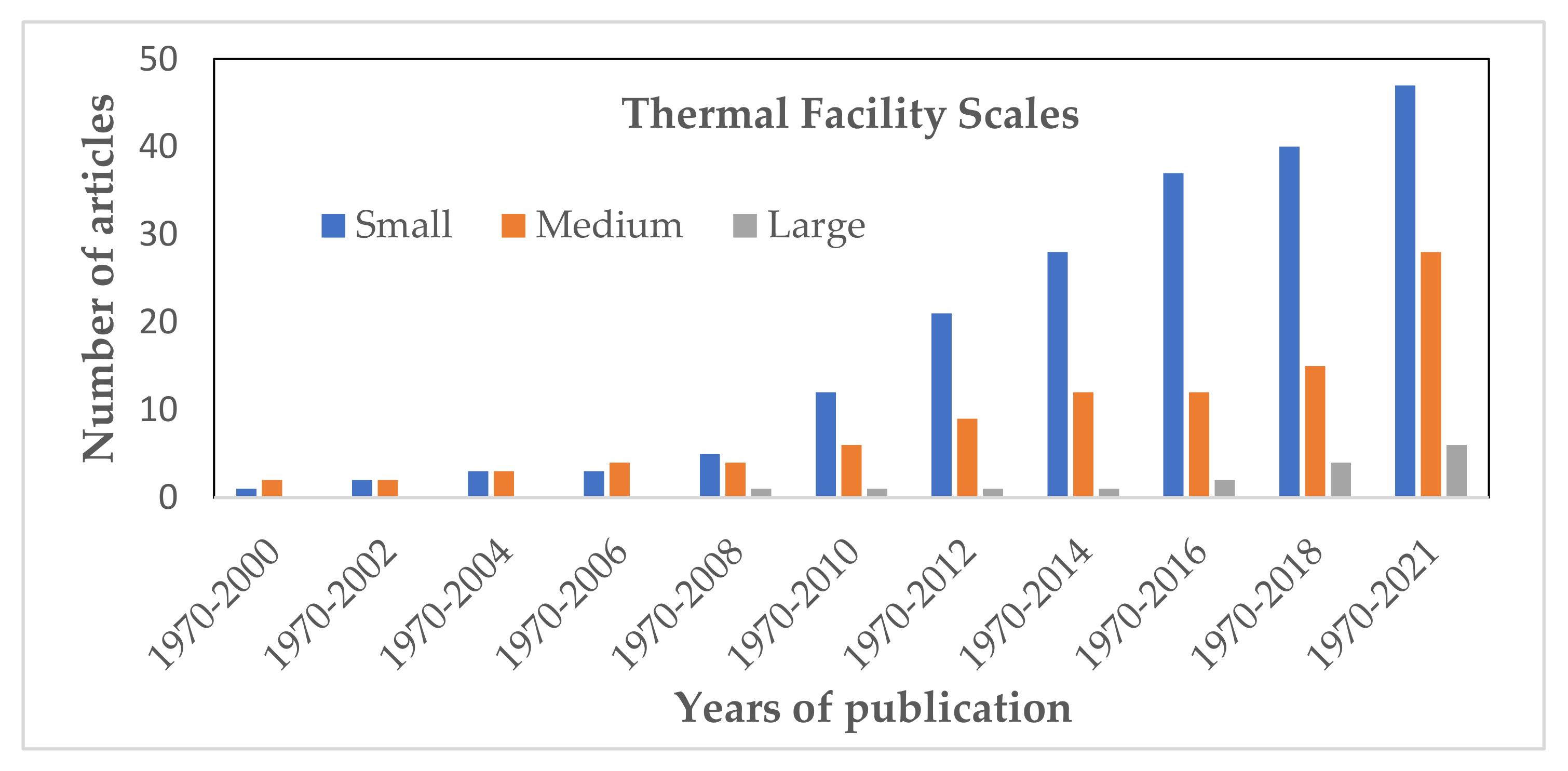
This section entails the research works reported by various research teams on the use of different classes of scales of the thermal and fire reaction devices to investigate and evaluate the fire hazard risk and both the qualitative and quantitative assessment of the gas emission species during the combustion reaction of the polymer composite materials.
3.1. Small Scale
The small-scale devices are utilised in the characterisation of the intrinsic materials properties and to evaluate the reaction mechanism of the material e.g., TGA, MCC, PFC, etc. In addition, the FTIR and mass spectrometry techniques are employed for the gas emission examination.
Table 2 highlighted some vital results of the thermal tests conducted by researchers using the small-scale facilities.
The blends of cotton/alginate fibres were prepared using alginate fibres. Alginate fibres improved the thermal stability of specimens at higher temperatures when compared to cotton fibres, as evidenced by an increase in residual chars. A Pyrolysis Combustion Fire Calorimeter (PCFC) was utilised to study the flame retardancy of the specimens [
18].
Bin et al. discovered that the inclusion of alginate fibres significantly reduces the heat release of combustion (HRC) and thermal heat release (THR) values of cotton/alginate blended fibres with various cotton/alginate fibre weight ratios. This finding suggests that alginate fibres limit the emission of combustible gases in cotton/alginate blended fibres, lowering pHRR values.
In 2010, Craig et al. developed a methodology of assessing the comparative hazard from the thermal degradation products of polymer materials using the animal technique methodology. The acute lethality and histopathological examinations were studied, and the rankings of the polymeric materials evaluated are as follows: PTFE ˃ PVC ˃ PU ˃ GFRP ˃ Copper-coated wire with mineral insulation [
10].
In 2018, Chuanmei et al. [
37] studied the flame-retardant and thermal decomposition properties of thermoplastic polyurethane (TPU) polymer composites based on HGM@[EOOEMIm][BF
4], which is prepared by the modification of hollow glass microsphere (HDM) with ionic liquid [EOOEMIm][BF
4].
The smoke-density test results revealed that the ionic liquid can remarkably decrease the amount of smoke production. The TGA results also revealed that the ionic liquid can improve the thermal stability of the TPU composites by promoting char formation in the combustion process. The flame retardancy and thermal stability of UV-curable epoxy acrylate (EA) nanocomposites based on organophilic α-ZnP (OZnP) have been studied by Weiyi et al. [
107].
Ding et al. [
108] evaluated the effect of ‘green’ carbon sources—for example starch, distarch phosphate (DSP), with acid source ammonium polyphosphate (APP)—on the flame retardancy of reconstituted tobacco sheets (RST).
The TGA results showed that the starch-based fire-retardant coating promoted the char formation and inhibited char combustion due to the mechanism of the cross-linked phosphate ester groups in the carbon sources. While the FTIR and MS analysis of the gaseous products shows that the modified RTSs release comparative harmful compounds such as CO and carbonyl compounds [
22].
The results of the MCC tests revealed a notable reduction in the heat release rate (HRR) and the peak of heat release rate (pHRR) relative to pure EA and the flame-retardant film without OZnP. In the course of thermal degradation, EA1 was easily degraded and released more gaseous compounds compared with EA2. The presence of OZnP could retard the motion and breakdown of the parent chain of the matrix resin during the thermal decomposition process.
In 2015, Lei et al. investigated the thermal degradation of hydroquinone bis (di-2-methylphenylphosphate) (HMP) by thermogravimetry (TG) coupled with Fourier Transform Infrared Spectroscopy (FTIR). The HMP disintegrated in two steps under air, according to semi-quantitative TG-FTIR studies. The HMP degraded into benzyl alcohol, hydroquinone phosphate step, carbon dioxide, water, and alkyne in the first step from 385 to 452 °C, and carbon dioxide, water, and alkyne in the second step from 491 to 800 °C [
47].
While examining the effects of interspace distance and the potential chemical reaction between PLA and the montmorillonite modifier (cloisite 15A, 20A, and 30B), Esra et al. (2016) discovered that the method of polymer incorporation into the nanocomposite is highly dependent on the mixing technique, the interspacing between the clay layers, and, most importantly, the type of organic modifier used [
46]. In addition, Esra et al. showed evidently from the result of the TGA that as the amount of cloisite increased, a slight increase in the char yield mainly due to the clay particles incorporated was detected.
The relative yield of products due to trans-esterification processes was reduced in the presence of water vapour. As the partial pressure of water was increased, the thermal degradation of PLA nanocomposites was changed to a low temperature.
Kun et al. [
65] used a reaction between trisilanolisobutyl-POSS and triglycidyl isocy-anurate to make a functional polyhedral oligomeric silsequioxane (NPOSS) with an epoxy ring linked, and then, they made a halogen-free epoxy composite containing silicon/nitrogen. According to the findings of the MCC, the presence of NPOSS (10% weight ratio) in epoxy resin (EP) can reduce its peak heat release rate by roughly 30%. DSC, TG, and TG-FTIR were used to characterise the thermal oxidation and degradation behaviour of EP and EP/NPOSS composites. In the condensed phase, NPOSS can slow down the movement and scission of EP polymeric chains, forming a solid charred layer that protects the underlying materials from further combustion.
Maria et al. [
81] examined the thermal degradation and fire behaviour of an unsaturated polyester resin with phosphate-based fire-retardant additives and its corresponding glass fibre composites in 2012. Three phosphate-based fire retardants were added to the commercial polyester resin at a ratio of 35%
w/
w: ammonium polyphosphate, silane-coated ammonium polyphosphate, and melamine pyrophosphate.
Then, the effects of the fire retardants on polymer thermal decomposition and small-scale fire behaviour were investigated utilising dynamic thermogravimetric tests (DTG) at various heating rates and microcalorimetric measurements using a FAA micro-calorimeter in accordance with ASTM D07309-07. According to the findings of Maria et al., the ammonium polyphosphate-containing resin outperformed the other systems in terms of thermal and fire performance. A vacuum infusion process was utilised to make unidirectional glass fibre composites.
Karen et al. (1997) examined the decomposition products of fire-retarded glass fibre-reinforced epoxy composites using FTIR spectroscopy.
This bench-scale study focused on the identification and quantification of toxic species. The FTIR spectroscopy identified toxic effluent species over a wide range of composite exposure temperatures (100 to 1000 °C), during pyrolysis and combustion [
64].
Formaldehyde, methanol, carbon mono oxide, nitric oxide, methane, and benzene were identified by the spectral analysis. In addition, as the thermal decomposition progressed, their oxides concentrations were observed. Thus, the material fire behaviour profile created by the decomposition mechanisms combined by the physical observations provides an evaluation of product fire safety performance and a basis for early fire detection.
Ku et al. [
92] examined the thermal degradation and flame retardancy of epoxy (EP) composites containing micro-crystalline cellulose whiskers (MCW) and micro-encapsulated ammonium polyphosphate (MFAPP).
When compared to EP and EP/MFAPP, the MCC test at 6 wt % loading revealed that its heat release rate lowers. The explanation for this is that EP composites with MCW have a higher charring capability in a fire.
Ku et al. also revealed from the TG and TG-FTIR results that at lower temperature, MFAPP stimulates the dehydration of MCW and EP and releases gases that are useful for the formation of an intumescent char.
Furthermore, at high temperatures, the residue does not emit any dangerous gas and acts as an effective insulation layer on the sample’s surface, protecting the underlying material from fire.
3.2. Medium Scale
Researchers have performed quite a number of studies on the fire behaviour and the protection of polymer composite materials used especially as vital components in the transport sectors and building element under fire condition [
109]. The bench-scale platform of the medium scale has provided significant fire testing parameters that indicate the fire reaction and fire resistance of the materials assessed (as shown in
Table 3,
Table 4,
Table 5 and
Table 6) e.g., a cone calorimeter test (CCT), limiting oxygen index (LOI), and underwriter’s laboratories (UL-94). Thus, the results have been a useful guide in the evaluation of the fire hazard risk and the smoke and toxic gases examination.
Gallo et al. (2013) prepared a flame-retarded styrene butadiene rubber composite. A well-dispersed spherically-shaped amorphous silicon dioxide with a broad size distribution (Sidistar) was used as the flame retardant in combination with two different kinds of aluminium trihydride (ATH): a finely precipitated form (ATH-A) and a coarse-grained form (ATH-B). Gallo et al. then investigated the thermal stability, gas transport, and fire properties with and without the Sidistar [
21].
The addition of Sidistar (synergist) slowed the release of pyrolysis gases through the matrix, lengthening the time before ignition. The oxygen index of materials with Sidistar and those with enhanced ATH content increased slightly in the LOI test. When the ATH content was raised and Sidistar was added, the horizontal burning rate in UL 94 indicated flame retardancy. When Sidistar was added to the cone calorimeter experiments, fire hazards such as pHRR, TpHRR, and THR were reduced, as were fire risks when ATH-B was used instead of ATH-A and the ATH content was raised.
The flame-retardant Disflamoll TP LXS 51064 based on an ammonium polyphosphate (APP) ester was incorporated in wood biocomposite without the use of a carbonising agent due to the change in the chemical nature of the material. The heteroatoms of the cellulose and the aromatic rings of lignin act as carbonisation agents for residue formation. Its exact disintegration mechanism, as well as its fire-retardant mechanism, have yet to be explored.
Therefore, in 2020, Henrik and Ulrike studied the flame retardant relative to its thermal decomposition and its mode of action in wood–plastic composites (WPCs) by using cone calorimeter (fire tests) and FTIR (pyrolysis and gas emission investigation) [
13].
Basically, the addition of the non-flammable fire-retardant (FR) to the WPC material reduced the HRR. In fact, a numerical value of 347 kW/m2 was obtained with 3 wt % FR, and then, it was further reduced to 311 kW/m2 when 10 wt % FR was incorporated.
The mechanism of the flame retardant was found to act majorly in the condensed phase by increasing the quantity of residue formed by the wood component of the WPC. Further flame dilution is achieved by the production of water, ammonia, and carbon dioxide during the decomposition of the flame retardant [
108].
Using a cone calorimeter, Talal et al. [
1] investigated the effect of various layers (thickness) on the thermal degradation and fire reaction properties of carbon fibre-reinforced epoxy and fibre-reinforced phenolic resin samples. The key fire reaction parameters, such as time-to-ignition and the peak of heat release rates, were shown to be dependent on the number of layers, according to the researchers’ findings. In addition, the amount of smoke and the carbon dioxide emission levels during the thermal degradation were found to decrease as the number of layers increased [
7]. It can be noticed for epoxy-reinforced composite that the total smoke production (TSP) of the smoke released in case of the specimen 4.2 mm is much higher than in case of the specimen 2.1 mm. However, the smoke is released much quicker in case of the thinner composite (80 s), while the thicker composite has a peak around 120 s. Similarly, as anticipated, the quantity of smoke generated for phenolic composite was found to increase with an increase in the number of layers, except for the one with nine plies.
Blended fibres of cotton/alginate can be utilised as filling materials for the production of toys, pillows, and sleeping bags. According to the results obtained by Bin et al., the flammability of the fibre blend can meet the combustion requirements for its outdoor applications. Recently, Bin et al. (2020) prepared cotton/alginate blends fibres [
18]. Relative to cotton fibres, alginate fibres enhanced the thermal stabilities of the specimens at higher temperatures, showing in the increase in the char residues.
It also enhanced the flame-retardant properties and fire behaviour. Relative to that of cotton fibres, there are 26% (cotton4/alginate1), 43% (cotton5/alginate5), and 68% (cotton1/alginate4) reductions in FIGRA, respectively. As revealed in
Table 4, the lower FIGRA values of cotton/alginate blended fibres mean the lower fibre hazard of the materials [
109].
The alginate fibres clearly reduced the total heat release, peak heat release rate, total smoke production, and the CO2 production.
In 2008, Albert et al. enhanced the flame retardancy of polypropylene (PP) via the chemical activity (catalytic effects) of carbon nanotubes and polyhedral oligomeric silsesquioxane (POSS) in the course of thermal degradation and combustion.
The inclusion of nanotubes was discovered to accelerate the oxidative dehydrogenation in PP, resulting in the formation of a stable carbon char surface layer that acts as an efficient oxygen barrier. A similar action was performed by metal-containing polysilsequioxanes dispersed in PP [
3,
47].
Glass fibre-reinforced polymer composites’ substantial fire dangers have severely hampered their research and applications. Wei et al. [
4,
39] treated the composites with flame retardants to minimise their flammability. The usage of three trivalent metal hypophosphite halogen-free flame retardants (Al, La, Ce) is investigated in this study. The thermal and combustion behaviour of glass fibre-reinforced polybutylene terephthalate (GRPBT) composites were quantitatively investigated using cone calorimeter and FTIR techniques. The results showed that the pHRR and TSP of GRPBT/cerium hypophosphite (CHp) composites were reduced by 76% and 44%, respectively.
Table 5 reveals the work of Hongxia et al. [
58,
60], who examined the effects and mechanisms of aluminium phosphinate (AlPi) on the flame retardancy of three polymers: thermoplastic polyurethane (TPU), poly (2,6-dimethyl-1,4-phenylene oxide) (PPO), and polypropylene (PP).
Their fire reaction properties were assessed using the cone calorimeter test (CCT), vertical burning tests (UL-94), and limiting oxygen index (LOI).
The results revealed that the amount of AlPi needed to reach a V-0 UL-94 rating in the TPU, PPO, and PP matrices were 0 wt %, 30 wt %, and 50 wt %, respectively. Moreover, the addition of AlPi significantly increased the LOI values of PPO and PP. AlPi also decreased the pHHR of PPO and TPU, whereas that of PP increased. The flame inhibition process for AlPi/TPU and AlPi/PP could be attributed to the synergistic reaction of phosphorus and nitrogen in the gas phase during the burning process, therefore increasing the CO production.
As a result of its strong chemical resistance and mechanical qualities, epoxy resin (EP) is a widely utilised thermosetting resin in different industrial domains such as paints and electronic goods. EP, on the other hand, is extremely combustible, limiting its use. Therefore, Zong-Min et al. [
58,
110] synthesised a novel flame retardant of P/N-containing oligomer poly (piperazine phenyl-phosphamide) (BPOPA), which was used to improve the flame retardancy of EP resin/ammonium polyphosphate (APP).
The LOI value of EP containing 10% APP alone was 30.2%; however, the UL-94 received no rating.
EP/7.5APP/2.5BPOPA received a UL-94 V-0 grade with an LOI value of 33.1% when both 7.5 wt % APP and 2.5 wt % BPOPA were added to it.
Furthermore, when compared to EP/10APP, EP/7.5APP/2.5BPOPA had significantly reduced pHRR and TSP values.
Salasniska et al. [
111] presented a novel intumescent fire retardant (FR) system based on ground hazelnut shell (HS) and L-histidinium dihydrogen phosphate-phosphonic acid (LHP) into epoxy resin (EP) in order to test their performance as fire retardants and their synergistic impact in 2020. Cone calorimeter and UL-94 tests were used to assess the burning behaviour and smoke production. FTIR was also used to analyse the volatile compounds that formed during the thermal decomposition and burning processes. Salasniska et al. found that the system containing 5 wt % of LHP led to the formation of a swollen char with numerous closed cells, which reduced the burning process as well as smoke emission.
Recently, Haowei et al. (2020) [
50,
88] produced PLA/IFM composites with Ti
3C
2 MXene nanosheets by melt blending and then investigated the synergistic effects of MXene on the fire performance of the PLA/IFR system by substituting parts of the IFM with MXene.
It was revealed by Haowei et al. that the addition of 1 wt % MXene and 11 wt % IFR leads to an apparent increase in LOI (160.4%) and an obvious reduction in pHRR (64.6%), achieving a V-0 rating in UL-94. Furthermore, the cone calorimeter test results and other analysis of the carbon residues of PLA/IFR/MXene composites revealed that the nano TiO2 catalyst and two-dimensional nanosheet barrier efficiently enhanced the tendency of intumescent char formation by the PLA/IFR systems, thereby inhibiting further flame propagation and the spread of fire hazard.
Rigid poly(urethane-isocyanurate) (PIR) foams have been used in everyday life, and insulation materials play a vital role in the modern built environment, transportation, and industrial applications. However, the fire reactivity (thermal and chemical) of this kind of product remains one of the most limiting factors for their wider applications in some sectors.
Therefore, in order to increase the thermal stability of these types of foam, Damien et al. [
15,
98] investigated at medium scale the effects of a reduced oxygen atmosphere on the reaction of fire of flame-retardant PIR foams. This experiment was carried out using a standard controlled-atmosphere cone calorimeter (ISO 5660) coupled with a Fourier Transform Infrared Spectrometer. Damien et al. presented and discussed the dependence of the results on both oxygen concentration (from 0 up to ambient condition) and irradiance level (20 to 50 kW/m
2). At the end, the chemical compositions of the identified gaseous products were qualified and quantified during the decomposition and combustion processes. The combustion reaction in the gaseous phase releases mainly CO
2, CO, and H
2O as well as other species such as HCN and HCL.
In 2015, Zhuoli et al. [
72,
97] investigated the thermo-oxidative degradation behaviour and fire performance of both homogenous and heterogenous high-impact polystyrene (HIPS). The high-impact polystyrene (HIPS)-based composite was alternatively filled by magnesium hydroxide (MH) and microencapsulated red phosphorus (MRP) flame retardants and pure HIPS sheets; thus, a HIP/MH/MRP composite with an alternating layered structure was successfully prepared through layer-on-layer melt laminating.
In comparison to its homogeneous counterpart containing the same quantity of flame retardant, the alternating composite demonstrates higher thermo-oxidative stability, flame retardancy, smoke suppression, and decreased toxic gas emission. The alternating composite can produce not only more charred residue but also more continuous and compact residue, which acts as an insulator on the composite surface and prevents heat transmission and mass transfer between the flame in the gas phase and the underlying polymers in the condensed phase.
Furthermore, the remarkable decreased smoke and toxic gas release revealed by the alternating composite in the cause of the combustion process is extremely important to reduce the harm to people in case of fires.
Zhuoli et al. summarised on the whole that the fire safety performance of the alternating composite improves to a large extent. This research work (as shown in
Table 6) focus presents another technique to further increase the flame retardancy and smoke inhibition of polymer composites. The thermal decomposition and fire behaviour of an unsaturated polyester resin and the corresponding glass fibre (GF) composites were investigated.
In order to improve the fire behaviour of the polyester resin, different phosphate fire retardants, ammonium polyphosphate (APP), silane-coated ammonium polyphosphate (S-APP), and melamine pyrophosphate (MPP) were dispersed within the resin.
According to Maria et al. [
81,
112], using the cone calorimeter, a reduction of pHRR (21%) and THR (39%) for the fire-retarded composite were measured.
Thus, the findings of the researchers indicated that APP is efficient as a flame retardant of polyester-based glass fibre-reinforced composites due to the capacity to form a good-quality char.
Compared with poly (butylene terephthalate) (PBT), glass fibre-reinforced (butylene terephthalate) (GF-PBT) is difficult to flame retard with halogen-free flame retardants. In the investigation by Li et al. [
58], aluminium salt of hypophosphorous acid (AP) was used as a flame retardant for GF-PBT. The addition of AP to the composites could result in an increase in LOI value, a UL-94 V-0 (1.6 mm) classification, and a better fire performance in CCT. Furthermore, the mechanical properties of the flame-retardant composites were not deteriorated, retaining an acceptable level.
Furan resin nanocomposites packed with silica nanoparticles were synthesised by Marco et al. [
42,
108]. A commercially available water-based suspension of silica particles was used to develop a dispersion method that could be easily scaled up to the industrial level. To improve the interaction between the silica particles and the matrix, various treatments with silanes were carried out. Although furan resin has a minimal fire danger by itself, the introduction of silica nanoparticles improves its performance in both flaming combustion and thermal resistance of the combustion char. This is owing to the protective silica skin that forms on the deteriorating furan resin as it burns.
3.3. Large Scale
The Federal Aviation Administration (FAA) developed the NexGen burner to replace the traditional oil burners used for mandatory fire certification tests on power plant-related components. Since it has the capacity to adjust both air and fuel flow rates, this is conceivable. The time to failure and mass loss of several composites during combustion are shown in
Table 7.
NexGen can simulate the fire condition specified by the FAA standard (ISO2685:1998 and AC20-135). However, according to Yi-Huan et al. (2017) [
113], the current calibration criteria may not guarantee repeatability, necessitating the study of the sensitivity of performance of the burner to air and fuel flow rate, as measured by the temperature and heat flux for calibration purposes.
It was demonstrated that different burner operating settings might satisfy current calibration standards, resulting in differing outcomes during/after tests.
Using smaller thermocouples during calibration resulted in a test with less severe burner damage, allowing the test sample to survive longer.
Horold et al. [
25] examined the fire stability of polymer composite materials in structural application. A large-scale fire test procedure under fully developed fire was applied to a series of glass fibre composites (GFCs) and a fire-retardant glass fibre composite (GFC-FR) with the dimensions of 500 × 500 × 24 mm. Particularly, the effect was on the altered core structure (polyvinylchloride foam, polyisocyanurate foam reinforced with stitching the two skins together and balsa wood).
Some physical deformation of the composites was observed by Horold et al., such as cracks and delamination. The results obtained reveal that the fire resistance depends on the core material, which was able to prolong the time to failure by 2.1–3.4 times compared to the control sample. It was also observed that the core materials enhanced the performance even more than the flame retardants by a factor of 1.2–2.1; however, the intumescent flame retardant crucially improved the performance of each material, and the best result was achieved in the case of FR-GFC (stitched) (4.4 times the control sample).
The shell specimen that are representative of a typical carbon-reinforced polymer in modern civil aviation were exposed to a fully developed fire with direct flame impingement to one side at a heat flux of 182 kW/m
2. Sebastian et al. [
24] looked at the time to failure of CFRP shells with and without flame-retarding designs. The unprotected CFRP specimens broke after only 27 s, while the specimens with integrated protective layers that had low heat of conductivity and strong burn through resistance showed the best results. An integrated titanium foil decelerated the decomposition of the epoxy matrix and increased the time to failure by 68% compared to the unprotected CFRP shell.
Grange et al. [
79] investigated the fire behaviour of three carbon-reinforced composites in a large-scale experiment. The carbon–polyetheretherketone (carbon/PEKK) composite has greater thermal stability, with less temperature variation and a moderate backside face temperature, according to their findings. Furthermore, these materials have a limited HRR value, which is up to five times lower than carbon-phenolic and three times lower than carbon-bismaleimide, limiting fire spread.
Eliot et al. [
30] developed a kerosene flame test bench in order to analyse and compare the fire behaviour of composites mainly for aeronautic applications.
Two carbon-reinforced polymer composites based on thermosetting carbon/epoxy and thermoplastic carbon/polyphenylene sulphide with the same thickness and stacking sequence were studied. The results obtained revealed that the thermosetting-based laminate decomposed very fast and reached higher final temperature at the back face.
After 15 min, the thermoplastic-based composite was not fully decomposed and a significant swelling was observed, which seems to induce an important thermal protection effect.