CO2 Recycling in the Iron and Steel Industry via Power-to-Gas and Oxy-Fuel Combustion
Abstract
:1. Introduction
2. Description of Case Studies
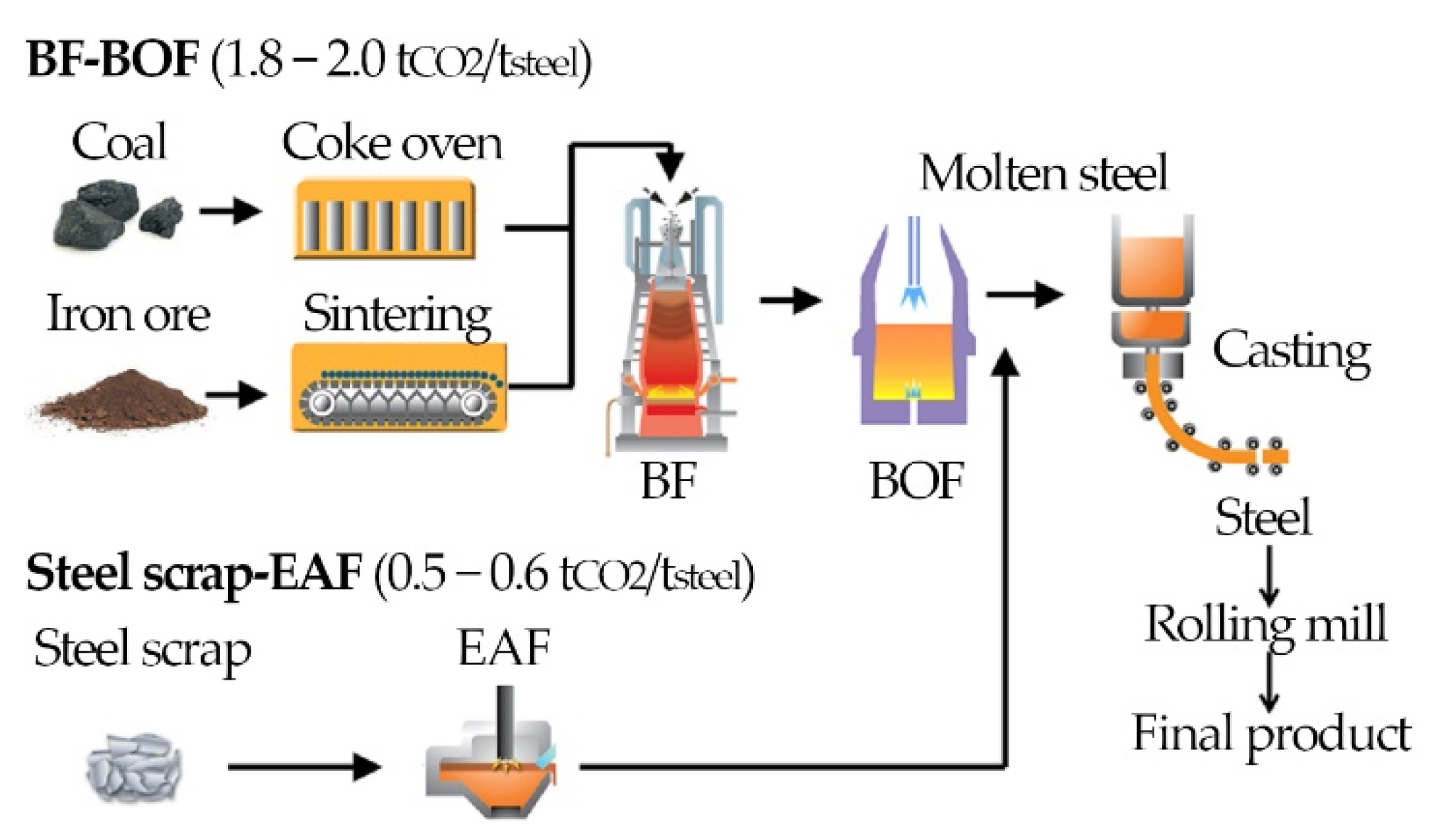
2.1. Case 0: Reference Plant for BF-BOF Ironmaking
2.2. Case 1: Power-to-Methane Integration in Ironmaking with Oxy-Fuel Combustion and TGR
2.3. Case 2: Methanation of COG Integration in Ironmaking with Oxy-Fuel Combustion and TGR
3. Methodology
3.1. Iron and Steel Plant
3.2. Power Plant
3.3. Power-to-Gas Plant
4. Results and Discussion
4.1. Case 0: Reference Plant for BF-BOF Ironmaking
4.2. Case 1: Power-to-Methane Integration in Ironmaking with Oxy-Fuel Combustion and TGR
4.3. Case 2: Methanation of COG Integration in Ironmaking with Oxy-Fuel Combustion and TGR
4.4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASU | air separation unit |
| BAT | best available technology |
| BF | blast furnace |
| BFG | blast furnace gas |
| BOF | basic oxygen furnace |
| BOFG | basic oxygen furnace gas |
| CDQ | coke dry quenching |
| CO | coke oven |
| COG | coke oven gas |
| PtG | power-to-gas |
| SNG | synthetic natural gas |
| TGR | top gas recycling |
Appendix A. Stream Data
| Stream | cp | m | T | Stream | cp | m | T | Stream | cp | m | T |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (kJ/kg.K) | (kg/kgsteel) | (°C) | (kJ/kg.K) | (kg/kgsteel) | (°C) | (kJ/kg.K) | (kg/kgsteel) | (°C) | |||
| 1 | 0.473 | 1.426 | 25 | 49 | 0.907 | 0.085 | 25 | 180 | 4.18 | 0.08955 | 25 |
| 2 | 0.835 | 0.0713 | 25 | 101 | 0.473 | 1.426 | 25 | 181 | 4.18 | 0.2414 | 25 |
| 3 | 0.473 | 1.426 | 800 | 102 | 0.835 | 0.0713 | 25 | 182 | 14.34 | 0.02701 | 25 |
| 4 | 0.473 | 1.426 | 150 | 103 | 0.473 | 1.426 | 800 | 183 | 0.914 | 0.2144 | 25 |
| 5 | 1.005 | 0.6232 | 25 | 104 | 0.473 | 1.426 | 150 | 184 | 2.239 | 0.06506 | 25 |
| 6 | 1.126 | 0.6232 | 650 | 105 | 1.005 | 0.6232 | 25 | 185 | 4.18 | 0.1016 | 25 |
| 7 | 1.126 | 0.4762 | 650 | 106 | 1.126 | 0.6232 | 650 | 186 | 4.18 | 2.665 | 25 |
| 8 | 1.126 | 0.147 | 650 | 107 | 1.126 | 0.4762 | 650 | 187 | 4.18 | 2.665 | 80 |
| 9 | 1.426 | 0.08527 | 25 | 108 | 1.126 | 0.147 | 650 | 190 | 1.005 | 0.7772 | 25 |
| 10 | 1.012 | 0.2374 | 500 | 109 | 1.426 | 0.08527 | 25 | 191 | 1.038 | 0.5938 | 25 |
| 11 | 0.835 | 0.5238 | 25 | 110 | 1.012 | 0.2374 | 500 | 192 | 0.914 | 0.1803 | 25 |
| 12 | 0.836 | 0.4191 | 1100 | 111 | 0.835 | 0.4568 | 25 | 193 | 0.914 | 0.3947 | 25 |
| 13 | 0.836 | 0.4191 | 150 | 112 | 0.836 | 0.3654 | 1100 | 194 | 0.914 | 0.08873 | 25 |
| 14 | 9.035 | 0.1048 | 1350 | 113 | 0.836 | 0.3654 | 150 | 195 | 1.179 | 0.08873 | 1650 |
| 15 | 1.005 | 0.668 | 25 | 114 | 9.035 | 0.09136 | 1350 | 196 | 1.117 | 0.04026 | 25 |
| 16 | 9.035 | 0.04969 | 25 | 115 | 1.005 | 0.4562 | 25 | 197 | 1.005 | 0.05914 | 25 |
| 17 | 1.012 | 0.7408 | 500 | 116 | 1.117 | 0.3105 | 25 | 198 | 1.012 | 0.104 | 600 |
| 18 | 1.038 | 0.3646 | 25 | 117 | 1.012 | 0.8021 | 500 | 200 | 0.907 | 0.085 | 25 |
| 19 | 1.178 | 0.3646 | 800 | 118 | 1.038 | 0.318 | 25 | 201 | 0.749 | 0.085 | 1650 |
| 20 | 1.005 | 1.278 | 25 | 119 | 1.178 | 0.318 | 800 | 202 | 1.005 | 0.06183 | 25 |
| 21 | 1.208 | 1.278 | 1200 | 130 | 0.914 | 0.3059 | 25 | 203 | 1.154 | 0.06183 | 800 |
| 22 | 9.035 | 0.05062 | 25 | 131 | 1.142 | 0.3059 | 1200 | 204 | 0.749 | 0.085 | 460 |
| 23 | 1.005 | 0.6805 | 25 | 132 | 0.842 | 0.5324 | 25 | 205 | 1.426 | 0.1346 | 1650 |
| 24 | 1.012 | 0.7547 | 300 | 133 | 1.323 | 0.5324 | 1200 | 206 | 0.5 | 1 | 1650 |
| 25 | 0.907 | 0.283 | 25 | 134 | 1.117 | 0.4782 | 25 | 207 | 1.412 | 0.04795 | 25 |
| 26 | 1.412 | 2.084 | 200 | 135 | 1.005 | 0.7025 | 25 | 208 | 1.005 | 0.08269 | 25 |
| 27 | 0.749 | 0.283 | 1200 | 136 | 1.012 | 1.235 | 600 | 209 | 1.012 | 0.1335 | 850 |
| 28 | 0.749 | 0.283 | 460 | 137 | 1.037 | 0.53 | 25 | 210 | 0.5 | 1 | 25 |
| 29 | 1.005 | 0.1754 | 25 | 138 | 1.254 | 0.53 | 1200 | 334 | 1.412 | 0.4782 | 25 |
| 30 | 1.154 | 0.1754 | 800 | 140 | 2.239 | 0.06506 | 25 | 335 | 1.005 | 0.7025 | 25 |
| 31 | 0.48 | 1.039 | 1200 | 141 | 5.632 | 0.06506 | 1200 | 336 | 1.012 | 1.235 | 600 |
| 32 | 1.005 | 0.3825 | 25 | 142 | 1.117 | 0.1019 | 25 | 342 | 1.412 | 0.1019 | 25 |
| 33 | 0.914 | 0.08873 | 25 | 143 | 1.005 | 0.1498 | 25 | 343 | 1.005 | 0.1498 | 25 |
| 34 | 1.038 | 0.2922 | 25 | 144 | 1.012 | 0.2633 | 600 | 344 | 1.012 | 0.2633 | 600 |
| 35 | 1.179 | 0.08873 | 1650 | 150 | 0.907 | 0.283 | 25 | 374 | 1.412 | 0.01384 | 25 |
| 36 | 1.005 | 0.07301 | 25 | 151 | 0.749 | 0.283 | 1200 | 376 | 1.098 | 1.059 | 25 |
| 37 | 9.035 | 0.00543 | 25 | 152 | 0.749 | 0.283 | 460 | 382 | 9.035 | 0.09106 | 25 |
| 38 | 1.012 | 0.08096 | 600 | 153 | 1.005 | 0.1754 | 25 | 385 | 4.18 | 0.00883 | 25 |
| 39 | 1.426 | 0.1346 | 1650 | 154 | 1.154 | 0.1754 | 800 | 386 | 4.18 | 0.8714 | 25 |
| 40 | 0.749 | 0.085 | 1650 | 155 | 0.48 | 1.039 | 1200 | 387 | 4.18 | 0.8714 | 80 |
| 41 | 0.749 | 0.085 | 460 | 156 | 1.117 | 2.186 | 200 | 390 | 1.005 | 1.701 | 25 |
| 42 | 1.005 | 0.0847 | 25 | 157 | 1.098 | 2.136 | 25 | 391 | 1.038 | 1.3 | 25 |
| 43 | 1.154 | 0.0847 | 800 | 158 | 4.18 | 0.05031 | 25 | 392 | 0.914 | 0.3947 | 25 |
| 44 | 0.5 | 1 | 1650 | 159 | 4.18 | 2.352 | 25 | 396 | 1.412 | 0.04026 | 25 |
| 45 | 0.5 | 1 | 25 | 160 | 4.18 | 2.352 | 80 | 397 | 1.005 | 0.05914 | 25 |
| 46 | 1.426 | 0.04795 | 25 | 174 | 1.098 | 0.1389 | 25 | 398 | 1.012 | 0.104 | 600 |
| 47 | 1.005 | 0.08269 | 25 | 175 | 1.098 | 1.062 | 25 | ||||
| 48 | 1.012 | 0.1335 | 850 | 176 | 1.098 | 0.9343 | 25 |
References
- Huitu, K.; Helle, H.; Helle, M.; Kekkonen, M.; Saxén, H. Optimization of steelmaking using fastmet direct reduced iron in the blast furnace. ISIJ Int. 2013, 53, 2038–2046. [Google Scholar] [CrossRef] [Green Version]
- Quader, M.A.; Ahmed, S.; Ghazilla, R.A.R.; Ahmed, S.; Dahari, M. A comprehensive review on energy efficient CO2 breakthrough technologies for sustainable green iron and steel manufacturing. Renew. Sustain. Energy Rev. 2015, 50, 594–614. [Google Scholar] [CrossRef]
- He, K.; Wang, L. A review of energy use and energy-efficient technologies for the iron and steel industry. Renew. Sustain. Energy Rev. 2017, 70, 1022–1039. [Google Scholar] [CrossRef]
- Ariyama, T.; Takahashi, K.; Kawashiri, Y.; Nouchi, T. Diversification of the Ironmaking Process Toward the Long-Term Global Goal for Carbon Dioxide Mitigation. J. Sustain. Metall. 2019, 5, 276–294. [Google Scholar] [CrossRef]
- Lisbona, P.; Bailera, M.; Peña, B.; Romeo, L.M. Integration of CO2 capture and conversion. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 503–522. ISBN 9780128196571. [Google Scholar]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M. Power to gas-oxyfuel boiler hybrid systems. Int. J. Hydrog. Energy 2015, 40, 10168–10175. [Google Scholar] [CrossRef] [Green Version]
- Romeo, L.M.; Bailera, M. Design configurations to achieve an effective CO2 use and mitigation through power to gas. J. CO2 Util. 2020, 39, 1–10. [Google Scholar] [CrossRef]
- Bailera, M.; Hanak, D.P.; Lisbona, P.; Romeo, L.M. Techno-economic feasibility of power to gas–oxy-fuel boiler hybrid system under uncertainty. Int. J. Hydrog. Energy 2019, 4, 9505–9516. [Google Scholar] [CrossRef] [Green Version]
- Eveloy, V. Hybridization of solid oxide electrolysis-based power-to-methane with oxyfuel combustion and carbon dioxide utilization for energy storage. Renew. Sustain. Energy Rev. 2019, 108, 550–571. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Peña, B.; Romeo, L.M. A review on CO2 mitigation in the Iron and Steel industry through Power to X processes. J. CO2 Util. 2021, 46, 101456. [Google Scholar] [CrossRef]
- Kirschen, M.; Risonarta, V.; Pfeifer, H. Energy efficiency and the influence of gas burners to the energy related carbon dioxide emissions of electric arc furnaces in steel industry. Energy 2009, 34, 1065–1072. [Google Scholar] [CrossRef]
- Rosenfeld, D.C.; Böhm, H.; Lindorfer, J.; Lehner, M. Scenario analysis of implementing a power-to-gas and biomass gasification system in an integrated steel plant: A techno-economic and environmental study. Renew. Energy 2020, 147, 1511–1524. [Google Scholar] [CrossRef]
- Steelmaking Technologies. CISDI Ingeniering CO. LTD. Available online: http://www.cisdigroup.com/4-steelmaking.html (accessed on 15 September 2021).
- He, H.; Guan, H.; Zhu, X.; Lee, H. Assessment on the energy flow and carbon emissions of integrated steelmaking plants. Energy Rep. 2017, 3, 29–36. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Pu, G.; Qi, H. Integrated assessment of exergy, energy and carbon dioxide emissions in an iron and steel industrial network. Appl. Energy 2016, 183, 430–444. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Lingiardi, O.; Molenaar, R.; van Opbergen, R.; Sha, Y.; Warren, P. Modern Blast Furnace Ironmaking an Introduction; IOS Press: Amsterdam, The Netherlands, 2020; ISBN 978-1-64368-123-8. [Google Scholar]
- Ariyama, T.; Sato, M.; Nouchi, T.; Takahashi, K. Evolution of blast furnace process toward reductant flexibility and carbon dioxide mitigation in steel works. ISIJ Int. 2016, 56, 1681–1696. [Google Scholar] [CrossRef] [Green Version]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Darde, A.; Prabhakar, R.; Tranier, J.P.; Perrin, N. Air separation and flue gas compression and purification units for oxy-coal combustion systems. Energy Procedia 2009, 1, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Biswas, A.K. Principles of Blast Furnace Ironmaking. Theory and Practice; Cootha Publishing House: Brisbane, Australia, 1981. [Google Scholar]
- IAE—Iron & Steel Roadmap. 2020. Available online: https://www.iea.org/reports/iron-and-steel-technology-roadmap (accessed on 24 October 2021).
- ITM Power to study feasibility of 100 MW P2G energy storage. Fuel Cells Bull. 2018, 2018, 11. [CrossRef]
- Arasto, A.; Tsupari, E.; Kärki, J.; Pisilä, E.; Sorsamäki, L. Post-combustion capture of CO2 at an integrated steel mill—Part I: Technical concept analysis. Int. J. Greenh. Gas Control 2013, 16, 271–277. [Google Scholar] [CrossRef]
- Tobiesen, F.A.; Svendsen, H.F.; Mejdell, T. Modeling of blast furnace CO2 capture using amine absorbents. Ind. Eng. Chem. Res. 2007, 46, 7811–7819. [Google Scholar] [CrossRef]
- Keys, A.; van Hout, M.; Daniels, B. Decarbonisation Options for the Dutch Steel Industry; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2019.
- Zuo, G.; Hirsch, A. The trial of the Top Gas Recycling Blast Furnace at LKAB’s EBF and Scale-Up. Rev. Métallurgie 2009, 2, 255. [Google Scholar] [CrossRef]
- Danloy, G.; Berthelemot, A.; Grant, M.; Borlée, J.; Sert, D.; van der Stel, J.; Jak, H.; Dimastromatteo, V.; Hallin, M.; Eklund, N.; et al. ULCOS—Pilot testing of the low-CO2 Blast Furnace process at the experimental BF in Lulea. Rev. Metall. Cah. D’Inform. Technol. 2009, 106, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Dai, J.; Li, C.; Yu, X.; Xue, Z.; Saxén, H. A Review on Explorations of the Oxygen Blast Furnace Process. Steel Res. Int. 2021, 92, 202000326. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Peña, B.; Romeo, L.M. Energy Storage; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-46526-1. [Google Scholar]






| Natural Gas | COG | BFG | BOFG | |
|---|---|---|---|---|
| H2 | 0 | 56 | 4 | 1.5 |
| CH4 | 100 | 30 | 0 | 0 |
| CO | 0 | 10 | 25 | 66.5 |
| CO2 | 0 | 5 | 20 | 20 |
| O2 | 0 | 1 | 0 | 2 |
| N2 | 0 | 5 | 51 | 10 |
| Stream | Bibl. | Case 0 | Case 1 | Case 2 | |
|---|---|---|---|---|---|
| Raw materials | Iron ore | 1550 | 1430 | 1430 | 1430 |
| Coal | 560 | 520 | 460 | 460 | |
| Coke oven | COG | 90 | 110 | 90 | 90 |
| Blast furnace (BF) | Sinter | 1550 | 1430 | 1430 | 1430 |
| Coke | 400 | 420 | 370 | 370 | |
| Air (hot blast) | 1210 | 1280 | - | - | |
| O2 (hot blast) | - | - | 310 | 310 | |
| BFG (hot blast) | - | - | 1060 | 1060 | |
| CH4 (hot blast) | - | - | 65 | 65 | |
| BFG | 2420 | 2080 | 2190 | 2190 | |
| Slag | 280 * | 280 | 280 | 280 | |
| Pig iron | 1040 * | 1040 | 1040 | 1040 | |
| Basic oxygen furnace (BOF) | O2 | 70 | 90 | 90 | 90 |
| Steel | 1000 | 1000 | 1000 | 1000 | |
| Slag | 80 * | 80 | 80 | 80 | |
| BOFG | 130 * | 130 | 130 | 130 | |
| Power-to-gas (PtG) | O2 | - | - | 210 | - |
| H2 | - | - | 27 | - | |
| BFG | - | - | 139 | 14 | |
| COG | - | - | - | 91 | |
| CH4 | - | - | 65 | 65 |
| Process | Case 0 | Case 1 | Case 2 | |
|---|---|---|---|---|
| Thermal energy consumption | Sintering | 523 | 523 | 523 |
| Coke oven | 1631 | 1442 | 1442 | |
| Air (hot blast) | 1814 | - | - | |
| O2 + BFG (hot blast) | - | 1239 | 1239 | |
| CH4 (hot blast) | - | 431 | 431 | |
| Blast furnace | 2915 | 3900 | 3900 | |
| O2 heating | 170 | 170 | 170 | |
| BOF | 1228 | 1228 | 1228 | |
| Casting, rolling | 300 | 300 | 300 | |
| Total | 8581 | 9233 | 9233 | |
| Electricity consumption | Sintering * | 180 | 180 | 180 |
| Coke oven * | 42 | 42 | 42 | |
| Blast furnace * | 376 | 376 | 376 | |
| ASU | 128 | 252 | 568 | |
| BOF * | 128 | 128 | 128 | |
| Electrolyser | - | 4991 | - | |
| H2 compressor | - | 96 | 32 | |
| CO2 compressor | - | 51 | 36 | |
| Other * | 20 | 20 | 20 | |
| Total | 874 | 6136 | 1382 | |
| Electricity production | Power plant | 1260 | 1443 | 652 |
| Case 0 | Case 1 | Case 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COG | BFG | BOFG | COG | BFG | BOFG | COG | BFG | BOFG | ||
| Mass flow (kg/t steel) | 110 | 2080 | 130 | 90 | 2140 | 130 | 90 | 2140 | 130 | |
| Energy content (MJ/kg) | 40.0 | 2.7 | 6.3 | 40.0 | 5.7 | 6.3 | 40.0 | 5.7 | 6.3 | |
| Utilization of the energy content of the gases by type of process (%) | Internal use | 99.1 | 0 | 99.0 | 0 | 42.2 | 99.0 | 0 | 43.6 | 99.0 |
| Power plant | 0.9 | 100 | 1.0 | 100 | 1.6 | 1.0 | 0.3 | 6.0 | 1.0 | |
| Methanation | - | - | - | 0 | 6.5 | 0 | 99.7 | 0.6 | 0 | |
| TGR | - | - | - | 0 | 49.7 | 0 | 0 | 49.7 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perpiñán, J.; Bailera, M.; Romeo, L.M.; Peña, B.; Eveloy, V. CO2 Recycling in the Iron and Steel Industry via Power-to-Gas and Oxy-Fuel Combustion. Energies 2021, 14, 7090. https://doi.org/10.3390/en14217090
Perpiñán J, Bailera M, Romeo LM, Peña B, Eveloy V. CO2 Recycling in the Iron and Steel Industry via Power-to-Gas and Oxy-Fuel Combustion. Energies. 2021; 14(21):7090. https://doi.org/10.3390/en14217090
Chicago/Turabian StylePerpiñán, Jorge, Manuel Bailera, Luis M. Romeo, Begoña Peña, and Valerie Eveloy. 2021. "CO2 Recycling in the Iron and Steel Industry via Power-to-Gas and Oxy-Fuel Combustion" Energies 14, no. 21: 7090. https://doi.org/10.3390/en14217090
APA StylePerpiñán, J., Bailera, M., Romeo, L. M., Peña, B., & Eveloy, V. (2021). CO2 Recycling in the Iron and Steel Industry via Power-to-Gas and Oxy-Fuel Combustion. Energies, 14(21), 7090. https://doi.org/10.3390/en14217090







