Abstract
Plastics are versatile materials used in a variety of sectors that have seen a rapid increase in their global production. Millions of tonnes of plastic wastes are generated each year, which puts pressure on plastic waste management methods to prevent their accumulation within the environment. Recycling is an attractive disposal method and aids the initiative of a circular plastic economy, but recycling still has challenges to overcome. This review starts with an overview of the current European recycling strategies for solid plastic waste and the challenges faced. Emphasis lies on the recycling of polyolefins (POs) and polyethylene terephthalate (PET) which are found in plastic packaging, as packaging contributes a signification proportion to solid plastic wastes. Both sections, the recycling of POs and PET, discuss the sources of wastes, chemical and mechanical recycling, effects of recycling on the material properties, strategies to improve the performance of recycled POs and PET, and finally the applications of recycled POs and PET. The review concludes with a discussion of the future potential and opportunities of recycled POs and PET.
1. Introduction
Plastic materials offer numerous advantages due to their low cost, high toughness, durability, property of being lightweight, easy processability, low thermal conductivity, and high environmental and corrosion resistance [1]. For all these reasons, the global production of plastics has been growing since the 1950s and the global annual primary plastic production is predicted to reach 1100 million tonnes in 2050 if similar production trends of the past 70 years continue [2]. However, since 2018, the European plastic production has been declining and this decline has been dramatised due to the COVID-19 pandemic [3]. The extensive use of plastics has led to the rapid consumption of fossil fuel resources and the generation of a vast amount of plastic wastes. Over 90% of plastics are manufactured from virgin fossil feedstocks and by 2050 the plastic industry will account for 20% of the total global oil consumption [4]. The management of plastic wastes is extremely important as it is predicted by 2050 there will be 12,000 million tonnes of plastic wastes in landfills and/or the natural environment globally, due to plastics’ slow degradability in the ambient environment [5]. Degradation rates of plastics can vary from hundreds to thousands of years and are dependent on the environmental conditions [6,7]. During degradation, plastics release toxic, harmful chemicals such as bisphenol A and heavy metals, which can leach into the natural environment, causing ecological harm along with potential detrimental effects to human health [8,9,10,11,12]. A growing concern is the increasing accumulation of plastic wastes in the marine environment, contributing 50–80% of all marine debris, and its detrimental effect on marine life [12,13,14].
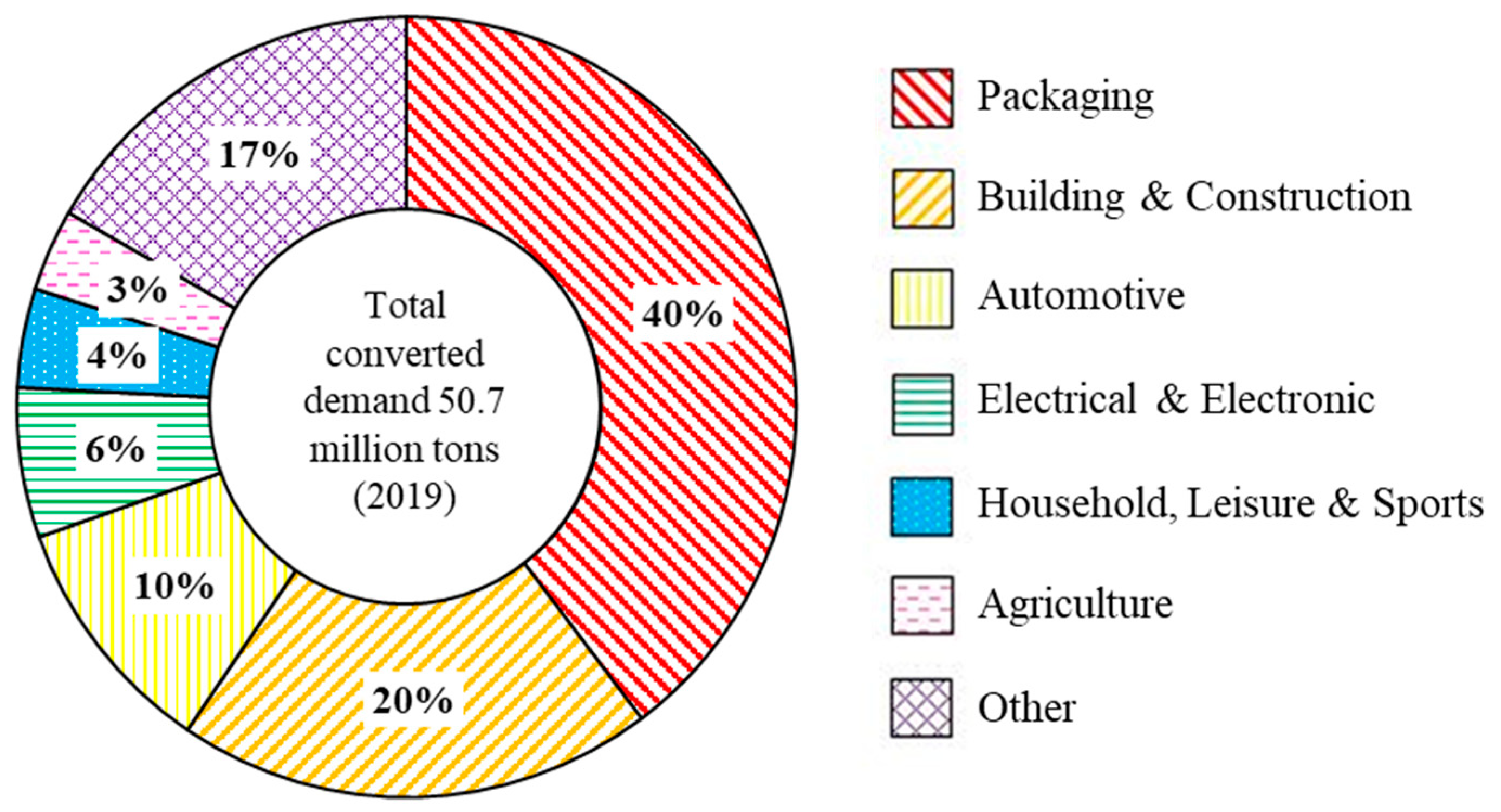
At the end of its life cycle, a plastic product can either undergo recycling, energy recovery, or be placed in landfill [15,16,17,18]. The waste management method implemented for plastic wastes varies from country to country [19]. Globally, we currently operate as a linear plastic waste economy where 24% of plastic wastes are incinerated, 58% are put in landfills or enter the environment, and only 18% of plastics are recycled [7]. Achieving a circular plastic economy where all plastic wastes are re-used, recycled, and recovered is the desired solution to prevent further plastic waste environmental contamination [20]. For a circular economy to be achieved, it is important to understand and analyse the life cycle of plastics from manufacturing through to reprocessing but also to design plastic products with end-of-life management in mind [3,4,21]. To tackle global climate change and the plastic waste crisis, there has been an increase in international agreements, e.g., the Paris Agreement and legislations and voluntary agreements such as the UK Plastics Pact [22,23]. The European Commission set out their “European Strategy for Plastics in a Circular Economy” in 2018 to reduce the impact of plastic wastes, which has resulted in an increase in European recycling rates [24,25]. Plastics are used in a variety of industries such as packaging, automotive, building, construction, etc. (Figure 1) [3].
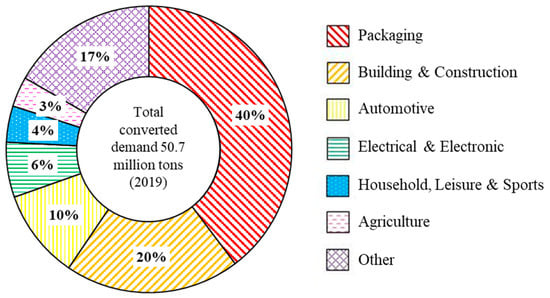
Figure 1.
European industry demand for all types of plastics in 2019 [3].
Plastic packaging is the largest end use market for plastics and accounts for nearly 70% of all plastic wastes [3,26]. In 2019, 17.8 million tonnes of plastic post-consumer packaging wastes were collected from household, industrial, and commercial sources, of which 42% was recycled, 18.5% was landfilled, and 39.5% underwent energy recovery [3]. Therefore, a key aim of the “European Strategy for Plastics in a Circular Economy” is to ensure all plastic packaging placed on the EU market is reusable or easily recycled by 2030 [25]. Several industries utilise plastic packaging to preserve and protect products of the industrial packing, pharmaceuticals, personal and household care, and electronics industries, but the food and beverage industry possess the largest share in the plastic packaging market [27,28]. Plastic packaging can be flexible or rigid [29], and possess a range of versatile properties: flexibility, strength, low weight, chemical stability, permeability, and ease of sterilisation [3,30]. The short lifetime, single use, and improper waste management of plastic packaging has led to detrimental environmental effects which are well documented in the literature [12,14,31]. However, the use of plastic packaging has beneficial environmental and economic impacts, for example, in food plastic packaging. Food packaging is lightweight, which reduces greenhouse emissions and costs during transportation, increases food shelf life which reduces food waste, and improves food hygiene and safety [32,33,34,35]. The prominent plastics found in plastic packaging are high density polyethylene (HDPE), low density polyethylene (LDPE), polypropylene (PP), and polyethylene terephthalate (PET) [21,30,36,37]. Plastics present in smaller quantities include polystyrene (PS), expanded polystyrene (EPS), polyvinyl chloride (PVC), and other polymers such as polyamide (PA), ethylene-vinyl acetate (EVA), and ethylene vinyl alcohol (EVOH) [36]. This review is focused on the mechanical and chemical recycling of the prominent plastics, LDPE, HDPE, PP, and PET, which are found in post-consumer plastic packaging wastes.
2. Recycling and Energy Recovery of Plastic Wastes
Recycling involves the conversion of waste materials into materials of commercial value for re-use and has been reviewed by several authors [18,38,39,40,41,42]. Recycling plastics has several environmental benefits: a net reduction in greenhouse gases and other potent molecules, net energy savings, and a reduction in the consumption of natural resources in comparison to other waste management methods [21,43]. Recycling is an attractive option to achieve a circular plastic economy as it is a viable method of dealing with plastic waste on an industrial scale with environmental benefits. However, the recycling industry faces several challenges related to high costs and the availability of recycling infrastructure to accommodate for the variation in the recyclate quality [41]. Moreover, the low cost of virgin plastics can hinder the use of recycled plastics by industries [44]. The major problems associated with recycled plastics are the different contaminants present in the plastic wastes, the reduction in their molecular weights on undergoing various recycling steps, and their degradation by oxygen, light, temperature, or water during their service life [45].
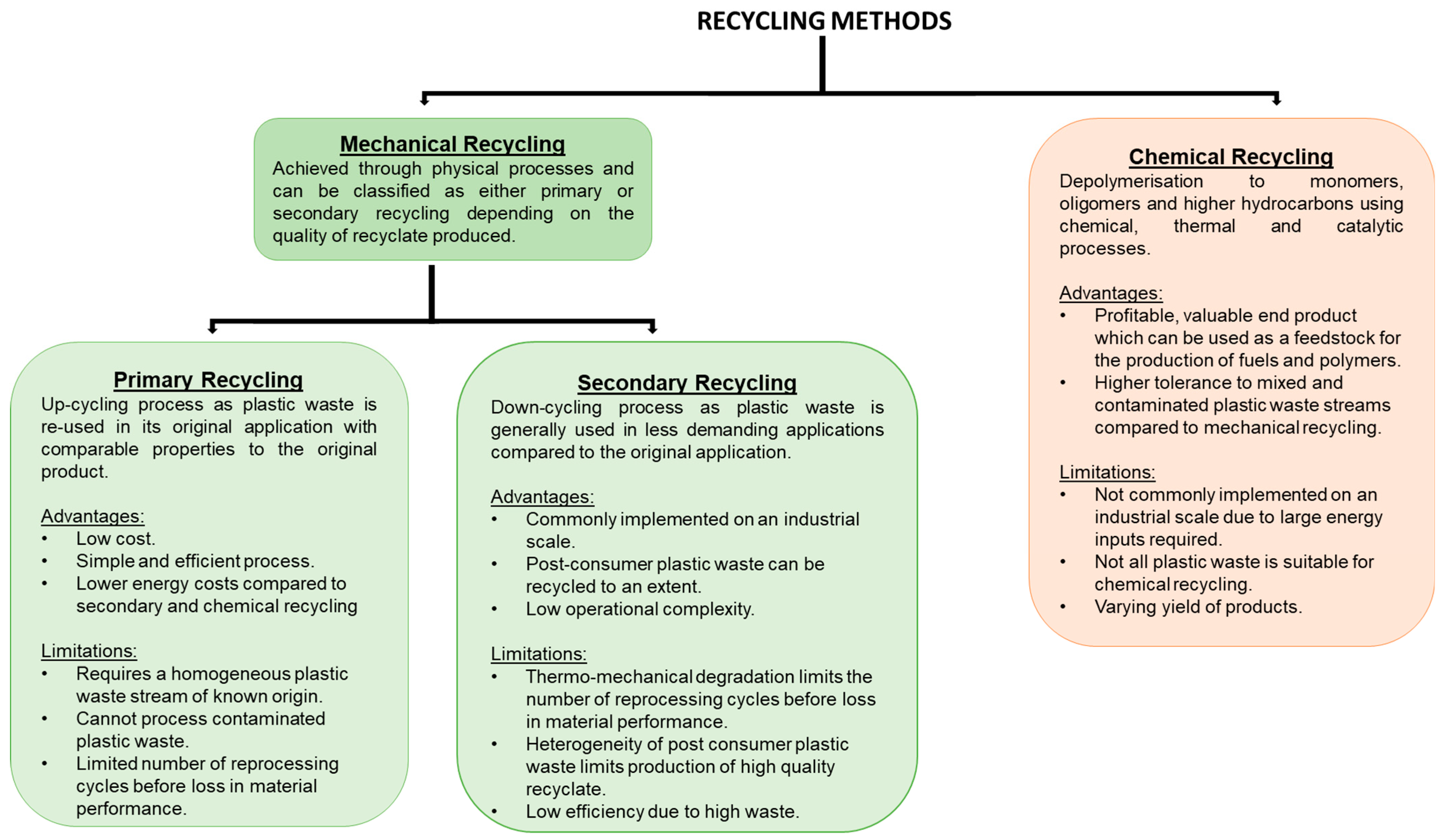
There are two forms of recycling: mechanical and chemical. Figure 2 summarises their advantages and current limitations.
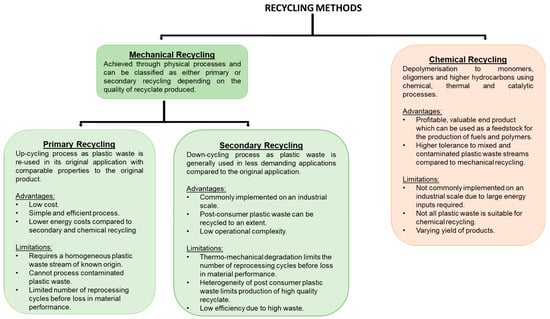
Figure 2.
A summary of the advantages and limitations of mechanical and chemical recycling [17,18,39,41,46,47,48,49,50,51].
2.1. Mechanical Recycling
Mechanical recycling is the process of recovering plastics through physical processes such as melting, grinding, shredding, and extrusion. There are two categories of mechanical recycling: primary and secondary. Both use mechanical processes to recover plastics, but they differ in the quality of the end product [52,53]. Primary recycling is a closed-loop process where pre-consumer polymers are put back into the reprocessing cycle and extruded. This process produces recyclates of similar performance to the virgin material and can be used to manufacture the same product as the virgin material or for new products for demanding applications [38,51,54,55]. Primary recycling requires knowledge of the waste source origin, previous product application, and history, which ensures low levels of molecular contamination and high polymeric purity [49,56]. PET bottles are an example which typically undergo primary recycling [57]. Primary recycling is desirable due to the production of high quality recyclates, reduction in energy costs, and conservation of natural resources. Secondary recycling involves the recovery of plastics from municipal solid waste (MSW) and is described as down-cycling due to the recyclate demonstrating a reduction in properties, and consequently it are used in less demanding environments compared to the virgin material [39,55]. For example, recycled polyolefins (rPOs) can be used to manufacture floor tiles [54]. Post-consumer plastic packaging commonly undergoes secondary recycling as it is contaminated by residue, additives, and the presence of composite materials and laminate structures [52,58].
In the United Kingdom, secondary recycling begins with the kerbside collection of household wastes, MSW [59,60]. Other collection schemes include household waste recycling centres and bring sites/banks [60,61]. After collection, the MSW is taken to a material recovery facility. At this facility, the plastic solid waste (PSW) is sorted from the MSW, e.g., paper and cardboard, by either manual or automated means [62]. The PSW is then transported to a plastic recycling facility where further sortation occurs into single polymer type waste streams, as PSW is a mixture of different plastics, e.g., PP, PE, PVC, PS, and PET [63]. Plastics are sorted and undergo a size reduction by cutting and shredding mechanisms; a washing process involving a hot wash in alkaline and detergent solutions; and drying and reprocessing steps: compounding and pelletizing [39,59,64]. The sorting process is extremely important to limit contamination entering the recycled plastics stream to improve recyclate quality and to reduce the waste of target and non-target plastics which will ultimately end up in landfill sites [21,59,64]. Luijsterburg et al. [64] found that the final quality of the recyclate was dependent on the sorting process but also the reprocessing steps. The sorting and processing will vary greatly from location to location due to different plastic waste collection processes, waste stream composition, recycling infrastructures, available equipment, and capacity, but also by the recycled plastic type market demand [21]. There are several separation and identification techniques which can be used, such as sink–float [65,66], froth flotation [67], near/mid infrared spectroscopy [68,69,70], X-ray fluorescence spectroscopy [71,72], hydrocyclones [73,74,75], tribo-electrostatic [76,77,78], magnetic density sorting [79,80], magnetic levitation [81,82], hyperspectral imaging [83,84,85], laser induced breakdown spectroscopy [86,87,88], hybrid jig [89], and many others. There are several reviews in the literature discussing the techniques, principles, advantages, and drawbacks in detail [18,46,79,90,91,92,93,94].
2.2. Chemical Recycling
Chemical recycling involves the chemical conversion of polymers to monomers or oligomers which can be achieved through depolymerisation reactions [17,38,95,96,97]. Chemical recycling can be categorised as either a homogenous or heterogeneous process [50]. Homogenous processes include methanolysis, glycolysis, and alcoholysis [50]. Typical heterogeneous processes for plastic wastes include pyrolysis [98,99,100] and gasification [98,101], but other novel processes exist such as catalytic cracking [47,102] or microwave-assisted pyrolysis [47,103,104]. These processes result in monomers and other low molecular weight oligomers usually in the form of a liquid oil and syngas, which is a mixture consisting of carbon monoxide, carbon dioxide, and hydrogen [48,98,105]. These molecules can be used as feedstock for the production of new polymers and composites [106], valuable chemicals, or fuel [40], and hence contribute towards a circular plastic economy. Chemical recycling of plastic waste has numerous advantages compared to mechanical recycling: higher tolerance to waste contamination, avoidance of recyclate performance losses, and formation of products with economic value, and sortation is not always required; for example, in gasification, all MSW can be treated together without prior sortation [47,98,107,108]. Currently, chemical recycling is not practiced on an industrial scale due to the large energy costs and limited technology [46,98,109,110]. Some of the processes are unselective, so the yield and composition of products can vary greatly depending upon the waste composition and processing conditions [110,111], but the use of catalysts has been shown to help produce selective products and require lower temperatures [110,112].
2.3. Energy Recovery
Energy recovery is the process of recovering the energy content within plastic wastes through incineration [46,97]. The energy recovery process generates heat which can be used to generate electricity, providing an alternative to burning fossil fuels [113]. Plastics generate a significant amount of energy due to their high lower calorific value (LCV) [114] and can provide a high electricity-to-heat ratio [113]. For example, polyethylene (PE) has an LCV of 43 MJ kg−1, which is the same LCV as diesel fuel [114]. Different plastics possess slightly different LCVs: PA has an LCV of 31.4 MJ kg−1 whereas PS has an LCV of 41.6 MJ kg−1, due to their chemical structures [115]. Combining a high proportion of plastic wastes into the waste MSW feedstock can improve its combustibility [116]. Energy recovery is a useful method to dispose of large quantities of waste with its infrastructure requiring less space compared to landfills [117]. Energy recovery is the preferred management method when plastic sorting is too difficult or too expensive during recycling, as mixed plastic wastes still provide a high LCV of 30–40 MJ kg−1, which is comparable to the LCV of coal [50,114,118]. Incineration produces waste products, bottom ash, and fly ash, and their amounts and compositions can vary depending on the waste composition and the incineration technology used [119,120]. Typically, these waste products are placed in landfills due to their toxicity, but bottom ash has been re-used in road reconstruction [119,121]. A major concern surrounding the incineration of plastic wastes is the production and release of persistent organic pollutants, particulates, and hazardous toxic compounds such as dioxins and furans into the environment [10,115,116]. Recent research has also shown microplastics are present within the waste products, bottom ash, and fly ash, caused by incomplete combustion, which is concerning due to the ecological harm caused by microplastics [117]. There are strict European regulations for incineration as stated by the EU Hazardous Waste Incineration Directive. Incineration has environmental concerns, so recycling is the preferred route for waste management.
3. Recycled Plastics
3.1. Thermodynamics of Recycled Plastic Blends
During the recycling process, the complete separation of each plastic is challenging. The resulting recycled plastic will therefore contain a low fraction of other plastics and can be considered a polymer blend [122]. A polymer blend is a mixture of two or more polymers or copolymers which forms a new material with different physical properties [123]. Recycled blends are immiscible due to their heterogeneous nature which affects their final properties.
The immiscibility can be determined by the Gibbs free energy of mixing, ΔGmix, which is calculated by the following equation (Equation (1)) [124]:
where ∆Hmix is the enthalpy of mixing, T is the temperature, and ∆Smix is the entropy of mixing.
The ∆Smix is usually negligible, so ∆Hmix is the main contributor to ∆Gmix. The blend is immiscible when the ∆Gmix is positive, but this does not mean that a negative ∆Gmix is a sufficient condition for miscibility. Additionally, the second derivative shown by Equation (2) needs to be positive for a system to be miscible [125]:
where is the volume fraction of the second polymer in a two-component polymer blend. Immiscible blends result in multi-phase morphology and poor interfacial adhesion between the phases, leading to poor mechanical properties [123]. Recycled blends have poor mechanical and physical properties due to polymer degradation through the recycling process and inhomogeneity.
3.2. Compatibilization of Recycled Plastic Blends
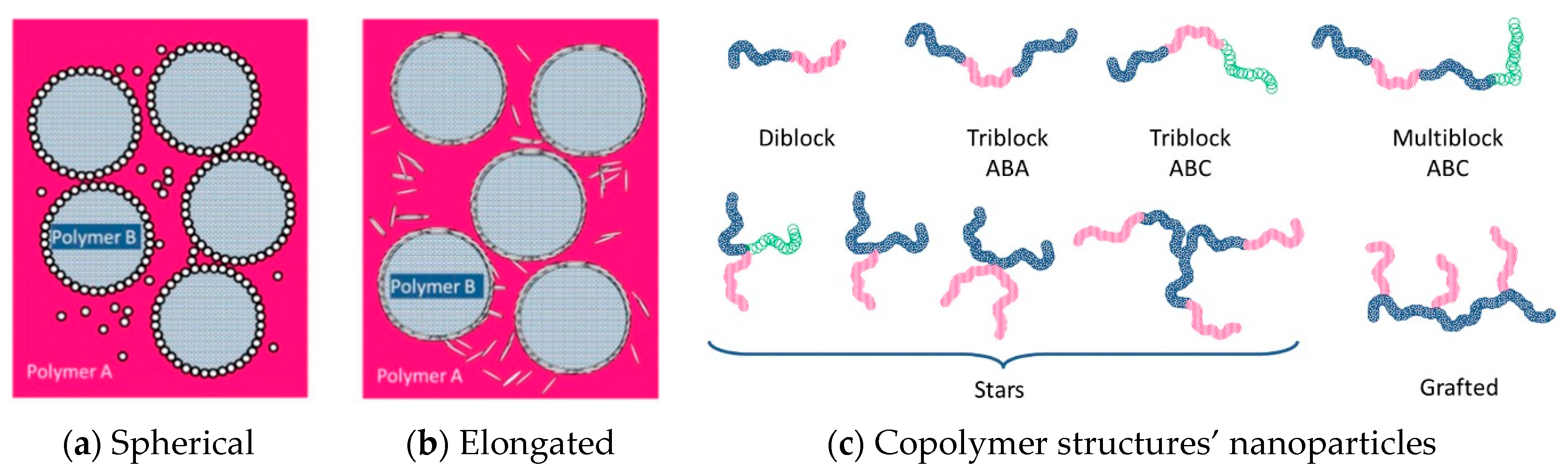
To improve the performance of recycled plastics, compatibilization is required. Compatibilization improves interfacial properties between the different constituents by stabilizing a desired morphology, reducing interfacial tension, and enhancing phase adhesion, thus leading to the improvement of mechanical properties [122]. Compatibilizers can be graft/block copolymers, nanoparticles, or ionomers [40]. Their efficiency depends on their structure/shape, molecular architecture, chemical composition, and concentration (Figure 3). Thus, spherical nanoparticles are more efficient than elongated ones and longer branched copolymers are more effective than shorter diblock copolymers [40]. However, it is still hard to predict the type and amount of compatibilizer required for recycled blends due to the inhomogeneity of plastic wastes.
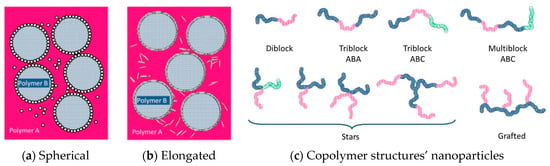
Figure 3.
Presentation of compatibilizer shapes and molecular architectures for (a) spherical nanoparticles (b) elongated nanoparticles (c), copolymer structures: diblock copolymer consisting of two homopolymer blocks, triblock copolymers consisting of three homopolymer blocks, multiblock copolymer consisting of four or more homopolymer blocks, star copolymers consisting of polymeric chains attached to a central core, and grafted copolymers consisting of main homopolymer chain with different polymeric side chains. Reproduced with permission [40].
4. Recycled Polyolefins (rPOs)
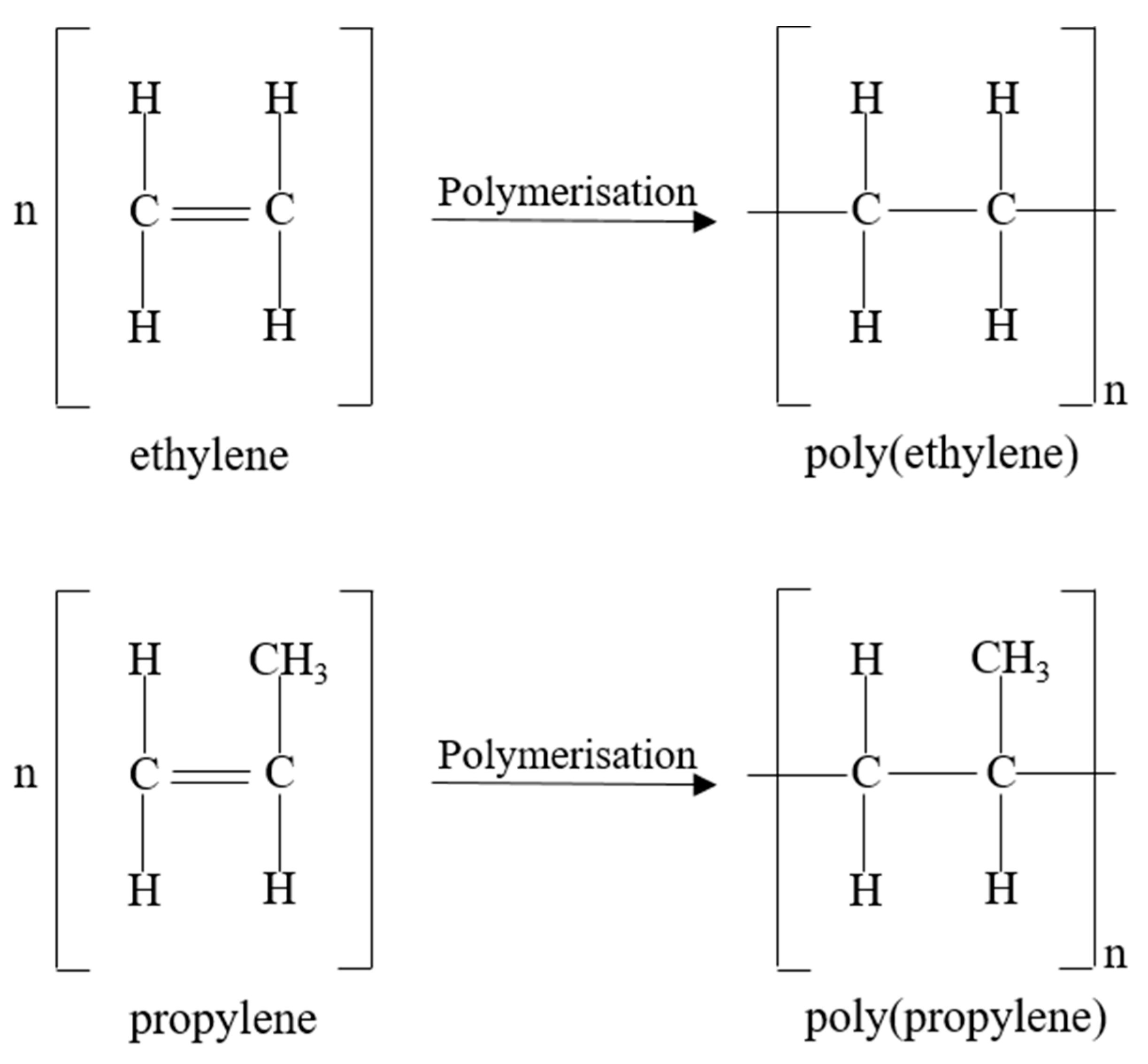
POs, such as PP and PE, are attractive due to their low cost, high abundancy, good mechanical properties and chemical resistance, low density, and ease of processability [126]. For these reasons, 9.8 million tons of PP and 15.1 million tons of PE were manufactured in 2019, which accounted for 49.2% of the total converted plastic European demand (50.7 million tonnes) [3]. POs are fully saturated hydrocarbons synthesised by the polymerisation of an olefinic monomer in the presence of a catalyst [127]. The olefinic monomers, ethylene and propylene, depicted in Figure 4, are obtained from the cracking of petroleum feeds or the dehydrogenation of alkanes [126]. Different grades of PP and PE exist, such as blow moulding or injection moulding grades, which differ in melt viscosity and can be obtained through the use of co-monomers such as hexane or butane during olefinic polymerisation [127].
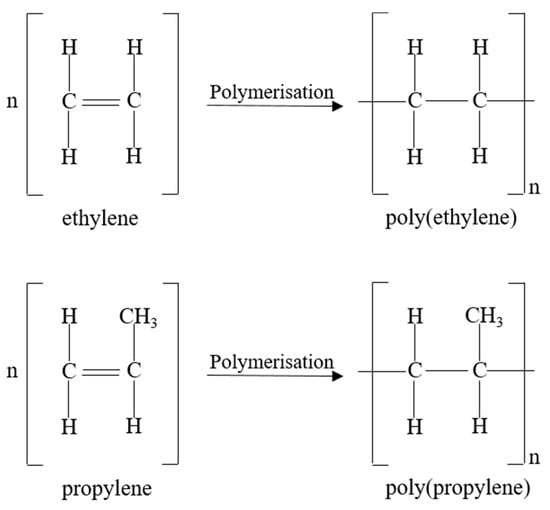
Figure 4.
Ethylene and propylene monomers for the formation of PE and PP, respectively.
PP is a semi-crystalline polymer which is widely used in packaging films and household and electrical appliances [128,129,130]. For example, biaxially orientated polypropylene (BOPP) is commonly used in the manufacturing of flexible and light packaging [131]. PP possesses good mechanical properties and processability; however, its applications are limited by UV degradation, flammability, and poor impact strength at low temperatures [130,131]. There are three different types of PP: atactic PP, syndiotactic PP, and isotactic PP (iPP) [128,129,132]. In iPP, the methyl groups are all located on one side of the main chain and it is typically used in research papers due to its stability, homogeneity, and high crystallinity [128,132].
PE is a semi-crystalline polymer used in a wide range of applications from packaging to medical devices due to its diverse properties [128,129,133]. Depending on the polymerisation mode, three different PEs can form: high density PE (HDPE), low density PE (LDPE), and linear low density PE (LLDPE) [128]. The branching degree and the molecular weight influence their physical properties in the melt and solid state [134,135,136]. There are many other types of PEs which exist on the PO market: for example, ultrahigh molecular weight PE (UHMWPE) and very low-density PE (VLDPE), which are used in very specific applications [134]. UHMWPE is typically incorporated in biomedical applications such as artificial joints [137], whereas VLDPE is typically used to manufacture films for food packaging [138]. Generally, PEs are limited by poor environmental stress cracking resistance (ESCR) [139]. ESCR is affected by molecular weight, molecular weight distribution, density, number of tie molecules between crystalline and amorphous domains, and the testing conditions [140,141,142]. Typically, the higher the level of crystallinity, the lower the ESCR [143]. Table 1 summarises the structure, properties, and applications of HDPE, LDPE, and LLDPE.

Table 1.
Structure, properties, and applications of HDPE, LDPE, and LLDPE. The list of properties and applications is non-exhaustive [53,133,134,138,144,145,146,147].
4.1. Mechanical Recycling of POs
Mechanical secondary recycling is a commonly used method for recycling POs due to its low cost and reliability [42]. Due to the heterogeneity of PSW and difficulty in 100% separation of different plastic types, maintaining a constant recyclate quality or performance is challenging. The complete separation of PP and PE during recycling is challenging and expensive due to their similar physical properties and densities [144,145]. PP/PE blends are commonly found in recycled wastes and are of great commercial interest. The blends display poor mechanical performance due to immiscibility, thermo-mechanical degradation during reprocessing, and the presence of contaminants [146]. During reprocessing, recycled PO (rPO) blends undergo the addition of stabilizers, compatibilizers, and fillers to improve performance, but this can cause issues in processability and cost.
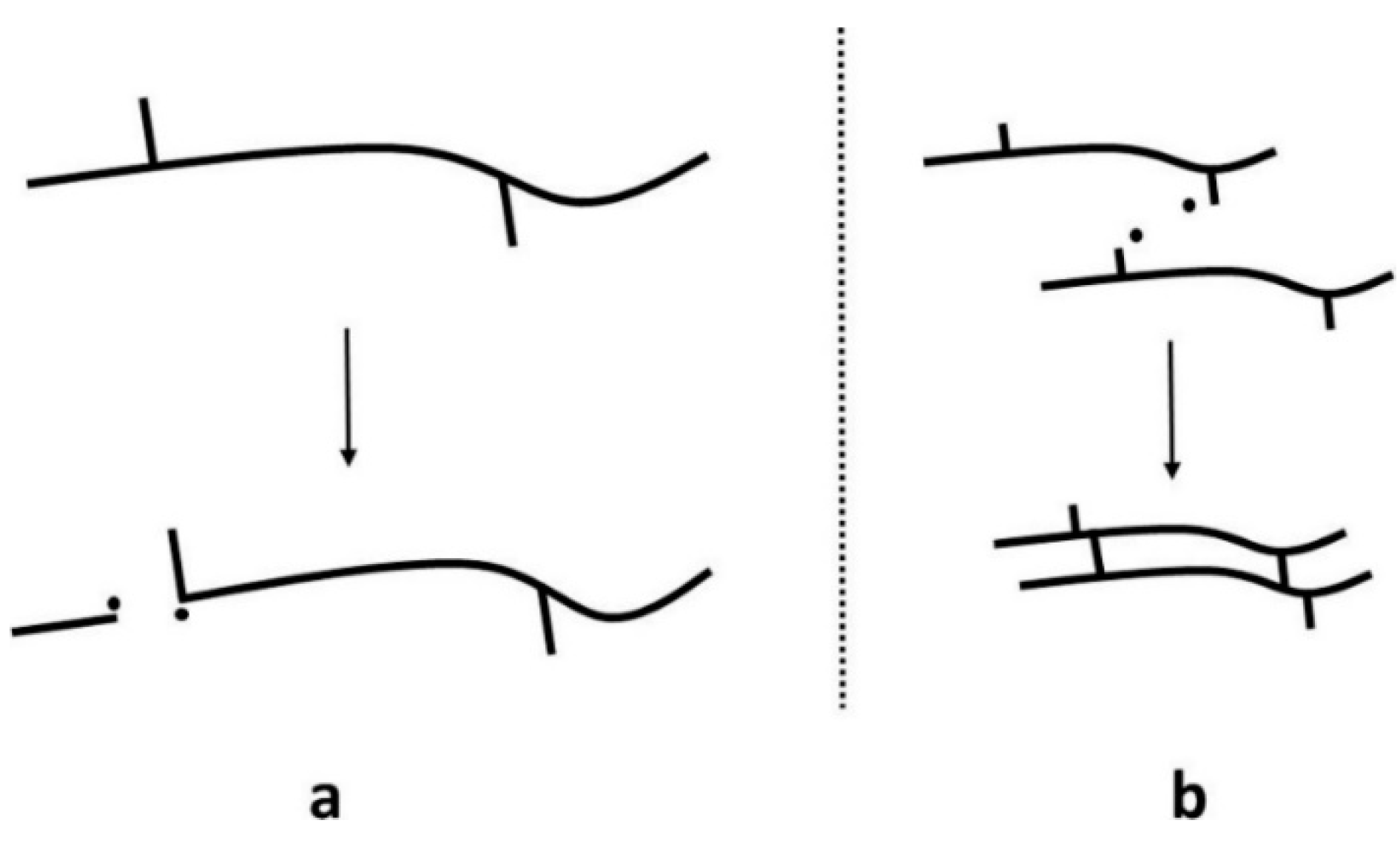
A major challenge of mechanical recycling is the occurrence of thermo-mechanical and thermo-oxidative degradation caused by high temperatures and shear in the presence of oxygen and impurities during reprocessing [147,148]. Degradation mechanisms cause irreversible changes within the polymer structure, causing the deterioration of the thermal, mechanical, and physical performance of the recycled materials [149,150]. During mechanical recycling, two competing degradation mechanisms occur: random chain scission and chain crosslinking (Figure 5) [151,152]. Random chain scission is the process of breaking bonds in the polymer backbone chain, leading to the formation of reactive free radicals. Chain crosslinking occurs when free radicals react, forming a crosslink between polymer chains to form a network structure.
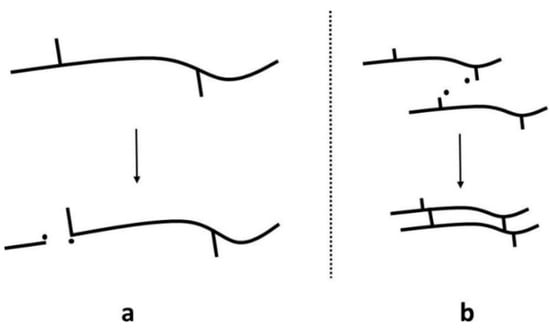
Figure 5.
Degradation mechanisms: (a) random chain scission and (b) crosslinking. Reproduced with permission [18].
Chain scission is considered to be the dominant mechanism and results in a decrease in the polymer molecular weight and an increase in polydispersity showing the presence of different chain lengths [122]. The presence of chain crosslinking, however, increases the molecular weight due to the formation of longer chains and crosslinking [152]. The extent of degradation is dependent upon several factors: the number of re-processing cycles, polymer chemical structure, thermal-oxidative stability of the polymer, and the re-processing conditions [128,152,153,154]. For example, Nait-Ali et al. [155] studied the influence of oxygen concentration on this competition between chain scission and chain crosslinking. They concluded that a well-oxygenated environment favours chain scission while a low-oxygenated environment provokes chain crosslinking. The presence of oxygen leads to the formation of oxygenated functional groups on the polymer chain, such as ketones, which influence the final performance.
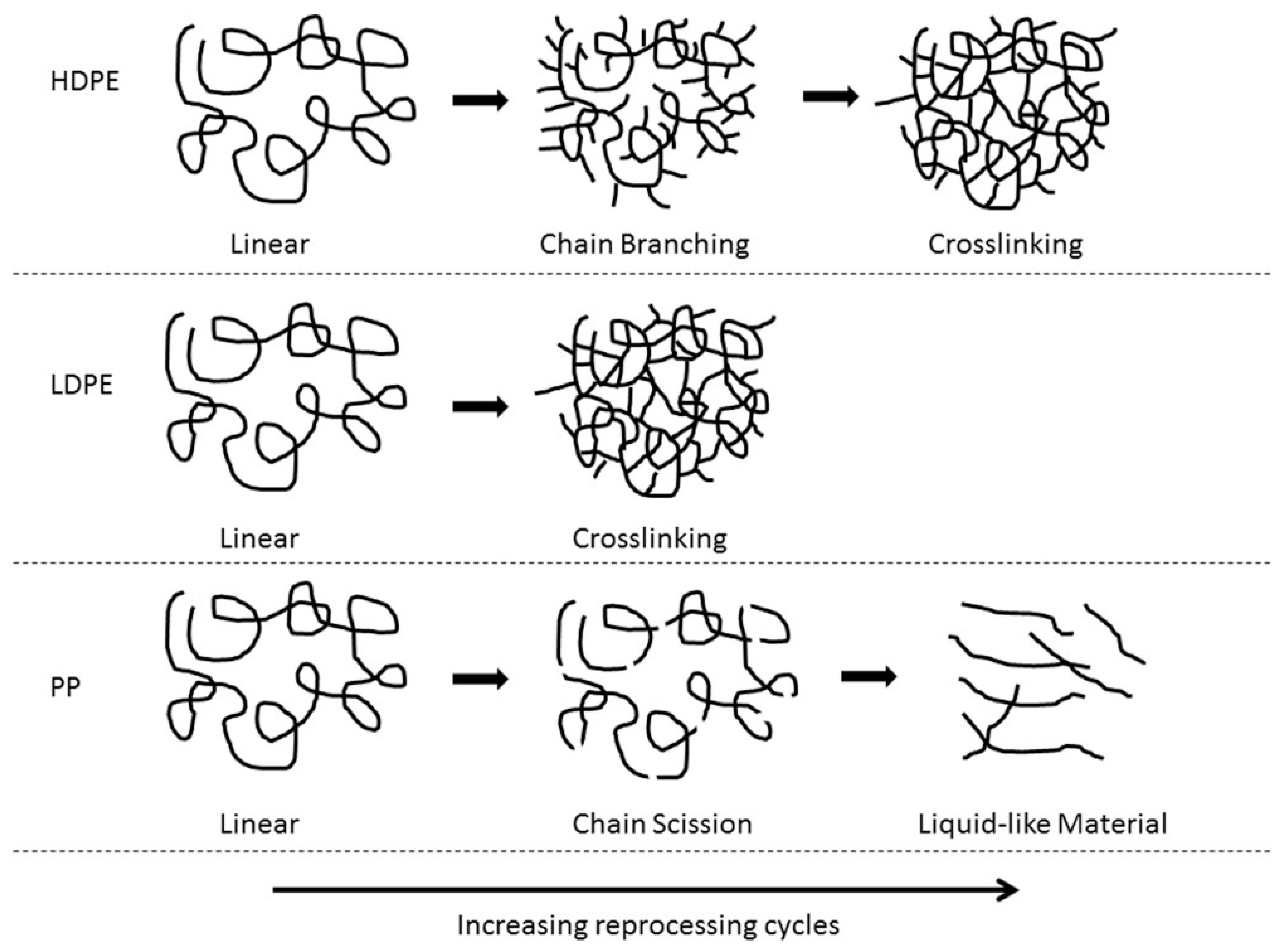
HDPE, LDPE, and PP have been found to have different degradation behaviours during mechanical reprocessing (Figure 6) [154]. HDPE and LDPE have high thermal stability, can be subjected to a high number of extrusion cycles before degradation, and typically undergo chain scission and chain branching/crosslinking. Chain scission has been shown to be the dominant degradation mechanism in HDPE by Abad et al. [156], further supported by Pinherio et al. [152], who both studied HDPE subjected to five extrusion cycles. However, Oblak et al. [157] subjected HDPE to 100 consecutive extrusion cycles at 220–270 °C and found that the chain scission was dominant up to the 30th extrusion cycle but upon further increase, chain branching dominated. Eventually, crosslinking occurred after the 60th cycle as determined through the melt flow index (MFI), rheological behaviour, and gas permeation chromatography (GPC). Jin et al. [158] found that when virgin LDPE (vLDPE) was subjected to 100 extrusion cycles at 240 °C to simulate the recycling process, chain scission and crosslinking occurred simultaneously, determined by rheological and MFI measurements. However, even though both chain scission and crosslinking were occurring, it was suggested that one mechanism was usually dominant. At a low number of extrusion cycles, chain scission dominated, whereas at a higher number of extrusion cycles, crosslinking dominated. Similar results were found by Dostál et al. [159], in which vLDPE was subjected to 20 extrusion cycles at 150–170 °C. Chain scission dominated at cycles 1 and 2 followed by crosslinking occurring from cycles 3–20 followed by the formation of a microgel. In comparison to HDPE and LDPE, PP has been found to only undergo chain scission [160]. Aurrekoetxea et al. [161] studied the effect of recycling on iPP by subjecting iPP to 10 injection moulding cycles at 200 °C. They found that the MFI increased but the chemical structure remained unchanged by FTIR, suggesting that chain scission was the dominant degradation mechanism. Costa et al. [162] also found that chain scission was the dominant degradation mechanism for PP. They found that, after 19 reprocessing cycles at 270 °C, PP underwent chain scission resulting in a molecular weight reduction and the presence of entanglements, causing the formation of a low viscosity liquid-like material (Figure 6). This low viscosity liquid-like material could cause issues in processability during further reprocessing cycles and limit the recyclate value.
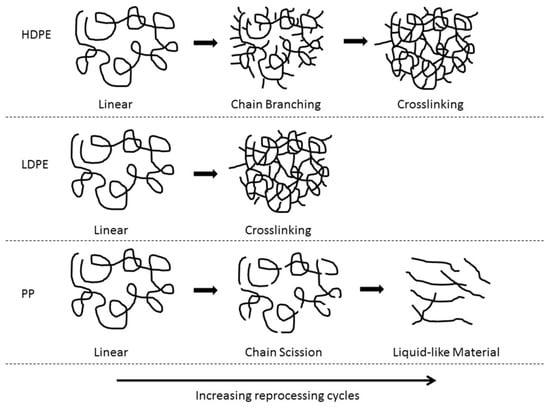
Figure 6.
Degradation mechanisms of HPDE, LDPE, and PP as the number of reprocessing cycles is increased. Reproduced with permission [154].
The MFI which can also be denoted as the melt flow rate (MFR) is a key parameter to understand the processability of materials, as any change in MFI can cause difficulties during reprocessing and manufacturing [154,157]. Several researchers have shown MFI to be affected during recycling due to a change in the polymer molecular weight and distribution caused by degradation [122,148,157,163]. Kartalis et al. [163] reported a reduction in MFI with an increasing number of extrusion cycles that was caused by random chain scission of a 75/25 weight ratio (wt%) LDPE/medium density PE (MDPE) blend. To prevent a significant deterioration in MFI, stabilizers were added during reprocessing. Upon stabilizer addition, an improvement in molecular weight retention was observed, resulting in processing stability. Heat stabilizers along with other additives are typically added during reprocessing. On the other hand, an increase in MFI was reported with increase in recycling cycles which could be caused by chain crosslinking [159,161]. If the MFI changes significantly after each recycling cycle, the reprocessing conditions such as temperature may have to be altered, which can be time consuming and costly. Predicting the MFI of recycled blends has been shown to be possible by Hubo et al. [164], who compared the experimental MFI of different compositions of post-consumer PP/HDPE blends to the rule of mixtures. Little deviation was found between the experimental and theoretical values. However, predicting the change in MFI with increasing recycling cycles is challenging due to degradation and the presence of contaminants. Rytöluoto et al. [165] investigated the effects of three reprocessing cycles on the performance of biaxially oriented silica polypropylene nanocomposite (SiO2-BOPP) films containing 4.5 wt% silica particles. They found that reprocessing cycles had a detrimental effect on the steady shear viscosity and film processability, but silica particle agglomeration remained mostly unaffected.
During a recycling cycle, thermal degradation can result in a decrease in the molecular weight, causing the mechanical properties to decrease [160]. The number of recycling cycles has been shown to affect rheological and mechanical properties due to their dependence upon the molecular weight [157,162,166,167]. The polymer structure and thermal stability will determine the number of cycles a polymer can endure before a reduction in mechanical performance is observed. PP and PE are semi-crystalline polymers and their degradation in the solid state typically occurs in the amorphous phase [159]. Degradation affects the crystallinity and thus the mechanical properties [162]. Interestingly, the recycled PP (rPP) crystallinity has been found to be higher than that of virgin PP (vPP) by several authors [161,162,166]. Costa et al. [162] suggested that a higher value of crystallinity of rPP compared to vPP was caused by a decrease in the molecular weight, which resulted in an increase in chain mobility. Increased chain mobility improved the ability of chains to fold into thicker lamella, and hence an increased crystallisation rate and crystallinity. An increase in crystallinity can result in improvements in tensile properties, Young’s modulus, and yield stress, but causes the elongation at break to decrease.
Aurrekoetxea et al. [161] subjected PP to 10 successive injection moulding cycles at 200 °C and found that the degree of crystallinity increased with each cycle. This caused an increase in the Young’s modulus and yield stress. On the other hand, Oliveira et al. [160], who subjected PP to seven successive cycles at 175–190 °C, suggested a decrease in Young’s modulus and yield stress after the third cycle, which was caused by the reduction in tie molecules between the crystalline and amorphous phases (Table 2). Additionally, impact strength was also observed to decrease after the fifth cycle, which was caused by chain scission increasing the crystallinity. Conflicting observations by Aurrekoetxea et al. [161] and Oliveira et al. [160] for the Young’s modulus and yield stress of rPP could be due to differences in the processing methodology. Aurrekoetxea et al. [161] used injection moulding whereas Oliveira et al. [160] opted for a single screw extruder followed by compression moulding. This highlights the importance of the reprocessing methodology but also demonstrates the difficulty of comparing the performance of recycled materials in the literature.

Table 2.
Elastic modulus, yield stress, and impact strength of PP subjected to multiple extrusion cycles. Reproduced with permission [160].
PE can be subjected to a higher number of extrusion cycles before any deterioration in the mechanical properties is observed. Jin et al. [158] found no significant change in crystallinity and hence in mechanical properties of LDPE up to the 40th extrusion cycle. However, a decrease in crystallinity was observed between the 40–50th cycles, either caused by short side branches in the backbone chain, side groups, or by crosslinking. Through creep experiments it was found that the time-dependent mechanical properties were affected after the 40th extrusion cycle, which can be related to the decrease in crystallinity. Oblak et al. [157] subjected HDPE to 100 consecutive extrusion cycles at 220–270 °C. They found that chain branching and chain scission, which occurred up to the 60th cycle resulted, in a decrease in crystallinity and Young’s modulus. However, crystallinity and Young’s modulus remained stable after the 60th extrusion cycle due to crosslinking. After the 100th cycle, the Young’s modulus of recycled HDPE (rHDPE) had only reduced by 20% compared to that of the virgin HDPE (vHDPE).
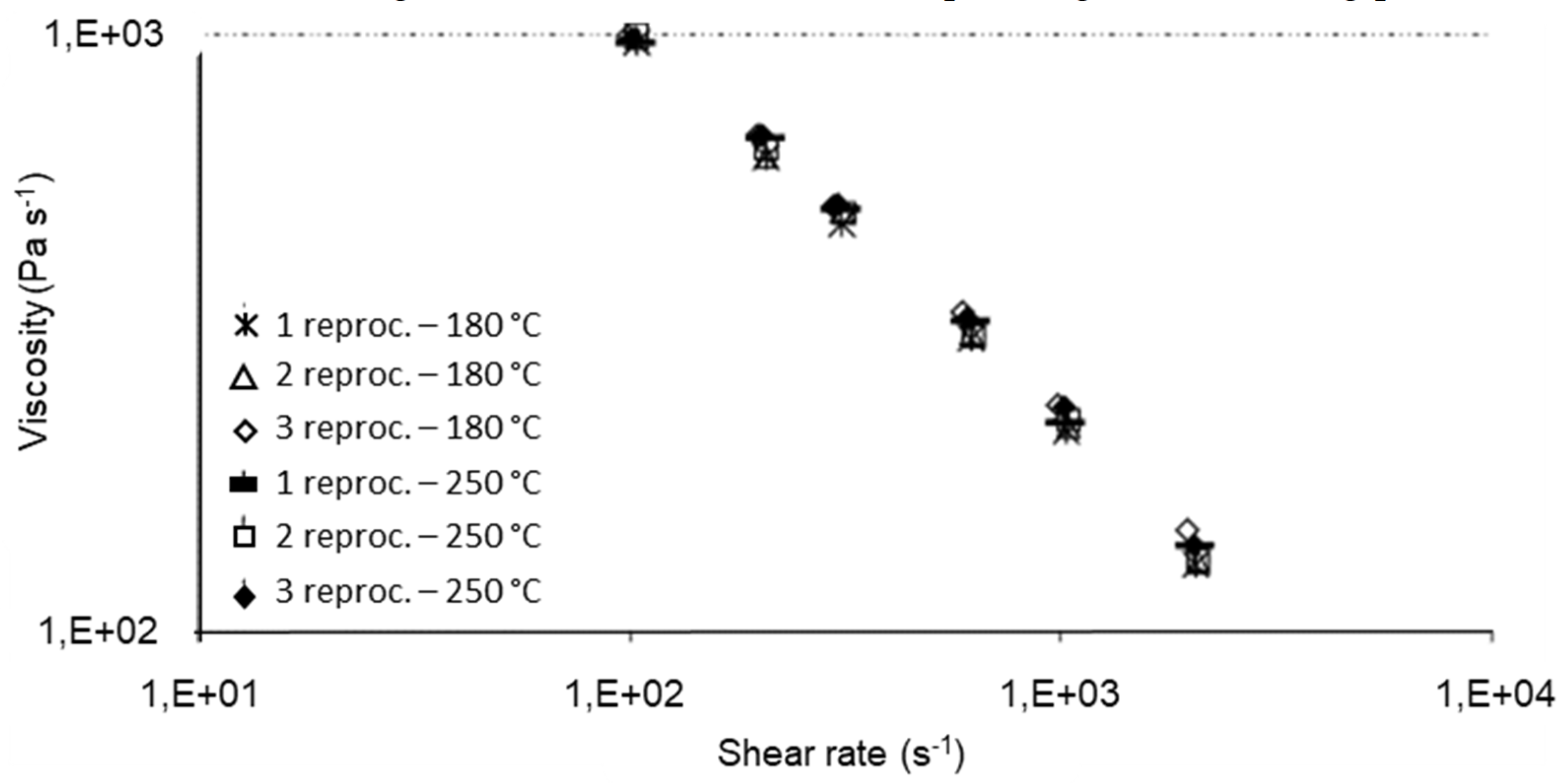
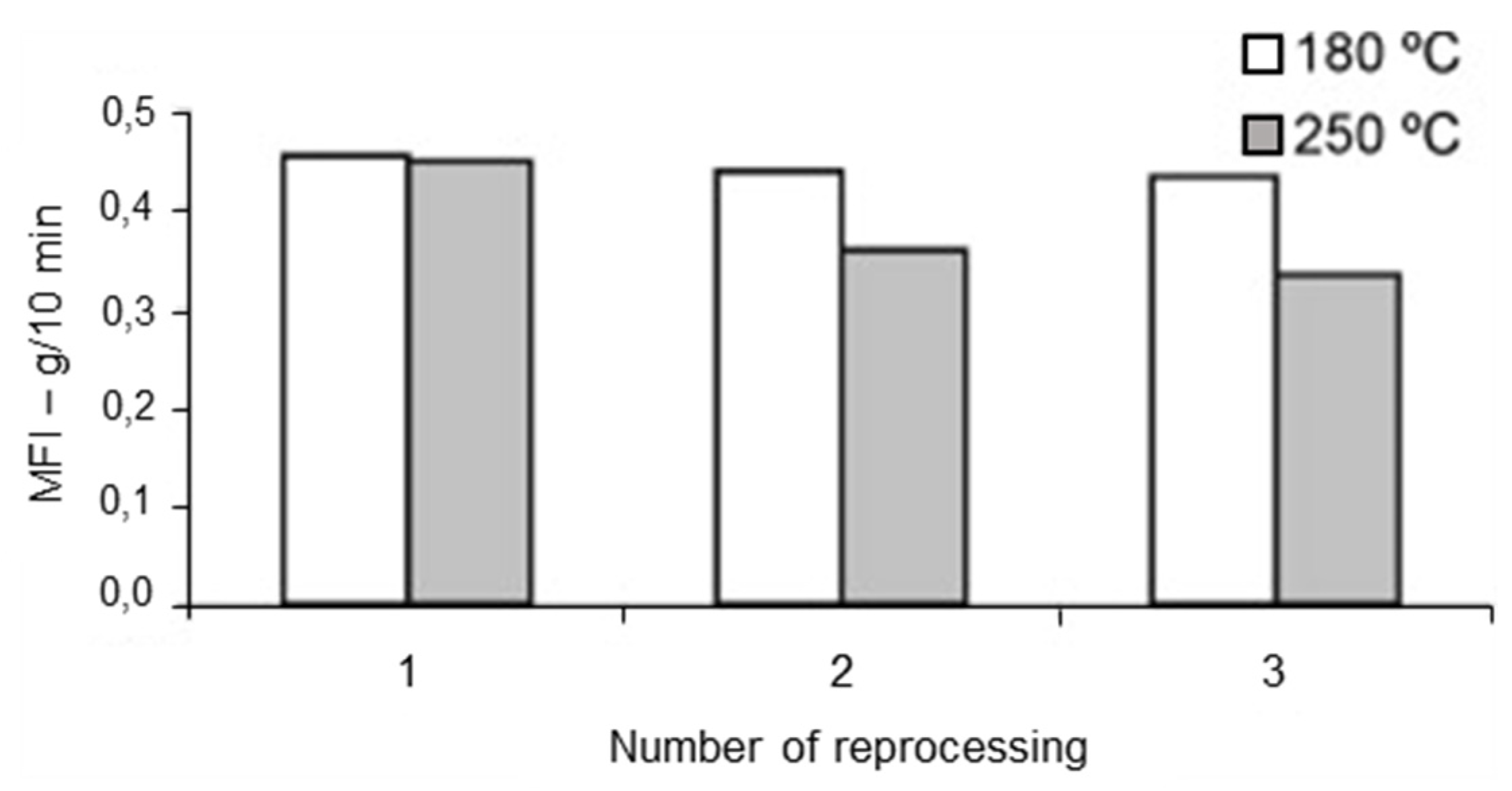
The processing conditions at which recycling is carried out affect the polymer degradation and thus the physical and mechanical properties of the recycled materials. The processing temperature has been found to be of importance by Santos et al. [148], who investigated the rheological, structural, and physiochemical properties of a municipal plastic waste blend extruded at conventional (150–180 °C) and aggressive temperatures (210–250 °C) three times. Rheological and structural properties remained unaltered up to the third cycle at conventional temperatures (Figure 7 and Figure 8). On the other, at higher (aggressive) temperatures, degradation occurred from the second extrusion cycle and required the addition of an antioxidant. This work highlighted the importance of processing temperature—the higher the temperature, the higher the rate of degradation—but it was limited to only one blend ratio. Guillén-Mallette et al. [168] investigated using recycled printed BOPP labels and printed PO caps as a chemical foaming agent in extruded products. Gases were produced as the inks on the BOPP labels degraded and these gases acted as the foaming agent in the PO matrix. They determined that the processing conditions, screw angular velocity and temperature, affected the cellular morphology. The crystallinity and melt strength of the PO matrix varied depending on the cooling process.
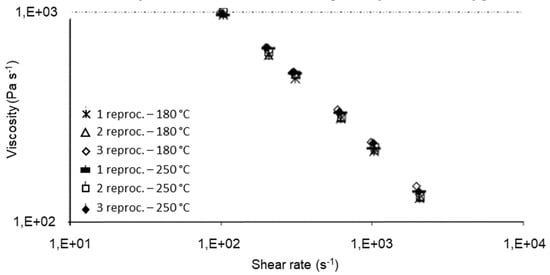
Figure 7.
Rheological curves of rPP/rHPDE 90/10 wt% under conventional and aggressive temperature conditions. Reproduced with permission [148].
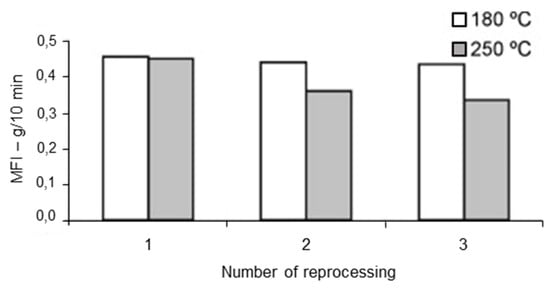
Figure 8.
MFI of rPP/rHPDE 90/10 wt% blend reprocessed under conventional and aggressive temperature conditions. Reproduced with permission [148].
4.2. Chemical Recycling of POs
Chemical recycling is not so easily achievable for POs as for other polymers such as PET due to POs’ chemical inertness, which requires harsh conditions to break the C–C backbone chain and C–H bonds [169,170]. The preferable chemical recycling routes for POs on large scales are thermochemical ones such as pyrolysis and catalytic cracking [112]. Several researchers have investigated the pyrolysis of POs [96,105,171,172,173,174,175,176], where above 700 °C the recycling products are olefin mixtures and aromatic compounds, while around 400–500 °C they are calorific gas, hydrocarbon oil, waxes, and chars [170,177]. Pyrolysis is a temperature-dependent, energy intensive process and results in a wide range of molecular weight hydrocarbons which are low value products [96,97]. The use of catalysts of suitable acidity and shape selectivity in pyrolysis narrows the molecular weight range of hydrocarbons in the crude oil product at lower temperatures [175,178,179]. Catalytic cracking of PP and PE is commonly carried out using zeolite [178,180,181,182,183,184] and FCC [185,186] catalysts, but Ziegler–Natta [187] catalysts have also been used. The use of catalysts has drawbacks such as recovery costs of the catalysts after use and catalyst deactivation over time [184]. Even with the addition of a catalyst, the temperatures required to process POs are still elevated, so work has begun on a tandem catalytic approach to degrade under mild conditions [188,189,190,191]. A limitation of most literature concerning catalytic cracking is that it has not been applied to real plastic waste streams, but work has begun on developing strategies for mixed plastic waste streams [172,192,193,194]. Sivagami et al. [195] assessed the catalytic pyrolysis of multi-layered plastics wastes: BOPP, metalized biaxial oriented polypropylene (MET/BOPP), PET, metalized polyethylene terepthlate (MET/PET), PET combined polyethylene (PET/PE), and mixed PO plastic waste, in the presence of a zeolite catalysts at pilot scale. They found that BOPP-based plastics gave a higher oil yield and calorific values compared to PET-based multi-layered plastics. By combining multi-layered plastics such as BOPP with PET or PO mixed plastic waste, a consistent oil yield and quality can be achieved. For example, mixing PET-based multi-layered plastics and BOPP/MET/BOPP in the range of 40–60% doubled the liquid yield.
Gasification of POs waste is also a promising technology [18,101]. Gasification involves the conversion of plastic waste into a mixture of carbon monoxide, carbon dioxide, methane, and hydrogen gases [196]. The desirable product from gasification is syngas, a mixture of hydrogen and carbon monoxide, which can be used to synthesise fuel chemicals, in particular methanol [98]. However, a concern surrounding the gasification of plastic waste is the production of harmful pollutants [98]. Gasification is usually carried out in conjunction with pyrolysis in the presence of catalysts and has been reported for POs [197].
4.3. Improving the Performance of rPO Blends
4.3.1. Composition of rPE and rPP Blends
The performance of PO blends is dependent upon the composition and the characteristics of the individual components. Strapasson et al. [145] investigated the tensile and impact properties of vPP/vLDPE blends at five different blend ratios. Upon addition of PP, the Young’s modulus and yield strength increased, but elongation at break and impact strength decreased. Hassan Awad et al. [198] observed similar tensile property trends in rPP/rHDPE blends upon rPP addition. The crystallisation behaviour of PO blends is also affected by the recycling process. Aumnate et al. [199] studied the effect of recycling on the crystallisation and mechanical behaviour of five PP/LDPE blend ratios: PP, LDPE, and PP/LDPE at 25:75 wt%, 50:50 wt%, and 75:25 wt%. An increased rate of crystallisation and formation of imperfect crystallites were found in the rPP/rLDPE blends compared to the virgin blend, which was caused by the decrease in molecular weight during the recycling process. Interestingly, they found that the tensile properties of the rPP/rLDPE blend showed only a slight change from the virgin blend.
4.3.2. Addition of Virgin Polymers
The addition of virgin polymers to recycled polymer wastes has been shown to be an effective method of improving the performance of recycled materials [144,200,201]. For industry, the quantity of virgin polymer required is of key interest to strike a balance between cost and material performance. Typically, the same type of polymer is added to the recycled waste, which can minimise the change in melt viscosity. Meran et al. [200] added vPP to rPP, vLDPE to rLDPE, and vHDPE to rHDPE. They found that the recycled polymer tensile strength and elongation at break improved upon the addition of virgin polymer. The addition of virgin polymers to rPO blends has also been investigated by several authors. Madi [144] investigated the thermal and mechanical behaviour of rHDPE and vPP blends which contained up to 30 wt% of vPP. They found that the dispersion of vPP through the rHDPE was poor and the presence of vPP affected crystallite formation, resulting in a decrease in crystallinity and heat of fusion. The tensile strength was observed to improve as the vPP content was increased, but elongation at break decreased, which was caused by HDPE potentially decreasing the plasticity of PP. Lovinger et al. [202] suggested that the mechanical properties of vPP/rHDPE blends were determined by the morphology. rPOs can also be blended with other polymers such as polyamide to improve their performance [203,204].
4.3.3. Addition of Compatibilizers
Non-reactive compatibilizers such as graft and block copolymers are located at the interface between phases and contain a component that is miscible in one phase and another component that is miscible in the other phase [124,204]. The effectiveness of compatibilization on the performance of PO blends is influenced by the chemical structure of the compatibilizer, the amount of compatibilizer added and the method of blending. Commonly used compatibilizers for rPP/rPE blends are ethylene–propylene rubber monomer (EPRM) and ethylene propylene diene monomer (EPDM), as they are cheap, readily available, and easily processed. EPDM and EPRM are used as impact modifiers to improve the toughness of recycled blends. Bertin and Robin [205] investigated an rPP/rLDPE blend prepared by single and twin screw extruders with the addition of different compatibilizers: EPRM, EPDM, and a PE-g-(2-methyl-1,3-butadiene) graft copolymer. All rPP/rLDPE/compatibilizer blends exhibited improved elongation at break and impact strength, but the extent of improvement was dependent upon the structure of the compatibilizer. The chemical structure of the copolymers, such as the ratio of ethylene to propylene or the use of block versus random copolymer, affects the resulting morphology and mechanical properties. Bertin and Robin [205] found that random copolymers performed as more efficient compatibilizers than graft copolymers, giving enhanced mechanical properties. Radonjič and Gubeljak [204] investigated the compatibilization effect of two different EPRM copolymers upon the mechanical properties of rPP/rHDPE and rPP/rLDPE blends at 80/20 wt%. The EPRM block copolymers differed in ethylene content: EPRM-1 had 68% and EPRM-2 had 59% ethylene, and the EPRM content in the blends remained at 10 wt%. They found that EPMR-1 and EPRM-2 both decreased the size of the dispersed phase in the phase separated morphology upon addition. The effectiveness of the EPRM compatibilizer was affected by the ethylene monomer content. The notched impact strength and the elongation at break improved upon the addition of EPRM-1/2 in the rPP/rLDPE blend, whereas the elongation at yield and Young’s modulus improved marginally. The improvements in the rPP/rLDPE blend were greater upon addition of the higher ethylene containing EPRM-1. However, no significant improvements were observed with the exception of notched impact strength for the rPP/rHDPE blend upon the addition of EPRM.
Maleated POs are also used as compatibilizers in the literature [204,206]. Atiqah et al. [206] used a maleated PP (MAPP) to improve the tensile properties of rPP/rHDPE blends. They observed an increase in tensile strength, Young’s modulus, and elongation at break with the presence of MAPP, which was attributed to the improvement in interfacial adhesion between the rPP and rHDPE phases. Similar results were reported by Radonjič and Gubeljak [204] who found the presence of the 10 wt% compatibilizer EPRM improved the phase adhesion by reducing the size of the dispersed rPP phase in 20/80 wt% rPP/rHDPE and 20/80 wt% rPP/rLDPE blends. The MFI was found to decrease upon the addition of compatibilizers, which was attributed to the improvement in phase adhesion.
The amount of compatibilizer added to a system will be effective up to an optimum level, at which point the interface becomes saturated. Hanna [207] investigated the mechanical properties of rPP/rPE blends with and without the compatibilizer EPDM prepared by a designed mixing-injection moulding machine. It was observed that the addition of 4 wt% EPDM to rPP/rPE blend increased the tensile strength. Upon further increase to 6 wt%, EPDM tensile strength was not affected. This is most likely due to the saturation of the interface with EPDM. The amount of EPDM did not have a significant effect on the elongation at break, flexural strength, and modulus, but minor improvements were observed. Batch mixing followed by compression moulding or single/twin screw extrusion followed by injection moulding were the methods used to produce PO blends. Bertin and Robin [205] found that blends of rPP/rLDPE/compatibilizer prepared by twin screw extrusion were more homogeneous with improved mechanical properties compared to single screw prepared blends. A uniform dispersion of compatibilizers is important for consistent performance.
4.3.4. Production of Composites from rPOs and Inorganic/Organic Fillers
To further enhance the performance of recycled materials at low cost, particular fillers can be added along with or instead of compatibilizers to form composite materials. Nano-fillers can stabilize immiscible blend morphology, leading to a finer morphology and improved material performance [40]. Recyclates contain additives such as heat stabilizers which are added during reprocessing. Therefore, the presence of additives in recyclates needs to be taken into account when adding nano-fillers and/or compatibilizers. It is essential that a balance between the quantity of filler and compatibilizer is struck as nano-fillers can affect compatibilizer efficiency if located at the interface [146]. POs are hydrophobic in nature and the addition of hydrophilic fillers results in a poor performing composite caused by a lack of bonding between the matrix and filler [208]. To improve the adhesion between the polymer matrix and filler, a coupling agent can be added and/or the surface of the filler can be modified. Coupling agents are bifunctional molecules which act as a bridge between the matrix and filler and usually have a polar and nonpolar functionality [209]. Common examples of coupling agents are copolymers containing maleic anhydride (MA) such as maleated polypropylene (MAPP), silanes, and titanates. Surface modification of the filler can occur through either chemical or physical routes. Inorganic fillers such as minerals can be added to improve the mechanical and thermal properties of the rPOs. Calcium carbonate (CaCO3) is a cheap mineral which can improve the impact strength, hardness, and Young’s modulus, but was found to accelerate polymer degradation [210]. Brachet et al. [210] studied the effect of the addition of compatibilizer ethylene–octene rubber (EOR) at either 5 wt% or 10 wt% and CaCO3 at either 10 wt% or 20 wt% for rPP. Improvements were observed in Young’s modulus at 23 °C upon the addition of CaCO3, whereas yield stress and strain increased with EOR and CaCO3 + EOR (Table 3). Only minor changes occurred in mechanical performance in comparison to those reported in the literature. Additionally, it was found that the mechanical properties were dependent upon the quantity of EOR and CaCO3. For example, Young’s modulus decreased upon addition of 5 wt% of EOR, but was found to increase upon the addition of 20 wt% of CaCO3. A ratio of 10 wt% EOR and 10 wt% CaCO3 gave the optimum balance for mechanical performance.

Table 3.
Trends in the mechanical properties of rPP with the addition of elastomer EOR and CaCO3 or both. Reproduced with permission [210].
CaCO3 can be surface treated to improve dispersion and the interactions between the filler and polymer matrix. Elloumi et al. [211] compared the effects of the addition of CaCO3 in vPP and rPP. The CaCO3 was surface treated with stearic acid but was found to have a non-uniform dispersion through vPP. The dispersion was improved in rPP due to the lower viscosity of the matrix. The mechanical properties of both vPP/CaCO3 and rPP/CaCO3 were investigated. The Young’s modulus of vPP and rPP increased by 17% and 9.5%, respectively, with 10 wt% CaCO3. The yield strength for both the vPP and rPP composites decreased upon increasing the CaCO3 loading.
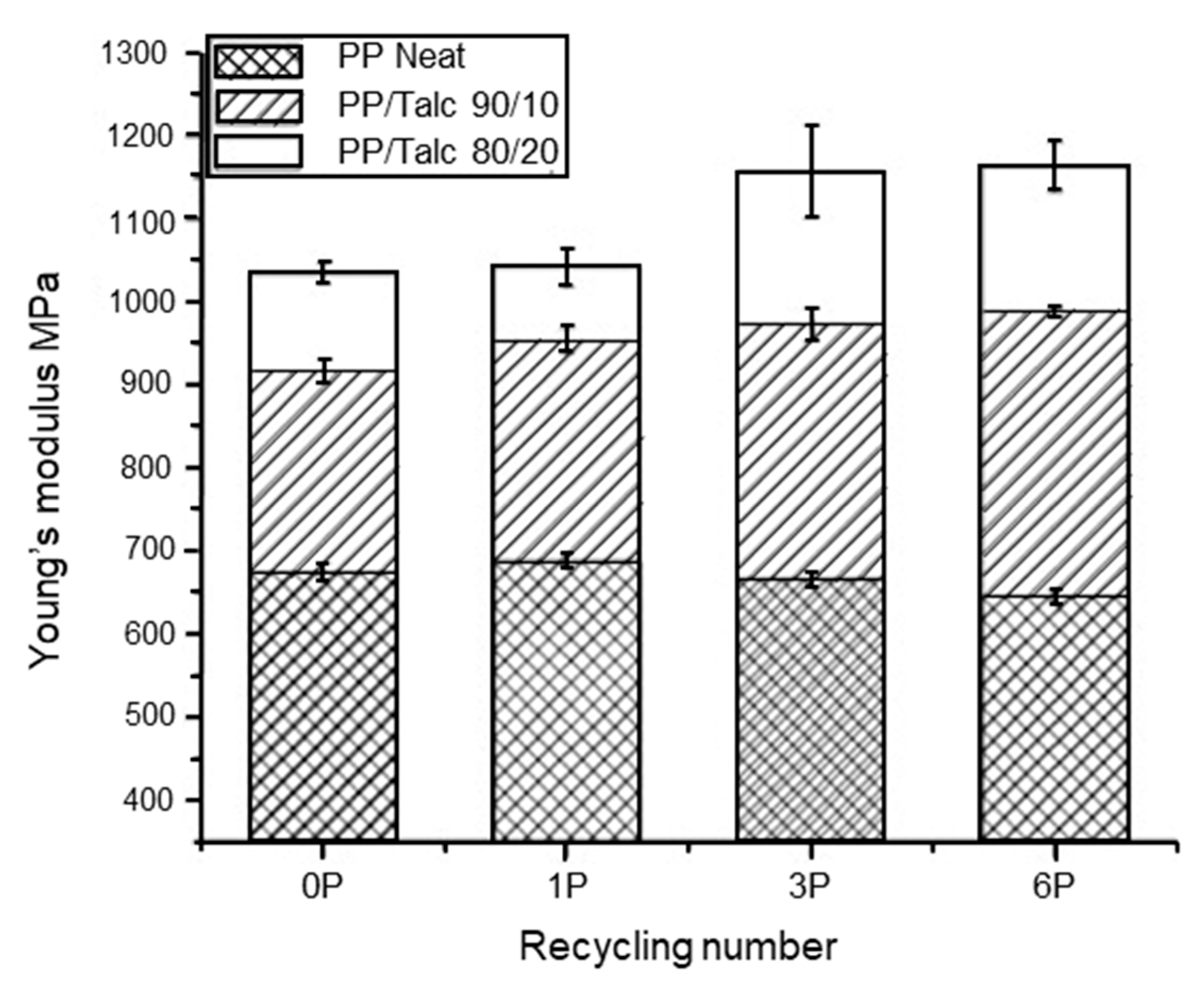
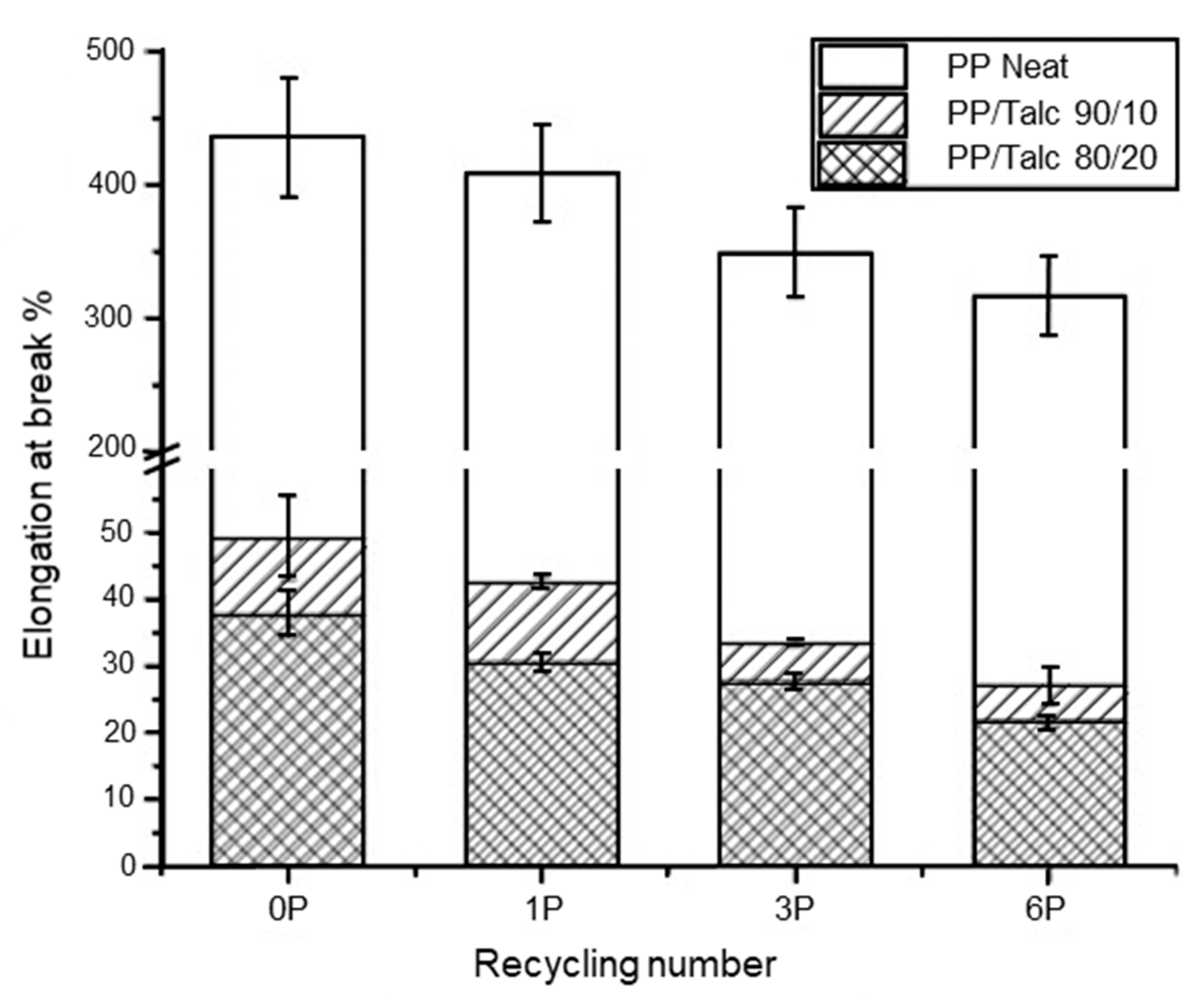
Talc is another commonly used inorganic filler. At high loadings (10–40 wt%), talc acts as rigid particles to increase the stiffness and Young’s modulus of the plastic, but causes a reduction in the elongation at break and impact strength [212]. This was observed by Wang et al. [213], who injection moulded PP with talc at 10 and 20 wt% loading. The PP/talc compound underwent six reprocessing cycles at 200 °C. They observed an increase in the Young’s modulus (Figure 9) and a decrease in the elongation at break (Figure 10) with reprocessing cycles for both PP/talc 90/10 wt% and PP/talc 80/20 wt% compositions. However, an increase in yield stress was observed for the PP/talc 80/20 wt% with recycling cycles, but with 10 wt% talc yield stress remained constant. Thermal properties were also investigated. The presence of talc stabilized the melting and crystallisation temperature of PP during the recycling processes, but the glass transition temperature (Tg) was found to decrease.
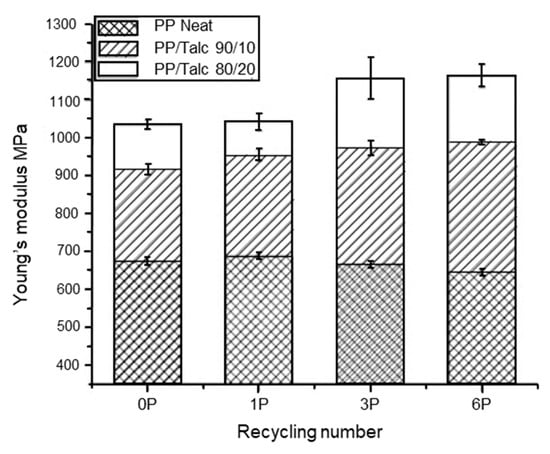
Figure 9.
Young’s modulus of non-recycled and recycled neat PP and PP/talc composites. Reproduced with permission [213].
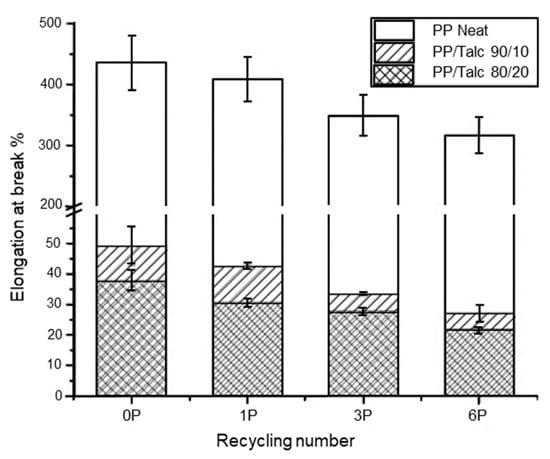
Figure 10.
Elongation at break of non-recycled and recycled neat PP and PP/talc composites. Reproduced with permission [213].
At low (below 10%) loading levels, talc can act as a nucleating agent to promote heterogeneous crystallisation, causing a change in the material performance. Sánchez-Soto et al. [214] investigated a blend of rHDPE from two different sources, industrial moulding scraps and post-consumer bottles, with talc prepared by a twin-screw extruder. The Young’s modulus increased and strain at break decreased with a smaller talc particle size (2 μm) at a higher loading (10–20 wt%), which was caused by an increase in the number of small regular crystallites in the matrix caused by the nucleation effect of talc.
Fly ash (FA) is a waste by-product from thermal power plants and has been shown to be an effective mineral filler in rHPDE [215] and rPP composites [216] to enhance the mechanical properties. As with other fillers, FA is surface modified with coupling agents due to weak interfacial bonding between the FA particles and polymer matrix. Green couplings agents, stearic acid [217], lauric acid [218], and palmitic acid [219], were shown to be effective coupling agents in rPP/FA composites. The use of green coupling agents instead of conventional chemicals can improve the sustainability and reduce the cost of rPO/FA composites.
Organoclays are layered silicates which are modified with alkylammonium groups to improve their interaction with hydrophobic polymers [220,221]. A commonly used organoclay is organically modified montmorillonite (OMMT) [221]. Organoclays have excellent thermal stability, modulus, barrier properties, and flame retardancy, which can enhance the performance of a polymer matrix [220]. This has been shown by Phuong et al. [222], who found a significant improvement in the mechanical performance of rPP which was comparable to vPP in the presence of 4 wt% nanoclay and a MAPP compatibilizer. Melt intercalation is a convenient and popular method to produce organoclay reinforced composites which involves the dispersion of the organoclay in the polymer melt. However, during processing, alkylammonium surfactants were found to degrade as the temperature increased beyond 180 °C [223]. Touati et al. [224] found that, after a recycling cycle, the mechanical and thermal properties of PP/OMMT/PP grafted maleic anhydride (PP-g-MA) composite decreased significantly but remained constant upon further recycling cycles. The dispersion of the OMMT was found to increase with recycling cycles due to the decrease in complex viscosity caused by a reduction in molecular weight.
4.3.5. Production of Composites from rPOs and Fibres
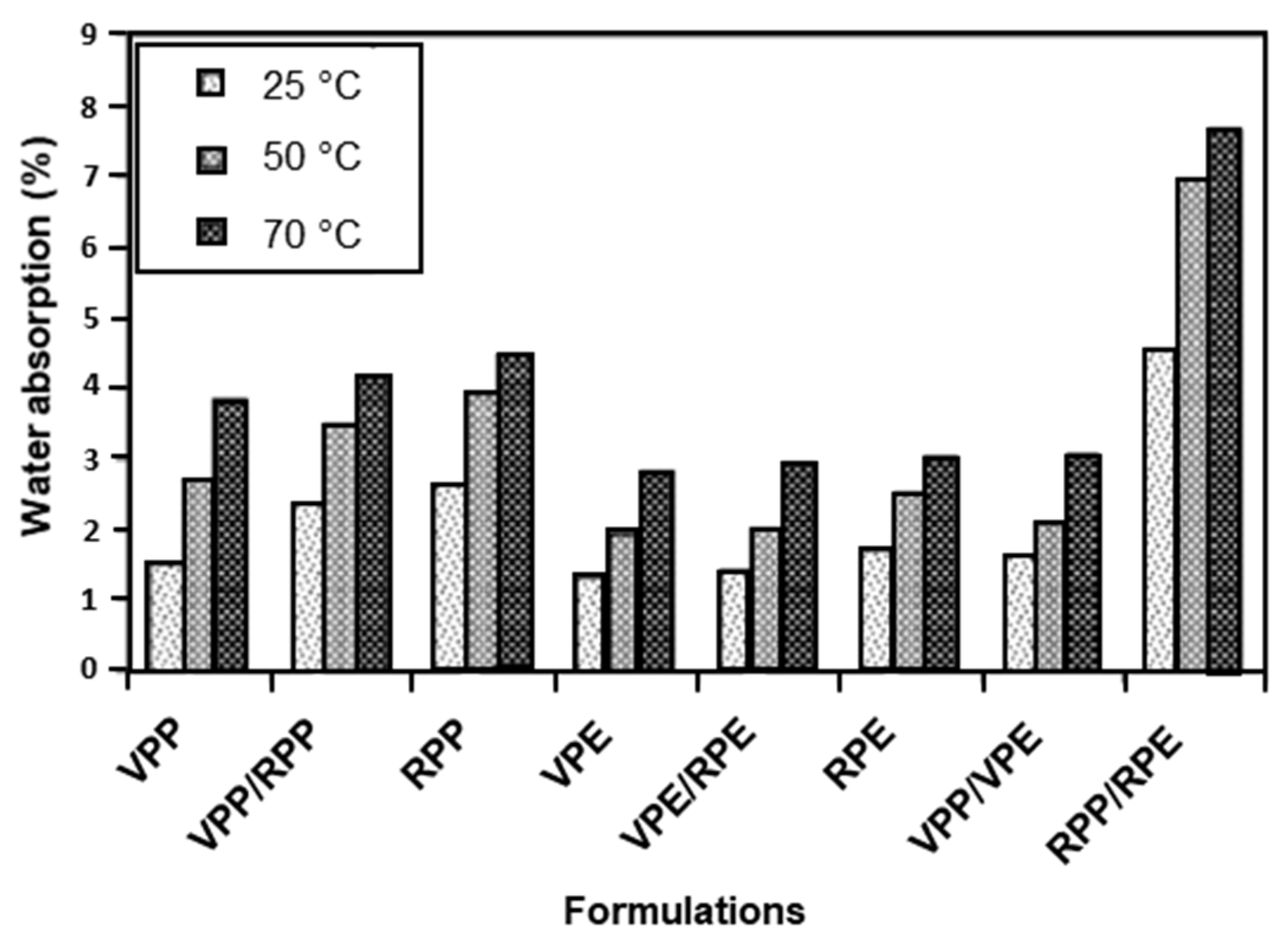
Composites consisting of rPOs and glass fibres [225,226] or carbon nanotubes [227] have been reported in the literature. However, the addition of natural plant-based fibres to recycled plastics to form wood–plastic composites (WPC) is gaining popularity as they are considered to be a sustainable and green material. Examples of plant-based fibres include wood, bamboo, pine, kenaf, cellulose, pineapple fibre, and hemp [228,229,230,231,232,233,234]. Plant fibres are cheap, readily available, and biodegradable [234], but they do possess challenges in processability due to fibre agglomeration and fibre breakage, degradation during processing and the WPC lifetime, incompatibility with hydrophobic matrices, and lifetime, which results in poor composite performance. However, the limitations can be overcome through the addition of coupling agents and/or fibre surface modification and by finding optimum processing conditions. WPCs have the potential to be used in the automotive and construction industries due to their superior strength/weight and stiffness/weight ratios [228]. Rohit et al. [235] studied the mechanical and morphological properties of a composite consisting of a recycled LDPE:LLDPE:BOPP matrix reinforced with sisal fibres at 5, 10, 15, and 20 wt%. The optimum fibre content was found to be 15 wt%. The tensile strength, flexural strength, and modulus increased with increased fibre content up to the optimum of 15 wt%. However, poor adhesion was found between the fibres and matrix and therefore required the addition of a compatibilizer. Inácio et al. [236] suggested that bamboo fibre reinforced rPP/talc/EPDM composites with a PP-g-MA compatibilizer have the potential to be used in the automotive industry. They found that the addition of the bamboo fibres and PP-g-MA resulted in an increase in the tensile strength and modulus, flexural strength, and fatigue life of the composites. Impact strength and elongation at break were found to decrease. Improvements in impact strength are possible through the use of impact modifiers, such as EPDM and ethylene vinyl acetate (EVA). The durability and longevity of the composite is crucial for its use in interior and exterior applications within the automotive industry. Therefore, more research into improving the adhesion between the natural fibre and recycled matrix is required to ensure fibre debonding does not occur, along with the effects of the environmental conditions on the degradation of natural fibres within WPCs. Due to plant fibres’ hydrophilic nature, they absorb water from the surrounding environment [237]. This results in thickness swelling, resulting in a dimensional instability in WPCs and limiting their possible end use. Kazemi Najafi et al. [238] compared the properties of WPCs made from wood flour and virgin and recycled plastics, PP and HDPE, after immersion in distilled water after 2 and 24 h at three temperatures: 25, 50, and 70 °C. They found that WPCs containing recycled plastics had a higher water absorption compared to WPCs containing virgin plastics caused by the poor wetting of the wood flour by the recycled plastics. Weak bonding between the wood flour and the recycled plastics caused the rate and the amount of water absorbed to increase. WPCs with mixed rPP/rPE had the greatest water absorption. Additionally, the immersion temperature was found to influence the extent of water absorption with higher temperatures leading to higher levels of water absorption (Figure 11).
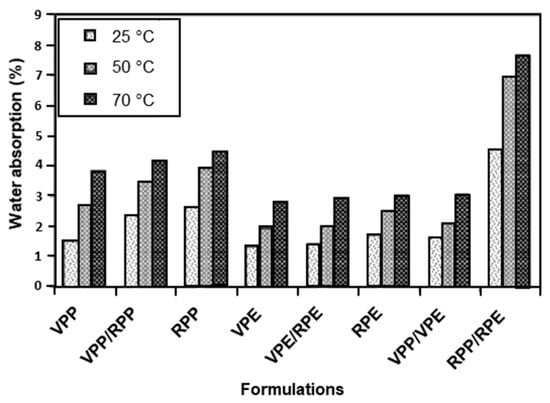
Figure 11.
Water absorption of virgin and recycled plastic/wood flour composites after 2 h of immersion at three immersion temperatures: 25 °C, 50 °C, and 70 °C. Reproduced with permission [238].
Ashori and Nourbakhsh [239] investigated the addition of recycled old newsprint in rHDPE/rPP blends. They suggested that water absorption in fibrous composites was dependent upon several factors: temperature, fibre loading, permeability and orientation, void content, diffusivity, area of exposed surfaces, and the hydrophilicity of the components. A concern for potential industries is WPCs’ high risk of flammability. However, Zhang et al. [240] found an improvement in the flame retardancy of vPP/wood composites through the use of ammonium polyphosphate and silica as fire retardants. Das et al. [241] showed that organosilanes are an effective coupling agent in rPP/jute caddy composites to enhance their thermal stability.
4.4. Applications of rPOs
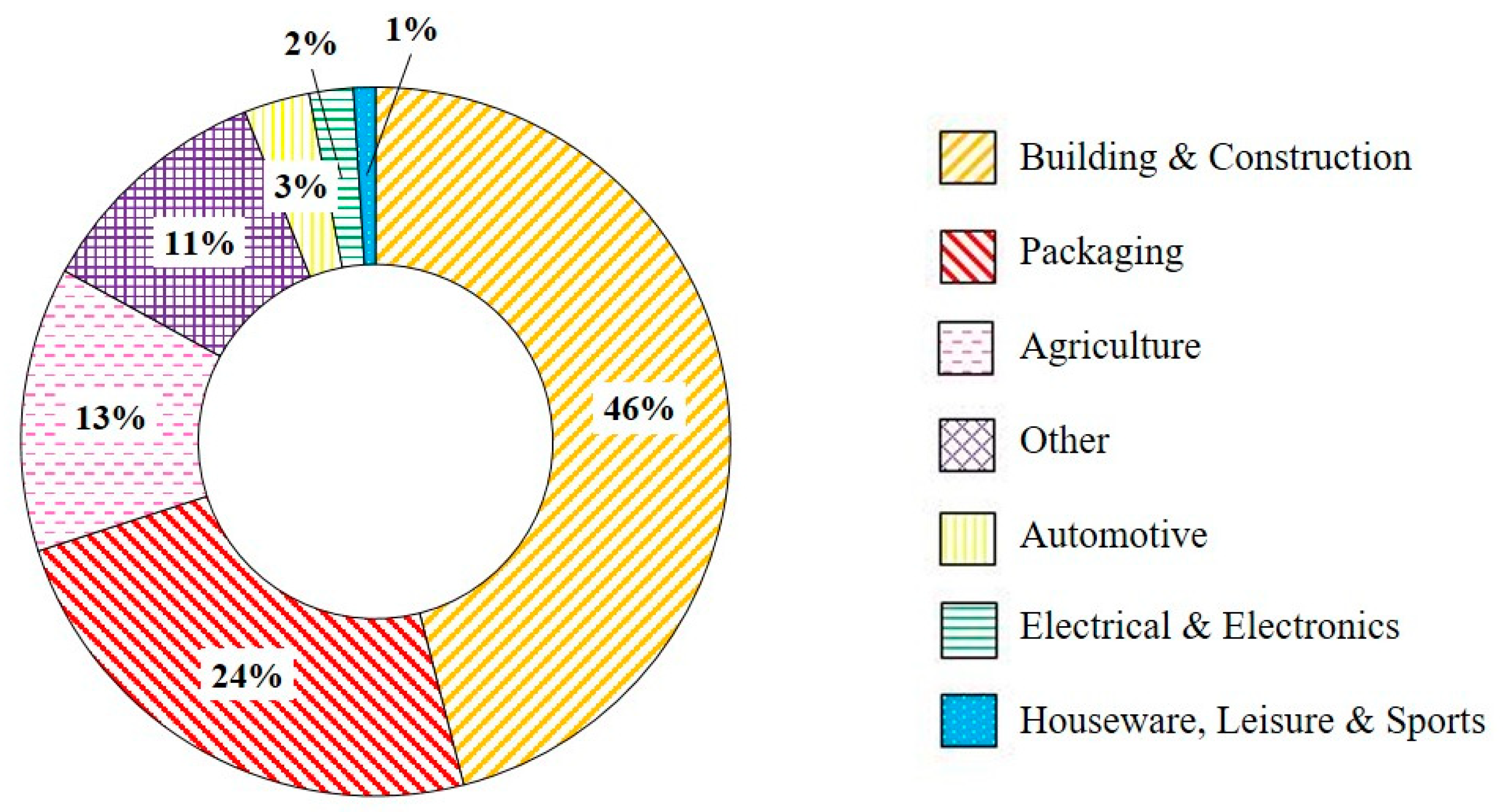
The price of POs is determined by the oil price as they are sourced from petrochemical derivatives, ethylene and propylene. As the price of oil changes, so does the cost of virgin polymers [17]. rPOs have the potential of being used in a wide range of applications if a control and improvement in waste quality could be achieved without a large increase in cost. Plastics Europe [30] found that post-consumer recyclates have been used in a variety of industries, as shown in Figure 12, with the building and construction sector using the highest proportion of recyclates [242]. Jubinville et al. [62] discussed several potential applications for rPO from WPCs, which were discussed in Section 4.3.5, as a fuel either via pyrolysis or energy recovery, to aggregates within concrete. Andoh et al. [243] proposed a 75 wt% rHDPE:25 wt% bamboo fibre composite which could potentially be used in wind blade manufacturing. Post-consumer PO waste can additionally be used in the manufacturing of plastic lumber. Plastic lumber is used in the construction of docks, marine piling, fences, and park benches [244]. Grammatikos et al. [245] demonstrated a WPC consisting of rHDPE with 30, 40, and 50 wt% wood fibre flour which had adequate mechanical performance for use as flooring in ship containers.
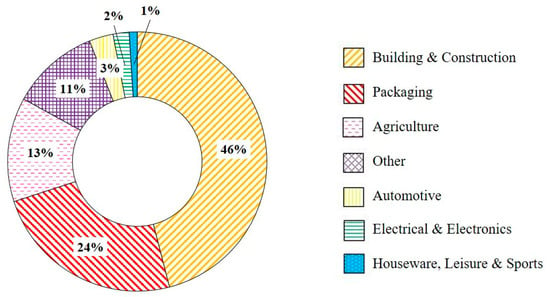
Figure 12.
Use of post-consumer recyclates in different industries in 2019 [30].
5. Recycled Polyethylene Terephthalate (rPET)
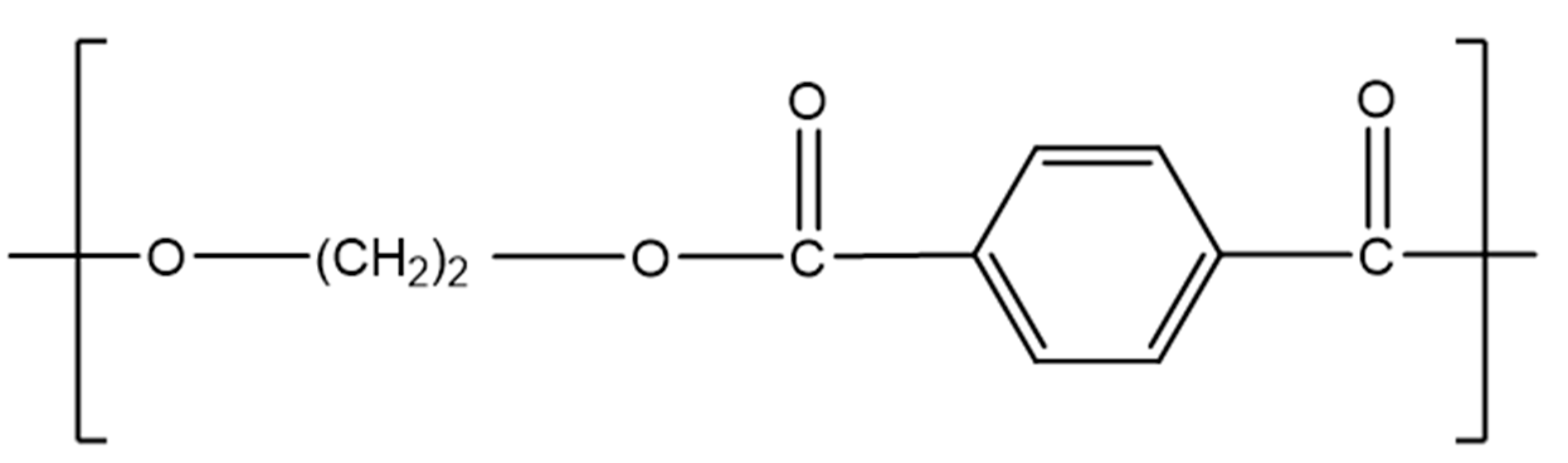
Currently, polyethylene terephthalate (PET) is mainly used for fibre production and in the packaging sector for bottles and trays, due to its resistance to shrinkage, heat stability, high stiffness, strength, and high barrier property against oxygen [57]. For these reasons, 96% of its European production is dedicated to the packaging industry [246] and its use for drinking bottles expanded worldwide from 300 billion bottles in 2000 to 480 billion in 2016 [247]. The PET quantity produced globally each year is approximately 70 million tonnes compared to 33 million tonnes in 2015 [248,249]. PET is a part of the polyester family, and its monomer unit is presented in Figure 13.
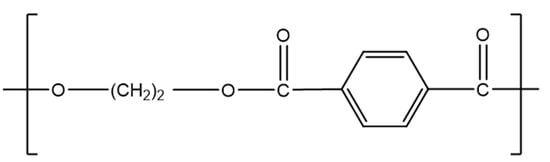
Figure 13.
PET unit. Reproduced with permission [118].
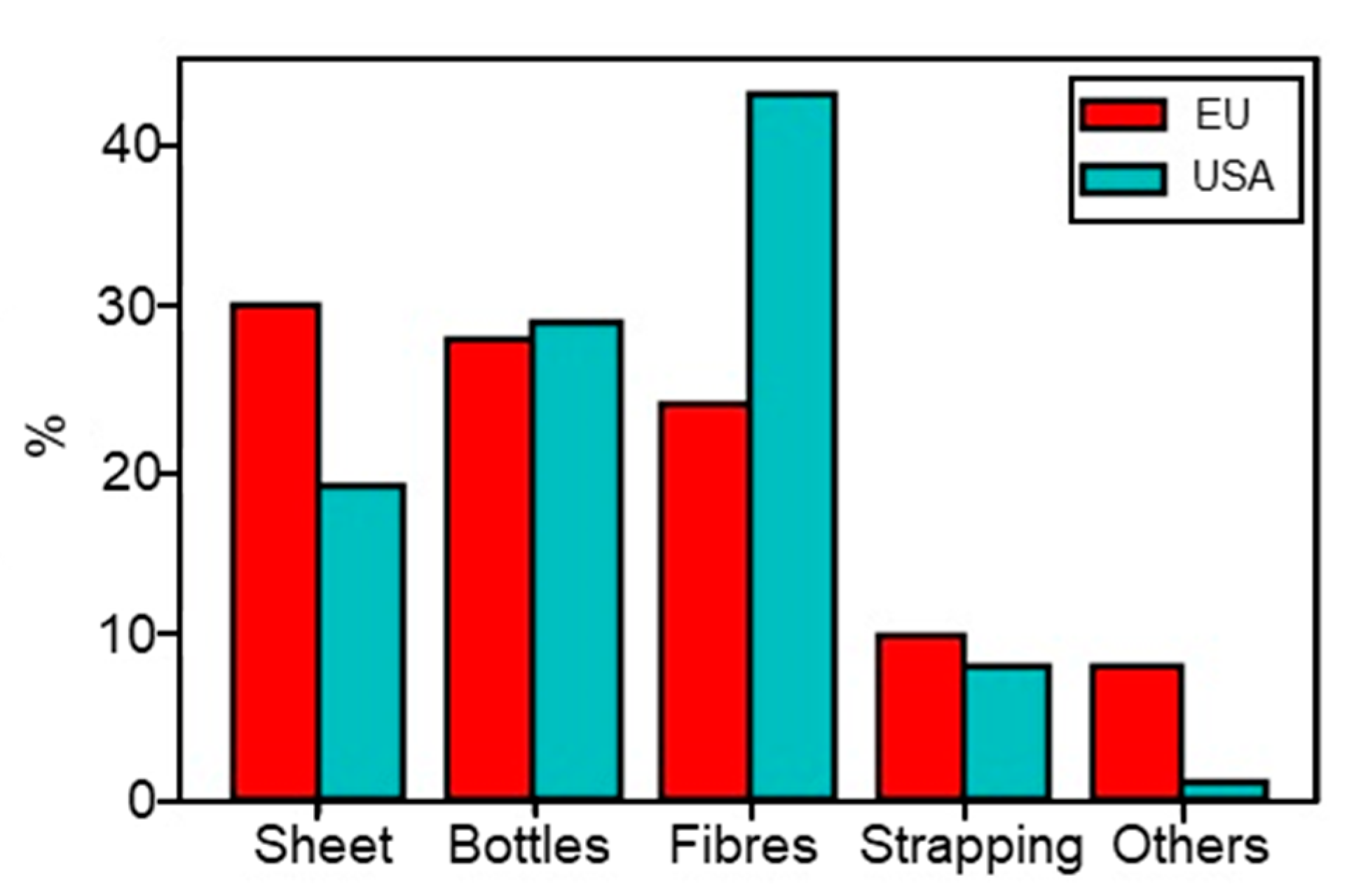
PET can be produced either by an esterification reaction between ethylene glycol (EG) and terephthalic acid (TPA) or by a transesterification of dimethyl terephthalate (DMT) with EG [250]. Both reactions create a prepolymer: bis (hydroxyethyl) terephthalate (BHET) [250]. Different grades of PET are used for different applications such as bottles, geotextiles, clothing, etc. Properties such as intrinsic viscosity (IV), molecular weight, or optical properties vary for all these grades [251]. The unrestrained PET production presents a real threat for the environment, animals, and human health if no recycling policy is implemented. As an example, one million PET bottles are discarded worldwide per minute (2018) [252,253], and the full decomposition of PET in soil requires over 300 years [44]. For these reasons, many countries are taking more and more interest in PET recycling, which can reduce the waste quantity and the exploitation of fuel resources. India, Germany, Japan, and South Africa appear to be leaders in this sector [254]. Thus, PET is one of the most recycled plastics with about 18.5% of PET wastes recycled in 2016 [255]. Even if the applications of recycled PET (rPET) can vary depending on the countries, today rPET is mainly used for fibres and plastic bottle production as presented in Figure 14 [246,254]. However, rPET can also be found in electrical and lighting components, housewares, sports equipment, automotive parts [256], and in bitumen roads and pavement [242]. rPET presents some advantages in the construction industry, such as raising the ductility of concrete or reducing the weight of aggregates [242,257].
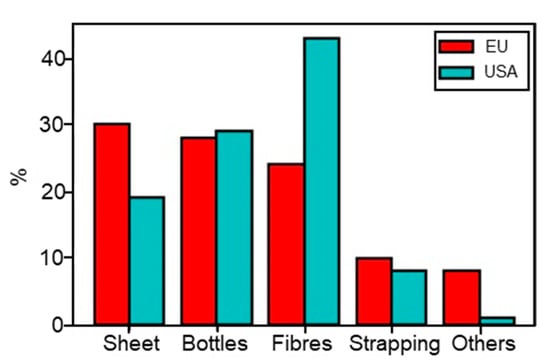
Figure 14.
Applications of rPET in the EU and USA [246,254].
Meanwhile, PET recycling represents a real economic and technical challenge. Economically, the low cost of vPET can hinder the use of rPET in industries even if PET is one of the most cost-efficient plastics to recycle [44,249]. Technically, the different contaminants present in PET waste can highly reduce its molecular weight and hence its mechanical properties [45]. Different waste management techniques are employed for PET: mechanical recycling, chemical recycling, and energy recovery [258,259,260,261,262]. Primary recycling requires high quality waste and energy recovery is selected when sorting is too difficult or too expensive. Neither of these appear like promising solutions for PET; therefore, major research is focused upon chemical and mechanical recycling [118].
5.1. Chemical Recycling of PET
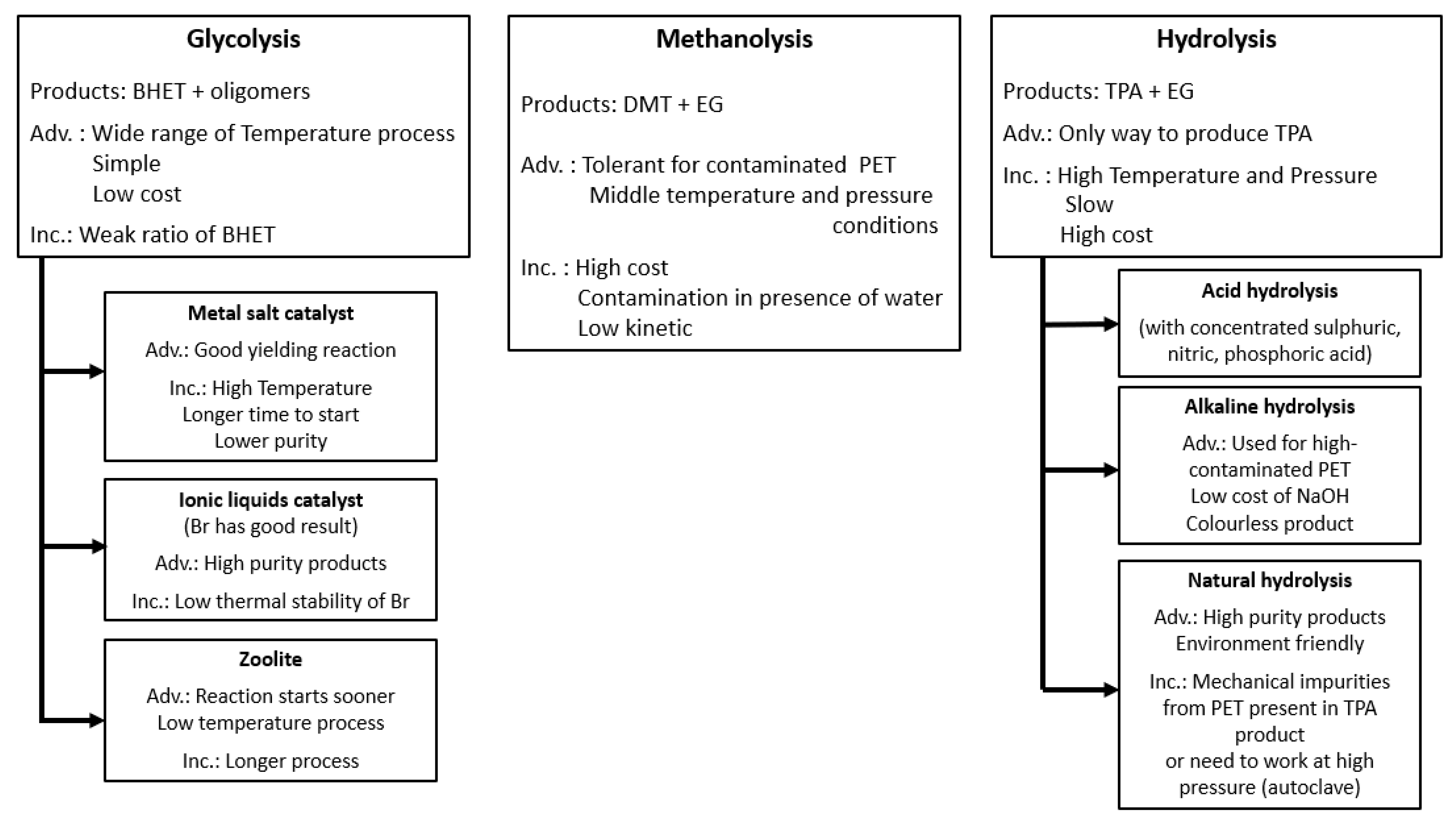
Different treatments have been developed in order to depolymerise PET waste [259,262,263]. The three major chemical routes for depolymerisation are glycolysis [264,265,266,267,268,269], methanolysis [270,271,272,273,274], and hydrolysis [270,274,275,276,277,278,279,280,281], summarised in Figure 15. For glycolysis and hydrolysis transformations, researchers focussed on the selection of catalysts, which could offer a high yield, short process time, and more environmental friendliness [106,249,282,283]. Thus, for glycolysis, Chiaie et al. [249] underlined the importance of tuning the strength of Lewis acid-base catalysts. López-Fonseca et al. [282] recommended the use of sodium bicarbonate as a catalyst, which can be as efficient as zinc acetate in higher concentrations but is more respectful towards the environment. For methanolysis, Yang et al. [283] obtained a good degree of depolymerisation under the supercritical state of methanol for different PET colours and waste origins. The different products of chemically recycled PET can be used to produce new materials. For example, Viante et al. [106] developed magnetic microsphere composites from magnetic nanoparticles and BHET produced by glycolysis and modified by glycidyl methacrylate (GMA).
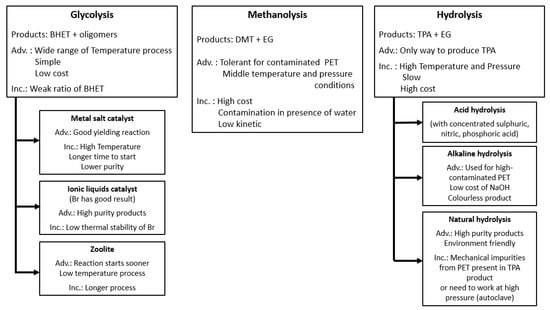
Figure 15.
Presentation of major chemical treatments for PET recycling. The abbreviation “Adv” stands for advantages and “Inc” stands for limitations [249,250,251,282,283,284,285,286].
Two other chemical treatments were reported: ammonolysis and aminolysis [249,250,251,282,283,284,285,286]. They correspond to a reaction of PET with anhydrous ammonia and with amine in aqueous solution, respectively. Moreover, Kárpáti et al. [287,288] recently developed a new chemical route for PET recycling by acido-alcoholysis which allowed the production of high quality oligoesters by combining depolymerisation and polycondensation in one step. Chemical treatments appear to be a very promising way to recycle waste PET because they offer the possibility to produce new virgin PET with good properties. However, the use of chemicals, which poses a threat to the environment and incurs high costs, present challenges that still have to be solved.
5.2. Mechanical Recycling of PET
Subjecting PET, in particular multi-layered PET packaging, to several mechanical reprocessing cycles produces lower quality rPET products because of the degradation of PET during the re-melting process [256,282,289,290]. The breakdown of the backbone chain causes a decrease in the molecular weight and intrinsic viscosity (IV) with a rise of the end groups’ (carboxyl and hydroxyl) number [282,289,291]. These phenomena are accompanied by a change in the PET colour, which becomes slightly browner or greyer [292]. This degradation of PET is also linked to the trace acidic contaminants during the melting step of the recycling [258,293]. Acids such as acetic acids from EVA or hydrochloric acid from PVC induced chain scission of the molten PET, causing degradation [251]. The presence of acidic contaminants is a major issue for PET recycling. PVC is a commonly used plastic which possesses low thermal stability and undergoes significant thermo-oxidative degradation by chain scission during reprocessing [294,295]. Szarka et al. [294] illustrated the importance of understanding the effect of thermo-oxidative degradation on PVC thermal stability. They found at shorter degradation times partially oxidized PVCs formed and at longer PVC degradation times (after 3 h) oily products were formed, which could increase the number of routes for PVC recycling. Awaja and Pavel [118] underlined the impossibility of removing more than 90% of PVC during the manual sorting step. Moreover, the contamination by metal ions can also create transesterification and polycondensation reactions [251]. Finally, an amount of water even in the range of 0.01–0.02 wt%, incites hydrolysis of the ester groups in PET during melting, leading to a decrease in molecular weight [155]. Without a drying step, Eriksen et al. [296] noticed an extreme decrease in the mechanical properties of rPET from packaging waste in comparison to vPET. Tensile and impact strengths were reduced by more than 55% and 90%, respectively. On the contrary, Qin et al. [297] underlined that with dried copolymer rPET from clear flakes, melt-spun fibres have a tenacity and a strain at break very close to melt-spun fibres from vPET. Likewise, Frounchi [298] noticed only a very slight decrease of tensile and impact strength of dried rPET even when the molecular weight was reduced by around 26%. He also studied the impact of the change in chain length on crystallisation. Cold crystallisation was evident in rPET and not in vPET, and the crystallisation rate increased with an increase in the number of recycling cycles.
To minimise rPET degradation, multiple solutions have been investigated, such as solid-state polymerisation (SSP) [285,299], adding chemical stabilisers, or chain extenders [300,301,302,303,304]. The SSP process is commonly used in industry to improve the quality of rPET by aiming to increase the molecular weight of rPET [305]. The SSP process corresponds to heating PET at temperatures between the Tg and melting temperature (Tm) and under low pressure [306,307,308]. These conditions increase the molecular weight by transesterification reactions and improve the mechanical properties of rPET. Chain extenders have been used in industry with PET to counter the degradation caused by mechanical recycling, but there are concerns of their use in food grade packaging due to migrations [309]. Pyromellitic dianhydride (PMDA) is a commonly used chain extender for rPET. According to Kossentini-Kallel et al. [310], the addition of 0.3 wt% PMDA increased the molecular weight and IV by approximately 150% and 75%, respectively. Awaja et al. [311] reported that IV and die pressure were raised with an increasing amount of PMDA. They also highlighted the possibility to achieve improved elastic modulus and tensile strength for reactively extruded rPET compared to vPET. Awaja and Pavel [118] studied the impact of PMDA on injection stretch blow moulding (ISBM) of rPET and vPET blends. Other types of chain extenders have also been investigated, such as diisocyanates [312], diphosphates [313], and silicones [314]; the last ones are particularly effective to increase the flexibility of rPET, facilitating the extrusion process.
Effects of Mechanical Recycling on PET Properties
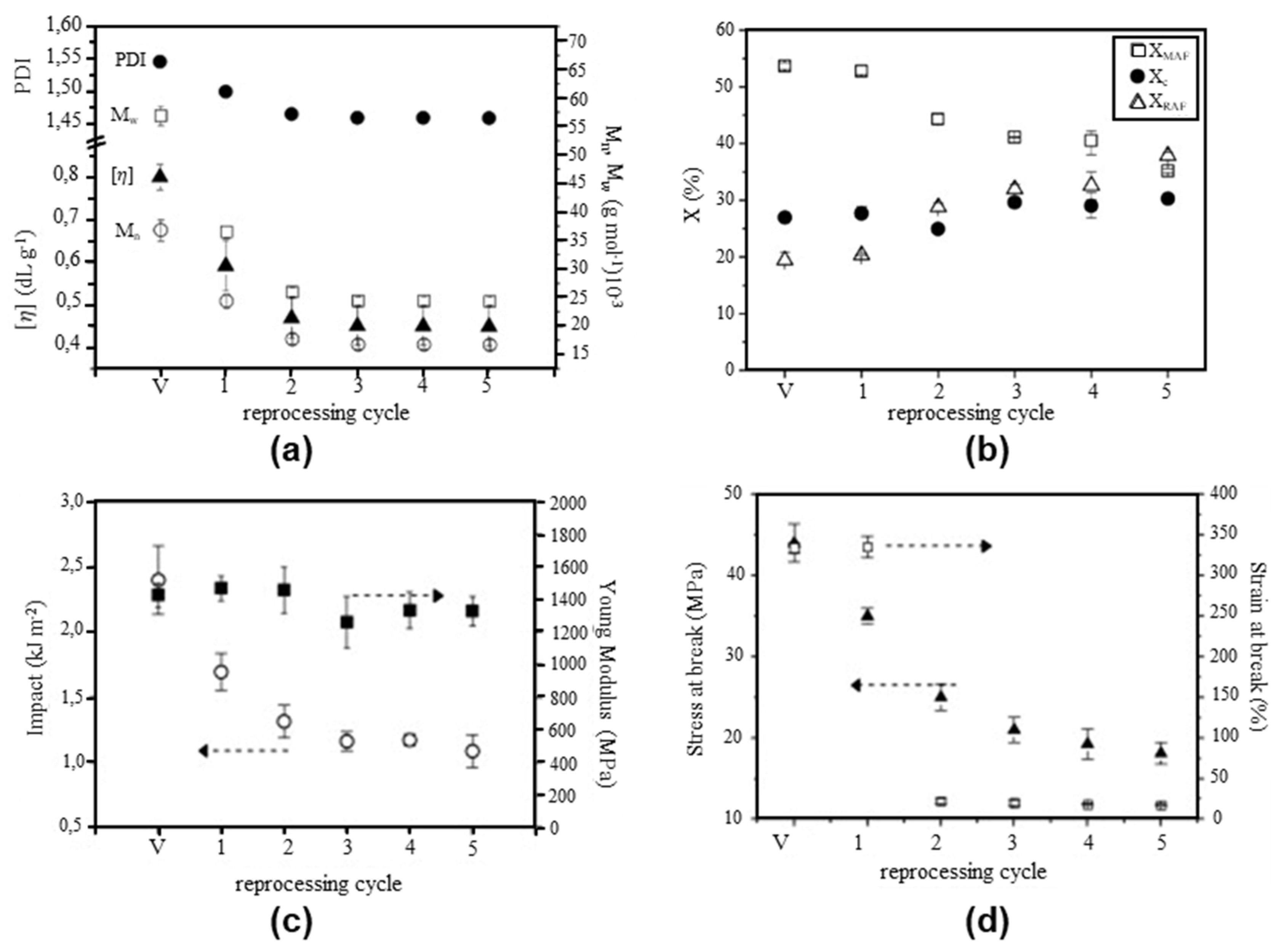
The thermal and mechanical properties of rPET are difficult to define because they strongly depend on the sources of the PET waste, the presence of contaminants, and the recycling conditions. Badia et al. [315] investigated the influence of mechanical recycling on the vPET properties. They emphasised the importance of chain scissions, which were revealed by a higher number of -OH groups, which explains the yellowing aspects of rPET. They also studied the influence of recycling cycle number on the viscosity and polydispersity index (PDI) (Figure 16a), crystallinity (Figure 16b), and mechanical properties (Figure 16c,d) of PET.
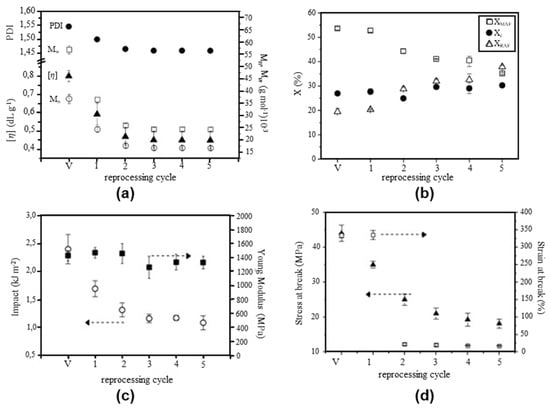
Figure 16.
Effect of mechanical reprocessing cycles on (a) PDI and viscosity, (b) crystallinity, (c) impact and Young’s modulus, and (d) stress and strain at break of PET. Reproduced with permission [315], 2012, Elsevier.
Some authors also studied the influence of contaminant polymers on the final properties of rPET [45,316]. Itim and Philip [316] investigated the influence of 5 wt% of PP contamination in bottle grade PET multicolour waste. With this contaminant, the crystallinity degree and crystallisation rate were reduced in comparison to neat rPET and that decreased further with the number of recycling cycles. They also highlighted that under these conditions crosslinking predominated over chain scissions. Torres et al. [45] compared the properties of vPET and rPET from homogeneous blue bottles, and rPET from heterogeneous wastes contaminated by PVC. Their different results are summarised in Table 4. They reported that rPET was more sensitive to hydrolytic degradation than vPET due to the presence of contaminants and moisture.

Table 4.
Comparative properties of vPET and rPET. Reproduced with permission [45].
5.3. Compatibilization of rPO and rPET Blends
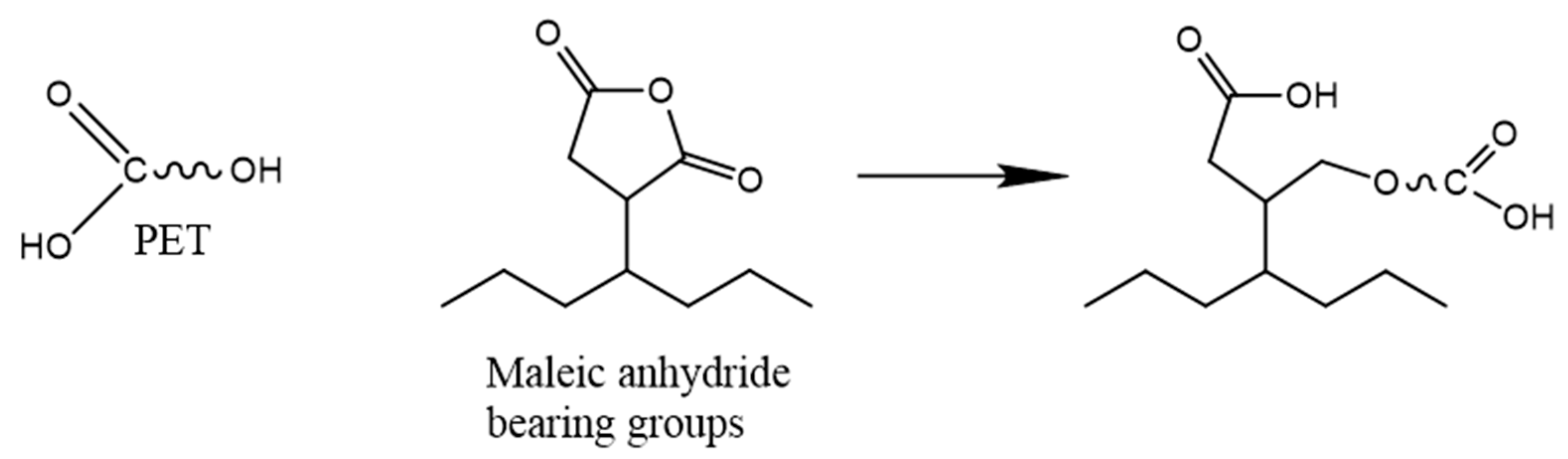
The blending of rPET with other virgin/recycled polymers such as POs can produce a material with beneficial versatile mechanical and barrier properties and processability, overcoming the rPET limitations and reducing the need for plastic waste sortation during mechanical recycling [317,318]. rPET has a generally higher tensile and flexural strength and modulus than rPO, while rPOs offer higher impact strength. Thus, the blends of these materials appear less brittle than neat rPET and stiffer than neat rPO [319,320,321]. Therefore, their blends can represent very promising materials. However, their processing gives rise to numerous difficulties. Firstly, due to a large difference between their melting temperatures (Tm_PET ≈ 250 °C, Tm_PE ≈ 100–140 °C, Tm_PP ≈ 160 °C), POs and the adhesion between PO and PET can be degraded at temperatures corresponding to rPET processing temperatures (around 270 °C). Acceptable mechanical properties can be obtained for an injection moulding temperature close to 185 °C [320]. As mentioned before, the crystallinity of recycled polymers is higher than that of virgin ones due to their shorter molecular chains and decrease in chain entanglement [199,322]. Zander et al. [323] noticed that the relative crystallinity of each phase and more particularly of the dispersed phase decreased by blending in comparison to neat materials. On the other hand, numerous studies reported the nucleating effect of the rPET phases in the rPO matrix [319,324] as well as the rPO phases in the rPET matrix [325]. Due to a large difference in polarity between POs (nonpolar) and PET (polar) structures, they are immiscible [325]. Therefore, the production of their blend requires a compatibilization step [40]. The addition of compatibilizers is the most widely employed method, but other approaches such as radical processing via the use of an initiator or irradiation have also been investigated [40]. For rPET and rPO, the most common compatibilizers are the ones with MA or GMA functions (Table 5). Some of the compatibilizers such as EPDM, EVA, poly(styrene-butadiene-styrene) (SBS), and poly (styrene-b-(ethylene-co-butylene)-b-styrene) (SEBS) also have a rubbery function which improves the toughness of the blends but might reduce its stiffness [323,326,327,328,329]. The MA function can react with the hydroxyl group of rPET by the esterification reaction (Figure 17). These compatibilizers allow for an improvement of the impact strength and elongation at break of the blends, higher than those of the neat rPET.

Table 5.
Literature summary on preparing PO/rPET blends.
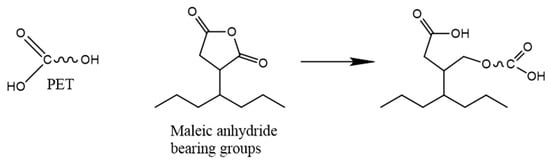
Figure 17.
Chemical reaction between rPET and MA function of the compatibilizer Reproduced under terms of the CC-BY license with permission [335].
Taghavi et al. [330] reported optimal compositions of blends: 75/10/15 wt% and 70/15/15 wt%, respectively, for rPET/vHDPE/SEBS-grafted-MA (SEBS-g-MA) and rPET/vHDPE/Maleic anhydride-grafted-polyethylene (MAPE). Their comparative study concluded that the rPET/vHDPE samples with MAPE were smoother and had a lower number of voids than the ones with SEBS-g-MA. Lei et al. [319] studied the influence of the introduction of rPET into the rHDPE matrix. The dispersed and matrix phases were inverted in this work in comparison to the work of Taghavi et al. [330]. In order to improve the dispersion of rPET in the rHDPE matrix, they added 2 wt% of PE-g-MA as a compatibilizer and 5 wt% of SEBS as an impact modifier in a 70/30 wt% blend. Under these conditions, the impact strength of the blend became higher than that of neat rPET and its phases were well dispersed, but the tensile strength was reduced. According to Araujo et al. [325], an enhanced impact strength value was obtained with the 80/20 wt% for rPP/rPET blend and 20 phr of SEBS-MA, which acted as a compatibilizer as well as an impact modifier. Moreover, they underlined that SEBS-MA was more efficient at dispersing rPET particles in the rPP matrix than at dispersing rPP particles in the rPET matrix. This phenomenon was caused by the lower viscosity of rPET, whose droplets could be more easily dispersed than the ones of rPP. Zander et al. [323] studied the effect of 5 wt% of SEBS-MA on an rPP/rPET blend. They observed the presence of two distinct melting peaks which revealed that the miscibility is not enhanced by the compatibilizer. SEBS-MA was also used to improve the compatibility between rPET and vLLDPE. A blend of 80/20 wt% rPET/vLLDPE showed an increase in the elongation at break from 10.4% (without compatibilizer) to 267.5% upon the addition of 10 wt% of SEBS-MA compatibilizer [328]. All the blending conditions (compatibilizers and temperatures) presented above are summarised in Table 5.
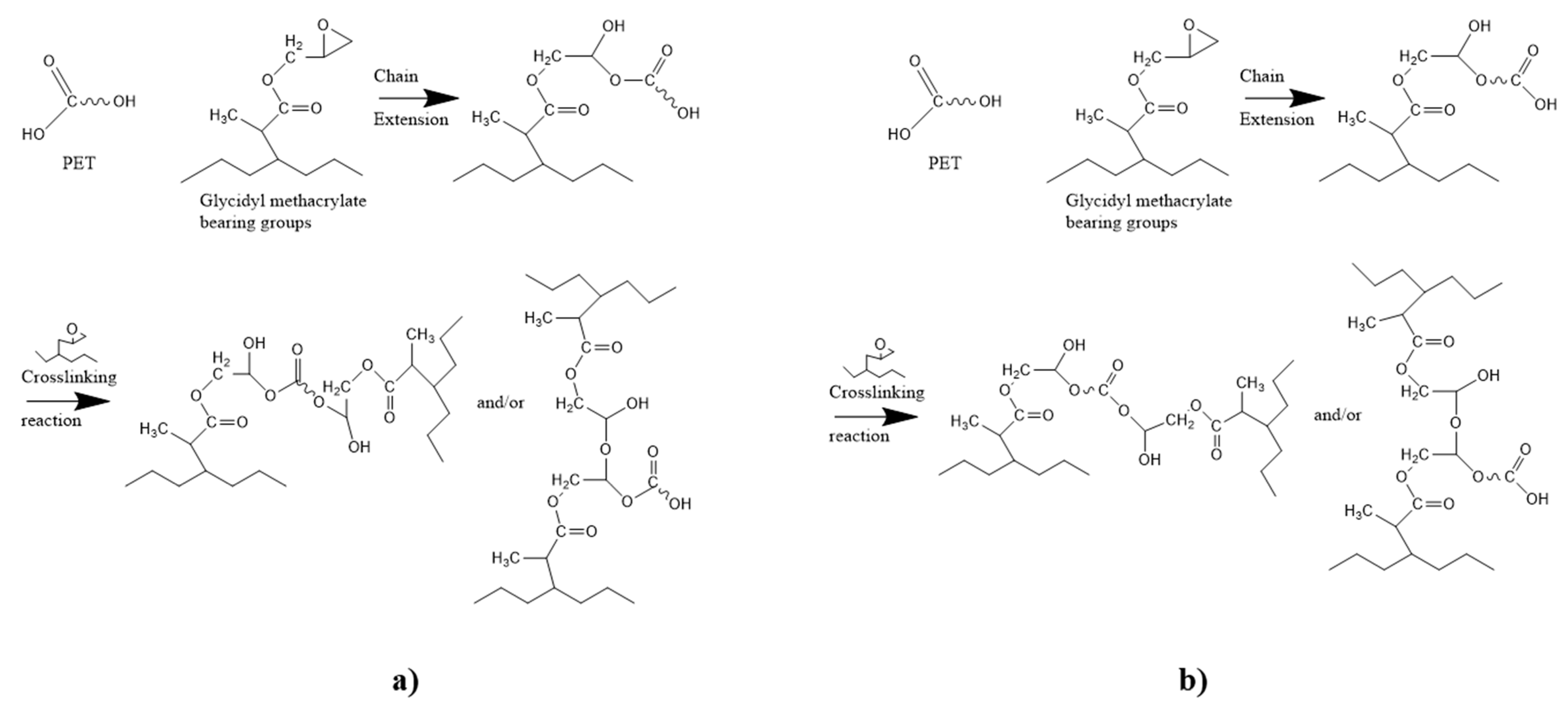
The GMA compatibilizers appear more promising to improve the compatibility between rPET and rPOs as their epoxy group can react with both hydroxyl and carboxyl groups of rPET (Figure 18) [331].
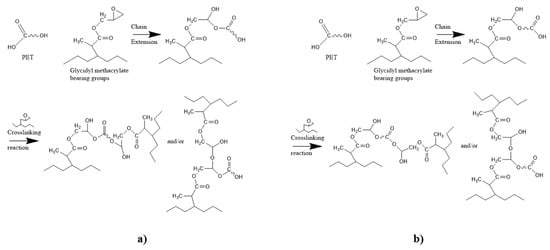
Figure 18.
Chemical reactions between rPET and GMA function. (a) Chain extension with OH end group and crosslinking (b) chain extension with COOH end group and crosslinking. Reproduced under terms of the CC-BY license with permission [335].
Pracella et al. [332] showed that a very good dispersion was possible with very low amounts of ethylene-glycidyl methacrylate (E-GMA) (<5 pph) for the 75/25 wt% rPET/rPE blend and the 75/25 wt% vHDPE/rPET blend. In the case of the second blend, 5 wt% of E-GMA produced a blend of higher impact strength, tensile strength, and modulus compared to pure rHDPE [331,333]. Kalfoglou et al. [336] demonstrated that among the E-GMA, ethylene-ethylacrylate glycidyl methacrylate (E-EA-GMA), SEBS-g-MA, and ethylene-methyl acrylate copolymer (E-MeA-g-MA), the highest impact strength and tensile properties were obtained with a 70/20 wt% rPET/rHDPE blend having 10 parts of E-GMA. Imamura et al. [334] highlighted a very promising effect of E-GMA on rPET/rPE/rPP/rPS blends, which increased the impact strength and the elongation at break and offered higher tensile properties than an α-olefin modified LLDPE copolymer compatibilizer. Researchers have studied the effect of varying compatibilizer dosage on material performance, such as rHDPE/rPET blends and rPET/LLDPE blends [328,333]. Generally, a compatibilizer dosage above 5–10% within the blend has been found to have a negative impact on the mechanical properties due to the formation of agglomerates and voids. Compatibilizers in high dosages can also reduce the crystallinity of the polymers by increasing the adhesion between them [328,333].
Less generic compatibilizers for the use in rPET and rPO blends have also been studied. Choudhury et al. [337] evaluated the efficiency of an ionomeric compatibilizer, Surlyn 1650, which is a zinc salt of the ethylene-methacrylic acid copolymer. The carboxylate function of this compatibilizer can react with the carbonyl and hydroxyl groups of rPET. This study underlined an improved compatibilization of the rLLDPE/rLDPE/rPET compared to the one observed with PE-g-MA. The effect of gamma irradiation was examined by Abdel Tawab et al. [338]. This treatment showed two antagonist effects: the enhancement of crosslinking, which improved the adhesion of polymers, but also an oxidative chain scission. By coupling a gamma irradiation dose of 100 kGy and 10 wt% amount of EVA, Abdel Tawab et al. [338] produced a blend with improved mechanical properties.
5.4. Production of Composites from rPET and/or rPO
As described in Section 5.3, rPET and rPO can be combined to produce blends, but they can also be used to produce composites. Both rPET and rPO can be employed as fillers to improve matrices which are used for the production of composites. The fillers help to improve the tensile strength, creep resistance, heat deflection temperature, and the shrinkage of the matrix [339,340,341]. rPET microfibrils were added into the rHDPE matrix by Lei et al. [320], where E-GMA proved to be an efficient compatibilizer. rPET particles and fibres can also reinforce other matrices such as unsaturated polyester resin (UPR). With both being polyester, a good adhesion between the particles and matrix can be achieved. Fidanovski et al. [252] showed that for bio-based UPR and rPET particles, the mechanical properties were lower than those of non-bio-based UPR and glass fibres (GF). However, due to their cost-efficiency and eco-friendliness, they remain very promising materials for future applications. The incorporation of rPET fibres into the PP matrix without compatibilizer was studied by Santos and Pezzin [257]. They showed an increase of impact strength with rPET fibre volume fraction (Vf) between 3 and 7% and suggested the use of a compatibilizer to produce composites with higher rPET Vf. The properties of reinforcing rPET fibres can be improved in different ways, such as by modifying their viscosity during the extrusion process by adding 0.5 wt% of hyper-branched PET (HBPET). This treatment increases the tenacity and initial modulus of the fibres due to the lubricant property of HBPET [342]. rPET fibres can also be designed to reinforce a lower melting PET copolymer matrix as proposed by Romhány et al. [343]
Other forms of rPET can also be used to produce composites. PET char, which is a product from PET waste pyrolysis, can be added in epoxy resin to produce semi-conductor composites, whose mechanical properties (surface hardness and tensile strength) depend on the pyrolysis conditions [344]. rPET is also widely used to reinforce concrete. It improves tensile strength and fracture energy in comparison to normal concrete. Khalid et al. [345] studied the influence of rPET particle shapes on the final properties of the concrete. They reported that ring-shaped rPET (cross-sectional diameter of 60 ± 5 mm and a thickness of 10 ± 1 mm) reinforcement improved the strength of the first crack by one-third in comparison to normal concrete. On the contrary, irregularly shaped fibres (different length and width) have the lowest results. However, the addition of rPET in concrete presents a real challenge for the post-fire properties as discussed by Nematzadeh et al. [346].
Composites can also be developed with the rPET matrix and can be reinforced with GF, CaCO3, talc, and plant-based organic fillers. In order to improve tensile strength, flexural strength, and modulus, GF are more efficient [327,347]. Scelsi et al. [348] revealed that for recycled plastic blends, the addition of GF did not reduce the elongation at break as it did for virgin plastic blends. GF produces new products with recycled plastics which exhibit properties, such as specific strength and thermal expansion, close to those of steel [348]. Moreover, GF/rPET (from the SSP recycling process) composites offer tensile, flexural, and impact strengths in the same order of magnitude as the ones of GF/vPET composites, when aminosilane or epoxysilane coupling agents are used as fibre sizings [299]. Aminosilane coupling agents can react with the carboxyl group of rPET, enhancing the fibre/matrix interface [349]. De Moura Giraldi et al. [349] studied the influence of process parameters on the final mechanical properties of rPET reinforced with 30 wt% GF. The composites with recycled plastics mainly had fibre lengths lower or equal to 5–6 mm. The results of Santos and Pezzin [257] for a PP matrix reinforced with short rPET fibres are very encouraging in terms of Izod impact strength even without a compatibilizer. Moreover, rPET fibres present multiple advantages. One is their low cost in comparison to E-glass fibres, which is between $1450 and $3300/ton [350,351]. Furthermore, their elongation at break is around 10 times higher than that of E-glass fibres [352,353,354,355]. Their properties are particularly interesting to develop composites with high impact resistance as required in the automotive sector or for wind turbine blades production. In the rPET matrix, different PO fillers can be added and the adhesion between the filler and matrix can be ensured with the same compatibilizers as discussed in Section 5.3 and summarised in Table 5. However, other types of fillers in the rPET matrix have also been studied in the literature. Graphene nanoplatelets (GNP), employed as reinforcing fillers in the rPET matrix, improved the crystallinity of rPET by their nucleating effects, but without modifying its crystalline structure [356]. The stability and storage moduli of rPET increased with increasing GNP content and its damping behaviour was improved at low temperatures. Montmorillonite is another filler which can be added in recycled thermoplastic blends to increase Young’s modulus and the tensile strength of the blends [326]. Talc or glass beads can also play the role of fillers in the rPET matrix. They reduce the moisture absorption in comparison to neat rPET due to higher crystallinity, which might be attributed to the nucleating effects of the fillers [357]. Fibres can be added in rPET/rPO blends in order to enhance their properties. Marzuki et al. [358] reported that rPET/rPP/PP-MA blends reinforced with kenaf fibres showed an increase in impact strength, but the stress concentration increased in the material due to low adhesion between these hydrophilic fibres and less hydrophilic plastics. The stress concentration points caused a decrease in the tensile strength and elongation at break in comparison to the blends.
5.5. Applications of rPET
rPET up-cycling has potential as shown by several recycling companies such as Indorama, who manufacture PET resins with up to 30% rPET [359], and Charpak Ltd., who manufactures rPET packaging with a minimum of 50% post-consumer wastes and up to 40% post-industrial wastes [360]. Additionally, the household brand Coca-Cola has invested in the rPET company CuRe to help accelerate Coca-Cola’s sustainability goals and aim of 100% rPET for bottle manufacturing [361]. Additionally, rPET has been used in a variety of lower performance applications as a substitute for wood or concrete: outdoor furniture, decking, traffic barriers, house flooring [362], water pipes [321], and in the packaging sector [338]. An interesting use of rPET has been within the textile industry as fibres [363]. The use of rPET in fabrics has been highlighted by the sports industry, for example, the US men’s basketball team uniforms have been made from r-PET bottles [256]. The development of rPOs and rPET microfibril composites which can be further reinforced with GF or natural fibres has led to the possibility of rPET being used in higher performance applications such as scaffolding, railroad ties, or car bumpers [256]. The drawback of this use is the loss of recyclability caused by the introduction of fibres in the blends. The reprocessed materials will exhibit lower mechanical properties due to the reduction in fibre length by shredding. The outlets of rPET fibres are always more diversified, as is shown by the new production of honeycomb made from 95% rPET from the packaging industry [364]. Moreover, PET bottle wastes have also been used to produce the cores of wind turbine blades by Armacell [365].
6. Future Application Potential of rPOs and rPET
As previously discussed, the use of rPO and rPET is very diverse both in terms of their structures (neat plastics, blends, fibres, composites) and their applications (packaging industry, construction sector, furniture). Their use in producing a diverse range of sustainable products can contribute immensely to the circular economy and to the reduction of landfills. Due to the lowering of material properties during recycling, most applications of rPET and rPO are of low value. However, through innovations, new novel applications of plastic packaging wastes are emerging, for example, as carbon chip anodes for rechargeable batteries [366] or as aggregates in concrete [367].
A report by Hundertmark et al. [368] suggested that recycled plastics would represent nearly a third of the plastics entering the market by 2030. Large variations in recyclate quality caused by the presence of other plastics and additives occur between different batches and waste streams. The determination of the recyclate composition is of key importance in order to maximise the performance and minimise the performance variabilities. Identifying the optimal amount of compatibilizer/filler to improve the performance is extremely challenging, time consuming, and potentially costly. In the literature, there is a lack of research focusing on the performance of different recyclate batches with different compositions without the addition of additional components. Ideally, the recycling industry would require a portfolio of recyclate compositions, a few key properties (viscosity, basic mechanical properties, and degradation behaviour), and suggested lists of compatibilizers/stabilizers/fillers that can be used with the recyclates to enhance their processability and performance as industrial products. A regulation to bring more control of the type and composition of the virgin plastic packaging products could also help in identifying the composition of the waste streams.
Another poorly investigated topic is the processability of continuous fibre reinforced composites using recycled plastics as the matrix for medium to high strength applications. To the best of our knowledge, very few articles such as the study of Wu and Lai [352] have been published in this area. Wu and Lai [352] manufactured self-reinforced rPET composites by film stacking from fabrics composed of uncommingled yarns. They found that self-reinforced rPET composites had extremely high tensile strength and breaking strength in comparison to a composite reinforced with non-recycled technical fibres. For further developments in continuous rPET fibre reinforced composites, the selection of compatibilizers to reinforce the adhesion between the continuous recycled rPET fibres and recycled plastic is paramount. Additionally, extensive research should focus on the quantity of compatibilizer required and the point at which the compatibilizer should be added during the manufacturing, for example, during fibres extrusion or mixed with the polymer matrix before fibre addition. The interaction between compatibilizer and fibre sizing should be investigated. A work on fibre design as a core/binding structure as proposed by Romhány et al. [343], or as a core/sheath structure could also improve the adhesion between continuous fibres and matrices. This knowledge would help to define the optimal conditions to manufacture medium to high performance recycled thermoplastics-based composites.
There is a global growing demand for the use of composite materials in the wind energy sector. The wind composites material market is expected to be worth $12 billion in 2023, an annual growth rate (CAGR) of 9.6% [369]. This sector represents a very promising market for composites and a major fraction could be from recycled plastics. In the renewable energy sector, the use of recycled-plastics-based composites can be highly beneficial in order to produce greener energy and introduce recyclability into the products. However, significant research efforts are needed to develop innovative technologies that can deliver high performing composites using recycled plastics and reinforcement fibres. Various virgin POs and PET-based composite technologies exist in the market which are used for a diverse range of medium to high performance applications [370,371,372]. They can serve as guidelines for future innovations with recycled polymers in a similar direction, producing continuous-fibre reinforced composites with higher mechanical performance.
The ban of single use plastics has driven the need for a “design for recycling” approach for the sustainability of plastic packaging [367]. Future ideas include designing plastics with possible sites for depolymerisation reactions, which would aid the chemical recycling process [373]. An alternative emerging research area is the use of abiotic and biotic environments to degrade plastic wastes to monomers and small oligomers [367]. However, extensive research is still required in this area on processing conditions, dealing with contaminants, controlling the variation in recyclate quality, and bringing in more reliability of recycled-plastics-based products.
7. Conclusions
We have given an overview of the mechanical and chemical recycling of plastic packaging wastes. POs and PET are commonly found in plastic wastes due to their high use in consumer goods. Plastic wastes are heterogeneous in nature and complete separation of individual plastics from a mixed waste stream is extremely difficult. The presence of contaminants in the recycled plastics introduces variability in their properties and maintaining a constant recyclate performance becomes challenging.
POs and PET are mechanically recycled; however, this method is inefficient and results in degradation during reprocessing, causing poor final performance. Chemical recycling, on the other hand, can deal with plastic wastes’ inhomogeneity as complete separation is not always required. Chemical recycling is an attractive method to achieve monomers and is considered to be the most sustainable solution. The yield and composition of products can vary greatly depending upon the waste composition and recycling conditions. PET, having chain scission sites in its backbone at the ester linkages, undergoes chemical recycling via three main routes: glycolysis, methanolysis, and hydrolysis. Although chemical recycling is a highly desired method of recycling, it is important to note that energy is consumed in every step: for example, in producing a polymer from a monomer, chemically depolymerising the polymer back into its monomer form at end-of-life, and again repolymerising the monomer into polymer. Hence, innovative technologies should also be developed where the waste or recycled polymers can be directly converted into value-added products, for example, monomers or polymers with desired properties with minimum required modification. POs mainly undergo mechanical recycling due to the absence of chain scission sites in their backbone. The compatibilizers and fillers such as plant-based fibres, organoclays, and calcium carbonate, play key roles in enhancing the properties of recycled plastics. Improvements in performance have been demonstrated for recycled POs and PET, with some being comparable to their virgin properties. Focus is shifting onto the formation of green composites, which consist of green coupling agents and fillers, as the demand for sustainability and biodegradability increases. This review shows that recycled POs and PET are used in low demanding applications, but the introduction of innovative technologies can broaden the future application areas, achieving medium to high performance products.
Author Contributions
Conceptualization, H.J., F.S., D.R., V.K.; investigation, H.J., F.S., V.K., D.R.; writing—original draft preparation, H.J., F.S.; writing- review and editing, V.K., D.R.; supervision, V.K., D.R.; project administration, H.J., D.R., V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the EPSRC and the SOFI CDT (Grant ref. No. EP/L015536/1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Shrivastava, A. Plastic Properties and Testing; Shrivastava, A., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; ISBN 978-0-323-39500-7. [Google Scholar]
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 13–32. ISBN 978-0-12-817880-5. [Google Scholar]
- PlasticEurope Plastics—The Facts. 2020. Available online: https://www.plasticseurope.org/en/resources/market-data (accessed on 4 January 2021).
- Ellen MacArthur Foundation; McKinsey & Co. The New Plastics Economy: Rethinking the Future of Plastics. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/EllenMacArthurFoundation_TheNewPlasticsEconomy_Pages.pdf (accessed on 11 November 2020).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, uses, and fate of all plastics ever made. Sci. Adv. 2017, 3, 5. [Google Scholar] [CrossRef]
- Welden, N.A. The environmental impacts of plastic pollution. In Plastic Waste and Recycling; Letcher, T.M., House, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–222. ISBN 978-0-12-817880-5. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Kubowicz, S.; Booth, A.M. Biodegradability of plastics: Challenges and misconceptions. Environ. Sci. Technol. 2017, 51, 12058–12060. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic pollutants from plastic waste—A review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yasuhara, A.; Shiraishi, H.; Nakasugi, O. Bisphenol A in hazardous waste landfill leachates. Chemosphere 2001, 42, 415–418. [Google Scholar] [CrossRef]
- Cook, C.R.; Halden, R.U. Ecological and health issues of plastic waste. In Plastic Waste and Recycling; Letcher, T.M., House, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 513–527. ISBN 9780128178805. [Google Scholar]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of various plastics in the environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Takada, H., Karapanagioti, H.K., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 71–92. ISBN 978-3-319-95568-1. [Google Scholar]
- Cordier, M.; Uehara, T. How much innovation is needed to protect the ocean from plastic contamination? Sci. Total Environ. 2019, 670, 789–799. [Google Scholar] [CrossRef]
- Ilyas, M.; Ahmad, W.; Khan, H.; Yousaf, S.; Khan, K.; Nazir, S. Plastic waste as a significant threat to environment—A systematic literature review. Rev. Environ. Health 2018, 33, 383–406. [Google Scholar] [CrossRef]
- Potrykus, M.; Redko, V.; Głowacka, K.; Piotrowicz-Cieślak, A.; Szarlej, P.; Janik, H.; Wolska, L. Polypropylene structure alterations after 5 years of natural degradation in a waste landfill. Sci. Total Environ. 2021, 758, 143649. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Garciá, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 46. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- d’Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Sci. Rep. J. Field Actions 2019, 12–21. [Google Scholar]
- Winans, K.; Kendall, A.; Deng, H. The history and current applications of the circular economy concept. Renew. Sustain. Energy Rev. 2017, 68, 825–833. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Iacovidou, E. Closing the loop on plastic packaging materials: What is quality and how does it affect their circularity? Sci. Total Environ. 2018, 630, 1394–1400. [Google Scholar] [CrossRef]
- EuropeanCommission Paris Agreement. Available online: https://ec.europa.eu/clima/policies/international/negotiations/paris_en (accessed on 6 May 2021).
- WRAP The UK Plastics Pact. Available online: https://wrap.org.uk/taking-action/plastic-packaging/the-uk-plastics-pact (accessed on 6 May 2021).
- Eurostat Record Recycling Rates and Use of Recycled Materials in the EU. Available online: https://ec.europa.eu/eurostat/documents/2995521/9629294/8-04032019-BP-EN.pdf/295c2302-4ed1-45b9-af86-96d1bbb7acb1 (accessed on 2 February 2021).
- EuropeanCommission A European Strategy for Plastics in a Circular Economy. Available online: https://ec.europa.eu/info/research-and-innovation/research-area/environment/plastics-circular-economy_en# (accessed on 4 May 2021).
- WRAP Plastic Packaging. Available online: https://wrap.org.uk/taking-action/plastic-packaging (accessed on 5 May 2021).
- Research, G.V. Plastic Packaging Market Trends and Growth 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/plastic-packaging-market (accessed on 21 April 2021).
- Dahlbo, H.; Poliakova, V.; Mylläri, V.; Sahimaa, O.; Anderson, R. Recycling potential of post-consumer plastic packaging waste in Finland. Waste Manag. 2018, 71, 52–61. [Google Scholar] [CrossRef]
- Lange, J.; Wyser, Y. Recent Innovations in Barrier Technologies for Plastic Packaging—A Review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- PlasticsEurope Plastics—The Facts. 2019. Available online: https://www.plasticseurope.org/en/resources/market-data (accessed on 2 May 2020).
- Kehinde, O.; Ramonu, O.J.; Babaremu, K.O.; Justin, L.D. Plastic wastes: Environmental hazard and instrument for wealth creation in Nigeria. Heliyon 2020, 6, e05131. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.; Deeney, M.; White, H.; Joy, E.; Kalamatianou, S.; Kadiyala, S. PROTOCOL: Plastics in the food system: Human health, economic and environmental impacts. A scoping review. Campbell Syst. Rev. 2019, 15, 1–12. [Google Scholar] [CrossRef]
- Galić, K.; Ščetar, M.; Kurek, M. The benefits of processing and packaging. Trends Food Sci. Technol. 2011, 22, 127–137. [Google Scholar] [CrossRef]
- Billiet, S.; Trenor, S.R. 100th anniversary of macromolecular science viewpoint: Needs for plastics packaging circularity. ACS Macro Lett. 2020, 9, 1376–1390. [Google Scholar] [CrossRef]
- Claudio, L. News focus packaging and public health. Environ. Health Perspect. 2012, 120. [Google Scholar] [CrossRef] [PubMed]
- Emblem, A. Plastics properties for packaging materials. In Packaging Technology; Emblem, A., Emblem, H., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 287–309. ISBN 978-0-85709-570-1. [Google Scholar]
- PlasticsEurope Plastics in Packaging. Available online: https://www.plasticseurope.org/en/about-plastics/packaging (accessed on 19 November 2020).
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Grigore, M.E. Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2017, 2, 24. [Google Scholar] [CrossRef]
- Maris, J.; Bourdon, S.; Brossard, J.M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of polymers: A review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Satapathy, S. An analysis of barriers for plastic recycling in the Indian plastic industry. Benchmarking Int. J. 2017, 24, 415–430. [Google Scholar] [CrossRef]
- Perera, S.; Arulrajah, A.; Wong, Y.C.; Horpibulsuk, S.; Maghool, F. Utilizing recycled PET blends with demolition wastes as construction materials. Constr. Build. Mater. 2019, 221, 200–209. [Google Scholar] [CrossRef]
- Torres, N.; Robin, J.J.; Boutevin, B. Study of thermal and mechanical properties of virgin and recycled poly(ethylene terephthalate) before and after injection molding. Eur. Polym. J. 2000, 36, 2075–2080. [Google Scholar] [CrossRef]
- da Silva, D.J.; Wiebeck, H. Current options for characterizing, sorting, and recycling polymeric waste. Prog. Rubber Plast. Recycl. Technol. 2020, 36, 284–303. [Google Scholar] [CrossRef]
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assessment. Waste Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef]
- Cagnetta, G.; Zhang, K.; Zhang, Q.; Huang, J.; Yu, G. Mechanochemical pre-treatment for viable recycling of plastic waste containing haloorganics. Waste Manag. 2018, 75, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Goodship, V. Plastic recycling. Sci. Prog. 2007, 90, 245–268. [Google Scholar] [CrossRef]
- Kumar, S.; Panda, A.K.; Singh, R.K. A review on tertiary recycling of high-density polyethylene to fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Iacovidou, E. An overview of the challenges and trade-offs in closing the loop of post-consumer plastic waste (PCPW): Focus on recycling. J. Hazard. Mater. 2019, 380, 120887. [Google Scholar] [CrossRef]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef]
- Merrington, A. Recycling of plastics. In Applied Plastics Engineering Handbook; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2011; pp. 177–192. ISBN 978-1-4377-3514-7. [Google Scholar]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Brouwer, M.T.; Thoden van Velzen, E.U.; Augustinus, A.; Soethoudt, H.; De Meester, S.; Ragaert, K. Predictive model for the Dutch post-consumer plastic packaging recycling system and implications for the circular economy. Waste Manag. 2018, 71, 62–85. [Google Scholar] [CrossRef]
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Pohjakallio, M.; Vuorinen, T.; Oasmaa, A. Chemical routes for recycling—Dissolving, catalytic, and thermochemical technologies. In Plastic Waste and Recycling; Letcher, T.M., House, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 359–384. ISBN 9780128178805. [Google Scholar]
- Kosior, E.; Mitchell, J. Current industry position on plastic production and recycling. In Plastic Waste and Recycling; Letcher, T.M., House, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–162. ISBN 978-0-12-817880-5. [Google Scholar]
- Cimpan, C.; Maul, A.; Jansen, M.; Pretz, T.; Wenzel, H. Central sorting and recovery of MSW recyclable materials: A review of technological state-of-the-art, cases, practice and implications for materials recycling. J. Environ. Manag. 2015, 156, 181–199. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Purnell, P.; Iacovidou, E.; Velis, C.A.; Atseyinku, M. Post-consumer plastic packaging waste in England: Assessing the yield of multiple collection-recycling schemes. Waste Manag. 2018, 75, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Jubinville, D.; Esmizadeh, E.; Saikrishnan, S.; Tzoganakis, C.; Mekonnen, T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustain. Mater. Technol. 2020, 25, e00188. [Google Scholar] [CrossRef]
- Faraca, G.; Astrup, T. Plastic waste from recycling centres: Characterisation and evaluation of plastic recyclability. Waste Manag. 2019, 95, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, B.; Goossens, H. Assessment of plastic packaging waste: Material origin, methods, properties. Resour. Conserv. Recycl. 2014, 85, 88–97. [Google Scholar] [CrossRef]
- Bauer, M.; Lehner, M.; Schwabl, D.; Flachberger, H.; Kranzinger, L.; Pomberger, R.; Hofer, W. Sink–float density separation of post-consumer plastics for feedstock recycling. J. Mater. Cycles Waste Manag. 2018, 20, 1781–1791. [Google Scholar] [CrossRef]
- Mumbach, G.D.; de Sousa Cunha, R.; Machado, R.A.F.; Bolzan, A. Dissolution of adhesive resins present in plastic waste to recover polyolefin by sink-float separation processes. J. Environ. Manag. 2019, 243, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Wang, L.; Wang, H. Application of froth flotation in the separation of polyvinyl chloride and polycarbonate for recycling of waste plastic based on a novel surface modification. Waste Manag. 2020, 110, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, J.; Yao, L.; Xu, Z. Auto-sorting commonly recovered plastics from waste household appliances and electronics using near-infrared spectroscopy. J. Clean. Prod. 2020, 246, 118732. [Google Scholar] [CrossRef]
- Signoret, C.; Caro-Bretelle, A.S.; Lopez-Cuesta, J.M.; Ienny, P.; Perrin, D. MIR spectral characterization of plastic to enable discrimination in an industrial recycling context: II. Specific case of polyolefins. Waste Manag. 2019, 98, 160–172. [Google Scholar] [CrossRef]
- Rozenstein, O.; Puckrin, E.; Adamowski, J. Development of a new approach based on midwave infrared spectroscopy for post-consumer black plastic waste sorting in the recycling industry. Waste Manag. 2017, 68, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Adel Ismael Chaqmaqchee, F. Comparison of Various plastics wastes using X-ray fluorescence. Am. J. Mater. Synth. Process. 2017, 2, 24. [Google Scholar] [CrossRef]
- Sharkey, M.; Abdallah, M.A.E.; Drage, D.S.; Harrad, S.; Berresheim, H. Portable X-ray fluorescence for the detection of POP-BFRs in waste plastics. Sci. Total Environ. 2018, 639, 49–57. [Google Scholar] [CrossRef]
- Fu, S.; Hua, W.; Yuan, H.; Ling, J.; Shi, Q. Study on the light medium separation of waste plastics with hydrocyclones. Waste Manag. 2019, 91, 54–61. [Google Scholar] [CrossRef]
- Fu, S.; Qian, Y.; Yuan, H.; Wu, M.; Hua, W.; Jiang, M. Study on the performance of a hydrocyclone used for recycling the waste SCR catalyst. J. Environ. Chem. Eng. 2020, 8, 104349. [Google Scholar] [CrossRef]
- Yuan, H.; Fu, S.; Tan, W.; He, J.; Wu, K. Study on the hydrocyclonic separation of waste plastics with different density. Waste Manag. 2014, 45, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.V.M.; Cella, M.; Tanabe, E.H.; Bertuol, D.A. Application of tribo-electrostatic separation in the recycling of plastic wastes. Process. Saf. Environ. Prot. 2018, 114, 219–228. [Google Scholar] [CrossRef]
- Dascalescu, L.; Zeghloul, T.; Iuga, A. Electrostatic separation of metals and plastics from waste electrical and electronic equipment. In WEEE Recycling: Research, Development, and Policies; Chagnes, A., Cote, G., Ekberg, C., Nilsson, M., Retegan, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 75–106. ISBN 9780128033647. [Google Scholar]
- Dizdar, T.O.; Kocausta, G.; Gülcan, E.; Gülsoy, Ö.Y. A new method to produce high voltage static electric load for electrostatic separation—Triboelectric charging. Powder Technol. 2018, 327, 89–95. [Google Scholar] [CrossRef]
- Serranti, S.; Luciani, V.; Bonifazi, G.; Hu, B.; Rem, P.C. An innovative recycling process to obtain pure polyethylene and polypropylene from household waste. Waste Manag. 2015, 35, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Luciani, V.; Bonifazi, G.; Rem, P.; Serranti, S. Upgrading of PVC rich wastes by magnetic density separation and hyperspectral imaging quality control. Waste Manag. 2015, 45, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, F.; Xie, J.; Zhang, C.; Fu, J.; Zhao, P. Magnetic projection: A novel separation method and its first application on separating mixed plastics. Waste Manag. 2019, 87, 805–813. [Google Scholar] [CrossRef]
- Zhao, P.; Xie, J.; Gu, F.; Sharmin, N.; Hall, P.; Fu, J. Separation of mixed waste plastics via magnetic levitation. Waste Manag. 2018, 76, 46–54. [Google Scholar] [CrossRef]
- Mehrubeoglu, M.; Van Sickle, A.; Turner, J. Detection and identification of plastics using SWIR hyperspectral imaging. In Proceedings of the SPIE Optical Engineering + Applications, San Diego, CA, USA, 24–28 April 2020; SPIE-Intl Soc Optical Eng: San Diego, CA, USA, 2020; p. 15. [Google Scholar]
- Bonifazi, G.; Capobianco, G.; Palmieri, R.; Serranti, S. Hyperspectral imaging applied to the waste recycling sector. Spectrosc. Eur. 2019, 31, 8–11. [Google Scholar]
- Serranti, S.; Cucuzza, P.; Bonifazi, G. Hyperspectral imaging for VIS-SWIR classification of post-consumer plastic packaging products by polymer and color. In Proceedings of the SPIE 11525, SPIE Future Sensing Technologies, Online, 9–13 November 2020; p. 30. [Google Scholar]
- Zeng, Q.; Sirven, J.-B.; Gabriel, J.-C.P.; Tay, C.Y.; Lee, J.-M. Laser induced breakdown spectroscopy for plastic analysis. TrAC Trends Anal. Chem. 2021, 116280. [Google Scholar] [CrossRef]
- Costa, V.C.; Aquino, F.W.B.; Paranhos, C.M.; Pereira-Filho, E.R. Use of laser-induced breakdown spectroscopy for the determination of polycarbonate (PC) and acrylonitrile-butadiene-styrene (ABS) concentrations in PC/ABS plastics from e-waste. Waste Manag. 2017, 70, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.C.; Castro, J.P.; Andrade, D.F.; Victor Babos, D.; Garcia, J.A.; Sperança, M.A.; Catelani, T.A.; Pereira-Filho, E.R. Laser-induced breakdown spectroscopy (LIBS) applications in the chemical analysis of waste electrical and electronic equipment (WEEE). TrAC-Trends Anal. Chem. 2018, 108, 65–73. [Google Scholar] [CrossRef]
- Ito, M.; Takeuchi, M.; Saito, A.; Murase, N.; Phengsaart, T.; Tabelin, C.B.; Hiroyoshi, N.; Tsunekawa, M. Improvement of hybrid jig separation efficiency using wetting agents for the recycling of mixed-plastic wastes. J. Mater. Cycles Waste Manag. 2019, 21, 1376–1383. [Google Scholar] [CrossRef]
- Wang, C.Q.; Wang, H.; Fu, J.G.; Liu, Y.N. Flotation separation of waste plastics for recycling—A review. Waste Manag. 2015, 41, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, C.; Longhurst, P.J.; Wagland, S.T. Critical review of real-time methods for solid waste characterisation: Informing material recovery and fuel production. Waste Manag. 2017, 61, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tian, D.; Li, C.; Li, Y.; Yang, G.; Ding, Y. A review of laser-induced breakdown spectroscopy for plastic analysis. TrAC-Trends Anal. Chem. 2019, 110, 327–334. [Google Scholar] [CrossRef]
- Wu, G.; Li, J.; Xu, Z. Triboelectrostatic separation for granular plastic waste recycling: A review. Waste Manag. 2013, 33, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Gundupalli, S.P.; Hait, S.; Thakur, A. A review on automated sorting of source-separated municipal solid waste for recycling. Waste Manag. 2017, 60, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Sharmila, V.G.; Ushani, U.; Amudha, V.; Kumar, G. Impervious and influence in the liquid fuel production from municipal plastic waste through thermo-chemical biomass conversion technologies—A review. Sci. Total Environ. 2020, 718, 137287. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.M.; D’hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Ajibola, A.A.; Omoleye, J.A.; Efeovbokhan, V.E. Catalytic cracking of polyethylene plastic waste using synthesised zeolite Y from Nigerian kaolin deposit. Appl. Petrochem. Res. 2018, 8, 211–217. [Google Scholar] [CrossRef]
- Aishwarya, K.N.; Sindhu, N. Microwave assisted pyrolysis of plastic waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef]
- Rosi, L.; Bartoli, M.; Frediani, M. Microwave assisted pyrolysis of halogenated plastics recovered from waste computers. Waste Manag. 2018, 73, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Adrados, A.; de Marco, I.; Caballero, B.M.; López, A.; Laresgoiti, M.F.; Torres, A. Pyrolysis of plastic packaging waste: A comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manag. 2012, 32, 826–832. [Google Scholar] [CrossRef]
- Viante, M.F.; Zanela, T.M.P.; Stoski, A.; Muniz, E.C.; Almeida, C.A.P. Magnetic microspheres composite from poly(ethylene terephthalate) (PET) waste: Synthesis and characterization. J. Clean. Prod. 2018, 198, 979–986. [Google Scholar] [CrossRef]
- Aznar, M.P.; Caballero, M.A.; Sancho, J.A.; Francés, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes—The environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]
- Garcia, J.M.; Robertson, M.L. The future of plastics recycling. Science (80-) 2017, 358, 870–872. [Google Scholar] [CrossRef]
- Worch, J.C.; Dove, A.P. 100th anniversary of macromolecular science viewpoint: Toward catalytic chemical recycling of waste (and future) plastics. ACS Macro Lett. 2020, 9, 1494–1506. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- de Camargo, R.V.; Saron, C. Mechanical–Chemical recycling of low-density polyethylene waste with polypropylene. J. Polym. Environ. 2020, 28, 794–802. [Google Scholar] [CrossRef]
- Eriksson, O.; Finnveden, G. Plastic waste as a fuel—CO2-neutral or not? Energy Environ. Sci. 2009, 2, 907–914. [Google Scholar] [CrossRef]
- Bujak, J.W. Thermal utilization (treatment) of plastic waste. Energy 2015, 90, 1468–1477. [Google Scholar] [CrossRef]
- Nagy, A.; Kuti, R. The environmental impact of plastic waste. AARMS 2016, 15. [Google Scholar] [CrossRef]
- Makarichi, L.; Jutidamrongphan, W.; Techato, K. Anan the evolution of waste-to-energy incineration: A review. Renew. Sustain. Energy Rev. 2018, 91, 812–821. [Google Scholar] [CrossRef]
- Shen, M.; Hu, T.; Huang, W.; Song, B.; Qin, M.; Yi, H.; Zeng, G.; Zhang, Y. Can incineration completely eliminate plastic wastes? An investigation of microplastics and heavy metals in the bottom ash and fly ash from an incineration plant. Sci. Total Environ. 2021, 779, 146528. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Reijnders, L. Hazardous waste incineration ashes and their utilization. Encycl. Sustain. Sci. Technol. 2018, 1–17. [Google Scholar] [CrossRef]
- Kanhar, A.H.; Chen, S.; Wang, F. Incineration fly ash and its treatment to possible utilization: A review. Energies 2020, 13, 6681. [Google Scholar] [CrossRef]
- Joseph, A.M.; Snellings, R.; Van den Heede, P.; Matthys, S.; De Belie, N. The use of municipal solidwaste incineration ash in various building materials: A Belgian point of view. Materials 2018, 11, 141. [Google Scholar] [CrossRef]
- Vilaplana, F.; Karlsson, S. Quality concepts for the improved use of recycled polymeric materials: A review. Macromol. Mater. Eng. 2008, 293, 274–297. [Google Scholar] [CrossRef]
- Utracki, L.A. Compatibilization of polymer blends. Can. J. Chem. Eng. 2002, 80, 1008–1016. [Google Scholar] [CrossRef]
- Ajitha, A.R.; Thomas, S. Introduction: Polymer Blends, Thermodynamics, Miscibility, Phase Separation, and Compatibilization; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128160060. [Google Scholar]
- Manias, E.; Utracki, L.A. Polymer blends handbook. In Polymer Blends Handbook; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 171–280. ISBN 9789400760646. [Google Scholar]
- Lohse, D.J. Polyolefins. In Applied Polymer Science: 21st Century; Craver, C., Carraher, C., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2000; pp. 73–91. ISBN 9788578110796. [Google Scholar]
- Posch, W. Polyolefins. In Applied Plastics Engineering Handbook; Ebnesajjad, S., Ed.; William Andrew Publishing: Norwich, NY, USA, 2011; pp. 23–48. ISBN 9781437735147. [Google Scholar]
- Agboola, O.; Sadiku, R.; Mokrani, T.; Amer, I.; Imoru, O. Polyolefins and the environment, 2nd ed.; Ugbolue, S.C.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780081012512. [Google Scholar]
- Manjula, B.; Reddy, A.B.; Sadiku, E.R.; Sivanjineyulu, V.; Molelekwa, G.F.; Jayaramudu, J.; Raj Kumar, K. Use of polyolefins in hygienic applications. Polyolefin Fibres Struct. Prop. Ind. Appl. Second Ed. 2017, 539–560. [Google Scholar] [CrossRef]
- Kupolati, W.K.; Sadiku, E.R.; Ibrahim, I.D.; Adeboje, A.O.; Kambole, C.; Ojo, O.O.S.; Eze, A.A.; Paige-Green, P.; Ndambuki, J.M. The Use of Polyolefins in Geotextiles and Engineering Applications, 2nd ed.; Ugbolue, S.C.O., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081012512. [Google Scholar]
- Rodrigues, M.; Quevedo, S.; Souza, J.; Farias, J. Waste recycling in biaxially oriented polypropylene—BOPP. J. Eng. 2020, 10, 11–15. [Google Scholar] [CrossRef]
- Graziano, A.; Jaffer, S.; Sain, M. Review on modification strategies of polyethylene/polypropylene immiscible thermoplastic polymer blends for enhancing their mechanical behavior. J. Elastomers Plast. 2019, 51, 291–336. [Google Scholar] [CrossRef]
- Kim, Y.K. The use of polyolefins in industrial and medical applications. In Polyolefin Fibres: Structure, Properties and Industrial Applications, 2nd ed.; Ugbolue, S.C.O., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 135–155. ISBN 9780081012512. [Google Scholar]
- McKeen, L.W. 8—Polyolefins. In The Effect of UV Light and Weather on Plastics and Elastomers; McKeen, L.W., Ed.; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 223–249. ISBN 978-0-12-816457-0. [Google Scholar]
- He, F.A.; Zhang, L.M. Study on branching structure, melting, and crystallization of polyethylene prepared by nickel a-diimine catalyst covalently intercalated inside OapPOSS-modified laponite clay gallery. Polym. Test. 2014, 35, 80–86. [Google Scholar] [CrossRef]
- Grause, G.; Chien, M.F.; Inoue, C. Changes during the weathering of polyolefins. Polym. Degrad. Stab. 2020, 181, 109364. [Google Scholar] [CrossRef]
- Patil, N.A.; Njuguna, J.; Kandasubramanian, B. UHMWPE for biomedical applications: Performance and functionalization. Eur. Polym. J. 2020, 125, 109529. [Google Scholar] [CrossRef]
- PolymerDatabase Polymer Properties Database: Very Low Density Polyethylene. Available online: http://www.polymerdatabase.com/PolymerBrands/VLDPE.html (accessed on 26 March 2021).
- Cheng, J.J.; Polak, M.A.; Penlidis, A. Influence of micromolecular structure on environmental stress cracking resistance of high density polyethylene. Tunn. Undergr. Sp. Technol. 2011, 26, 582–593. [Google Scholar] [CrossRef]
- Shirkavand, M.J.; Azizi, H.; Ghasemi, I. Effect of molecular structure parameters on crystallinity and environmental stress cracking resistance of high-density polyethylene/TiO2 nanocomposites. Adv. Polym. Technol. 2018, 37, 1–8. [Google Scholar] [CrossRef]
- Wypych, G. Microscopic mechanisms of damage caused by degradants. In Atlas of Material Damage; ChemTec Publishing: Scarborough, ON, Canada, 2012; pp. 105–283. ISBN 978-1-927885-26-0. [Google Scholar]
- INEOS Olefins & Polymers USA Environmental Stress Crack Resistance of Polyethylene. Available online: https://www.ineos.com/globalassets/ineos-group/businesses/ineos-olefins-and-polymers-usa/products/technical-information--patents/ineos-environmental-stress-crack-resistance-of-pe.pdf (accessed on 24 May 2021).
- Ebnesajjad, S. Effect of chemicals on plastics. In Chemical Resistance of Commodity Thermoplastics; Baur, E., Ruhrberg, K., Woishnis, W., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Madi, N.K. Thermal and mechanical properties of injection molded recycled high density polyethylene blends with virgin isotactic polypropylene. Mater. Des. 2013, 46, 435–441. [Google Scholar] [CrossRef]
- Strapasson, R.; Amico, S.C.; Pereira, M.F.R.; Sydenstricker, T.H.D. Tensile and impact behavior of polypropylene/low density polyethylene blends. Polym. Test. 2005, 24, 468–473. [Google Scholar] [CrossRef]
- Kazemi, Y.; Ramezani Kakroodi, A.; Rodrigue, D. Compatibilization efficiency in post-consumer recycled polyethylene/polypropylene blends: Effect of contamination. Polym. Eng. Sci. 2015, 55, 2368–2376. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Martín-Alfonso, J.E. The effect of thermal and thermo-oxidative degradation conditions on rheological, chemical and thermal properties of HDPE. Polym. Degrad. Stab. 2017, 141, 11–18. [Google Scholar] [CrossRef]
- Santos, A.S.F.; Agnelli, J.A.M.; Trevisan, D.W.; Manrich, S. Degradation and stabilization of polyolefins from municipal plastic waste during multiple extrusions under different reprocessing conditions. Polym. Degrad. Stab. 2002, 77, 441–447. [Google Scholar] [CrossRef]
- Saikrishnan, S.; Jubinville, D.; Tzoganakis, C.; Mekonnen, T.H. Thermo-mechanical degradation of polypropylene (PP) and low-density polyethylene (LDPE) blends exposed to simulated recycling. Polym. Degrad. Stab. 2020, 182, 109390. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S. The effect of process and structural parameters on the stability, thermo-mechanical and thermal degradation of polymers with hydrocarbon skeleton containing PE, PP, PS, PVC, NR, PBR and SBR. J. Therm. Anal. Calorim. 2021, 143, 2867–2882. [Google Scholar] [CrossRef]
- Loultcheva, M.K.; Proietto, M.; Jilov, N.; Mantia, F.P.L. Recycling of high density polyethylene containers. Polym. Degrad. Stab. 1997, 57, 77–81. [Google Scholar] [CrossRef]
- Pinheiro, L.A.; Chinelatto, M.A.; Canevarolo, S.V. The role of chain scission and chain branching in high density polyethylene during thermo-mechanical degradation. Polym. Degrad. Stab. 2004, 86, 445–453. [Google Scholar] [CrossRef]
- AlMa’adeed, M.A.-A. Polyolefin Compounds and Materials; Kalia, S., Dehradun, I., Eds.; Springer: Berlin/Heidelberg, Germany; ISBN 78-3-319-25980-2.
- Yin, S.; Tuladhar, R.; Shi, F.; Shanks, R.A.; Combe, M.; Collister, T. Mechanical reprocessing of polyolefin waste: A review. Polym. Eng. Sci. 2015, 55, 2899–2909. [Google Scholar] [CrossRef]
- Nait-Ali, L.K.; Colin, X.; Bergeret, A. Kinetic analysis and modelling of PET macromolecular changes during its mechanical recycling by extrusion. Polym. Degrad. Stab. 2011, 96, 236–246. [Google Scholar] [CrossRef]
- Abad, M.J.; Ares, A.; Barral, L.; Cano, J.; Díez, F.J.; García-Garabal, S.; López, J.; Ramírez, C. Effects of a mixture of stabilizers on the structure and mechanical properties of polyethylene during reprocessing. J. Appl. Polym. Sci. 2004, 92, 3910–3916. [Google Scholar] [CrossRef]
- Oblak, P.; Gonzalez-Gutierrez, J.; Zupančič, B.; Aulova, A.; Emri, I. Processability and mechanical properties of extensively recycled high density polyethylene. Polym. Degrad. Stab. 2015, 114, 133–145. [Google Scholar] [CrossRef]
- Jin, H.; Gonzalez-Gutierrez, J.; Oblak, P.; Zupančič, B.; Emri, I. The effect of extensive mechanical recycling on the properties of low density polyethylene. Polym. Degrad. Stab. 2012, 97, 2262–2272. [Google Scholar] [CrossRef]
- Dostál, J.; Kašpárková, V.; Zatloukal, M.; Muras, J.; Šimek, L. Influence of the repeated extrusion on the degradation of polyethylene. Structural changes in low density polyethylene. Eur. Polym. J. 2008, 44, 2652–2658. [Google Scholar] [CrossRef]
- Oliveira, T.A.; Oliveira, R.R.; Barbosa, R.; Azevedo, J.B.; Alves, T.S. Effect of reprocessing cycles on the degradation of PP/PBAT-thermoplastic starch blends. Carbohydr. Polym. 2017, 168, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Aurrekoetxea, J.; Sarrionandia, M.A.; Urrutibeascoa, I.; Maspoch, M.L. Effects of recycling on the microstructure and the mechanical properties of isotactic polypropylene. J. Mater. Sci. 2001, 36, 2607–2613. [Google Scholar] [CrossRef]
- Costa, H.M.; Ramos, V.D.; de Oliveira, M.G. Degradation of polypropylene (PP) during multiple extrusions: Thermal analysis, mechanical properties and analysis of variance. Polym. Test. 2007, 26, 676–684. [Google Scholar] [CrossRef]
- Kartalis, C.N.; Papaspyrides, C.D.; Pfaendner, R. Recycling of post-used PE packaging film using the restabilization technique. Polym. Degrad. Stab. 2000, 70, 189–197. [Google Scholar] [CrossRef]
- Hubo, S.; Delva, L.; Van Damme, N.; Ragaert, K. Blending of recycled mixed polyolefins with recycled polypropylene: Effect on physical and mechanical properties. In Proceedings of the AIP Conference Proceedings, Graz, Austria, 31 October 2016; American Institute of Physics Inc: Graz, Austria, 2016; Volume 1779. [Google Scholar]
- Rytöluoto, I.; Ritamaki, M.; Lahti, K.; Pasanen, S.; Pelto, J. Dielectric breakdown properties of mechanically recycled SiO2-BOPP nanocomposites. In Proceedings of the IEEE International Conference on Dielectrics (ICD), Montpellier, France, 23 August 2016; pp. 288–292. [Google Scholar]
- Ha, K.H.; Kim, M.S. Application to refrigerator plastics by mechanical recycling from polypropylene in waste-appliances. Mater. Des. 2012, 34, 252–257. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, J.; Yan, S.; Xie, Y.; Ma, J.; Chen, X. Biodegradable poly(l-lactide)/poly(ε-caprolactone)-modified montmorillonite nanocomposites: Preparation and characterization. Polymer (Guildf) 2007, 48, 6439–6447. [Google Scholar] [CrossRef]
- Guillén-Mallette, J.; González-Chi, P.I.; Cruz-Estrada, R.H.; y, R.N.M.-F.; Rivero-Ayala, M.A. Recycling printed polypropylene labels and polyolefins caps as chemical foaming agent to produce foam products. J. Cell. Plast. 2021, 57, 733–756. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Achilias, D.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, E. V Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Velghe, I.; Carleer, R.; Yperman, J.; Schreurs, S. Study of the pyrolysis of municipal solid waste for the production of valuable products. J. Anal. Appl. Pyrolysis 2011, 92, 366–375. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Valorization of packaging plastic waste by slow pyrolysis. Resour. Conserv. Recycl. 2018, 128, 69–77. [Google Scholar] [CrossRef]
- Park, K.B.; Jeong, Y.S.; Guzelciftci, B.; Kim, J.S. Characteristics of a new type continuous two-stage pyrolysis of waste polyethylene. Energy 2019, 166, 343–351. [Google Scholar] [CrossRef]
- Harmon, R.E.; SriBala, G.; Broadbelt, L.J.; Burnham, A.K. Insight into polyethylene and polypropylene pyrolysis: Global and mechanistic models. Energy Fuels 2021, 35, 6765–6775. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Larrain, M.; Van Passel, S.; Thomassen, G.; Kresovic, U.; Alderweireldt, N.; Moerman, E.; Billen, P. Economic performance of pyrolysis of mixed plastic waste: Open-loop versus closed-loop recycling. J. Clean. Prod. 2020, 270, R713–R715. [Google Scholar] [CrossRef]
- Achilias, D.S.; Andriotis, L.; Koutsidis, I.A.; Louka, D.A.; Nianias, N.P.; Siafaka, P.; Tsagkalias, I.; Tsintzou, G. Recent advances in the chemical recycling of polymers (PP, PS, LDPE, HDPE, PVC, PC, Nylon, PMMA). Mater. Recycl.-Trends Perspect. 2012, 3, 64. [Google Scholar] [CrossRef]
- Chandrasekaran, S.R.; Kunwar, B.; Moser, B.R.; Rajagopalan, N.; Sharma, B.K. Catalytic Thermal Cracking of Postconsumer Waste Plastics to Fuels. 1. Kinetics and Optimization. Energy Fuels 2015, 29, 6068–6077. [Google Scholar] [CrossRef]
- Burange, A.S.; Gawande, M.B.; Lam, F.L.Y.; Jayaram, R.V.; Luque, R. Heterogeneously catalyzed strategies for the deconstruction of high density polyethylene: Plastic waste valorisation to fuels. Green Chem. 2015, 17, 146–156. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Lima, R.B.; Neto, M.M.S.; Oliveira, D.S.; Santos, A.G.D.; Souza, L.D.; Caldeira, V.P.S. Obtainment of hierarchical ZSM-5 zeolites by alkaline treatment for the polyethylene catalytic cracking. Adv. Powder Technol. 2021, 32, 515–523. [Google Scholar] [CrossRef]
- Susastriawan, A.A.P.; Purnomo; Sandria, A. Experimental study the influence of zeolite size on low-temperature pyrolysis of low-density polyethylene plastic waste. Therm. Sci. Eng. Prog. 2020, 17, 100497. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Bilbao, J.; Olazar, M. Cracking of high density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind. Eng. Chem. Res. 2013, 52, 10637–10645. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Almeida, D.; de Marques Fatima, V.M.; Henriques, C.A. Petrochemical feedstock from pyrolysis of waste polyethylene and polypropylene using different catalysts. Fuel 2018, 215, 515–521. [Google Scholar] [CrossRef]
- Rodríguez, E.; Gutiérrez, A.; Palos, R.; Vela, F.J.; Arandes, J.M.; Bilbao, J. Fuel production by cracking of polyolefins pyrolysis waxes under fluid catalytic cracking (FCC) operating conditions. Waste Manag. 2019, 93, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Palos, R.; Gutiérrez, A.; Vela, F.J.; Arandes, J.M.; Bilbao, J. Effect of the FCC equilibrium catalyst properties and of the cracking temperature on the production of fuel from HDPE pyrolysis waxes. Energy Fuels 2019, 33, 5191–5199. [Google Scholar] [CrossRef]
- Donaj, P.J.; Kaminsky, W.; Buzeto, F.; Yang, W. Pyrolysis of polyolefins for increasing the yield of monomers’ recovery. Waste Manag. 2012, 32, 840–846. [Google Scholar] [CrossRef]
- Jia, X.; Qin, C.; Friedberger, T.; Guan, Z.; Huang, Z. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci. Adv. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Beckham, G.T.; Román-Leshkov, Y. Conversion of Polyolefin Waste to Liquid Alkanes with Ru-Based Catalysts under Mild Conditions. JACS Au 2021, 1, 8–12. [Google Scholar] [CrossRef]
- Nakaji, Y.; Tamura, M.; Miyaoka, S.; Kumagai, S.; Tanji, M.; Nakagawa, Y.; Yoshioka, T.; Tomishige, K. Low-temperature catalytic upgrading of waste polyolefinic plastics into liquid fuels and waxes. Appl. Catal. B Environ. 2021, 285, 119805. [Google Scholar] [CrossRef]
- Jiao, X.; Zheng, K.; Hu, Z.; Zhu, S.; Sun, Y.; Xie, Y. Conversion of waste plastics into value-added carbonaceous fuels under mild conditions. Adv. Mater. 2021, 2005192. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.M.I.; Nizami, A.S. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.S. Catalytic pyrolysis of plastic waste: Moving toward pyrolysis based biorefineries. Front. Energy Res. 2019, 7, 1–17. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process. Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Sivagami, K.; Divyapriya, G.; Selvaraj, R.; Madhiyazhagan, P.; Sriram, N.; Nambi, I. Catalytic pyrolysis of polyolefin and multilayer packaging based waste plastics: A pilot scale study. Process. Saf. Environ. Prot. 2021, 149, 497–506. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Singh, P.; Déparrois, N.; Burra, K.G.; Bhattacharya, S.; Gupta, A.K. Energy recovery from cross-linked polyethylene wastes using pyrolysis and CO2 assisted gasification. Appl. Energy 2019, 254, 113722. [Google Scholar] [CrossRef]
- Hassan Awad, A.; El Gamasy, R.; Abd El Wahab, A.; Hazem Abdellatif, M. Mechanical and physical properties of PP and HDPE. Eng. Sci. 2019, 4, 34. [Google Scholar] [CrossRef]
- Aumnate, C.; Rudolph, N.; Sarmadi, M. Recycling of polypropylene/polyethylene blends: Effect of chain structure on the crystallization behaviors. Polymers 2019, 11, 1456. [Google Scholar] [CrossRef]
- Meran, C.; Ozturk, O.; Yuksel, M. Examination of the possibility of recycling and utilizing recycled polyethylene and polypropylene. Mater. Des. 2008, 29, 701–705. [Google Scholar] [CrossRef]
- Curtzwiler, G.W.; Schweitzer, M.; Li, Y.; Jiang, S.; Vorst, K.L. Mixed post-consumer recycled polyolefins as a property tuning material for virgin polypropylene. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Williams, M.L.; Telephone, B. Tensile properties and morphology of blends of polyethylene and polypropylene. J. Appl. Polym. Sci. 1980, 25, 1703–1713. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Capizzi, L. Recycling of compatibilized and uncompatibilized nylon/polypropylene blends. Polym. Degrad. Stab. 2001, 71, 285–291. [Google Scholar] [CrossRef]
- Radonjič, G.; Gubeljak, N. The use of ethylene/propylene copolymers as compatibilizers for recycled polyolefin blends. Macromol. Mater. Eng. 2002, 287, 122–132. [Google Scholar] [CrossRef]
- Bertin, S.; Robin, J.J. Study and characterization of virgin and recycled LDPE/PP blends. Eur. Polym. J. 2002, 38, 2255–2264. [Google Scholar] [CrossRef]
- Atiqah, A.A.S.M.; Salmah, H.; Firuz, Z.; Lan, D.N.U. Properties of recycled high density polyethylene/recycled polypropylene blends: Effect of maleic anhydride polypropylene. Key Eng. Mater. 2014, 594–595, 837–841. [Google Scholar] [CrossRef]
- Hanna, E.G. Recycling of waste mixed plastics blends (PE/PP). J. Eng. Sci. Technol. Rev. 2019, 12, 87–92. [Google Scholar] [CrossRef]
- Saba, N.; Tahir, P.M.; Jawaid, M. A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- DeArmitt, C.; Rothon, R. Dispersants and coupling agents. In Applied Plastics Engineering Handbook; Kutz, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 441–454. ISBN 9781437735147. [Google Scholar]
- Brachet, P.; Høydal, L.T.; Hinrichsen, E.L.; Melum, F. Modification of mechanical properties of recycled polypropylene from post-consumer containers. Waste Manag. 2008, 28, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Elloumi, A.; Pimbert, S.; Bourmaud, A.; Bradai, C. Thermomechanical properties of virgin and rcycled polypropylene impact copolymer/CaCO3 nanocomposites. Polym. Eng. Sci. 2010, 50, 1904–1913. [Google Scholar] [CrossRef]
- Ammar, O.; Bouaziz, Y.; Haddar, N.; Mnif, N. Talc as reinforcing filler in polypropylene compounds: Effect on morphology and mechanical properties. Polym. Sci. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Wang, K.; Bahlouli, N.; Addiego, F.; Ahzi, S.; Rémond, Y.; Ruch, D.; Muller, R. Effect of talc content on the degradation of re-extruded polypropylene/talc composites. Polym. Degrad. Stab. 2013, 98, 1275–1286. [Google Scholar] [CrossRef]
- Sánchez-Soto, M.; Rossa, A.; Sánchez, A.J.; Gámez-Pérez, J. Blends of HDPE wastes: Study of the properties. Waste Manag. 2008, 28, 2565–2573. [Google Scholar] [CrossRef]
- Atikler, U.; Basalp, D.; Tihminlioǧlu, F. Mechanical and morphological properties of recycled high-density polyethylene, filled with calcium carbonate and fly ash. J. Appl. Polym. Sci. 2006, 102, 4460–4467. [Google Scholar] [CrossRef]
- Pardo, S.G.; Bernal, C.; Ares, A.; Abad, M.J.; Cano, J. Rheological, thermal, and mechanical characterization of fly ash-thermoplastic composites with different coupling agents. Polym. Compos. 2010, 31, 1722–1730. [Google Scholar] [CrossRef]
- Sengupta, S.; Maity, P.; Ray, D.; Mukhopadhyay, A. Stearic acid as coupling agent in fly ash reinforced recycled polypropylene matrix composites: Structural, mechanical, and thermal characterizations. J. Appl. Polym. Sci. 2013, 130, 1996–2004. [Google Scholar] [CrossRef]
- Sengupta, S.; Ray, D.; Mukhopadhyay, A.; Sengupta, S.; Kar, T. Lauric acid coated fly ash as a reinforcement in recycled polymer matrix composites. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Sengupta, S.; Ray, D.; Mukhopadhyay, A. Sustainable materials: Value-added composites from recycled polypropylene and fly ash using a green coupling agent. ACS Sustain. Chem. Eng. 2013, 1, 574–584. [Google Scholar] [CrossRef]
- de Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Zhao, Q.; Choo, H.; Bhatt, A.; Burns, S.E.; Bate, B. Review of the fundamental geochemical and physical behaviors of organoclays in barrier applications. Appl. Clay Sci. 2017, 142, 2–20. [Google Scholar] [CrossRef]
- Phuong, N.; Gilbert, V.; Chuong, B. Preparation of recycled polypropylene/ organophilic modified layered silicates nanocomposites part I: The recycling process of polypropylene and the mechanical properties of recycled polypropylene/organoclay nanocomposites. J. Reinf. Plast. Compos. 2008, 27, 1983–2000. [Google Scholar] [CrossRef]
- Shah, R.K.; Paul, D.R. Organoclay degradation in melt processed polyethylene nanocomposites. Polymer 2006, 47, 4075–4084. [Google Scholar] [CrossRef]
- Touati, N.; Kaci, M.; Bruzaud, S.; Grohens, Y. The effects of reprocessing cycles on the structure and properties of isotactic polypropylene/Cloisite 15A nanocomposites. Polym. Degrad. Stab. 2011, 96, 1064–1073. [Google Scholar] [CrossRef]
- Abdelhaleem, A.M.M.; Megahed, M.; Saber, D. Fatigue behavior of pure polypropylene and recycled polypropylene reinforced with short glass fiber. J. Compos. Mater. 2018, 52, 1633–1640. [Google Scholar] [CrossRef]
- Annandarajah, C.; Langhorst, A.; Kiziltas, A.; Grewell, D.; Mielewski, D.; Montazami, R. Hybrid cellulose-glass fiber composites for automotive applications. Materials (Basel) 2019, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Svensson, S.; Åkesson, D.; Bohlén, M. Reprocessing of high-density polyethylene reinforced with carbon nanotubes. J. Polym. Environ. 2020, 28, 1967–1973. [Google Scholar] [CrossRef]
- Ashori, A. Wood-plastic composites as promising green-composites for automotive industries! Bioresour. Technol. 2008, 99, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Panthapulakkal, S.; Zereshkian, A.; Sain, M. Preparation and characterization of wheat straw fibers for reinforcing application in injection molded thermoplastic composites. Bioresour. Technol. 2006, 97, 265–272. [Google Scholar] [CrossRef]
- Sood, M.; Deepak, D.; Gupta, V.K. Tensile properties of sisal fiber/recycled polyethylene (high density) composite: Effect of fiber chemical treatment. Mater. Today Proc. 2018, 5, 5673–5678. [Google Scholar] [CrossRef]
- Rohmat; Widiastuti, I.; Wijayanto, D.S. Characteristics of recycled HDPE/bamboo fibre composite. IOP Conf. Ser. Earth Environ. Sci. 2021, 1808, 012010. [Google Scholar] [CrossRef]
- Kim, J.; Cho, D. Effects of waste expanded polypropylene as recycled matrix on the flexural, impact, and heat deflection temperature properties of kenaf fiber/polypropylene composites. Polymers 2020, 12, 2578. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Kumar, J.; Pramod Naik, T.; Pabla, B.S.; Singh, I. Processing and characterization of pineapple fiber reinforced recycled polyethylene composites. Mater. Today Proc. 2021, 44, 2153–2157. [Google Scholar] [CrossRef]
- Koohestani, B.; Ganetri, I.; Yilmaz, E. Effects of silane modified minerals on mechanical, microstructural, thermal, and rheological properties of wood plastic composites. Compos. Part B Eng. 2017, 111, 103–111. [Google Scholar] [CrossRef]
- Rohit, K.; Dixit, S. Tensile and impact behaviour of thermoplastic BOPP/Milk pouches blends reinforced with sisal fibers. Prog. Rubber Plast. Recycl. Technol. 2017, 33, 139–152. [Google Scholar] [CrossRef]
- Inácio, A.L.N.; Nonato, R.C.; Bonse, B.C. Mechanical and thermal behavior of aged composites of recycled PP/EPDM/talc reinforced with bamboo fiber. Polym. Test. 2018, 72, 357–363. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. The hygroscopic behavior of plant fibers: A review. Front. Chem. 2014, 1, 1–12. [Google Scholar] [CrossRef]
- Kazemi Najafi, S.; Tajvidi, M.; Hamidina, E. Effect of temperature, plastic type and virginity on the water uptake of sawdust/plastic composites. Holz Als Roh-Und Werkst. 2007, 65, 377–382. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Characteristics of wood-fiber plastic composites made of recycled materials. Waste Manag. 2009, 29, 1291–1295. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, J.; Lu, B.X.; Xin, Z.X.; Kang, C.K.; Kim, J.K. Effect of flame retardants on mechanical properties, flammability and foamability of PP/wood-fiber composites. Compos. Part B Eng. 2012, 43, 150–158. [Google Scholar] [CrossRef]
- Das, K.; Adhikary, K.; Ray, D. Development of recycled polypropylene matrix composites reinforced with waste jute caddies. J. Reinf. Plast. Compos. 2009, 29, 201–208. [Google Scholar] [CrossRef]
- Azeez, T.O. Thermoplastic Recycling: Properties, Modifications, and Applications. In Thermosoftening Plastics; Evingür, G.A., Pekcan, Ö., Achilias, D.S., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Andoh, P.Y.; Sekyere, C.K.K.; Ayetor, G.K.K.; Sackey, M.N. Fabrication and testing of a low-cost wind turbine blade using bamboo reinforced recycled plastic. J. Appl. Eng. Technol. Sci. 2021, 2, 125–138. [Google Scholar]
- Nosker, T.; Lynch, J. Sustainability and Recycling of Polyethylene. In Handbook of Industrial Polyethylene Technology; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2016; pp. 1245–1252. ISBN 9781119159797. [Google Scholar]
- Grammatikos, S.; Bertin Jøsendal, H.; Papatzani, S.; Boura, O.; Hajmohammadian Baghban, M. Recycled wood plastic composites as floor panels for ship containers. IOP Conf. Ser. Mater. Sci. Eng. 2020, 842, 012006. [Google Scholar] [CrossRef]
- Lugal, L.; Grant, A.; Cordle, M.; Fletcher, D. PET Market in Europe—State of Play (Production, Collection and Recycling Data). Available online: https://www.eunomia.co.uk/reports-tools/pet-market-in-europe-state-of-play/ (accessed on 5 December 2020).
- Choudhary, K.; Sangwan, K.S.; Goyal, D. Environment and economic impacts assessment of PET waste recycling with conventional and renewable sources of energy. Procedia CIRP 2019, 80, 422–427. [Google Scholar] [CrossRef]
- Service, R. A Huge Step Forward. Mutant Enzyme Could Vastly Improve Recycling of Plastic Bottles. Available online: https://www.sciencemag.org/news/2020/04/huge-step-forward-mutant-enzyme-could-vastly-improve-recycling-plastic-bottles%0D (accessed on 4 May 2021).
- Chiaie, K.R.D.; McMahon, F.R.; Williams, E.J.; Price, M.J.; Dove, A.P. Dual-catalytic depolymerization of polyethylene terephthalate (PET). Polym. Chem. 2020, 11, 1450–1453. [Google Scholar] [CrossRef]
- Bartolome, L.; Imran, M.; Cho, B.; Al-Masry, W.; Kim, D. Recent Developments in the Chemical Recycling of PET. In Material Recycling—Trends and Perspectives; BoD–Books on Demand: Norderstedt, Germany, 2012; ISBN 978-953-51-0327-1. [Google Scholar]
- Park, S.H.; Kim, S.H. Poly (ethylene terephthalate) recycling for high value added textiles. Fash. Text. 2014, 1, 1. [Google Scholar] [CrossRef]
- Fidanovski, B.Z.; Popovic, I.G.; Radojevic, V.J.; Radisavljevic, I.Z.; Perisic, S.D.; Spasojevic, P.M. Composite materials from fully bio-based thermosetting resins and recycled waste poly(ethylene terephthalate). Compos. Part B Eng. 2018, 153, 117–123. [Google Scholar] [CrossRef]
- Snowden, S. New Enzyme Breaks Down Plastic in Hours and Enables High-Quality Recycling. Available online: https://www.forbes.com/sites/scottsnowden/2020/04/11/new-enzyme-breaks-down-plastic-in-hours/#2299a9125e4e (accessed on 3 July 2020).
- Gopalakrishna, K.G.; Reddy, N. Regulations on Recycling PET Bottles. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 23–35. ISBN 978-0-12-811361-5. [Google Scholar]
- Almeshal, I.; Tayeh, B.A.; Alyousef, R.; Alabduljabbar, H.; Mustafa Mohamed, A.; Alaskar, A. Use of recycled plastic as fine aggregate in cementitious composites: A review. Constr. Build. Mater. 2020, 253, 119146. [Google Scholar] [CrossRef]
- Rane, A.; Ajitha, A.R.; Aswathi, M.K.; Manju, P.; Kanny, K.; Thomas, S. Applications of waste poly(ethylene terephthalate) bottles. Recycl. Polyethyl. Terephthalate Bottles 2019, 169–189. [Google Scholar] [CrossRef]
- Santos, P.; Pezzin, S.H. Mechanical properties of polypropylene reinforced with recycled-pet fibres. J. Mater. Process. Technol. 2003, 143–144, 517–520. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Martin, E.J.P.; Oliveira, D.S.B.L.; Oliveira, L.S.B.L.; Bezerra, B.S. Life cycle comparative assessment of pet bottle waste management options: A case study for the city of Bauru, Brazil. Waste Manag. 2021, 119, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Chirayil, C.J.; Mishra, R.K.; Thomas, S. Materials Recovery, Direct Reuse and Incineration of PET Bottles. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 37–60. ISBN 9780128113615. [Google Scholar]
- Malik, N.; Kumar, P.; Shrivastava, S.; Ghosh, S.B. An overview on PET waste recycling for application in packaging. Int. J. Plast. Technol. 2017, 21, 1–24. [Google Scholar] [CrossRef]
- Thachnatharen, N.; Shahabuddin, S.; Sridewi, N. The waste management of polyethylene terephthalate (PET) plastic waste: A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1127, 012002. [Google Scholar] [CrossRef]
- Cano, I.; Martin, C.; Fernandes, J.A.; Lodge, R.W.; Dupont, J.; Casado-Carmona, F.A.; Lucena, R.; Cardenas, S.; Sans, V.; de Pedro, I. Paramagnetic ionic liquid-coated SiO2@Fe3O4 nanoparticles—The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET. Appl. Catal. B Environ. 2020, 260, 118110. [Google Scholar] [CrossRef]
- Park, R.; Sridhar, V.; Park, H. Taguchi method for optimization of reaction conditions in microwave glycolysis of waste PET. J. Mater. Cycles Waste Manag. 2020, 22, 664–672. [Google Scholar] [CrossRef]
- Guo, Z.; Adolfsson, E.; Tam, P.L. Nanostructured micro particles as a low-cost and sustainable catalyst in the recycling of PET fiber waste by the glycolysis method. Waste Manag. 2021, 126, 559–566. [Google Scholar] [CrossRef]
- Sert, E.; Yılmaz, E.; Atalay, F.S. Chemical recycling of polyethlylene terephthalate by glycolysis using deep eutectic solvents. J. Polym. Environ. 2019, 27, 2956–2962. [Google Scholar] [CrossRef]
- Sheel, A.; Pant, D. Chemical depolymerization of PET bottles via glycolysis. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 61–84. ISBN 9780128113615. [Google Scholar]
- Wang, L.; Nelson, G.A.; Toland, J.; Holbrey, J.D. Glycolysis of PET using 1,3-dimethylimidazolium-2-carboxylate as an organocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 13362–13368. [Google Scholar] [CrossRef]
- Han, M. Depolymerization of PET bottle via methanolysis and hydrolysis. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 85–108. ISBN 9780128113615. [Google Scholar]
- Genta, M.; Yano, F.; Kondo, Y.; Matsubara, W.; Oomoto, S. Development of chemical recycling process for post-consumer PET bottles by methanolysis in supercritical methanol. Mitsubishi Heavy Ind. Ltd. Tech. Rev. 2003, 40, 1–4. [Google Scholar]
- Pudack, C.; Stepanski, M.; Fässler, P. PET recycling—Contributions of crystallization to sustainability. Chemie-Ingenieur-Technik 2020, 92, 452–458. [Google Scholar] [CrossRef]
- Pham, D.D.; Cho, J. Low-energy catalytic methanolysis of poly(ethyleneterephthalate). Green Chem. 2021, 23, 511–525. [Google Scholar] [CrossRef]
- Padhan, R.K.; Sreeram, A. Chemical depolymerization of PET bottles via combined chemolysis methods. In Recycling of Polyethylene Terephthalate Bottles; Thomas, S., Rane, A., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 135–147. ISBN 9780128113615. [Google Scholar]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Sun, C.H.; Chen, X.P.; Zhuo, Q.; Zhou, T. Recycling and depolymerization of waste polyethylene terephthalate bottles by alcohol alkali hydrolysis. J. Cent. South. Univ. 2018, 25, 543–549. [Google Scholar] [CrossRef]
- Kandasamy, S.; Subramaniyan, A.; Ramasamy, G.; Ahamed, A.R.; Manickam, N.; Dhandapani, B. Study of alkaline hydrolysis of post consumed polyethylene terephthalate waste. AIP Conf. Proc. 2020, 2240, 110001. [Google Scholar] [CrossRef]
- Căta, A.; Ştefănuţ, M.N.; Ienaşcu, I.M.C.; Tănasie, C.; Miclău, M. Alkaline Hydrolysis of polyethylene terephthalate under microwave irradiation. Rev. Roum. Chim 2017, 62, 531–538. [Google Scholar]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- de Castro, A.M.; Carniel, A.; Nicomedes Junior, J.; da Conceição Gomes, A.; Valoni, É. Screening of commercial enzymes for poly(ethylene terephthalate) (PET) hydrolysis and synergy studies on different substrate sources. J. Ind. Microbiol. Biotechnol. 2017, 44, 835–844. [Google Scholar] [CrossRef] [PubMed]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Arnaiz, S.; Gutiérrez-Ortiz, J.I. Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts. Polym. Degrad. Stab. 2010, 95, 1022–1028. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Xiang, H.; Xu, Y.; Li, Y. Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym. Degrad. Stab. 2002, 75, 185–191. [Google Scholar] [CrossRef]
- Sinha, V.K.; Patel, M.R.; Patel, J. V PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karayannidis, G.P. The chemical recycling of PET in the framework of sustainable development. Water Air Soil Pollut. Focus 2004, 4, 385–396. [Google Scholar] [CrossRef]
- Khoonkari, M.; Haghighi, A.H.; Sefidbakht, Y.; Shekoohi, K.; Ghaderian, A. Chemical recycling of PET wastes with different catalysts. Int. J. Polym. Sci. 2015, 2015, 11. [Google Scholar] [CrossRef]
- Kárpáti, L.; Fogarassy, F.; Kovácsik, D.; Vargha, V. One-pot depolymerization and polycondensation of PET based random oligo- and polyesters. J. Polym Environ. 2019, 27, 2167–2181. [Google Scholar] [CrossRef]
- Kárpáti, L.; Szarka, G.; Hartman, M.; Vargha, V. Oligoester and polyester production via acido-alcoholysis of PET waste. Period. Polytech. Chem. Eng. 2018, 62, 336–344. [Google Scholar] [CrossRef]
- Del Mar Castro López, M.; Ares Pernas, A.I.; Abad López, M.J.; Latorre, A.L.; López Vilariño, J.M.; González Rodríguez, M.V. Assessing changes on poly(ethylene terephthalate) properties after recycling: Mechanical recycling in laboratory versus postconsumer recycled material. Mater. Chem. Phys. 2014, 147, 884–894. [Google Scholar] [CrossRef]
- Wu, H.; Lv, S.; He, Y.; Qu, J.-P. The study of the thermomechanical degradation and mechanical properties of PET recycled by industrial-scale elongational processing. Polym. Test. 2019, 77, 105882. [Google Scholar] [CrossRef]
- Elamri, A.; Lallam, A.; Harzallah, O.; Bencheikh, L. Mechanical characterization of melt spun fibers from recycled and virgin PET blends. J. Mater. Sci. 2007, 42, 8271–8278. [Google Scholar] [CrossRef]
- Alvarado Chacon, F.; Brouwer, M.T.; Thoden van Velzen, E.U. Effect of recycled content and rPET quality on the properties of PET bottles, part I: Optical and mechanical properties. Packag. Technol. Sci. 2020, 33, 347–357. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef]
- Szarka, G.; Domján, A.; Szakács, T.; Iván, B. Oil from poly(vinyl chloride): Unprecedented degradative chain scission under mild thermooxidative conditions. Polym. Degrad. Stab. 2012, 97, 1787–1793. [Google Scholar] [CrossRef]
- Szarka, G.; Iván, B. Thermal Properties, Degradation and stability of poly(vinyl chloride) predegraded thermooxidatively in the presence of dioctyl phthalate plasticizer. J. Macromol. Sci. Part A 2013, 50, 208–214. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Christiansen, J.D.; Daugaard, A.E.; Astrup, T.F. Closing the loop for PET, PE and PP waste from households: Influence of material properties and product design for plastic recycling. Waste Manag. 2019, 96, 75–85. [Google Scholar] [CrossRef]
- Qin, Y.; Qu, M.; Kaschta, J.; Schubert, D.W. Comparing recycled and virgin poly (ethylene terephthalate) melt-spun fibres. Polym. Test. 2018, 72, 364–371. [Google Scholar] [CrossRef]
- Frounchi, M. Studies on degradation of PET in mechanical recycling. Macromol. Symp. 1999, 144, 465–469. [Google Scholar] [CrossRef]
- Mondadori, N.M.L.; Nunes, R.C.; Bresciani Canto, L.; Zattera, A. Composites of Recycled PET Reinforced with Short Glass Fiber. J. Thermoplast. Compos. Mater. 2012, 25, 747–764. [Google Scholar] [CrossRef]
- Benvenuta-Tapia, J.J.; Vivaldo-Lima, E.; Guerrero-Santos, R. Effect of copolymers synthesized by nitroxide-mediated polymerization as chain extenders of postconsumer poly(ethylene terephthalate) waste. Polym. Eng. Sci. 2019, 59, 2255–2264. [Google Scholar] [CrossRef]
- Karsli, N.G. A study on the fracture, mechanical and thermal properties of chain extended recycled poly(ethylene terephthalate). J. Thermoplast. Compos. Mater. 2017, 30, 1157–1172. [Google Scholar] [CrossRef]
- Benvenuta-Tapia, J.J.; González-Coronel, V.J.; Soriano-Moro, G.; Martínez-De la Luz, I.; Vivaldo-Lima, E. Recycling of poly(ethylene terephthalate) by chain extension during reactive extrusion using functionalized block copolymers synthesized by RAFT polymerization. J. Appl. Polym. Sci. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Y.; Wang, K.; Xia, Y.; Gao, H.; Luo, K.; Cao, Z.; Qi, J. Effect of the trifunctional chain extender on intrinsic viscosity, crystallization behavior, and mechanical properties of poly(ethylene terephthalate). ACS Omega 2020, 5, 19247–19254. [Google Scholar] [CrossRef]
- Pandey, V.; Seese, M.; Maia, J.M.; Schiraldi, D.A. Thermo-rheological analysis of various chain extended recycled poly(ethylene terephthalate). Polym. Eng. Sci. 2020, 60, 2511–2516. [Google Scholar] [CrossRef]
- Nisticò, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Molnar, B.; Ronkay, F. Effect of solid-state polycondensation on crystalline structure and mechanical properties of recycled polyethylene-terephthalate. Polym. Bull. 2019, 76, 2387–2398. [Google Scholar] [CrossRef]
- Fitaroni, L.B.; de Oliveira, É.C.; Marcomini, A.L.; Paranhos, C.M.; Freitas, F.L.; Cruz, S.A. Reprocessing and solid state polymerization on contaminated post-consumer PET: Thermal and crystallization behavior. J. Polym. Environ. 2020, 28, 91–99. [Google Scholar] [CrossRef]
- Bocz, K.; Molnár, B.; Marosi, G.; Ronkay, F. Preparation of low-density microcellular foams from recycled PET modified by solid state polymerization and chain extension. J. Polym. Environ. 2019, 27, 343–351. [Google Scholar] [CrossRef]
- Benvenuta Tapia, J.J.; Hernández Valdez, M.; Cerna Cortez, J.; Díaz García, V.M.; Landeros Barrios, H. Improving the rheological and mechanical properties of recycled PET modified by macromolecular chain extenders synthesized by controlled radical polymerization. J. Polym. Environ. 2018, 26, 4221–4232. [Google Scholar] [CrossRef]
- Kossentini-Kallel, T.; Mnif, N.; Fourti, F.; Elleuch, B. PET recycling and chain extension during reactive processing in the presence of pyromellitic dianhydride (PMDA). Macromol. An. Indian J. 2008, 4. [Google Scholar]
- Awaja, F.; Daver, F.; Kosior, E. Recycled poly(ethylene terephthalate) chain extension by a reactive extrusion process. Polym. Eng. Sci. 2004, 44, 1579–1587. [Google Scholar] [CrossRef]
- Torres, N.; Robin, J.J.; Boutevin, B. Chemical modification of virgin and recycled poly(ethylene terephthalate) by adding of chain extenders during processing. J. Appl. Polym. Sci. 2001, 79, 1816–1824. [Google Scholar] [CrossRef]
- Bimestre, B.H.; Saron, C. Chain extension of poly (ethylene terephthalate) by reactive extrusion with secondary stabilizer. Mater. Res. 2012, 15, 467–472. [Google Scholar] [CrossRef]
- Wang, L.; Hao, T.; Huang, L.; Huang, J.; Huang, R. Silicone chain extender for recycled polyethylene-terephthalate with higher flexibility. J. Wuhan Univ. Technol.-Mat. Sci. Ed. 2019, 34, 1228–1232. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous and rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Itim, B.; Philip, M. Effect of multiple extrusions and influence of PP contamination on the thermal characteristics of bottle grade recycled PET. Polym. Degrad. Stab. 2015, 117, 84–89. [Google Scholar] [CrossRef]
- Ahmadlouydarab, M.; Chamkouri, M.; Chamkouri, H. Compatibilization of immiscible polymer blends (R-PET/PP) by adding PP-g-MA as compatibilizer: Analysis of phase morphology and mechanical properties. Polym. Bull. 2020, 77, 5753–5766. [Google Scholar] [CrossRef]
- Dimitrova, T.L.; La Mantia, F.P.; Pilati, F.; Toselli, M.; Valenza, A.; Visco, A. On the compatibilization of PET/HDPE blends through a new class of copolyesters. Polymer (Guildf.) 2000, 41, 4817–4824. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, Q.; Clemons, C.M.; Guo, W. Phase structure and properties of poly(ethylene terephthalate)/polyethylene based on recycled materials. J. Appl. Polym. Sci. 2009, 113, 1710–1719. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, Q.; Zhang, Q. Morphology and properties of microfibrillar composites based on recycled poly (ethylene terephthalate) and high density polyethylene. Compos. Part A Appl. Sci. Manuf. 2009, 40, 904–912. [Google Scholar] [CrossRef]
- Matias, Á.A.; Lima, M.S.; Pereira, J.; Pereira, P.; Barros, R.; Coelho, J.F.J.; Serra, A.C. Use of recycled polypropylene/poly(ethylene terephthalate) blends to manufacture water pipes: An industrial scale study. Waste Manag. 2020, 101, 250–258. [Google Scholar] [CrossRef]
- He, S.-S.; Wei, M.-Y.; Liu, M.-H.; Xue, W.-L. Characterization of virgin and recycled poly(ethylene terephthalate) (PET) fibers. J. Text. Inst. 2014, 106, 1–7. [Google Scholar] [CrossRef]
- Zander, N.E.; Gillan, M.; Burckhard, Z.; Gardea, F. Recycled polypropylene blends as novel 3D printing materials. Addit. Manuf. 2019, 25, 122–130. [Google Scholar] [CrossRef]
- Wang, D.; Yang, B.; Chen, Q.-T.; Chen, J.; Su, L.-F.; Chen, P.; Zheng, Z.-Z.; Miao, J.-B.; Qian, J.-S.; Xia, R.; et al. A facile evaluation on melt crystallization kinetics and thermal properties of low-density polyethylene (LDPE)/Recycled polyethylene terephthalate (RPET) blends. Adv. Ind. Eng. Polym. Res. 2019, 2, 126–135. [Google Scholar] [CrossRef]
- Araujo, L.M.G.; Morales, A.R.; Araujo, L.M.G.; Morales, A.R. Compatibilization of recycled polypropylene and recycled poly (ethylene terephthalate) blends with SEBS-g-MA. Polímeros 2018, 28, 84–91. [Google Scholar] [CrossRef]
- Hidayah Marzuki, N.; Irfiani, N.; Uzir Wahit, M.; Othman, N.; Izyan Syazana Mohd Yusoff, N. Mechanical properties of kenaf fiber and montmorillonite reinforced recycled polyethylene terephthalate/recycled polypropylene. Mater. Today Proc. 2018, 5, 21879–21887. [Google Scholar] [CrossRef]
- Scelsi, L.; Hodzic, A.; Soutis, C.; Hayes, S.A.; Rajendran, S.; AlMa’adeed, M.A.; Kahraman, R. A review on composite materials based on recycled thermoplastics and glass fibres. Plast. Rubber Compos. 2011, 40, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, W.; Yu, Y.; Li, B.; Wu, C. Structure and properties of compatibilized recycled poly(ethylene terephthalate)/linear low density polyethylene blends. Eur. Polym. J. 2007, 43, 3662–3670. [Google Scholar] [CrossRef]
- Delva, L.; Deceur, C.; Damme, N.; Ragaert, K. Compatibilization of PET-PE blends for the recycling of multilayer packaging foils. In Proceedings of the AIP Conference Proceedings, 2055, Dresden, Germany, 22 January 2019. [Google Scholar]
- Taghavi, S.K.; Shahrajabian, H.; Hosseini, H.M. Detailed comparison of compatibilizers MAPE and SEBS-g-MA on the mechanical/thermal properties, and morphology in ternary blend of recycled PET/HDPE/MAPE and recycled PET/HDPE/SEBS-g-MA. J. Elastomers Plast. 2018, 50, 13–35. [Google Scholar] [CrossRef]
- Chen, R.S.; Ghani, M.H.A.; Salleh, M.N.; Ahmad, S.; Gan, S. Influence of blend composition and compatibilizer on mechanical and morphological properties of recycled HDPE/PET blends. Mater. Sci. Appl. 2014, 5, 943–952. [Google Scholar] [CrossRef]
- Pracella, M.; Chionna, D.; Ishak, R.; Galeski, A. Recycling of PET and polyolefin based packaging materials by reactive blending. Polym.-Plast. Technol. Eng. 2004, 43, 1711–1722. [Google Scholar] [CrossRef]
- Salleh, M.N.; Ahmad, S.; Ghani, M.H.A.; Chen, R.S. Effect of compatibilizer on impact and morphological analysis of recycled HDPE/PET blends. AIP Conf. Proc. 2013, 1571, 70–74. [Google Scholar] [CrossRef]
- Imamura, N.; Sakamoto, H.; Higuchi, Y.; Yamamoto, H.; Kawasaki, S.; Yamada, K.; Nishimura, H.; Nishino, T. Effectiveness of compatibilizer on mechanical properties of recycled PET blends with PE, PP, and PS. Mater. Sci. Appl. 2014, 5, 548–555. [Google Scholar] [CrossRef][Green Version]
- Uehara, G.; França, M.; Canevarolo, S. Recycling assessment of multilayer flexible packaging films using design of experiments. Polímeros 2015, 25, 371–381. [Google Scholar] [CrossRef]
- Kalfoglou, N.K.; Skafidas, D.S.; Kallitsis, J.K.; Lambert, J.-C.; der Stappen, L. Comparison of compatibilizer effectiveness for PET/HDPE blends. Polymer (Guildf.) 1995, 36, 4453–4462. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukharjee, M.; Adhikari, B. Recycling of polyethylene/poly(ethylene terephthalate) post-consumer oil pouches using compatibiliser. Polym. Polym. Compos. 2006, 14, 635–646. [Google Scholar] [CrossRef]
- Abdel Tawab, K.; Ibrahim, S.M.; Magida, M.M. The effect of gamma irradiation on mechanical, and thermal properties of recycling polyethylene terephthalate and low density polyethylene (R-PET/LDPE) blend compatibilized by ethylene vinyl acetate (EVA). J. Radioanal Nucl. Chem 2013, 295, 1313–1319. [Google Scholar] [CrossRef]
- Ferreira, F.; Pinheiro, I.; de Souza, S.; Mei, L.; Lona, L. Polymer composites reinforced with natural fibers and nanocellulose in the automotive industry: A short review. J. Compos. Sci. 2019, 3, 51. [Google Scholar] [CrossRef]
- Rallini, M.; Kenny, J.M. Nanofillers in polymers. In Modification of Polymer Properties; Jasso-Gastinel, C.F., Kenny, J.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 47–86. ISBN 9780323443982. [Google Scholar]
- Kiran, M.D.; Govindaraju, H.K.; Jayaraju, T.; Kumar, N. Review-effect of fillers on mechanical properties of polymer matrix composites. Mater. Today Proc. 2018, 5, 22421–22424. [Google Scholar] [CrossRef]
- Saeed, H.A.M.; Eltahir, Y.A.; Xia, Y.; Yimin, W. Properties of recycled poly (ethylene terephthalate) (PET)/hyperbranched polyester (HBPET) composite fibers. J. Text. Inst. 2015, 106, 601–610. [Google Scholar] [CrossRef]
- Romhány, G.; Wu, C.M.; Lai, W.Y.; Karger-Kocsis, J. Fracture behavior and damage development in self-reinforced PET composites assessed by located acoustic emission and thermography: Effects of flame retardant and recycled PET. Compos. Sci. Technol. 2016, 132, 76–83. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yucel, A.; Yel, E.; Ahmetli, G. Production of epoxy composite from the pyrolysis char of washed PET wastes. Energy Procedia 2017, 118, 216–220. [Google Scholar] [CrossRef]
- Khalid, F.S.; Irwan, J.M.; Ibrahim, M.H.W.; Othman, N.; Shahidan, S. Performance of plastic wastes in fiber-reinforced concrete beams. Constr. Build. Mater. 2018, 183, 451–464. [Google Scholar] [CrossRef]
- Nematzadeh, M.; Shahmansouri, A.A.; Fakoor, M. Post-fire compressive strength of recycled PET aggregate concrete reinforced with steel fibers: Optimization and prediction via RSM and GEP. Constr. Build. Mater. 2020, 252, 119057. [Google Scholar] [CrossRef]
- Ronkay, F.; Czigány, T. Development of composites with recycled PET matrix. Polym. Adv. Technol. 2006, 17, 830–834. [Google Scholar] [CrossRef]
- Scelsi, L.; Mackley, M.R. Rheo-optic flow-induced crystallisation of polypropylene and polyethylene within confined entry-exit flow geometries. Rheol. Acta 2008, 47, 895–908. [Google Scholar] [CrossRef]
- de Moura Giraldi, A.L.F.; de Jesus, R.C.; Mei, L.H.I. The influence of extrusion variables on the interfacial adhesion and mechanical properties of recycled PET composites. J. Mater. Process. Technol. 2005, 162–163, 90–95. [Google Scholar] [CrossRef]
- Bourmaud, A.; Beaugrand, J.; Shah, D.U.; Placet, V.; Baley, C. Towards the design of high-performance plant fibre composites. Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Prasad, R.C. Composites, Science, and Technology; New Age International: New Delhi, India, 2000; ISBN 8122412513. [Google Scholar]
- Wu, C.-M.; Lai, W.-Y. Mechanical and open hole tensile properties of self-reinforced PET composites with recycled PET fiber reinforcement. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Telli, A.; Özdil, N. Effect of recycled PET fibers on the performance properties of knitted fabrics. J. Eng. Fiber. Fabr. 2015, 10, 47–60. [Google Scholar] [CrossRef]
- Fangueiro, R. Fibrous and Composite Materials for Civil. Engineering Applications; Fangueiro, R., Ed.; Woodhead Publishing: Cambridge, UK, 2011; ISBN 978-0-85709-252-6. [Google Scholar]
- Ma, K.-T.; Luo, Y.; Kwan, T.; Wu, Y. (Eds.) Hardware—off-vessel components. In Mooring System Engineering for Offshore Structures; Gulf Professional Publishing: Houston, TX, USA, 2019; pp. 175–198. ISBN 978-0-12-818551-3. [Google Scholar]
- Yang, B.; Chen, J.; Su, L.-F.; Miao, J.-B.; Chen, P.; Qian, J.-S.; Xia, R.; Shi, Y. Melt crystallization and thermal properties of graphene platelets (GNPs) modified recycled polyethylene terephthalate (RPET) composites: The filler network analysis. Polym. Test. 2019, 77, 105869. [Google Scholar] [CrossRef]
- Negoro, T.; Thodsaratpreeyakul, W.; Takada, Y.; Thumsorn, S.; Inoya, H.; Hamada, H. Role of crystallinity on moisture absorption and mechanical performance of recycled PET compounds. Energy Procedia 2016, 89, 323–327. [Google Scholar] [CrossRef]
- Marzuki, N.H.; Wahit, M.U.; Arsad, A.; Othman, N.; Yusoff, N.I.S.M. The effect of kenaf loading on the mechanical properties of kenaf-reinforced recycled poly(ethylene terephthalate)/recycled poly(propylene) (rPET/rPP) composite. Mater. Today Proc. 2020, 39, 959–964. [Google Scholar] [CrossRef]
- Ventures, I. Recycling. Available online: https://sustainability.indoramaventures.com/en/environmental/recycling/at-a-glance (accessed on 15 April 2021).
- Charpak PET Explained. Available online: https://www.charpak.uk/pet-plastic/ (accessed on 23 March 2021).
- Parker, T. Coca-Cola European Partners Invests in Recycled PET Innovator. Available online: https://www.nspackaging.com/news/company-news/coca-cola-european-partners-pet/ (accessed on 23 April 2021).
- Ávila, A.F.; Duarte, M. V A mechanical analysis on recycled PET/HDPE composites. Polym. Degrad. Stab. 2003, 80, 373–382. [Google Scholar] [CrossRef]
- Fibres, A. Recycled PET. Available online: https://www.ain-fibres.com/recycled-pet/ (accessed on 2 April 2021).
- Walker, T. Recycled PET Honeycomb Now Commercially Viable Using EconCore’s Technology. Available online: https://www.britishplastics.co.uk/materials/recycled-pet-honeycomb-now-commercially-viable-using-econcor/ (accessed on 4 June 2020).
- Reintjes, M. PET Bottles Find Second Life in Wind Turbine Rotor Blades. Available online: https://recyclinginternational.com/plastics/pet-bottles-find-second-life-in-wind-turbine-rotor-blades/2741/ (accessed on 25 June 2020).
- Engineering360. Packing Materials Find Second Use in Rechargable Batteries. Available online: https://insights.globalspec.com/article/730/packaging-materials-find-second-use-in-rechargeable-batteries (accessed on 2 May 2020).
- Drzyzga, O.; Prieto, A. Plastic waste management, a matter for the ‘community’. Microb. Biotechnol. 2019, 12, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, T.; Mayer, M.; McNally, C.; Simons, T.J.; Witte, C. How Plastics-Waste Recycling Could Transform the Chemical Industry; McKinsey & Company: New York, NY, USA, 2018. [Google Scholar]
- Energias Wind Turbine Composite Materials Market—Global Industry Analysis, Market Size, Opportunities and FORECAST, 2017–2023. Available online: https://www.energiasmarketresearch.com/global-wind-turbine-composite-materials-market-outlook/#1490956978512-aa4994a2-860cfa3f-f4e9 (accessed on 3 February 2020).
- Comfils Comfil Thermoplastic Composites. Available online: https://www.comfil.biz/ (accessed on 25 June 2020).
- Propex Furnishing Solutions CURV, made by Propex Furnishing Solutions. Available online: https://www.curvonline.com/ (accessed on 4 April 2020).
- DIT Thermoplastic Composite Material with Ultimate Properties. Available online: https://www.ditweaving.com/ (accessed on 25 June 2020).
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020, 5, 1–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).