Contribution of Oxide Supports in Nickel-Based Catalytic Elimination of Greenhouse Gases and Generation of Syngas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Synthesis
2.2. Catalytic Testing
2.3. Catalyst Characterization
3. Results
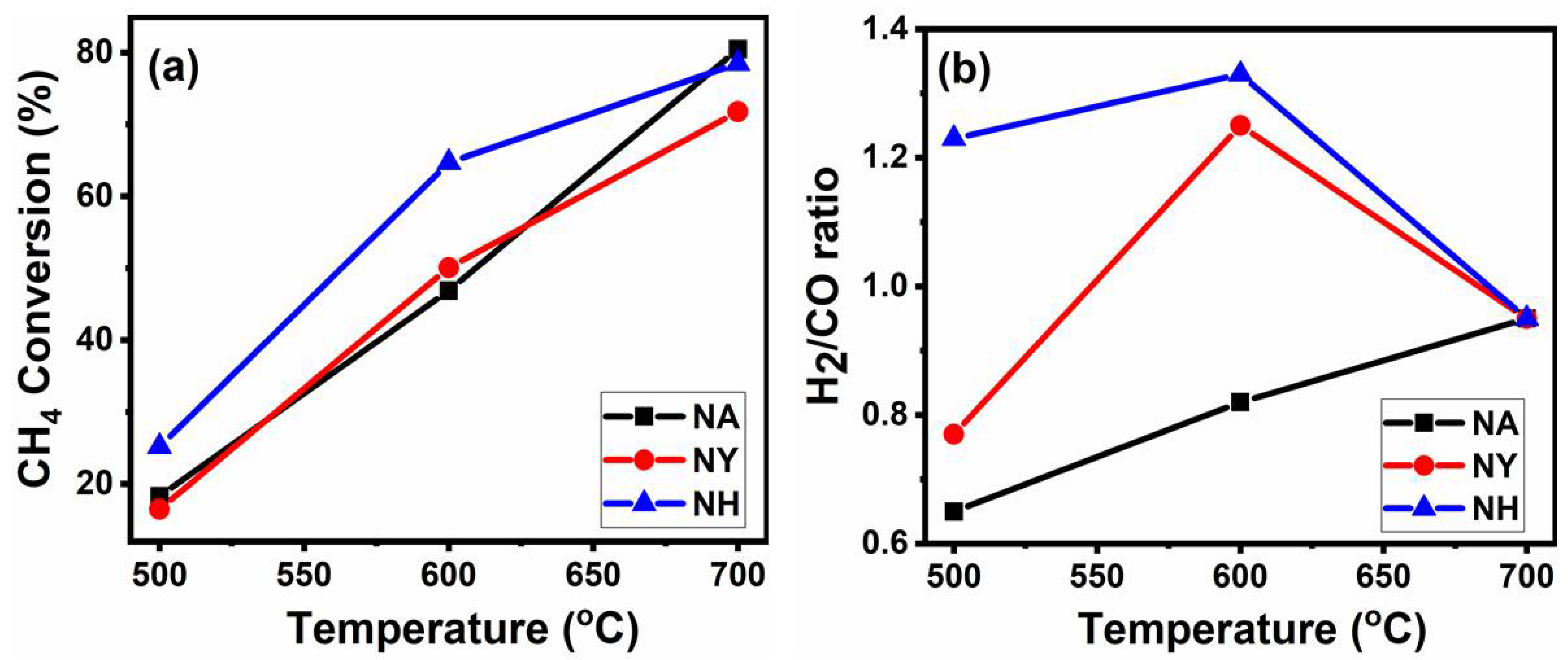
3.1. Catalytic Activity
3.2. Catalytic Stability
3.3. Catalysts Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, S.; Prasad, R. An overview of dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Hu, Y.H. Catalysts for CO2 reforming of CH4: A review. J. Mater. Chem. A 2021, 9, 12495–12520. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, F. Catalytic Conversion of Biogas to Syngas via Dry Reforming Process. In Advances in Bioenergy; Li, Y., Ge, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 43–76. [Google Scholar]
- Wang, X.; Economides, M. Advanced Natural Gas Engineering, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–368. [Google Scholar]
- Daza, C.E.; Gallego, J.; Mondragon, F.; Moreno, S.; Molina, R. High stability of Ce-promoted Ni/Mg–Al catalysts derived from hydrotalcites in dry reforming of methane. Fuel 2010, 89, 592–603. [Google Scholar] [CrossRef]
- Jeong, D.W.; Jang, W.J.; Shim, J.O.; Roh, H.S.; Son, I.H.; Lee, S.J. The effect of preparation method on the catalytic performance over superior MgO-promoted Ni–Ce0.8Zr0.2O2 catalyst for CO2 reforming of CH4. Int. J. Hydrogen Energy 2013, 38, 13649–13654. [Google Scholar] [CrossRef]
- Son, I.H.; Lee, S.J.; Song, I.Y.; Jeon, W.S.; Jung, I.; Yun, D.J.; Jeong, D.W.; Shim, J.O.; Jang, W.J.; Roh, H.S. Study on coke formation over Ni/γ-Al2O3, Co-Ni/γ-Al2O3, and Mg-Co-Ni/γ-Al2O3 catalysts for carbon dioxide reforming of methane. Fuel 2014, 136, 194–200. [Google Scholar] [CrossRef]
- Quiroga, M.M.B.; Luna, A.E.C. Catalytic activity and effect of modifiers on Ni-based catalysts for the dry reforming of methane. Int. J. Hydrogen Energy 2010, 35, 6052–6056. [Google Scholar] [CrossRef]
- Khan, A.; Sukonket, T.; Saha, B.; Idem, R. Catalytic activity of various 5 wt.% Ni/Ce0.5Zr0.33M0.17O2−δ catalysts for the CO2 reforming of CH4 in the presence and absence of steam. Energy Fuels 2012, 26, 365–379. [Google Scholar] [CrossRef]
- Chen, H.W.; Wang, C.Y.; Yu, C.H.; Tseng, L.T.; Liao, P.H. Carbon dioxide reforming of methane reaction catalyzed by stable nickel copper catalysts. Catal. Today 2004, 97, 173–180. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; Ferretti, O.A.; Resasco, D. Study of Ni catalysts on different supports to obtain synthesis gas. Int. J. Hydrogen Energy 2005, 30, 1399–1405. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrogen Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Catalytic conversion of methane to synthesis gas by partial oxidation and CO2 reforming. Adv. Catal. 2004, 48, 297–345. [Google Scholar]
- Luengnaruemitchai, A.; Kaengsilalai, A. Activity of different zeolite-supported Ni catalysts for methane reforming with carbon dioxide. Chem. Eng. J. 2008, 144, 96–102. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Jansen, J.; Bekkum, H.V. Introduction to Zeolite Science and Practice, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 1–1078. [Google Scholar]
- Csicsery, S.M. Shape-selective catalysis in zeolites. Zeolites 1984, 4, 202–213. [Google Scholar] [CrossRef]
- Khouw, C.B.; Dartt, C.B.; Labinger, J.A.; Davis, M.E. Studies on the Catalytic-Oxidation of Alkanes and Alkenes by Titanium Silicates. J. Catal. 1994, 149, 195–205. [Google Scholar] [CrossRef]
- Kareem, A.; Chand, S.; Mishra, I. Disproportionation of Toluene to Produce Benzene and p-Xylene: A Review. J. Sci. Ind. Res. 2001, 60, 319–327. [Google Scholar]
- Marcilly, C.R. Where and how shape selectivity of molecular sieves operates in refining and petrochemistry catalytic processes. Top. Catal. 2000, 13, 357–366. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Nimwattanakul, W.; Luengnaruemitchai, A.; Jitkarnka, S. Potential of Ni supported on clinoptilolite catalysts for carbon dioxide reforming of methane. Int. J. Hydrogen Energy 2006, 31, 93–100. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Ji, S.; Sun, S.; Ding, D.; Li, C. CO2 reforming of methane to syngas over Ni/SBA-15/FeCrAl. Stud. Surf. Sci. Catal. 2007, 167, 367–372. [Google Scholar]
- Wei, B.; Yang, H.; Hu, H.; Wang, D.; Jin, L. Enhanced production of light tar from integrated process of in-situ catalytic upgrading lignite tar and methane dry reforming over Ni/mesoporous Y. Fuel 2020, 279, 118533. [Google Scholar] [CrossRef]
- Albarazi, A.; Beaunier, P.; Da Costa, P. Hydrogen and syngas production by methane dry reforming on SBA-15 supported nickel catalysts: On the effect of promotion by Ce0.75ZrO0.25O2 mixed oxide. Int. J. Hydrogen Energy 2013, 38, 127–139. [Google Scholar] [CrossRef]
- Vafaeian, Y.; Haghighi, M.; Aghamohammadi, S. Ultrasound assisted dispersion of different amount of Ni over ZSM-5 used as nanostructured catalyst for hydrogen production via CO2 reforming of methane. Energy Convers. Manag. 2013, 76, 1093–1103. [Google Scholar] [CrossRef]
- Bawah, A.-R.; Malaibari, Z.O.; Muraza, O. Syngas production from CO2 reforming of methane over Ni supported on hierarchical silicalite-1 fabricated by microwave-assisted hydrothermal synthesis. Int. J. Hydrogen Energy 2018, 43, 13177–13189. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, S.; Zhang, A.; Song, C.; Shi, C.; Guo, X. Hollow zeolite encapsulated Ni–Pt bimetals for sintering and coking resistant dry reforming of methane. J. Mater. Chem. 2015, 3, 16461–16468. [Google Scholar] [CrossRef]
- Estephane, J.; Aouad, S.; Hany, S.; Khoury, B.E.; Gennequin, C.; Zakhem, H.E.; Nakat, J.E.; Aboukais, A.; Aad, E.A. CO2 reforming of methane over Ni–Co/ZSM5 catalysts. Aging and carbon deposition study. Int. J. Hydrogen Energy 2015, 40, 9201–9208. [Google Scholar] [CrossRef]
- Hambali, H.U.; Jalil, A.A.; Abdulrasheed, A.A.; Siang, T.J.; Vo, D.V.N. Enhanced dry reforming of methane over mesostructured fibrous Ni/MFI zeolite: Influence of preparation methods. J. Energy Inst. 2020, 93, 1535–1543. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Fatesh, A.S.A. Enhancing hydrogen production by dry reforming process with strontium promoter. Int. J. Hydrogen Energy 2014, 39, 1680–1687. [Google Scholar] [CrossRef]
- Fatesh, A.S.A.; Ibrahim, A.A.; Fakeeha, A.H.; Soliman, M.A.; Siddiqui, M.R.; Abasaeed, A.E. Coke formation during CO2 reforming of CH4 over alumina-supported nickel catalysts. Appl. Catal. A Gen. 2009, 364, 150–155. [Google Scholar] [CrossRef]
- Sarkar, B.; Tiwari, R.; Singha, R.K.; Suman, S.; Ghosh, S.; Acharyya, S.S.; Mantri, K.; Konathala, L.N.S.; Pendem, C.; Bal, R. Reforming of methane with CO2 over Ni nanoparticle supported on mesoporous ZSM-5. Catal. Today 2012, 198, 209–214. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Naeem, M.A.; Khan, W.U.; Fatesh, A.S.A. Syngas production via CO2 reforming of methane using Co-Sr-Al catalyst. J. Ind. Eng. Chem. 2014, 20, 549–557. [Google Scholar] [CrossRef]
- Naeem, M.A.; Fatesh, A.S.A.; Abasaeed, A.E.; Fakeeha, A.H. Activities of Ni-based nano catalysts for CO2–CH4 reforming prepared by polyol process. Fuel Process. Technol. 2014, 122, 141–152. [Google Scholar] [CrossRef]
- Fatesh, A.S.A.; Atia, H.; Dahrieh, J.K.A.; Ibrahim, A.A.; Eckelt, R.; Armbruster, U.; Abasaeed, A.E.; Fakeeha, A.H. Hydrogen production from CH4 dry reforming over Sc promoted Ni/MCM-41. Int. J. Hydrogen Energy 2019, 44, 20770–20781. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Sutthisripok, W.; Assabumrungrat, S. Synthesis gas production from dry reforming of methane over CeO2 doped Ni/Al2O3: Influence of the doping ceria on the resistance toward carbon formation. Chem. Eng. J. 2005, 112, 13–22. [Google Scholar] [CrossRef]
- de Sousa, F.F.D.; de Sousa, H.S.A.; Oliveira, A.C.; Junior, M.C.C.; Ayala, A.P.; Barros, E.B.; Viana, B.C.; Filho, J.M.; Oliveira, A.C. Nanostructured Ni-containing spinel oxides for the dry reforming of methane: Effect of the presence of cobalt and nickel on the deactivation behaviour of catalysts. Int. J. Hydrogen Energy 2012, 37, 3201–3212. [Google Scholar] [CrossRef]
- Moradi, G.; Khezeli, F.; Hemmati, H. Syngas production with dry reforming of methane over Ni/ZSM-5 catalysts. J. Nat. Gas Sci. Eng. 2016, 33, 657–665. [Google Scholar] [CrossRef]
- Najfach, A.J.; Almquist, C.B.; Edelmann, R.E. Effect of Manganese and zeolite composition on zeolite-supported Ni-catalysts for dry reforming of methane. Catal. Today 2021, 369, 41–47. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Atia, H.; Ibrahim, A.A.; Ha, Q.L.M.; Schneider, M.; M-Pohl, M.; Fakeeha, A.H. CO2-reforming of methane to produce syngas over Co-Ni/SBA-15 catalyst: Effect of support modifiers (Mg, La and Sc) on catalytic stability. J. CO2 Util. 2017, 21, 395–404. [Google Scholar] [CrossRef]






| Catalyst | % DF a | Coke (wt. %) b | SBET (m2/g) c | SBET (m2/g) d |
|---|---|---|---|---|
| NH | 2.0 | 3.7 | 335.3 | 318.4 |
| NY | 6.9 | 14.7 | 573.3 | 498.1 |
| NA | 2.9 | 8.5 | 209.7 | 171.7 |
| Catalyst | Reaction Temp. (°C) | Initial CH4 Conversion (%) | %DF | TOS (h) | Ref. |
|---|---|---|---|---|---|
| 7% Ni/ZSM-5 | 700 | 91 | 35.3 | 5 | [14] |
| 7% Ni/ Zeolite Y | 700 | 92 | 0.43 | 5 | |
| 1Co2Ni-ZSM5 | 800 | 66 | 13.6 | 12 | [28] |
| 5%Ni-ZSM | 800 | 96.2 | 24.2 | 9 | [32] |
| 2.25%Sr-10%Co/Al2O3 | 700 | 80.1 | 2.5 | 6 | [33] |
| Ni-Ce Imp | 700 | 81.1 | 3.7 | 6 | [34] |
| 5%Ni + 1%Sc/MCM41 | 800 | 65 | 19.2 | ~7 | [35] |
| NH | 700 | 78.5 | 2.0 | 9 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, W.U.; Khan, M.R.; Busquets, R.; Ahmad, N. Contribution of Oxide Supports in Nickel-Based Catalytic Elimination of Greenhouse Gases and Generation of Syngas. Energies 2021, 14, 7324. https://doi.org/10.3390/en14217324
Khan WU, Khan MR, Busquets R, Ahmad N. Contribution of Oxide Supports in Nickel-Based Catalytic Elimination of Greenhouse Gases and Generation of Syngas. Energies. 2021; 14(21):7324. https://doi.org/10.3390/en14217324
Chicago/Turabian StyleKhan, Wasim Ullah, Mohammad Rizwan Khan, Rosa Busquets, and Naushad Ahmad. 2021. "Contribution of Oxide Supports in Nickel-Based Catalytic Elimination of Greenhouse Gases and Generation of Syngas" Energies 14, no. 21: 7324. https://doi.org/10.3390/en14217324
APA StyleKhan, W. U., Khan, M. R., Busquets, R., & Ahmad, N. (2021). Contribution of Oxide Supports in Nickel-Based Catalytic Elimination of Greenhouse Gases and Generation of Syngas. Energies, 14(21), 7324. https://doi.org/10.3390/en14217324







