Metal-Air Batteries—A Review
Abstract
:1. Introduction
2. MABs’ Theoretical Considerations and Mechanisms
2.1. Cell Potential
2.2. Energy Efficiency
3. Electrochemical Performance of MABs
4. Anode of MABs
Progress Done in the Anode of MAB
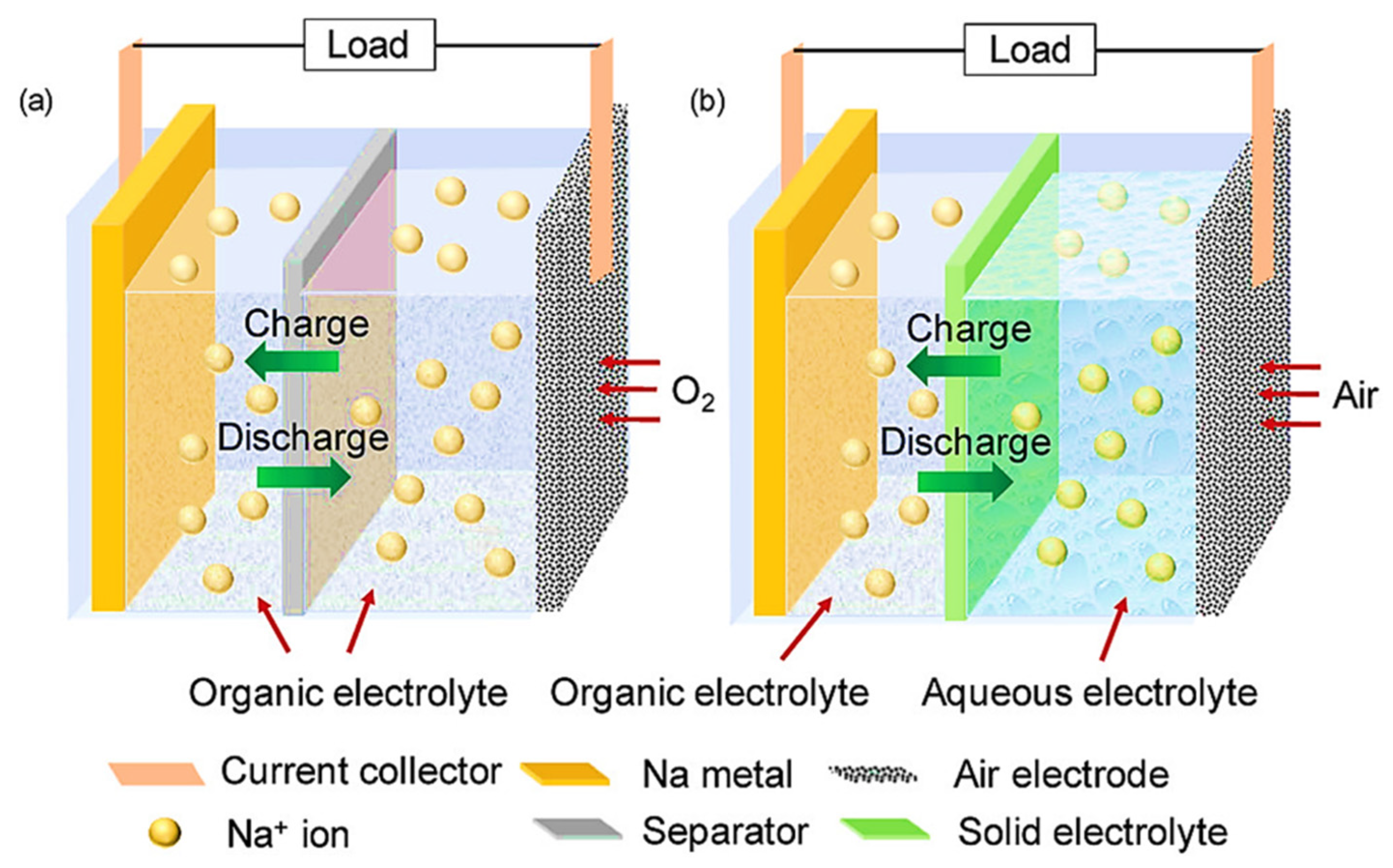
5. MABs Electrolyte
5.1. Aqueous Electrolyte
Electrolyte Additives
5.2. Non-Aqueous Electrolyte
5.3. Hybrid Electrolyte
Selecting the Electrolyte
6. Cathode of Metal-Air Batteries MABs
6.1. Air Cathode Components
6.2. Oxygen Electrochemical Reactions in MABs
6.3. Progress Done in the Cathode of MAB
7. Progress in the Design of the MABs
7.1. Classifications Cell Structure
7.1.1. Static MABs
Parallel-Plate Electrode Configuration
Coin-Type
In Situ Cell
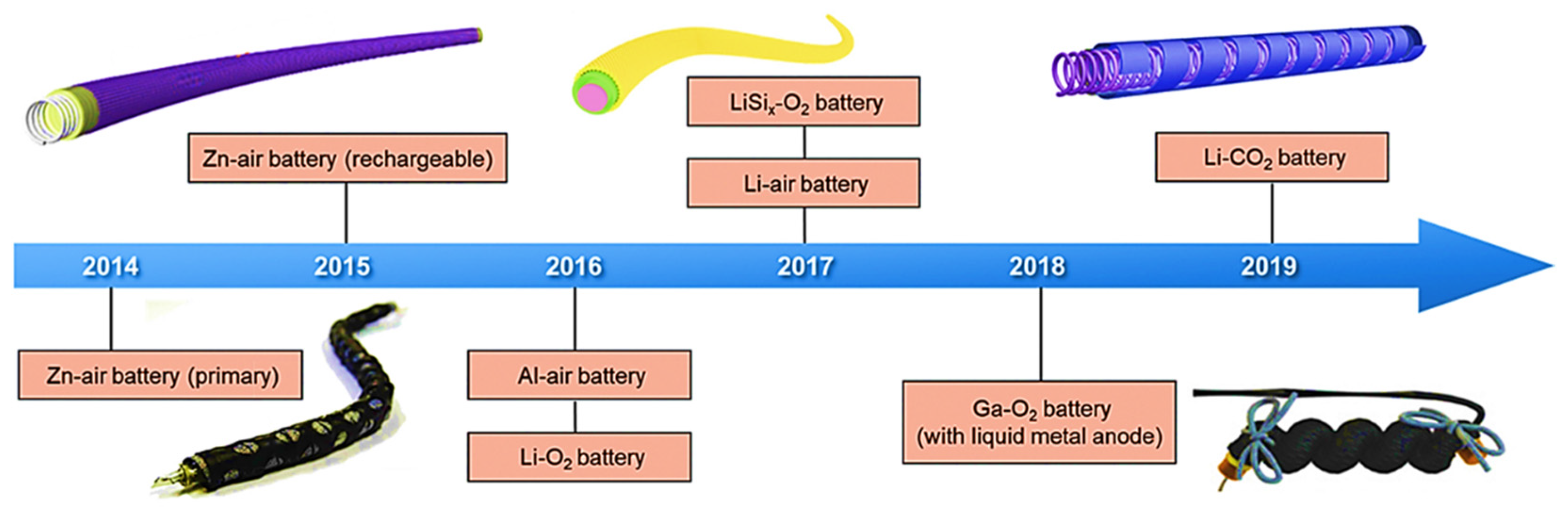
7.1.2. Flexible MABs
Fiber-Type
Sandwich-Type
Array
7.1.3. Flow MABs (MAFBs)
Anolyte Circulation
Electrolyte Cycling
Hybrid Electrolyte Flow Battery
Bidirectional Flow Battery with a Two-Layered Cathode
8. Large-Scale Metal-Air Batteries
9. Applications of MABs
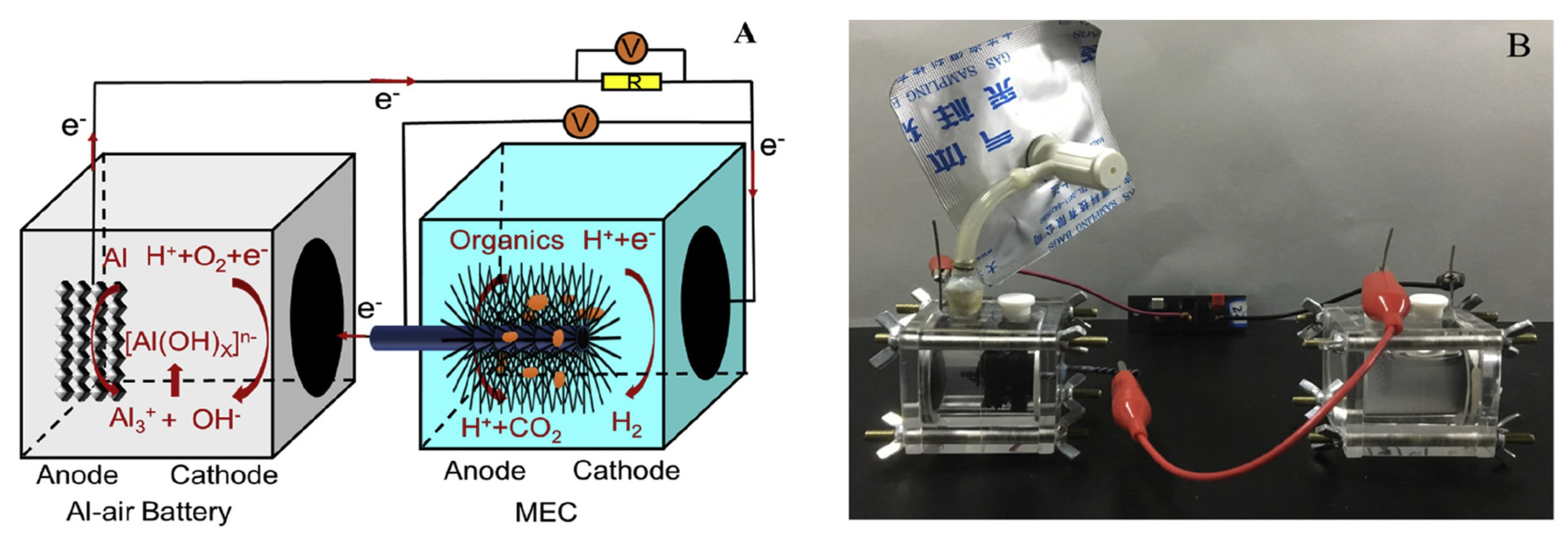
9.1. Wastewater Treatment Using MABs
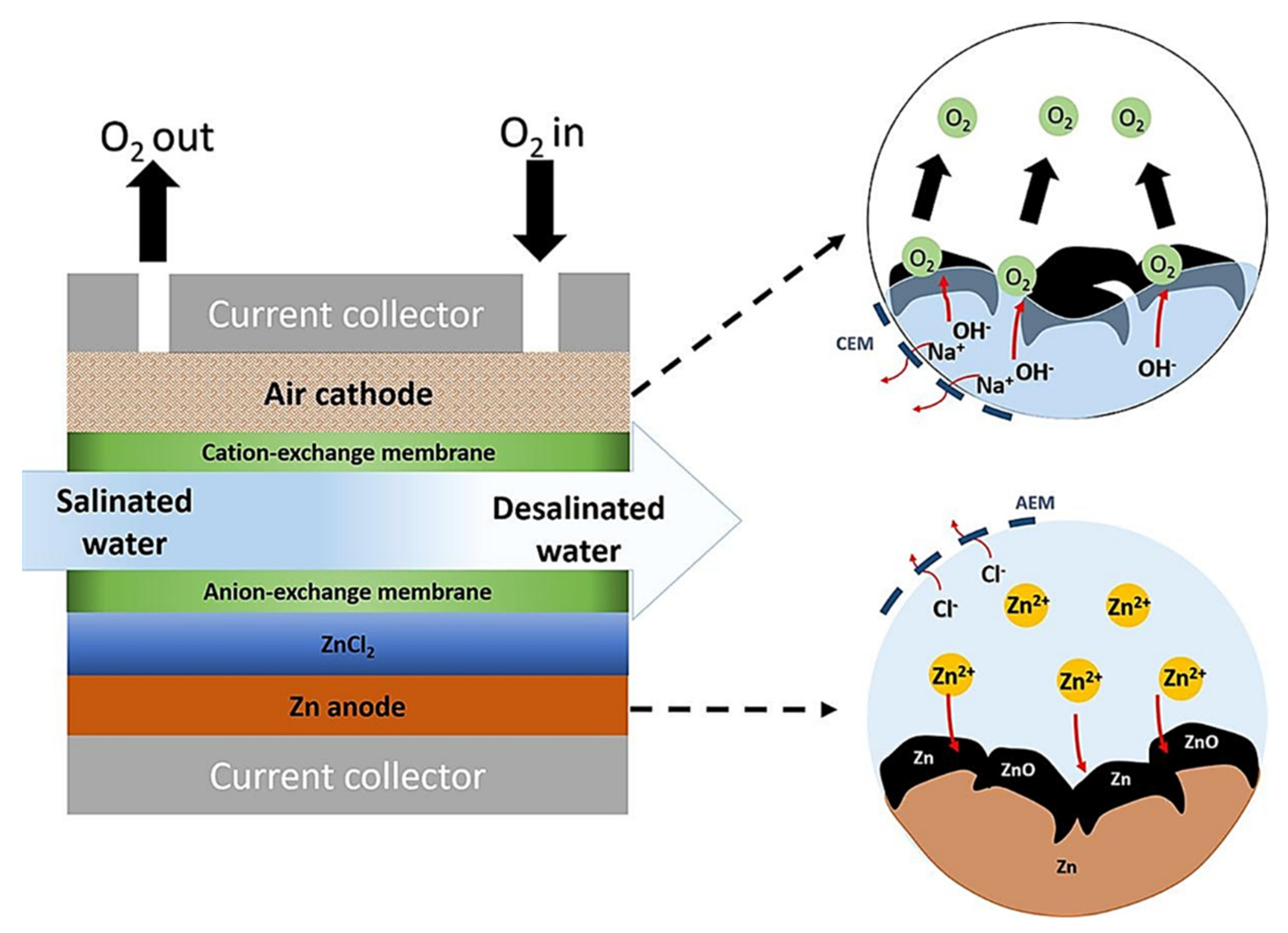
9.2. Water Desalination Using MABs
10. Challenges of MABs
10.1. Metallic Anode Challenges
10.2. Electrolyte Challenges
10.3. Cathode Challenges
11. Conclusions
- Various metals can be used as an anode of MABs. Each metal has its own advantages and disadvantages. Al is one of the promising electrodes due to its high energy density, low weight, good recyclability, environmental friendliness, and low cost.
- Despite the high ionic conductivity of the aqueous electrolytes, the leakage, stability, and thermodynamic limitations are challenges facing their application. Electrolyte additives or non-aqueous electrolytes can minimize or solve these problems and thus increase the durability and energy density of MABs.
- A thermally and mechanically porous cathode electrode that can perform ORR and OER effectively is essential for commercial MABs.
- Both the Al-air batteries and iron–air batteries are good candidates for the large-scale production of MABs
- Flow MABs are safe and have a long operational life because of the flow of anolyte/electrolyte, which minimizes side reactions. Flow MABs can be used for large-scale energy storage as well as stationary power plants.
- A few studies conducted on MABs in water desalination and wastewater treatment have shown promising results. However, more studies are still required.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, R. Focus: Business Models in Energy Storage. Available online: https://www.rolandberger.com/publications/publication_pdf/roland_berger_energy_storage_final.pdf. (accessed on 12 August 2021).
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, N.; Sun, C.; Luo, X. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery. Int. J. Energy Res. 2020, 44, 3839–3853. [Google Scholar] [CrossRef]
- Zablocki, A. Fact Sheet | Energy Storage. Available online: https://www.eesi.org/papers/view/energy-storage-2019 (accessed on 1 October 2021).
- Olabi, A.G.; Onumaegbu, C.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Al–Alami, A.H. Critical review of energy storage systems. Energy 2021, 214, 118987. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, H.; Liang, F.; Ma, W.; Lei, Y. Nanostructured arrays for metal–ion battery and metal–air battery applications. J. Power Sources 2021, 493, 229722. [Google Scholar] [CrossRef]
- Chen, X.; Ali, I.; Song, L.; Song, P.; Zhang, Y.; Maria, S.; Nazmus, S.; Yang, W.; Dhakal, H.N.; Li, H. A review on recent advancement of nano-structured-fiber-based metal-air batteries and future perspective. Renew. Sustain. Energy Rev. 2020, 134, 110085. [Google Scholar] [CrossRef]
- Kumar, P.; Goyal, S.K.; Singh, B.P. Application of bifunctional catalysts and metal organic frameworks in metal air batteries for renewable power conversion applications. Mater. Today Proc. 2021, 43, 2839–2842. [Google Scholar] [CrossRef]
- Cai, W.; Deng, J.; Lu, H.; Cao, Y. Performance of metal borides as anode in metal boride-air battery. Mater. Chem. Phys. 2020, 251, 123101. [Google Scholar] [CrossRef]
- Mladenova, E.; Slavova, M.; Mihaylova-Dimitrova, E.; Burdin, B.; Abrashev, B.; Krapchanska, M.; Raikova, G.; Vladikova, D. Monolithic carbon-free gas diffusion electrodes for secondary metal-air batteries. J. Electroanal. Chem. 2021, 887, 115112. [Google Scholar] [CrossRef]
- Gauthier, M.; Nguyen, M.H.; Blondeau, L.; Foy, E.; Wong, A. Operando NMR characterization of a metal-air battery using a double-compartment cell design. Solid State Nucl. Magn. Reson. 2021, 113, 101731. [Google Scholar] [CrossRef]
- Bo, W.; Ahmad, Z.; Alanzi, A.R.A.; Al-Omari, A.I.; Hafez, E.H.; Abdelwahab, S.F. The Current COVID-19 Pandemic in China: An Overview and Corona Data Analysis. Alex. Eng. J. 2021. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous metal-air batteries: Fundamentals and applications. Energy Storage Mater. 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Nagy, T.; Nagy, L.; Erdélyi, Z.; Baradács, E.; Deák, G.; Zsuga, M.; Kéki, S. Environmentally friendly Zn-air rechargeable battery with heavy metal free charcoal based air cathode. Electrochim. Acta 2021, 368, 137592. [Google Scholar] [CrossRef]
- Marini, E.; Jörissen, L.; Brimaud, S. Rational design of a low-cost, durable and efficient bifunctional oxygen electrode for rechargeable metal-air batteries. J. Power Sources 2021, 482, 228900. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent progress of metal–air batteries—A mini review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef] [Green Version]
- Hannan, M.A.; Lipu, M.S.H.; Hussain, A.; Mohamed, A. A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: Challenges and recommendations. Renew. Sustain. Energy Rev. 2017, 78, 834–854. [Google Scholar] [CrossRef]
- Li, J.; Du, Z.; Ruther, R.E.; An, S.J.; David, L.A.; Hays, K.; Wood, M.; Phillip, N.D.; Sheng, Y.; Mao, C. Toward low-cost, high-energy density, and high-power density lithium-ion batteries. Jom 2017, 69, 1484–1496. [Google Scholar] [CrossRef] [Green Version]
- Clemente, A.; Costa-Castelló, R. Redox flow batteries: A literature review oriented to automatic control. Energies 2020, 13, 4514. [Google Scholar] [CrossRef]
- Lopes, P.P.; Stamenkovic, V.R. Past, present, and future of lead–acid batteries. Science 2020, 369, 923–924. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Huang, S.; Samarakoon, W.; Xi, S.; Ji, Y.; Zhang, H.; Zhang, F.; Du, Y.; Feng, Z. Redox targeting-based vanadium redox-flow battery. ACS Energy Lett. 2019, 4, 3028–3035. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wei, L.; Wu, M.C.; Bai, B.F.; Zhao, T.S. Chloride ions as an electrolyte additive for high performance vanadium redox flow batteries. Appl. Energy 2021, 289, 116690. [Google Scholar] [CrossRef]
- Clark, S.; Latz, A.; Horstmann, B. A review of model-based design tools for metal-air batteries. Batteries 2018, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Gröger, O.; Gasteiger, H.A.; Suchsland, J.-P. Erratum: Review—Electromobility: Batteries or Fuel Cells? [J. Electrochem. Soc., 162, A2605 (2015)]. J. Electrochem. Soc. 2016, 163, X3. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. High energy density metal-air batteries: A review. J. Electrochem. Soc. 2013, 160, A1759. [Google Scholar] [CrossRef]
- Zhang, T.; Tao, Z.; Chen, J. Magnesium–air batteries: From principle to application. Mater. Horiz. 2014, 1, 196–206. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Li, H.-B.; Yoo, H.D.; Chi, X.; An, Q.; Liu, J.; Yu, M.; Wang, W.; Yao, Y. Mixed-phase mullite electrocatalyst for pH-neutral oxygen reduction in magnesium-air batteries. Nano Energy 2016, 27, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Li, C.S.; Sun, Y.; Gebert, F.; Chou, S.L. Current progress on rechargeable magnesium–air battery. Adv. Energy Mater. 2017, 7, 1700869. [Google Scholar] [CrossRef]
- Zhang, Z.; Zuo, C.; Liu, Z.; Yu, Y.; Zuo, Y.; Song, Y. All-solid-state Al–air batteries with polymer alkaline gel electrolyte. J. Power Sources 2014, 251, 470–475. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xue, J.; Zhu, C.; Li, X.; Wang, Z. High energy efficiency of Al-based anodes for Al-air battery by simultaneous addition of Mn and Sb. Chem. Eng. J. 2021, 417, 128006. [Google Scholar] [CrossRef]
- Mokhtar, M.; Talib, M.Z.M.; Majlan, E.H.; Tasirin, S.M.; Ramli, W.M.F.W.; Daud, W.R.W.; Sahari, J. Recent developments in materials for aluminum–air batteries: A review. J. Ind. Eng. Chem. 2015, 32, 1–20. [Google Scholar] [CrossRef]
- Bi, X.; Wang, R.; Yuan, Y.; Zhang, D.; Zhang, T.; Ma, L.; Lu, J. From sodium–oxygen to sodium–air battery: Enabled by sodium peroxide dihydrate. Nano Lett. 2020, 20, 4681–4686. [Google Scholar] [CrossRef]
- Sahgong, S.H.; Senthilkumar, S.T.; Kim, K.; Hwang, S.M.; Kim, Y. Rechargeable aqueous Na–air batteries: Highly improved voltage efficiency by use of catalysts. Electrochem. Commun. 2015, 61, 53–56. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef]
- Bansal, R.; Menon, P.; Sharma, R.C. Silicon–air batteries: Progress, applications and challenges. SN Appl. Sci. 2020, 2, 1–17. [Google Scholar] [CrossRef]
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y. Rechargeable Zn-air batteries: Recent trends and future perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium− air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Blurton, K.F.; Sammells, A.F. Metal/air batteries: Their status and potential—A review. J. Power Sources 1979, 4, 263–279. [Google Scholar] [CrossRef]
- Abraham, K.; Jiang, Z. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 1996, 143, 1. [Google Scholar] [CrossRef]
- McCloskey, B.; Speidel, A.; Scheffler, R.; Miller, D.; Viswanathan, V.; Hummelshøj, J.; Nørskov, J.; Luntz, A. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 2012, 3, 997–1001. [Google Scholar] [CrossRef]
- Monroe, C.W. Does oxygen transport affect the cell voltages of metal/air batteries? J. Electrochem. Soc. 2017, 164, E3547. [Google Scholar] [CrossRef]
- Horstmann, B.; Danner, T.; Bessler, W.G. Precipitation in aqueous lithium–oxygen batteries: A model-based analysis. Energy Environ. Sci. 2013, 6, 1299–1314. [Google Scholar] [CrossRef] [Green Version]
- Danner, T.; Horstmann, B.; Wittmaier, D.; Wagner, N.; Bessler, W.G. Reaction and transport in Ag/Ag2O gas diffusion electrodes of aqueous Li–O2 batteries: Experiments and modeling. J. Power Sources 2014, 264, 320–332. [Google Scholar] [CrossRef]
- Vegge, T.; Garcia-Lastra, J.M.; Siegel, D.J. Lithium–oxygen batteries: At a crossroads? Curr. Opin. Electrochem. 2017, 6, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Zheng, G.; Goh, F.T.; Hor, T.A.; Zong, Y.; Liu, Z. Durable rechargeable zinc-air batteries with neutral electrolyte and manganese oxide catalyst. J. Power Sources 2016, 332, 330–336. [Google Scholar] [CrossRef]
- Friesen, C.A.; Krishnan, R.; Friesen, G. Rechargeable Electrochemical Cell System with a Charging Electrode Charge/Discharge Mode Switching in the Cells. U.S. Patent Application No. 12/885,268, 24 March 2011. [Google Scholar]
- Liu, Q.; Chang, Z.; Li, Z.; Zhang, X. Flexible metal–air batteries: Progress, challenges, and perspectives. Small Methods 2018, 2, 1700231. [Google Scholar] [CrossRef]
- Zhang, X.-b. Metal-Air Batteries: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Durmus, Y.E.; Zhang, H.; Baakes, F.; Desmaizieres, G.; Hayun, H.; Yang, L.; Kolek, M.; Küpers, V.; Janek, J.; Mandler, D.; et al. Side by Side Battery Technologies with Lithium-Ion Based Batteries. Adv. Energy Mater. 2020, 10, 2000089. [Google Scholar] [CrossRef]
- McKerracher, R.; de Ponce Leon, C.; Wills, R.; Shah, A.; Walsh, F.C. A review of the iron–air secondary battery for energy storage. ChemPlusChem 2015, 80, 323–335. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [Green Version]
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Komilis, D.; Evangelou, A.; Giannakis, G.; Lymperis, C. Revisiting the elemental composition and the calorific value of the organic fraction of municipal solid wastes. Waste Manag. 2012, 32, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Gesser, H. Electrochemistry, Batteries and Fuel Cells. In Applied Chemistry; Springer: Berlin/Heidelberg, Germany, 2002; pp. 159–190. [Google Scholar]
- Yoshio, M.; Brodd, R.J.; Kozawa, A. Lithium-Ion Batteries; Springer: New York, NY, USA, 2009; Volume 1, pp. 2–3. [Google Scholar]
- Kang, J.; Yan, F.; Zhang, P.; Du, C. A novel way to calculate energy efficiency for rechargeable batteries. J. Power Sources 2012, 206, 310–314. [Google Scholar] [CrossRef]
- Weinrich, H.; Durmus, Y.E.; Tempel, H.; Kungl, H.; Eichel, R.-A. Silicon and iron as resource-efficient anode materials for ambient-temperature metal-air batteries: A review. Materials 2019, 12, 2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Z.; Parveen, N.; Ansari, S.A.; Senthilkumar, S.; Park, S.; Kim, Y.; Cho, M.H.; Ko, H. Three-dimensional SnS2 nanopetals for hybrid sodium-air batteries. Electrochim. Acta 2017, 257, 328–334. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Yang, X.; Liang, J.; Wang, B.; Koo, A.; Li, R.; Li, J.; Sun, X. High-performance and recyclable Al-air coin cells based on eco-friendly chitosan hydrogel membranes. ACS Appl. Mater. Interfaces 2018, 10, 19730–19738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, G.; Zhang, C.; Peng, C.; Pan, F. Effect of phosphate and vanadate as electrolyte additives on the performance of Mg-air batteries. Mater. Chem. Phys. 2018, 218, 256–261. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, J.; Wang, F.; Wang, L.; Liao, Z.; Zschech, E.; Müllen, K.; Feng, X. Cobalt-Based Metal–Organic Framework Nanoarrays as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn-Air Batteries. Chem. A Eur. J. 2018, 24, 18413–18418. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wang, J.; Lei, W.; Xuan, C.; Xiao, W.; Zhao, T.; Huang, T.; Chen, L.; Zhu, Y.; Wang, D. Restricting growth of Ni3Fe nanoparticles on heteroatom-doped carbon nanotube/graphene nanosheets as air-electrode electrocatalyst for Zn–air battery. ACS Appl. Mater. Interfaces 2018, 10, 38093–38100. [Google Scholar] [CrossRef]
- Figueredo-Rodríguez, H.; McKerracher, R.; Insausti, M.; Luis, A.G.; de Leόn, C.P.; Alegre, C.; Baglio, V.; Aricò, A.; Walsh, F. A rechargeable, aqueous iron air battery with nanostructured electrodes capable of high energy density operation. J. Electrochem. Soc. 2017, 164, A1148. [Google Scholar] [CrossRef]
- Sumathi, S.; Sethuprakash, V.; Basirun, W.; Zainol, I.; Sookhakian, M. Polyacrylamide-methanesulfonic acid gel polymer electrolytes for tin-air battery. J. Sol-Gel Sci. Technol. 2014, 69, 480–487. [Google Scholar] [CrossRef]
- Najam, T.; Shah, S.S.A.; Ding, W.; Deng, J.; Wei, Z. Enhancing by nano-engineering: Hierarchical architectures as oxygen reduction/evolution reactions for zinc-air batteries. J. Power Sources 2019, 438, 226919. [Google Scholar] [CrossRef]
- Egan, D.R.; Ponce de León, C.; Wood, R.J.K.; Jones, R.L.; Stokes, K.R.; Walsh, F.C. Developments in electrode materials and electrolytes for aluminium–air batteries. J. Power Sources 2013, 236, 293–310. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, R.N.; Ateş, S.; Yazıcı, B. Al-6013-T6 and Al-7075-T7351 alloy anodes for aluminium-air battery. Int. J. Hydrogen Energy 2017, 42, 23315–23325. [Google Scholar] [CrossRef]
- Chawla, N. Recent advances in air-battery chemistries. Mater. Today Chem. 2019, 12, 324–331. [Google Scholar] [CrossRef]
- Melhem, Z. Electricity Transmission, Distribution and Storage Systems; Woodhead Publishing Series in Energy: Number 38; Woodhead Publishing: Shaxton, UK, 2013. [Google Scholar]
- Imanishi, N.; Yamamoto, O. Perspectives and challenges of rechargeable lithium–air batteries. Mater. Today Adv. 2019, 4, 100031. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. A review of high energy density lithium–air battery technology. J. Appl. Electrochem. 2014, 44, 5–22. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Z.; Wang, D.; Wang, M.; Peng, Z.; Liu, Z.; He, H.; Wang, M.; Li, H. High-performance Li-air battery after limiting inter-electrode crosstalk. Energy Storage Mater. 2021, 39, 225–231. [Google Scholar] [CrossRef]
- Otaegui, L.; Rodriguez-Martinez, L.M.; Wang, L.; Laresgoiti, A.; Tsukamoto, H.; Han, M.H.; Tsai, C.L.; Laresgoiti, I.; López, C.M.; Rojo, T. Performance and stability of a liquid anode high-temperature metal–air battery. J. Power Sources 2014, 247, 749–755. [Google Scholar] [CrossRef]
- Wang, H.-F.; Xu, Q. Materials design for rechargeable metal-air batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Jung, K.-N.; Kim, J.; Yamauchi, Y.; Park, M.-S.; Lee, J.-W.; Kim, J.H. Rechargeable lithium–air batteries: A perspective on the development of oxygen electrodes. J. Mater. Chem. A 2016, 4, 14050–14068. [Google Scholar] [CrossRef]
- Christensen, J.; Albertus, P.; Sanchez-Carrera, R.S.; Lohmann, T.; Kozinsky, B.; Liedtke, R.; Ahmed, J.; Kojic, A. A critical review of Li/air batteries. J. Electrochem. Soc. 2011, 159, R1. [Google Scholar] [CrossRef]
- Gelman, D.; Shvartsev, B.; Ein-Eli, Y. Aluminum–air battery based on an ionic liquid electrolyte. J. Mater. Chem. A 2014, 2, 20237–20242. [Google Scholar] [CrossRef]
- Cohn, G.; Ein-Eli, Y. Study and development of non-aqueous silicon-air battery. J. Power Sources 2010, 195, 4963–4970. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Aslanbas, Ö.; Kayser, S.; Tempel, H.; Hausen, F.; De Haart, L.; Granwehr, J.; Ein-Eli, Y.; Eichel, R.-A.; Kungl, H. Long run discharge, performance and efficiency of primary Silicon–air cells with alkaline electrolyte. Electrochim. Acta 2017, 225, 215–224. [Google Scholar] [CrossRef]
- Reinsberg, P.; Bondue, C.J.; Baltruschat, H. Calcium–Oxygen Batteries as a Promising Alternative to Sodium–Oxygen Batteries. J. Phys. Chem. C 2016, 120, 22179–22185. [Google Scholar] [CrossRef]
- Shiga, T.; Hase, Y.; Kato, Y.; Inoue, M.; Takechi, K. A rechargeable non-aqueous Mg–O2 battery. Chem. Commun. 2013, 49, 9152–9154. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Lau, S.; Archer, L.A. Sodium–oxygen batteries: A new class of metal–air batteries. J. Mater. Chem. A 2014, 2, 12623–12629. [Google Scholar] [CrossRef]
- Narayanan, S.R.; Prakash, G.K.S.; Manohar, A.; Yang, B.; Malkhandi, S.; Kindler, A. Materials challenges and technical approaches for realizing inexpensive and robust iron–air batteries for large-scale energy storage. Solid State Ion. 2012, 216, 105–109. [Google Scholar] [CrossRef]
- Ren, X.; Wu, Y. A low-overpotential potassium–oxygen battery based on potassium superoxide. J. Am. Chem. Soc. 2013, 135, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Dincer, I. Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Wei, W.; Xu, Y.; Huang, J.; Zhu, J. Review of the application of metal-air battery principle in water treatment. In Proceedings of the 2019 International Conference on Building Energy Conservation, Thermal Safety and Environmental Pollution Control, Hefei, China, 1–3 November 2019; p. 06035. [Google Scholar]
- Han, X.; Qu, Y.; Li, D.; Dong, Y.; Chen, D.; Yu, Y.; Ren, N.; Feng, Y. Combined microbial electrolysis cell–iron-air battery system for hydrogen production and swine wastewater treatment. Process Biochem. 2021, 101, 104–110. [Google Scholar] [CrossRef]
- Han, X.; Qu, Y.; Dong, Y.; Zhao, J.; Jia, L.; Yu, Y.; Zhang, P.; Li, D.; Ren, N.; Feng, Y. Microbial electrolysis cell powered by an aluminum-air battery for hydrogen generation, in-situ coagulant production and wastewater treatment. Int. J. Hydrog. Energy 2018, 43, 7764–7772. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Palacin, M.R.; Croguennec, L. Rechargeable aqueous electrolyte batteries: From univalent to multivalent cation chemistry. J. Mater. Chem. A 2019, 7, 20519–20539. [Google Scholar] [CrossRef]
- Mori, R. Recent developments for aluminum–air batteries. Electrochem. Energy Rev. 2020, 3, 344–369. [Google Scholar] [CrossRef]
- Kar, M.; Simons, T.J.; Forsyth, M.; MacFarlane, D.R. Ionic liquid electrolytes as a platform for rechargeable metal–air batteries: A perspective. Phys. Chem. Chem. Phys. 2014, 16, 18658–18674. [Google Scholar] [CrossRef]
- Khan, Z.; Vagin, M.; Crispin, X. Can hybrid Na–air batteries outperform nonaqueous Na–O2 batteries? Adv. Sci. 2020, 7, 1902866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Manthiram, A. A voltage-enhanced, low-cost aqueous iron–air battery enabled with a mediator-ion solid electrolyte. ACS Energy Lett. 2017, 2, 1050–1055. [Google Scholar] [CrossRef]
- No More Trial-and Error When Choosing an Electrolyte for Metal-Air Batteries. Available online: https://phys.org/news/2019-07-trial-and-error-electrolyte-metal-air-batteries.html (accessed on 7 May 2021).
- Sankarasubramanian, S.; Kahky, J.; Ramani, V. Tuning anion solvation energetics enhances potassium–oxygen battery performance. Proc. Natl. Acad. Sci. USA 2019, 116, 14899–14904. [Google Scholar] [CrossRef] [Green Version]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; De Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Zha, Z.; Shen, C.; Wang, D.; Han, W. Review on air cathode in Li-air batteries. J. Technol. Innov. Renew. Energy 2013, 2, 293–305. [Google Scholar]
- Martin, J.; Neburchilov, V.; Wang, H.; Qu, W. Air Cathodes for Metal-air Batteries and Fuel Cells. In Proceedings of the 2009 IEEE Electrical Power & Energy Conference (EPEC), Montreal, QC, Canada, 22–23 October 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 1–6. [Google Scholar]
- Cai, X.; Lai, L.; Lin, J.; Shen, Z. Recent advances in air electrodes for Zn–air batteries: Electrocatalysis and structural design. Mater. Horiz. 2017, 4, 945–976. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, S.U. Theoretical basis of electrocatalysis. In Electrocatalysts for Fuel Cells and Hydrogen Evolution-Theory to Design; IntechOpen: London, UK, 2018; p. 13. [Google Scholar]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Sayed, E.T.; Mohamed, H.O.; Obaid, M.; Rezk, H.; Chae, K.-J. Nonprecious anodic catalysts for low-molecular-hydrocarbon fuel cells: Theoretical consideration and current progress. Prog. Energy Combust. Sci. 2020, 77, 100805. [Google Scholar] [CrossRef]
- Al-Dhaifallah, M.; Abdelkareem, M.A.; Rezk, H.; Alhumade, H.; Nassef, A.M.; Olabi, A.G. Co-decorated reduced graphene/titanium nitride composite as an active oxygen reduction reaction catalyst with superior stability. Int. J. Energy Res. 2021, 45, 1587–1598. [Google Scholar] [CrossRef]
- Wang, M.; Fang, Z.; Zhang, K.; Fang, J.; Qin, F.; Zhang, Z.; Li, J.; Liu, Y.; Lai, Y. Synergistically enhanced activity of graphene quantum dots/graphene hydrogel composites: A novel all-carbon hybrid electrocatalyst for metal/air batteries. Nanoscale 2016, 8, 11398–11402. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-N.; Hwang, S.M.; Park, M.-S.; Kim, K.J.; Kim, J.-G.; Dou, S.X.; Kim, J.H.; Lee, J.-W. One-dimensional manganese-cobalt oxide nanofibres as bi-functional cathode catalysts for rechargeable metal-air batteries. Sci. Rep. 2015, 5, 7665. [Google Scholar] [CrossRef]
- Wang, M.; Lai, Y.; Fang, J.; Qin, F.; Zhang, Z.; Li, J.; Zhang, K. Hydrangea-like NiCo2S4 hollow microspheres as an advanced bifunctional electrocatalyst for aqueous metal/air batteries. Catal. Sci. Technol. 2016, 6, 434–437. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, X.; Wang, G.; Jin, Y.; Du, X.; Du, W.; Sun, L.; Ji, C. PdCo bimetallic nano-electrocatalyst as effective air-cathode for aqueous metal-air batteries. Int. J. Hydrogen Energy 2018, 43, 5001–5011. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Q.A.; Yan, W.; Shao, Q.; Zhang, J. Design and fabrication of non-noble metal catalyst-based air-cathodes for metal-air battery. Can. J. Chem. Eng. 2019, 97, 2984–2993. [Google Scholar] [CrossRef]
- Tan, P.; Shyy, W.; Zhao, T.S.; Wei, Z.H.; An, L. Discharge product morphology versus operating temperature in non-aqueous lithium-air batteries. J. Power Sources 2015, 278, 133–140. [Google Scholar] [CrossRef]
- Gilligan, G.; Qu, D. Zinc-air and other types of metal-air batteries. In Advances in Batteries for Medium and Large-Scale Energy Storage; Elsevier: Amsterdam, The Netherlands, 2015; pp. 441–461. [Google Scholar]
- Han, X.; Li, X.; White, J.; Zhong, C.; Deng, Y.; Hu, W.; Ma, T. Metal–air batteries: From static to flow system. Adv. Energy Mater. 2018, 8, 1801396. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Bai, Z.; Jiang, G.; Li, M.; Feng, K.; Yang, L.; Ding, Y.; Yu, T.; Chen, Z. Controllable urchin-like NiCo2S4 microsphere synergized with sulfur-doped graphene as bifunctional catalyst for superior rechargeable Zn–air battery. Adv. Funct. Mater. 2018, 28, 1706675. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Chung, H.-J.; Ivey, D.G. A study of alkaline gel polymer electrolytes for rechargeable zinc–air batteries. Electrochim. Acta 2019, 327, 135021. [Google Scholar] [CrossRef]
- Jacas Biendicho, J.; Noréus, D.; Offer, C.; Svensson, G.; Smith, R.I.; Hull, S. New opportunities for air cathode batteries; in-situ neutron diffraction measurements. Front. Energy Res. 2018, 6, 69. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced architectures and relatives of air electrodes in Zn–air batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Hong, Y.; Liao, M.; Wang, B.; Wei, D.; Peng, H.; Ye, L.; Hong, Y.; Liao, M.; Wang, B.; et al. Recent advances in flexible fiber-shaped metal-air batteries. Energy Storage Mater. 2020, 28, 364–374. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Z.; An, X.; Yue, X.; Xiaokaiti, P.; Yoshida, A.; Abudula, A.; Guan, G. A sandwich-type composite polymer electrolyte for all-solid-state lithium metal batteries with high areal capacity and cycling stability. J. Membr. Sci. 2020, 596, 117739. [Google Scholar] [CrossRef]
- Qu, S.; Song, Z.; Liu, J.; Li, Y.; Kou, Y.; Ma, C.; Han, X.; Deng, Y.; Zhao, N.; Hu, W.; et al. Electrochemical approach to prepare integrated air electrodes for highly stretchable zinc-air battery array with tunable output voltage and current for wearable electronics. Nano Energy 2017, 39, 101–110. [Google Scholar] [CrossRef]
- Teabnamang, P.; Kao-ian, W.; Nguyen, M.T.; Yonezawa, T.; Cheacharoen, R.; Kheawhom, S. High-Capacity Dual-Electrolyte Aluminum–Air Battery with Circulating Methanol Anolyte. Energies 2020, 13, 2275. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, D.; Shi, L.; Xiang, Z. Efficient unitary oxygen electrode for air-based flow batteries. Nano Energy 2018, 47, 361–367. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Pichler, B.; Berner, B.S.; Rauch, N.; Zelger, C.; Pauling, H.-J.; Gollas, B.; Hacker, V. The impact of operating conditions on component and electrode development for zinc-air flow batteries. J. Appl. Electrochem. 2018, 48, 1043–1056. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tan, Y.; Luo, X.D.; Sun, C.Y.; Chen, N. Polarization Effects of a Rayon and Polyacrylonitrile Based Graphite Felt for Iron-Chromium Redox Flow Batteries. ChemElectroChem 2019, 6, 3175–3188. [Google Scholar] [CrossRef]
- grosse Austing, J.; Nunes Kirchner, C.; Hammer, E.-M.; Komsiyska, L.; Wittstock, G. Study of an unitised bidirectional vanadium/air redox flow battery comprising a two-layered cathode. J. Power Sources 2015, 273, 1163–1170. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Abdelkareem, M.A.; Lootah, M.A.; Sayed, E.T.; Wilberforce, T.; Alawadhi, H.; Yousef, B.A.A.; Olabi, A.G. Fuel cells for carbon capture applications. Sci. Total Environ. 2021, 769, 144243. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.T.; Abdelkareem, M.A.; Alawadhi, H.; Elsaid, K.; Wilberforce, T.; Olabi, A.G. Graphitic carbon nitride/carbon brush composite as a novel anode for yeast-based microbial fuel cells. Energy 2021, 221, 119849. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Gittleman, C.S.; Jia, H.; De Castro, E.S.; Chisholm, C.R.; Kim, Y.S. Proton conductors for heavy-duty vehicle fuel cells. Joule 2021, 5, 1660–1677. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspects of fuel cells: A review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef] [PubMed]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Maghrabie, H.M.; Abdelkareem, M.A. A review on Zero Energy Buildings–Pros and Cons. Energy Built Environ. 2021. [Google Scholar] [CrossRef]
- Maghrabie, H.M.; Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A.; Wilberforce, T.; Olabi, A.G. Building-integrated photovoltaic/thermal (BIPVT) systems: Applications and challenges. Sustain. Energy Technol. Assess. 2021, 45, 101151. [Google Scholar]
- Elavarasan, R.M.; Shafiullah, G.; Padmanaban, S.; Kumar, N.M.; Annam, A.; Vetrichelvan, A.M.; Mihet-Popa, L.; Holm-Nielsen, J.B. A comprehensive review on renewable energy development, challenges, and policies of leading Indian states with an international perspective. IEEE Access 2020, 8, 74432–74457. [Google Scholar] [CrossRef]
- Lu, Y.; Khan, Z.A.; Alvarez-Alvarado, M.S.; Zhang, Y.; Huang, Z.; Imran, M. A critical review of sustainable energy policies for the promotion of renewable energy sources. Sustainability 2020, 12, 5078. [Google Scholar] [CrossRef]
- Adebayo, T.S.; Kirikkaleli, D. Impact of renewable energy consumption, globalization, and technological innovation on environmental degradation in Japan: Application of wavelet tools. Environ. Dev. Sustain. 2021, 23, 16057–16082. [Google Scholar] [CrossRef]
- Rabaia, M.K.H.; Abdelkareem, M.A.; Sayed, E.T.; Elsaid, K.; Chae, K.-J.; Wilberforce, T.; Olabi, A.G. Environmental impacts of solar energy systems: A review. Sci. Total Environ. 2021, 754, 141989. [Google Scholar] [CrossRef] [PubMed]
- Sebestyén, V. Renewable and Sustainable Energy Reviews: Environmental impact networks of renewable energy power plants. Renew. Sustain. Energy Rev. 2021, 151, 111626. [Google Scholar] [CrossRef]
- Mohsin, M.; Kamran, H.W.; Nawaz, M.A.; Hussain, M.S.; Dahri, A.S. Assessing the impact of transition from nonrenewable to renewable energy consumption on economic growth-environmental nexus from developing Asian economies. J. Environ. Manag. 2021, 284, 111999. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. Thermal Energy Storage Systems and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Lai, C.S.; Locatelli, G. Economic and financial appraisal of novel large-scale energy storage technologies. Energy 2021, 214, 118954. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Salameh, T.; Abdelkareem, M.A.; Baroutaji, A. A Review on Failure Modes of Wind Turbine Components. Energies 2021, 14, 5241. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Salameh, T.; Sayed, E.T.; Husain, K.S.; Abdelkareem, M.A. Selection Guidelines for Wind Energy Technologies. Energies 2021, 14, 3244. [Google Scholar] [CrossRef]
- Ahmed, S.D.; Al-Ismail, F.S.; Shafiullah, M.; Al-Sulaiman, F.A.; El-Amin, I.M. Grid integration challenges of wind energy: A review. IEEE Access 2020, 8, 10857–10878. [Google Scholar] [CrossRef]
- Nazir, M.S.; Ali, N.; Bilal, M.; Iqbal, H.M. Potential environmental impacts of wind energy development: A global perspective. Curr. Opin. Environ. Sci. Health 2020, 13, 85–90. [Google Scholar] [CrossRef]
- Iqbal, A.; Mahmoud, M.S.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A.; Alawadhi, H.; Olabi, A.G. Evaluation of the nanofluid-assisted desalination through solar stills in the last decade. J. Environ. Manag. 2021, 277, 111415. [Google Scholar] [CrossRef] [PubMed]
- Maghrabie, H.M.; Abdelkareem, M.A.; Al-Alami, A.H.; Ramadan, M.; Mushtaha, E.; Wilberforce, T.; Olabi, A.G. State-of-the-Art Technologies for Building-Integrated Photovoltaic Systems. Buildings 2021, 11, 383. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, J.; Pu, Y.; Wang, P. Solar energy potential assessment: A framework to integrate geographic, technological, and economic indices for a potential analysis. Renew. Energy 2020, 149, 577–586. [Google Scholar] [CrossRef]
- Irfan, M.; Elavarasan, R.M.; Hao, Y.; Feng, M.; Sailan, D. An assessment of consumers’ willingness to utilize solar energy in China: End-users’ perspective. J. Clean. Prod. 2021, 292, 126008. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery technologies for grid-level large-scale electrical energy storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Salameh, T.; Sayed, E.T.; Abdelkareem, M.A.; Olabi, A.G.; Rezk, H. Optimal selection and management of hybrid renewable energy System: Neom city as a case study. Energy Convers. Manag. 2021, 244, 114434. [Google Scholar] [CrossRef]
- Shinde, P.A.; Chodankar, N.R.; Abdelkareem, M.A.; Han, Y.-K.; Olabi, A.G. Nitridation-induced in situ coupling of Ni-Co4N particles in nitrogen-doped carbon nanosheets for hybrid supercapacitors. Chem. Eng. J. 2022, 428, 131888. [Google Scholar] [CrossRef]
- Hameer, S.; van Niekerk, J.L. A review of large-scale electrical energy storage. Int. J. Energy Res. 2015, 39, 1179–1195. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Sayed, E.T.; Shehata, N.; Abdelkareem, M.A.; Atieh, M.A. Recent progress in environmentally friendly bio-electrochemical devices for simultaneous water desalination and wastewater treatment. Sci. Total Environ. 2020, 748, 141046. [Google Scholar] [CrossRef] [PubMed]
- Srimuk, P.; Wang, L.; Budak, Ö.; Presser, V. High-performance ion removal via zinc–air desalination. Electrochem. Commun. 2020, 115, 106713. [Google Scholar] [CrossRef]
- Sayed, E.T.; Al Radi, M.; Ahmad, A.; Abdelkareem, M.A.; Alawadhi, H.; Atieh, M.A.; Olabi, A.G. Faradic capacitive deionization (FCDI) for desalination and ion removal from wastewater. Chemosphere 2021, 275, 130001. [Google Scholar] [CrossRef] [PubMed]
- Al Radi, M.; Sayed, E.T.; Alawadhi, H.; Abdelkareem, M.A. Progress in energy recovery and graphene usage in capacitive deionization. Crit. Rev. Environ. Sci. Technol. 2021, 1–57. [Google Scholar] [CrossRef]
- Ghahari, M.; Rashid-Nadimi, S.; Bemana, H. Metal-air desalination battery: Concurrent energy generation and water desalination. J. Power Sources 2019, 412, 197–203. [Google Scholar] [CrossRef]
- Ghahari, M.; Nadimi, S.R. Integration of metal-air batteries with electrodialysis system to decrease of required energy for water desalination. In Proceedings of the 23rd Iranian Seminar of Analytical Chemistry, Tehran, Iran, 30 August–1 September 2016. [Google Scholar]
- Zhao, C.; Liu, G.; Sun, N.; Zhang, X.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Biomass-derived N-doped porous carbon as electrode materials for Zn-air battery powered capacitive deionization. Chem. Eng. J. 2018, 334, 1270–1280. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, X.; Jiang, Y.; Li, J.; Ding, J.; Hu, W.; Zhong, C. Recent advances and challenges in divalent and multivalent metal electrodes for metal–air batteries. J. Mater. Chem. A 2019, 7, 18183–18208. [Google Scholar] [CrossRef]





































| Battery Type | Power | Energy Density (Wh/Kg) | Operating Voltage (V) | Discharge Time, h | Cost ($/kWh) | Life Time at 80% DoD * Cycles | Main Advantage | Main Disadvantage | Energy Efficiency % | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Li–ion | 1 kW–1 MW | 100–200 (150–200 Wh/L) | 3.6–4 | 0.1–10 | 800 (2006) 268 (2015) | 4500–8000 | High energy | Poor safety | 93 | [17,18,19] |
| Lead–acid | 1 kW–10 MW | 20–40 (50–70 Wh/L) | 2.1 | 0.01–1 | 150 | 200–2000 | Low cost | Short life cycle | 85 | [17,19,20] |
| Redox flow | 100 kW–80/90 MW | 2–32 (25–30 Wh/L) | 1.4 | 1–10 | 223 | 2000–4000 | Flexible design | Low energy density | 82 | [2,21,22] |
| Metal Anode | Electrolyte | Anode Reaction | Cathode Reaction | Overall Reaction | Ref. |
|---|---|---|---|---|---|
| General | [16] | ||||
| Iron Fe | Alkaline aqueous | [51] | |||
| Aluminum Al | [32] | ||||
| Zinc Zn | [52] | ||||
| Lithium Li | Non-aqueous | [53] |
| Type | Calculated OCV (V) | Practical Energy Density (Wh/Kg) | Metal Cost ($/kg) | Efficiency (Discharge), % | Capacity Density (mA h/g) | Ref. |
|---|---|---|---|---|---|---|
| Fe/Air | 1.28 | 50–75 | 0.4 | 96 | 300–786 | [51] |
| Zn/Air | 1.65 | 350–500 | 1.85 | - | 300–875 | [52,66] |
| Mg/Air | 2.93 | 400–700 | 2.75 | - | 737−2131 | [28] |
| Al/Air | 2.71 | 300–500 | 1.75 | 70 | 260–2777 | [31,67,68] |
| Na/Air | 2.27 | 1600 | 1.7 | - | - | [69] |
| Li/Air | 2.96 | 1700 | 68 | 68–94 | 3842 | [70,71,72,73] |
| Sn/Air | 0.95 | - | 21 | 70–90 | - | [69,74] |
| MAB | Voltage (V) | Electrolyte | Ref. | ||
|---|---|---|---|---|---|
| Including O2 | Without O2 (Metal) | ||||
| Li/Air (Li2O product) | 5220 | 11,238 | 2.91 | Non aqueous | [76,77] |
| Al/Air | 2784 | 8091 | 2.7 | Aqueous | [67] |
| 3311 | 6258 | 2.1 | Non aqueous | [78] | |
| Si/Air | 3947 | 8461 | 2.21 | Non aqueous | [79] |
| 2334 | 8001 | 2.09 | Aqueous | [80] | |
| Ca/Air (CaO product) | 2996 | 4186 | 3.13 | Non aqueous | [81] |
| Mg/Air | 2848 | 6098 | 2.77 | Aqueous | [58] |
| 3919 | 6493 | 2.95 | Non aqueous | [82] | |
| Na/Air (Na2O2 product) | 1601 | 2716 | 2.33 | Non aqueous | [83] |
| Fe/Air | 764 | 1229 | 1.28 | Aqueous | [84] |
| Zn/Air | 1086 | 1352 | 1.65 | Aqueous | [52] |
| K/Air | 935 | 1700 | 2.48 | Non aqueous | [85] |
| Sn/Air (at 1000 K) | 860 | 6250 | 0.95 | Non aqueous | [69] |
| MAB | Discharge Product | Condition | Reversibility Cycles | Voltage (V) Ref. | |
|---|---|---|---|---|---|
| Fe/O2 | Fe | 453 | [b,c,d,e] | 3500 [b,d] | 1.28 |
| Zn/O2 | ZnO | >700 | [a,c,d] | >75 [a,c] | 1.65 |
| K/O2 | K | ~19,500 | [a,c,d] | >200 [a,c] | 2.48 |
| Na/O2 | ~18,300 | [a,c,d] | >20 [a,c] | 2.33 | |
| Na | 2.27 | ||||
| Mg/O2 | Mg( | ~2750 | [a,c,d,f] | <10 [a,c,d] | 2.77 |
| MgO | 2.95 | ||||
| Si/O2 | Si | ~1600 | [a,c,d] | Not yet | 2.09 |
| Si | 2.21 | ||||
| Al/O2 | Al | ~2300 | [a,c,d] | Limited | 2.71 |
| 2.1 | |||||
| Li/O2 | >11,050 | [a,c,d] | >250 [a,c] | 2.96 | |
| 2.91 |
| Electrolyte Solution | Aluminum Anode | Average Discharge Voltage (v) | Capacity Density (Ah/kg) | Efficiency (%) | Energy Density (Wh/kg) |
|---|---|---|---|---|---|
| 2 M NaCl | CG | 0.398 | 2751 | 92.3 | 1097 |
| UFG | 0.387 | 2726 | 91.6 | 958.4 | |
| 4 M KOH | CG | 1.38 | 2439 | 81.7 | 3363 |
| UFG | 1.44 | 2475 | 83.1 | 3593 | |
| 4 M NaOH | CG | 1.397 | 1647 | 55.2 | 2428 |
| UFG | 1.532 | 2307 | 77.3 | 3524 |
| Voltage (V) | Anode | Electric Capacity (C) | Electric Capacity/Discharge (C) | Performance (%) |
|---|---|---|---|---|
| 1 V | 4N grade Al | 1467.23 | 751.30 | 50.92 |
| 2N5 grade Al | 1991.22 | 377.47 | 18 | |
| 0.8 V | 4N grade Al | 2211.13 | 1590.70 | 71.76 |
| 2N5 grade Al | 2151.14 | 1625.61 | 75 |
| Na–Air | ||
|---|---|---|
| Barrier | Polymeric like Celgard 3501 | NASICON |
| Discharge products | NaO2 and/or Na2O2 | NaOH |
| Product solubility | Insoluble | Soluble |
| Overpotential gap | Higher | Lower |
| Overall performance (%) | ≥75 | ≤75 |
| Stability | No recommendable | Recommendable |
| Safety | Satisfactory | Reasonable |
| Membrane | Types | Merits | Demerits | Reference | |
|---|---|---|---|---|---|
| Aqueous | Alkaline | Potassium Hydroxide. Lithium Hydroxide. Sodium Hydroxide | Non-corrosive. Zn has rapid electrochemical kinetics as well as intrinsic electrochemical reversibility. Higher ionic conduction. ZAB has excellent performance at low temperatures. The solubility of zinc salts is very high. | CO2 sensitivity is a concern. Alkaline solutions are harmful to the environment. Dissolution of Zn, precipitation of insoluble CO32− hydrogen evolution, and electrolyte evaporation. | [16,75,97] |
| Neutral | Potassium Chloride Lithium Chloride Zinc Dichloride Magnesium Dichloride | Secondary ZABs have excellent cycle stability as well as a long calendar life. Less corrosion and discharge due to high activity of Al alloy Carbonization of the electrolyte should be avoided. Dendrite formation may be reduced. Reduce the solubility of zinc in ZAB. CO2 absorption is very low. | In industrial applications, this is a rare occurrence. | [16,58,97] | |
| Acidic | HCl HAc | Reduced the development of byproducts on the cathode and the formation of dendrites on the anode. | Rarely utilized in industrial applications. Some kinds of MABs are prone to corrosion issues. | [16,91] | |
| Non-aqueous | Ionic liquid | RTILs Lithium salts | Low volatility. Inflammability. High ionic conductivity. Excellent moisture resistance. | Carbonate crystallization. High purity is required. Synthesis is harmful to the environment. | [16,75,97] |
| Organic | Sodium-based salts | Sodium and lithium-air batteries are among the most often used types of batteries. Contributes to the development of SEI at the anode. | Costly. Combustible. A certain degree of toxicity is present. The discharge product is preventing air cathode pores from opening. | [75,93] | |
| Solid-state | Zr | Work in all MABs kinds without exception. Electrolyte leakage prevention, thermal stability, as well as robustness, are all important considerations. It is advantageous for increasing the energy density of MABs. Making wearable and flexible gadgets possible is made possible by this technology. | Increased resistance of the battery will result in a reduction in battery capacity. The wetting property is poor. | [16,75,91] | |
| Hybrid | Alkaline anolyte and acidic catholyte. | Higher performance. Cycling stability is excellent. | In industrial applications, this is a rare occurrence. | [16,93,97] | |
| Electrolyte | Oxygen Reaction | Reaction Pathway | ||
|---|---|---|---|---|
| Two-Step | Four-Step | |||
| Aqueous MABs | Alkaline | ORR |
|
|
| OER |
| |||
| Acidic | ORR |
| ||
| OER |
| |||
| Non-aqueous MABs | e.g., LAB in non-aqueous electrolyte | ORR |
| |
| OER |
| |||
| Temperature (°C) | ||
|---|---|---|
| 22 | 127 | 9.21 × |
| 30 | 158 | 1.03 × |
| 40 | 182 | 1.16 × |
| 50 | 205 | 1.30 × |
| 60 | 224 | 1.44 × |
| 70 | 238 | 1.55 × |
| Metal | Reducing Corrosion or HER Rate by |
|---|---|
| ZnO surface | Increasing ZnO reduces the self-discharge rate. |
| Al | Changing purity, properties, and temperature of the alkaline electrolyte. |
| Fe | Utilization of ionic membrane. Using an alloy as an anode instead of pure metal and additives such as sulfur or bismuth is a good way to save money. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-Air Batteries—A Review. Energies 2021, 14, 7373. https://doi.org/10.3390/en14217373
Olabi AG, Sayed ET, Wilberforce T, Jamal A, Alami AH, Elsaid K, Rahman SMA, Shah SK, Abdelkareem MA. Metal-Air Batteries—A Review. Energies. 2021; 14(21):7373. https://doi.org/10.3390/en14217373
Chicago/Turabian StyleOlabi, Abdul Ghani, Enas Taha Sayed, Tabbi Wilberforce, Aisha Jamal, Abdul Hai Alami, Khaled Elsaid, Shek Mohammod Atiqure Rahman, Sheikh Khaleduzzaman Shah, and Mohammad Ali Abdelkareem. 2021. "Metal-Air Batteries—A Review" Energies 14, no. 21: 7373. https://doi.org/10.3390/en14217373
APA StyleOlabi, A. G., Sayed, E. T., Wilberforce, T., Jamal, A., Alami, A. H., Elsaid, K., Rahman, S. M. A., Shah, S. K., & Abdelkareem, M. A. (2021). Metal-Air Batteries—A Review. Energies, 14(21), 7373. https://doi.org/10.3390/en14217373












