Phase Change Materials (PCMs) Based in Paraffin/Synthetic Saponite Used as Heat Storage Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Procedure
2.2. Characterization
3. Results
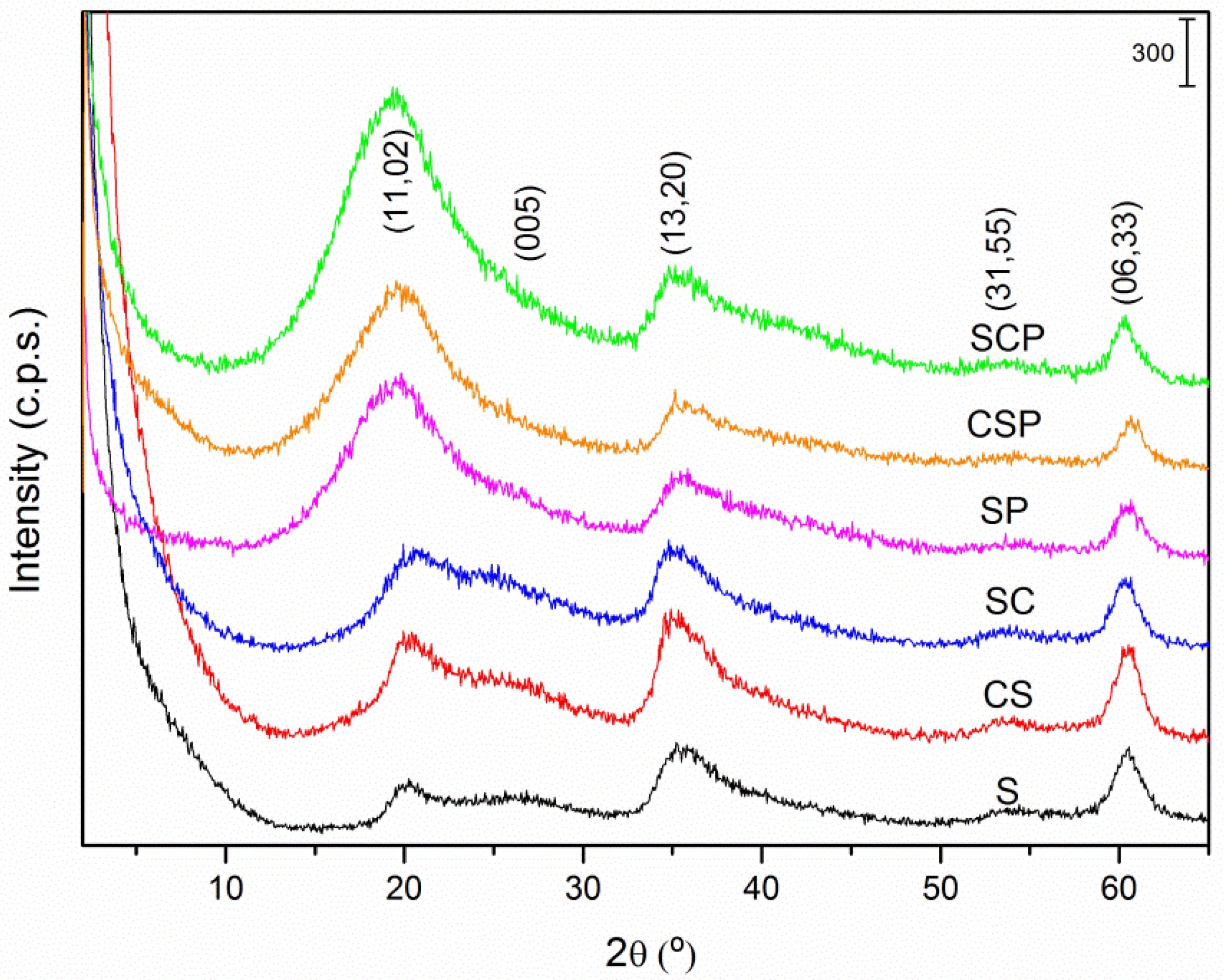
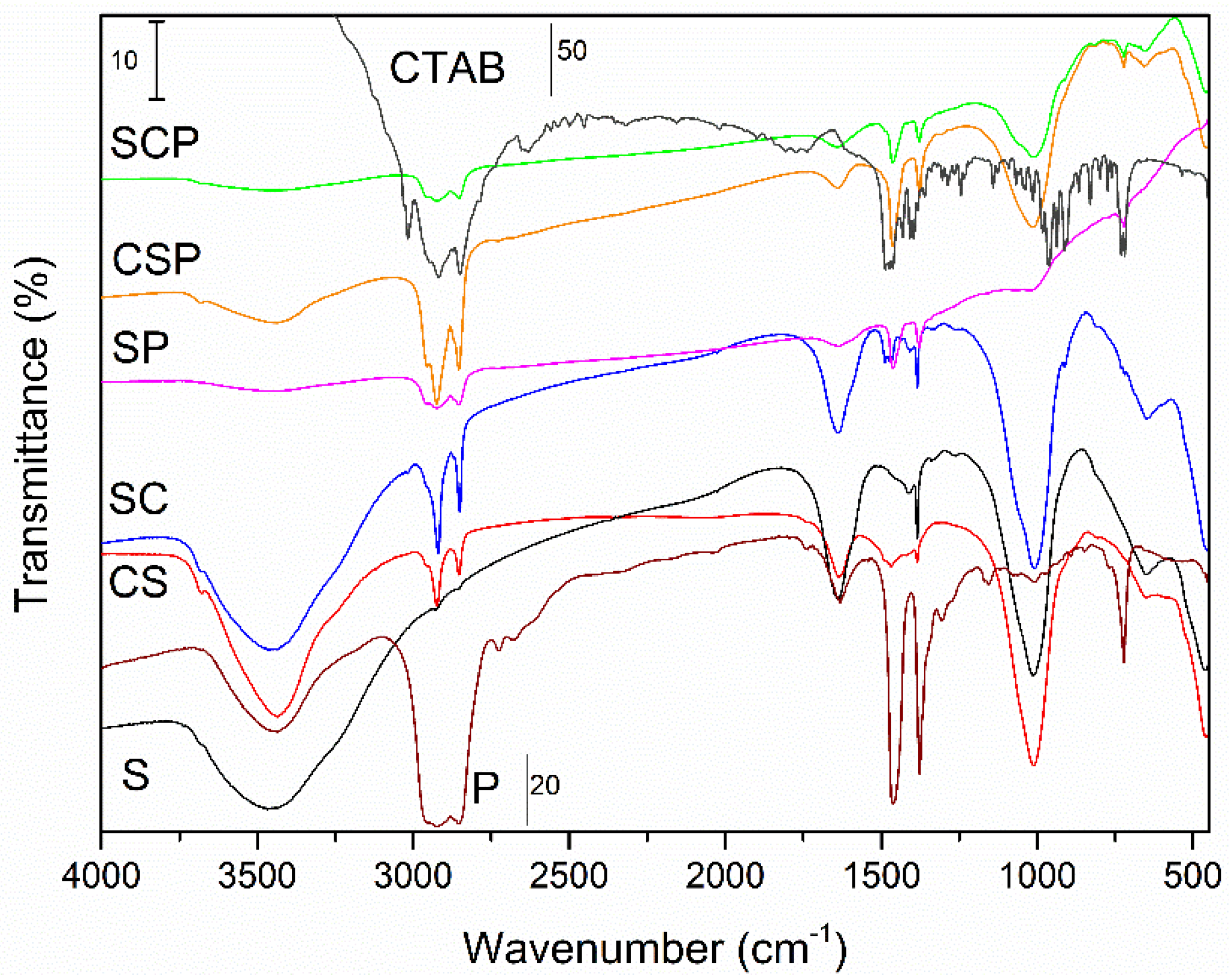
3.1. Characterization of Saponites and PCMs
3.2. Thermal Properties of the Paraffin/Saponite Composites
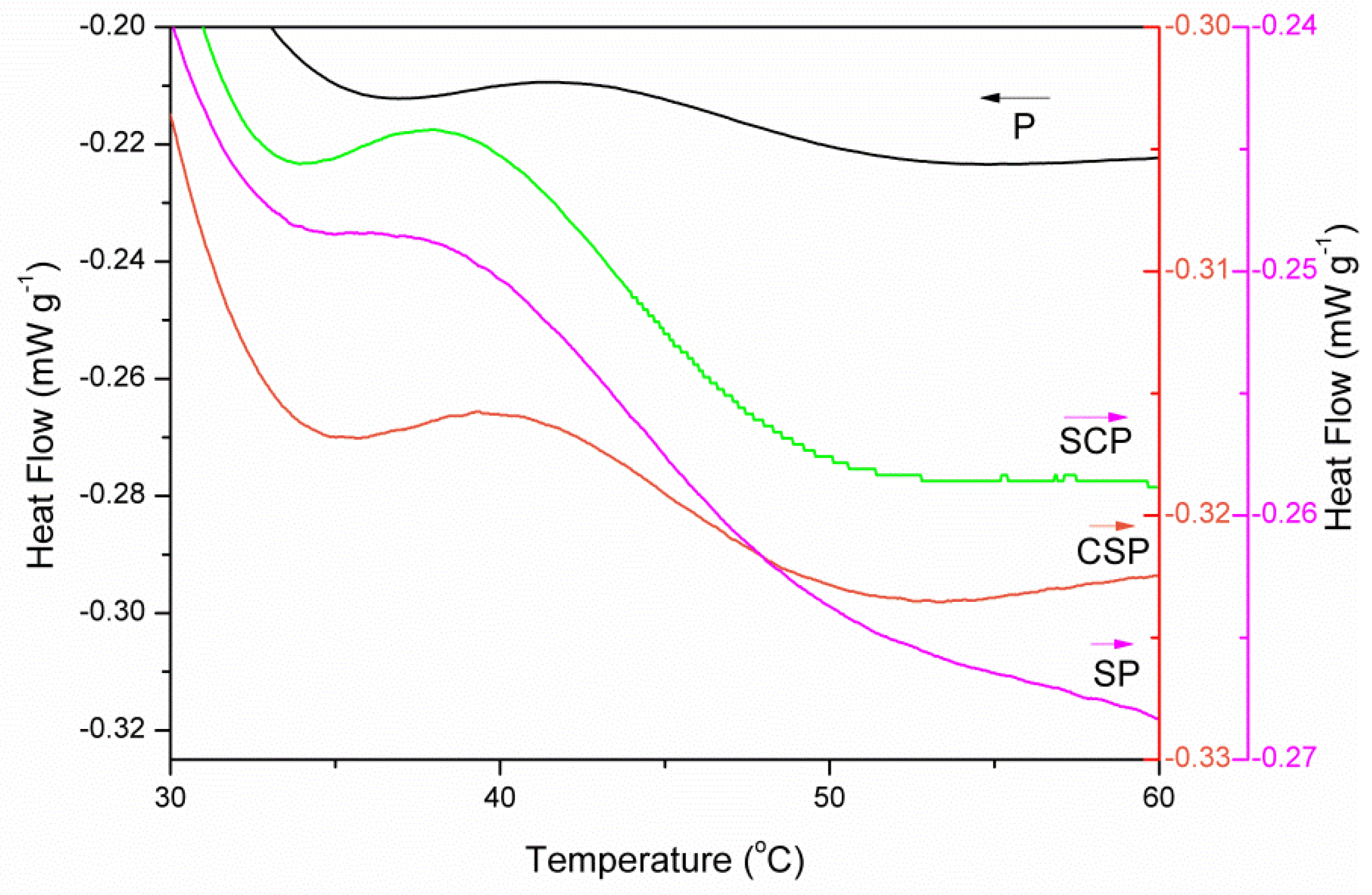
3.2.1. DSC Analysis
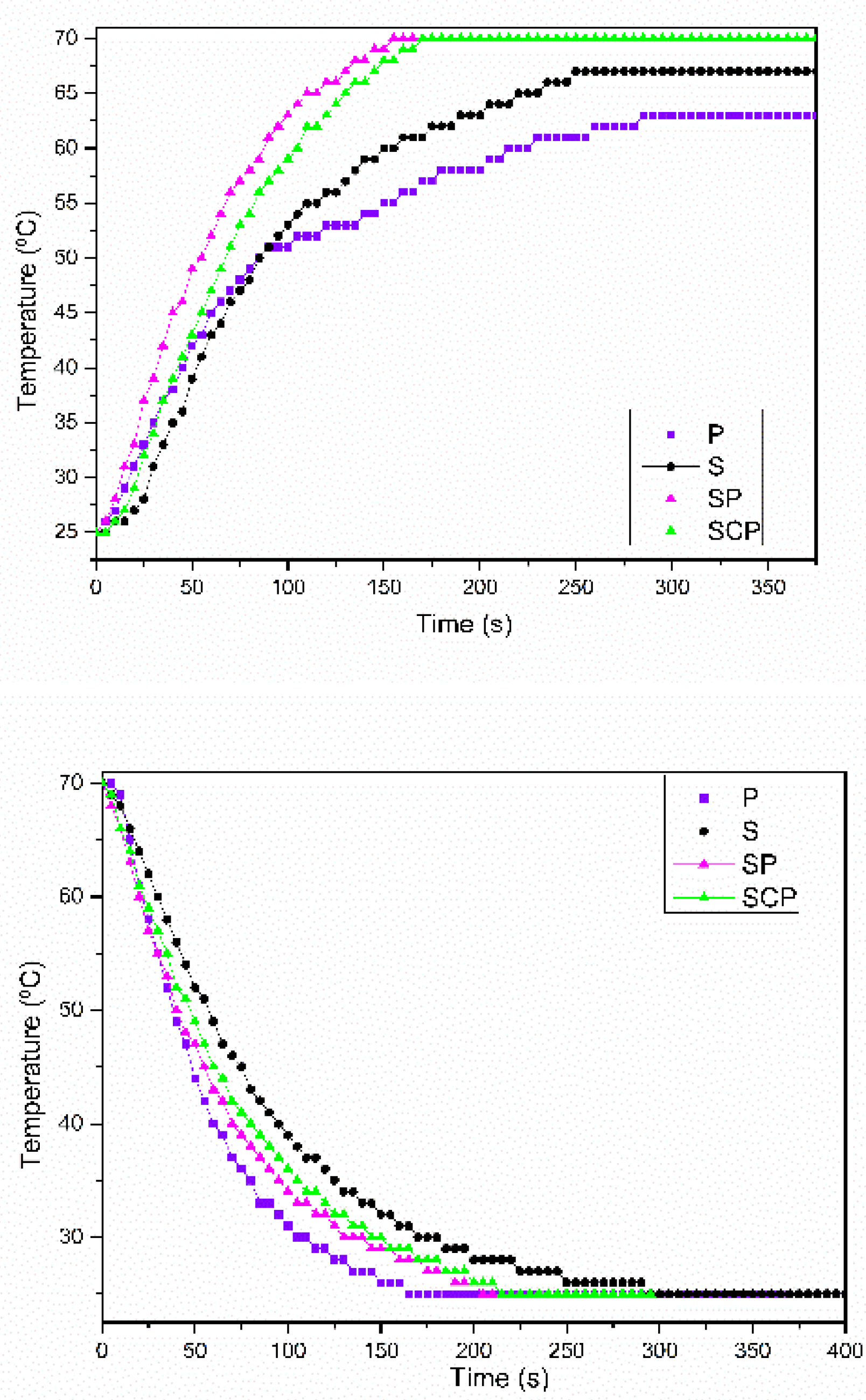
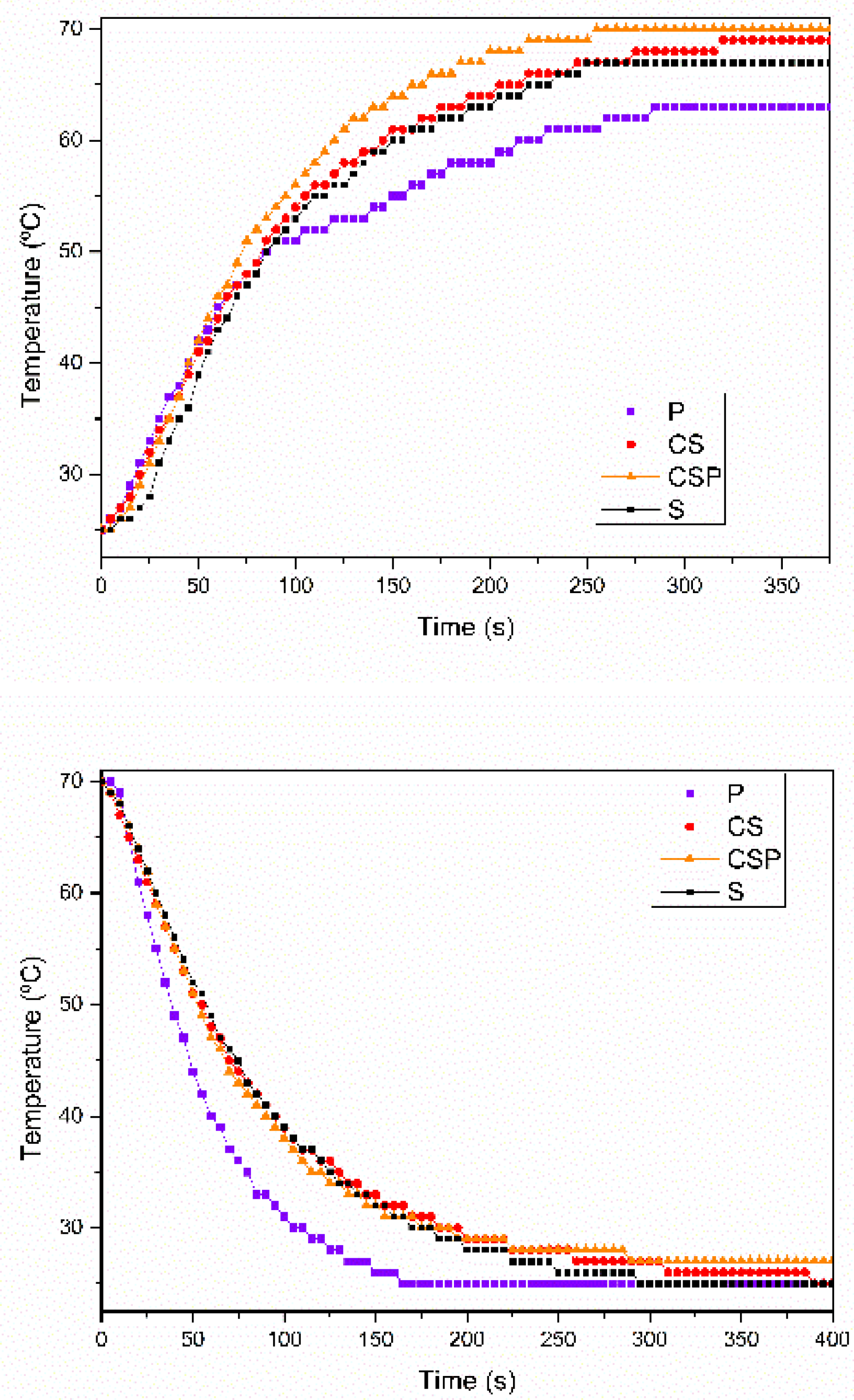
3.2.2. Heat Storage and Heat Release Properties of Paraffin/Synthetic Saponite PCM
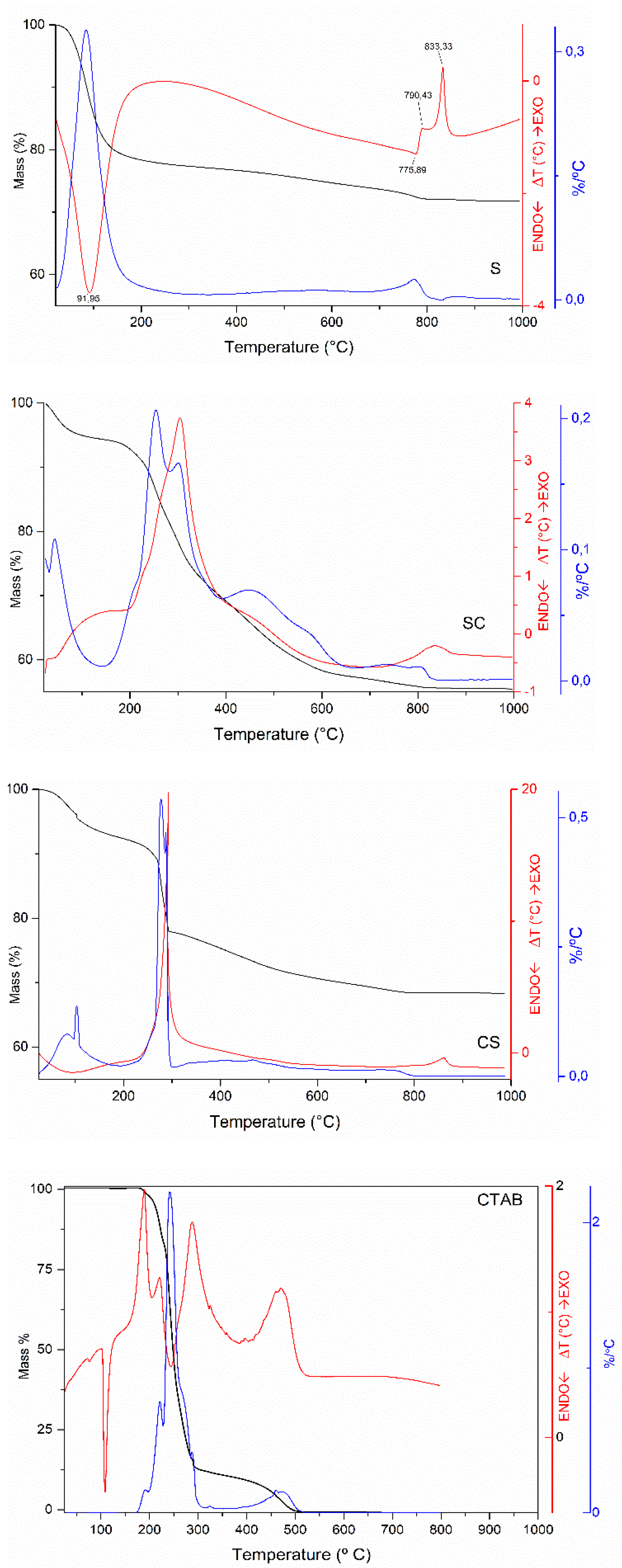
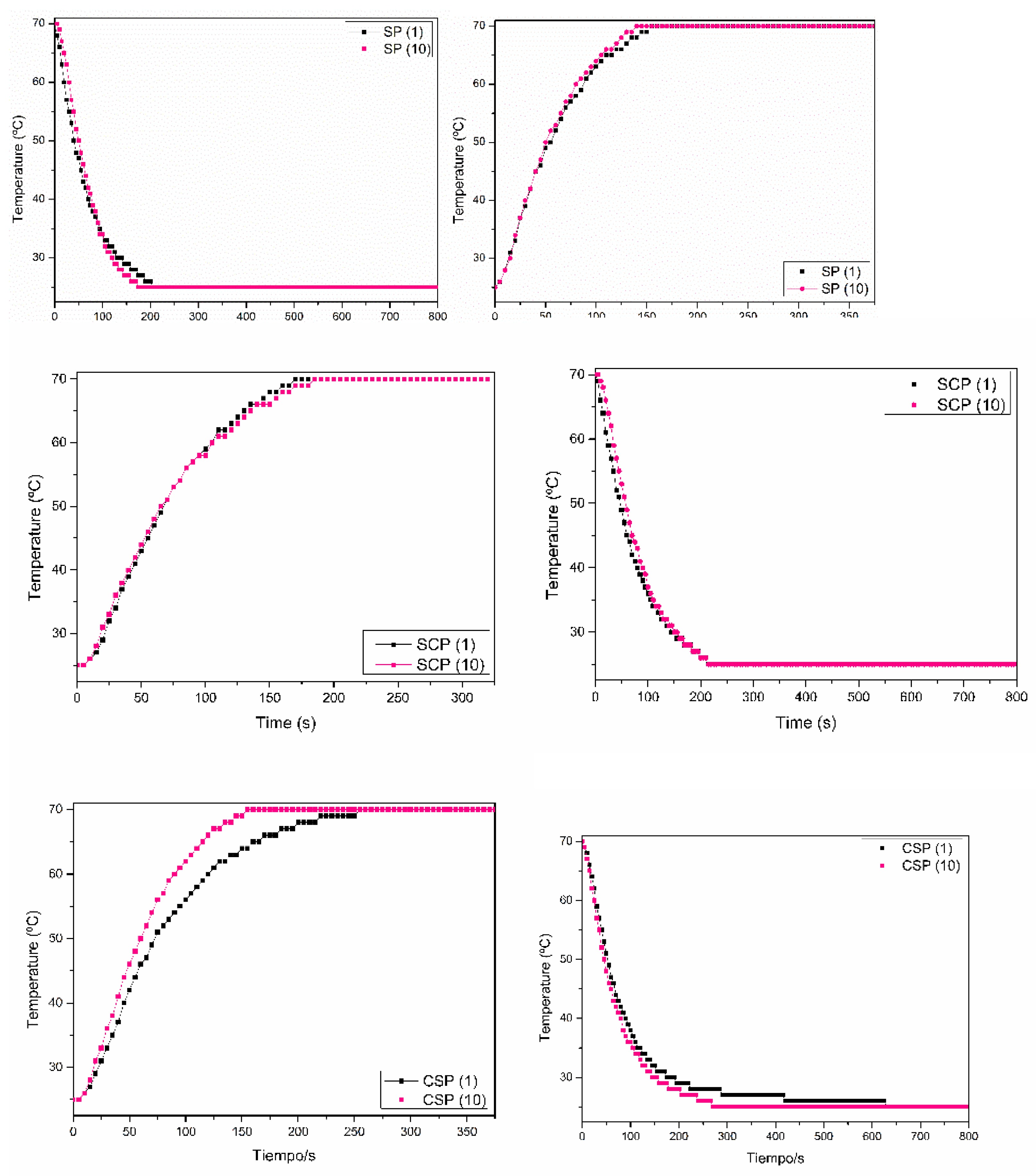
3.2.3. The Thermal Stability of the Paraffin/Synthetic Saponite PCM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, B.; Martin, V.; Setterwall, F. Phase transition temperature ranges and storage density of paraffin wax phase change materials. Energy 2004, 29, 1785–1804. [Google Scholar] [CrossRef]
- Li, C.; Fu, L.; Ouyang, J.; Tang, A.; Yang, H. Kaolinite stabilized paraffin composite phase change materials for thermal energy storage. Appl. Clay Sci. 2015, 115, 212–220. [Google Scholar] [CrossRef]
- Kang, P.; Liangjie, F.; Xiaoyu, L.; Jing, O.; Huaming, Y. Stearic acid modified montmorillonite as emerging microcapsules for thermal energy storage. Applied. Clay Sci. 2017, 138, 100–106. [Google Scholar]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Sari, A. Thermal energy storage characteristics of bentonite-based composite PCMs with enhanced thermal conductivity as novel thermal storage building materials. Energy Convers. Manag. 2016, 117, 132–141. [Google Scholar] [CrossRef]
- Sari, A. Thermal Energy Storage Properties and Laboratory-Scale Thermoregulation Performance of Bentonite/Paraffin Composite Phase Change Material for Energy-Efficient Buildings. J. Mater. Civil Eng. 2017, 29, 04017001. [Google Scholar] [CrossRef]
- Obaje, S.O.; Omada, J.L.; Dambatta, U.A. Clays and their Industrial Applications: Synoptic Review. Int. J. Sci. Technol. 2013, 3, 264–270. [Google Scholar]
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Clay Materials for Environmental Remediation; Springer: Cham, Switzerland, 2015; pp. 1–124. [Google Scholar]
- Okada, A.; Fukushima, Y.; Kawasumi, M.; Inagaki, S.; Usuki, A.; Sugiyama, S.; Kurauchi, T.; Kamigaito, O. Composite Material and Process for Manufacturing Same. U.S. Patent 4,739,007, 19 April 1988. [Google Scholar]
- Kawasumi, M.; Kohzaki, M.; Kojima, Y.; Okada, A.; Kamigaito, O. Process for Producing Composite Material. U.S. Patent 4810734A, 7 March 1989. [Google Scholar]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E.; Ozay, S.; Ozkilic, Y. Preparation of phase change material–montmorillonite composites suitable for thermal energy storage. Thermochim. Acta 2011, 524, 39–46. [Google Scholar] [CrossRef]
- Lim, S.C.; Gomes, C.; Kadir, M.Z.A.A. Preliminary grounding performance of bentonite mixed concrete encased steel cage under high soil resistivity condition. Int. J. Elec. Power 2013, 47, 117–128. [Google Scholar] [CrossRef]
- Sarı, A. Fabrication and thermal characterization of kaolin-based composite phase change materials for latent heat storage in buildings. Energy Build. 2015, 96, 193–200. [Google Scholar] [CrossRef]
- Yang, D.; Peng, F.; Zhang, H.; Guo, H.; Xiong, L.; Wang, C.; Shi, S.; Chen, X. Preparation of palygorskite paraffin nanocomposite suitable for thermal energy storage. Appl. Clay Sci. 2016, 126, 190–196. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Ali, S.; Razack, K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Gómez, M. Systematic review of encapsulation and shape-stabilization of phase change materials. J. Energy Storage 2020, 30, 101495. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. Enhanced thermophysical properties of organic PCM through shape stabilization for thermal energy storage in buildings: A state of the art review. Energy Build. 2021, 236, 110799. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Kao, H.; Tan, J. Experimental investigation of preparation and thermal performances of paraffin/bentonite composite phase change material. Energy Convers. Manag. 2011, 52, 3275–3281. [Google Scholar] [CrossRef]
- Gao, F. Clay/polymer composites: The story. Mater. Today 2004, 7, 50–55. [Google Scholar] [CrossRef]
- Trujillano, R.; Rico, E.; Vicente, M.A.; Rives, V.; Bergaoui, L.; Chaabene, S.B.; Ghorbel, A. Microwave-Assisted Synthesis of Fe3+ Saponites. Characterization by X-Ray Diffraction and FT-IR Spectroscopy. Macla 2009, 11, 189–190. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Absorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2010; pp. 1–338. [Google Scholar]
- Li, M.; Guo, Q. The preparation of the hydrotalcite-based composite phase change material. Appl. Energy 2015, 156, 207–212. [Google Scholar] [CrossRef]
- Suquet, H.; de la Calle, C.; Pezerat, H. Swelling and structural organization of saponite. Clays Clay Min. 1975, 23, 1–9. [Google Scholar] [CrossRef]
- Trujillano, R.; Rico, E.; Vicente, M.A.; Herrero, M.; Rives, V. Microwave radiation and mechanical grinding as new ways for preparation of saponite-like materials. Appl. Clay Sci. 2010, 48, 32–38. [Google Scholar] [CrossRef]
- Ahlrichs, J.L.; Serna, C.; Serratosa, J.M. Structural hydroxyls in sepiolites. Clays Clay Min. 1975, 23, 119–124. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vibr. Spect. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Su, G.; Yang, C.; Zhu, J.J. Fabrication of Gold Nanorods with Tunable Longitudinal Surface Plasmon Resonance Peaks by Reductive Dopamine. Langmuir 2015, 31, 817–823. [Google Scholar] [CrossRef]
- Cho, S.Y.; Fogle, H.S. Efforts on solving the problem of paraffin deposit. I: Using oil-soluble inhibitors. J. Ind. Eng. Chem. 1999, 5, 123–127. [Google Scholar]
- MacKenzie, R.C. Simple phyllosilicates based on gibbsite- and brucite-like sheets. In Differential Thermal Analysis; Academic Press: London, UK, 1973; Volume 1, pp. 498–538. [Google Scholar]
- Vicente, M.A.; Lambert, J.-F. Synthesis of Pt pillared clay nanocomposite catalysts from [PtII(NH3)4]Cl2 precursor. Phys. Chem. Chem. Phys. 2001, 3, 4843–4852. [Google Scholar] [CrossRef]
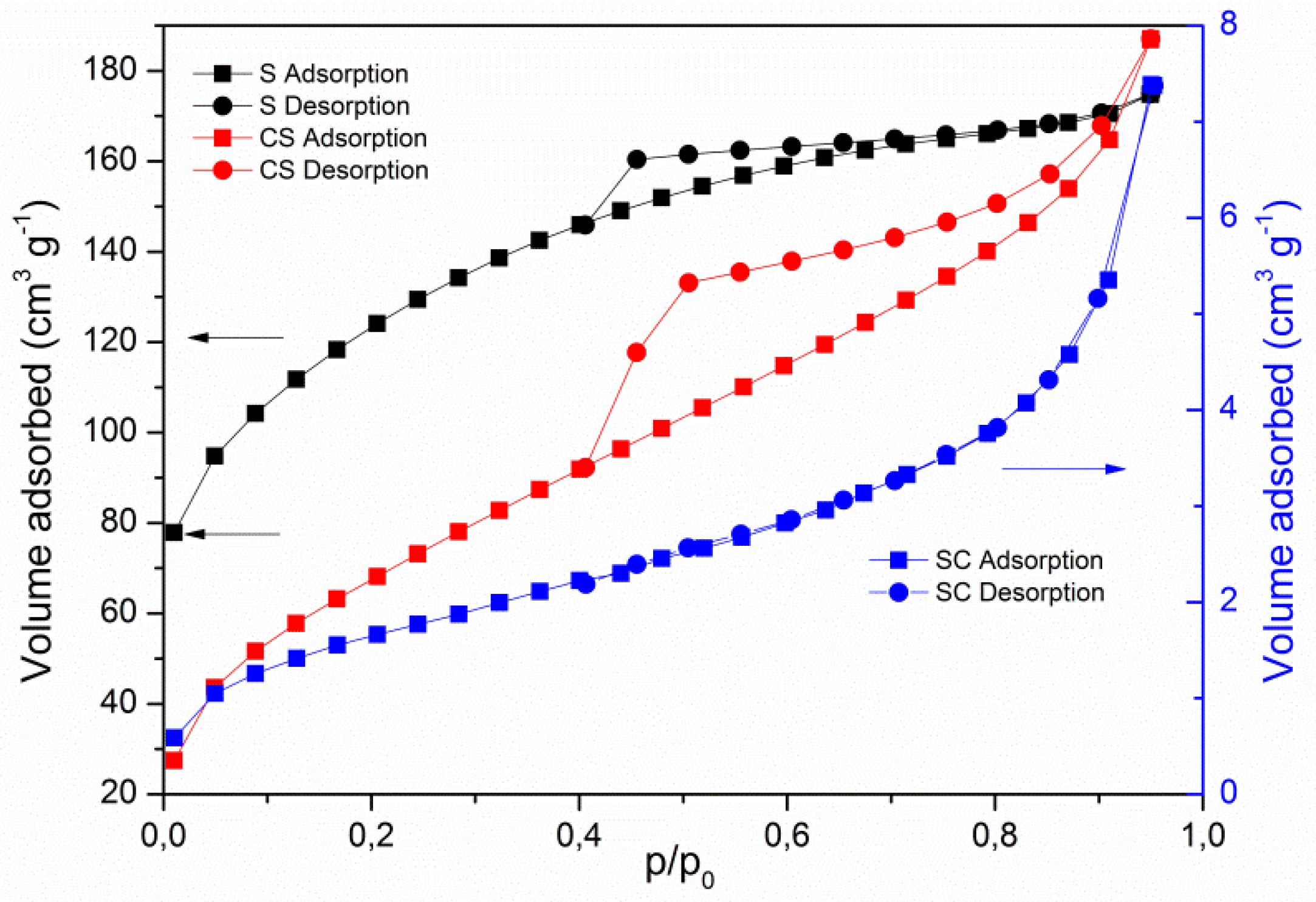
| Sample | SBET (m2/g) | St (m2/g) | Vmp (mm3/g) | Dp (nm) |
|---|---|---|---|---|
| S | 408 | 353 | 36 | 2.74 |
| CS | 252 | 252 | - | 4.14 |
| SC | 6 | 6 | - | 7.69 |
| S-S | S-L | |
|---|---|---|
| P | 36.2 | 53.5 |
| SP | 32.8 | 50.0 |
| SCP | 33.2 | 49.5 |
| CSP | 34.2 | 50.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillano, R.; González, B.; Rives, V. Phase Change Materials (PCMs) Based in Paraffin/Synthetic Saponite Used as Heat Storage Composites. Energies 2021, 14, 7414. https://doi.org/10.3390/en14217414
Trujillano R, González B, Rives V. Phase Change Materials (PCMs) Based in Paraffin/Synthetic Saponite Used as Heat Storage Composites. Energies. 2021; 14(21):7414. https://doi.org/10.3390/en14217414
Chicago/Turabian StyleTrujillano, Raquel, Beatriz González, and Vicente Rives. 2021. "Phase Change Materials (PCMs) Based in Paraffin/Synthetic Saponite Used as Heat Storage Composites" Energies 14, no. 21: 7414. https://doi.org/10.3390/en14217414
APA StyleTrujillano, R., González, B., & Rives, V. (2021). Phase Change Materials (PCMs) Based in Paraffin/Synthetic Saponite Used as Heat Storage Composites. Energies, 14(21), 7414. https://doi.org/10.3390/en14217414








