Remediation of Annular Gas Migration along Cemented Wellbores Using Reactive Mineral Fluids: Experimental Assessment of Sodium Bicarbonate and Sodium Silicate-Based Solutions
Abstract
:1. Introduction
2. Rationale for Selected Mineral Fluids
2.1. Sodium Bicarbonate
2.2. Sodium Silicate
3. Materials and Methods
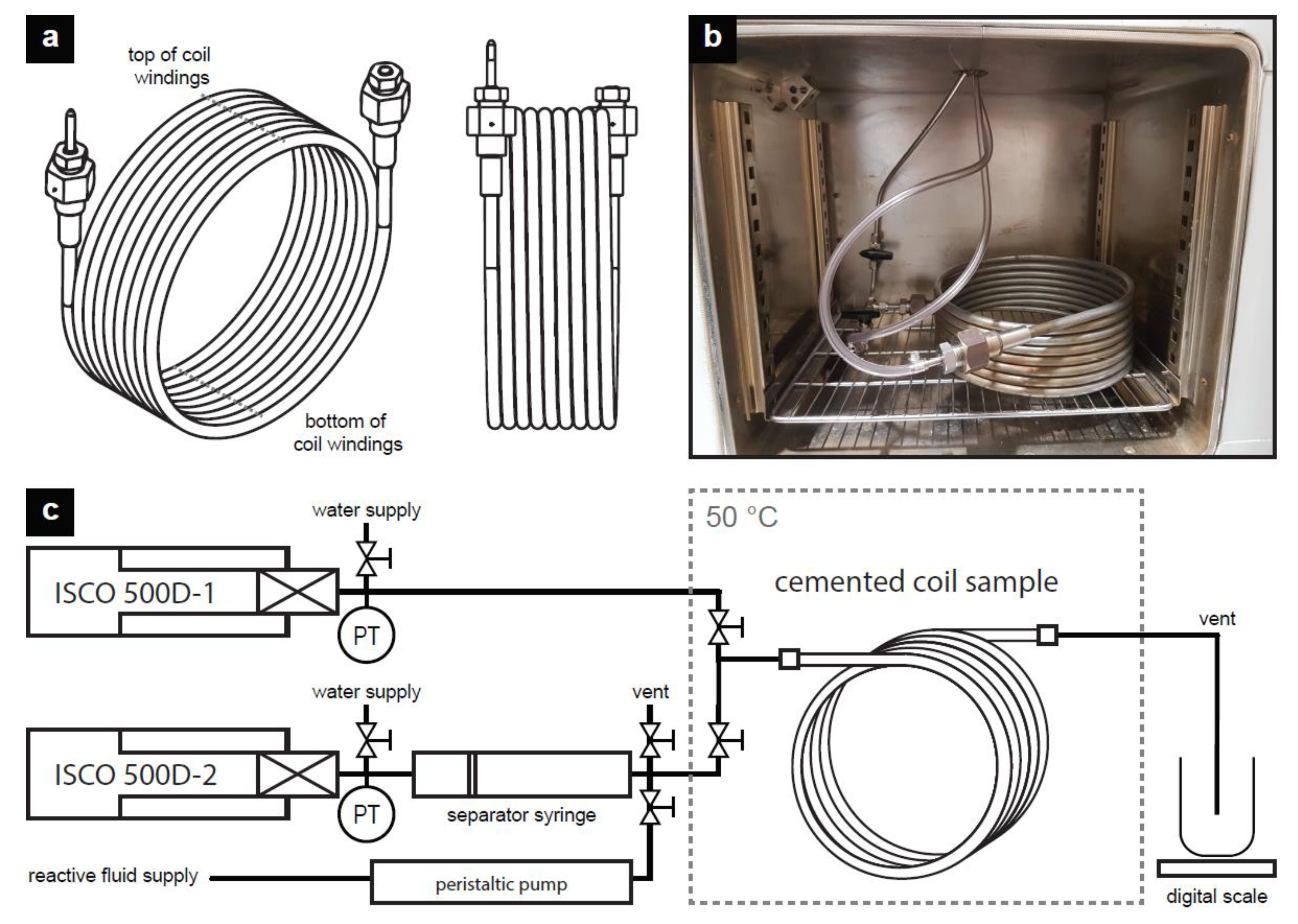
3.1. Materials and Preparation of Cemented Coil Samples
3.2. Materials and Preparation of Reactive Mineral Fluids
3.3. Reactive Flow-Through Permeameter System
3.4. Experimental Procedure
3.5. Data Acquisition and Processing
3.6. Post-Experiment Microstructural Analysis
4. Results and Analysis
4.1. Flow and Equivalent Permeability Evolution
4.1.1. Sample BC-1: Experiment with Sodium Bicarbonate Solution
4.1.2. Sample SIL-1: Experiment with Sodium Silicate Solution
4.2. Microstructural Observations
5. Discussion and Conclusions
5.1. Defect Sealing Potential of Sodium Bicarbonate-Based Solutions
5.2. Defect Sealing Potential of Sodium Silicate-Based Solutions
5.3. Conclusions and Implications for Applicability to Wellbores
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allahvirdizadeh, P. A review on geothermal wells: Well integrity issues. J. Clean. Prod. 2020, 275, 124009. [Google Scholar] [CrossRef]
- Huan, X.; Xu, G.; Zhang, Y.; Sun, F.; Xue, S. Study on Thermo-Hydro-Mechanical Coupling and the Stability of a Geothermal Wellbore Structure. Energies 2021, 14, 649. [Google Scholar] [CrossRef]
- Bo, Z.; Zeng, L.; Chen, Y.; Xie, Q. Geochemical reactions-induced hydrogen loss during underground hydrogen storage in sandstone reservoirs. Int. J. Hydrogen Energy 2021, 46, 19998–20009. [Google Scholar] [CrossRef]
- Zhang, M.; Bachu, S. Review of integrity of existing wells in relation to CO2 geological storage: What do we know? Int. J. Greenh. Gas Control 2011, 5, 826–840. [Google Scholar] [CrossRef]
- Vrålstad, T.; Saasen, A.; Fjær, E.; Øia, T.; Ytrehus, J.D.; Khalifeh, M. Plug & abandonment of offshore wells: Ensuring long-term well integrity and cost-efficiency. J. Pet. Sci. Eng. 2019, 173, 478–491. [Google Scholar]
- Barclay, I.; Pellenbarg, J.; Tettero, F.; Pfeiffer, J. The beginning of the end: A review of abandonment and decommissioning practices. Oilf. Rev. 2001, 13, 28–41. [Google Scholar]
- Kiran, R.; Teodoriu, C.; Dadmohammadi, Y.; Nygaard, R.; Wood, D.; Mokhtari, M.; Salehi, S. Identification and evaluation of well integrity and causes of failure of well integrity barriers (A review). J. Nat. Gas Sci. Eng. 2017, 45, 511–526. [Google Scholar] [CrossRef]
- Ahmed, S.; Salehi, S.; Ezeakacha, C. Review of gas migration and wellbore leakage in liner hanger dual barrier system: Challenges and implications for industry. J. Nat. Gas Sci. Eng. 2020, 78, 103284. [Google Scholar] [CrossRef]
- Dusseault, M.B.; Gray, M.N.; Nawrocki, P.A. Why Oilwells Leak: Cement Behavior and Long-Term Consequences. In Proceedings of the SPE International Oil and Gas Conference and Exhibition, SPE 64733, Beijing, China, 7–10 November 2000; p. 8. [Google Scholar]
- Agbasimalo, N.; Radonjic, M. Experimental Study of the Impact of Drilling Fluid Contamination on the Integrity of Cement–Formation Interface. J. Energy Resour. Technol. 2014, 136, 42908. [Google Scholar] [CrossRef] [Green Version]
- Tao, C.; Rosenbaum, E.; Kutchko, B.G.; Massoudi, M. A Brief Review of Gas Migration in Oilwell Cement Slurries. Energies 2021, 14, 2369. [Google Scholar] [CrossRef]
- Jandhyala, S.R.K.; Pangu, G.; Deshpande, A.; Wolterbeek, T.; Cornelissen, E.; van Eijden, J. Volume Change of Cement Plugs: Spotlight on the Role of Boundary Conditions Using an Improved Testing Method. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition; Society of Petroleum Engineers, Brisbane, Australia, 23–25 October 2018. [Google Scholar]
- Van Eijden, J.; Cornelissen, E.; Ruckert, F.; Wolterbeek, T. Development of Experimental Equipment and Procedures to Evaluate Zonal Isolation and Well Abandonment Materials. In Proceedings of the SPE/IADC Drilling Conference and Exhibition, The Hague, The Netherlands, 14–16 March 2017; SPE-184640-MS; Society of Petroleum Engineers: Hague, The Netherlands, 2017; pp. 14–16. [Google Scholar]
- Reddy, B.R.; Xu, Y.; Ravi, K.; Gray, D.W.; Pattillo, P. Cement Shrinkage measurement in oilwell cementing—A comparative study of laboratory methods and procedures. SPE Drill. Complet. 2009, 24, 104. [Google Scholar] [CrossRef]
- Chenevert, M.E.; Shrestha, B.K. Chemical Shrinkage Properties of Oilfield Cements (includes associated paper 23477). SPE Drill. Eng. 1991, 6, 37–43. [Google Scholar] [CrossRef]
- Wolterbeek, T.K.T.; Cornelissen, E.K.; Hangx, S.J.T.; Spiers, C.J. Impact of downhole pressure and fluid-access on the effectiveness of wellbore cement expansion additives. Cem. Concr. Res. 2021, 147, 106514. [Google Scholar] [CrossRef]
- Agofack, N.; Ghabezloo, S.; Sulem, J.; Garnier, A.; Urbanczyk, C. Experimental investigation of the early-age mechanical behaviour of oil-well cement paste. Cem. Concr. Res. 2019, 117, 91–102. [Google Scholar] [CrossRef]
- Meng, M.; Frash, L.P.; Carey, J.W.; Li, W.; Welch, N.J.; Zhang, W. Cement stress and microstructure evolution during curing in semi-rigid high-pressure environments. Cem. Concr. Res. 2021, 149, 106555. [Google Scholar] [CrossRef]
- Orlic, B. Some geomechanical aspects of geological CO2 sequestration. KSCE J. Civ. Eng. 2009, 13, 225–232. [Google Scholar] [CrossRef]
- Lecampion, B.; Bunger, A.; Kear, J.; Quesada, D. Interface debonding driven by fluid injection in a cased and cemented wellbore: Modeling and experiments. Int. J. Greenh. Gas Control 2013, 18, 208–223. [Google Scholar] [CrossRef]
- Wolterbeek, T.K.T.; Hangx, S.J.T.; Spiers, C.J. Effect of CO2-induced reactions on the mechanical behaviour of fractured wellbore cement. Geomech. Energy Environ. 2016, 7, 26–46. [Google Scholar] [CrossRef]
- De Andrade, J.; Sangesland, S.; Todorovic, J.; Vrålstad, T. Cement Sheath Integrity during Thermal Cycling: A Novel Approach for Experimental Tests of Cement Systems. In Proceedings of the SPE bergen one day seminar; Society of Petroleum Engineers, Bergen, Norway, 22 April 2015. [Google Scholar]
- Heathman, J.F.; Beck, F.E. Finite Element Analysis Couples Casing and Cement Designs for HTHP Wells in East Texas. In Proceedings of the IADC/SPE Drilling Conference; Society of Petroleum Engineers, Miami, FL, USA, 21–23 February 2006. [Google Scholar]
- Goodwin, K.J.; Crook, R.J. Cement sheath stress failure. SPE Drill. Eng. 1992, 7, 291–296. [Google Scholar] [CrossRef]
- Torsæter, M.; Todorovic, J.; Lavrov, A. Structure and debonding at cement–steel and cement–rock interfaces: Effect of geometry and materials. Constr. Build. Mater. 2015, 96, 164–171. [Google Scholar] [CrossRef]
- Dou, H.; Dong, X.; Duan, Z.; Ma, Y.; Gao, D. Cement Integrity Loss due to Interfacial Debonding and Radial Cracking during CO2 Injection. Energies 2020, 13, 4589. [Google Scholar] [CrossRef]
- Nelson, E.B.; Guillot, D. (Eds.) Well Cementing, 2nd ed.; Schlumberger: Sugar Land, TX, USA, 2006; ISBN 978-097885300-6. [Google Scholar]
- Lackey, G.; Rajaram, H. Modeling gas migration, sustained casing pressure, and surface casing vent flow in onshore oil and gas wells. Water Resour. Res. 2019, 55, 298–323. [Google Scholar] [CrossRef] [Green Version]
- Natural Resources Canada. Technology Roadmap to Improve Wellbore Integrity: Summary Report; Natural Resources Canada: Ottawa, ON, Canada, 2019; ISBN 978-0-660-29598-5.
- Ingraffea, A.R.; Wells, M.T.; Santoro, R.L.; Shonkoff, S.B.C. Assessment and risk analysis of casing and cement impairment in oil and gas wells in Pennsylvania, 2000–2012. Proc. Natl. Acad. Sci. USA 2014, 111, 10955–10960. [Google Scholar] [CrossRef] [Green Version]
- Brufatto, C.; Cochran, J.; Conn, L.; Power, D.; El-Zeghaty, S.Z.A.A.; Fraboulet, B.; Griffin, T.; James, S.; Munk, T.; Justus, F. From mud to cement—Building gas wells. Oilf. Rev. 2003, 15, 62–76. [Google Scholar]
- Davies, R.J.; Almond, S.; Ward, R.S.; Jackson, R.B.; Adams, C.; Worrall, F.; Herringshaw, L.G.; Gluyas, J.G.; Whitehead, M.A. Oil and gas wells and their integrity: Implications for shale and unconventional resource exploitation. Mar. Pet. Geol. 2014, 56, 239–254. [Google Scholar] [CrossRef] [Green Version]
- Slater, H.J. The Recommended Practice for Surface Casing Vent Flow and Gas Migration Intervention. In Proceedings of the SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers, Florence, Italy, 20–22 September 2010. [Google Scholar]
- Toor, I.A. Problems in Squeeze Cementing. In Proceedings of the Middle East Oil Technical Conference and Exhibition; Society of Petroleum Engineers, Manama, Bahrain, 14–17 March 1983. [Google Scholar]
- Fuller, G.A.; Mercado, S.; Mead, C. Engineered Solutions to Address Deepwater Remedial Cementing Challenges. In Proceedings of the IADC/SPE Drilling Conference and Exhibition; Society of Petroleum Engineers, Fort Worth, TX, USA, 1–3 March 2016. [Google Scholar]
- Cowan, M. Field Study Results Improve Squeeze Cementing Success. In Proceedings of the Production and Operations Symposium, Oklahoma City, OK, USA, 31 March–3 April 2007; Society of Petroleum Engineers: Richardson, TX, USA, 2007. [Google Scholar]
- Saponja, J. Surface casing vent flow and gas migration remedial elimination-new technique proves economic and highly successful. J. Can. Pet. Technol. 1999, 38, PETSOC-99-13-06. [Google Scholar] [CrossRef]
- Wojtanowicz, A.K.; Nishikawa, S.; Rong, X. Diagnosis and Remediation of Sustained Casing Pressure in Wells, Final Report Submitted to the US Department of Interior, Minerals Management Service; Louisiana State University: Baton Rouge, LA, USA, 2001. [Google Scholar]
- Winarga, K.; Dewanto, C.W. Surfactant Treatment Has Amazingly Improved Squeeze Cementing Result. In Proceedings of the Nigeria Annual International Conference and Exhibition; Society of Petroleum Engineers, Calabar, Nigeria, 31 July–7 August 2010. [Google Scholar]
- Clarke, W.J.; McNally, A.C. Ultrafine Cement for Oilwell Cementing. In Proceedings of the Low Permeability Reservoirs Symposium, Denver, CO, USA, 26–28 April 1993. [Google Scholar]
- Moghadam, A.; Castelein, K.; ter Heege, J.; Orlic, B. A study on the hydraulic aperture of microannuli at the casing–cement interface using a large-scale laboratory setup. Geomech. Energy Environ. 2021, 100269, 100269. [Google Scholar] [CrossRef]
- Al-Houti, N.; Al-Othman, M.; Al-Qassar, K.; Al-Ebrahim, A.; Matar, K.; Al Hamad, A. An Alternative Method for Cement Squeeze in North Kuwait: Case Study. In Proceedings of the SPE/ICoTA Coiled Tubing and Well Intervention Conference and Exhibition, Houston, TX, USA, 21–22 March 2017. [Google Scholar]
- Sanders, G.S.; Chambers, M.J.; Lane, R.H. Successful Gas Shutoff with Polymer Gel Using Temperature Modeling and Selective Placement in the Prudhoe Bay Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LO, USA, 25–28 September 1994. [Google Scholar]
- Todorovic, J.; Raphaug, M.; Lindeberg, E.; Vrålstad, T.; Buddensiek, M.-L. Remediation of Leakage through Annular Cement Using a Polymer Resin: A Laboratory Study. Energy Procedia 2016, 86, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Genedy, M.; Kandil, U.F.; Matteo, E.N.; Stormont, J.; Taha, M.M.R. A new polymer nanocomposite repair material for restoring wellbore seal integrity. Int. J. Greenh. Gas Control 2017, 58, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Beharie, C.; Francis, S.; Øvestad, K.H. Resin: An Alternative Barrier Solution Material. In Proceedings of the SPE Bergen One Day Seminar, Bergen, Norway, 22 April 2015. [Google Scholar]
- Wasch, L.J.; Wollenweber, J.; Tambach, T.J. Intentional salt clogging: A novel concept for long-term CO2 sealing. Greenh. Gases Sci. Technol. 2013, 3, 491–502. [Google Scholar] [CrossRef]
- Wolterbeek, T.K.T.; Peach, C.J.; Raoof, A.; Spiers, C.J. Reactive transport of CO2-rich fluids in simulated wellbore interfaces: Flow-through experiments on the 1–6 m length scale. Int. J. Greenh. Gas Control 2016, 54, 96–116. [Google Scholar] [CrossRef]
- Chavez Panduro, E.A.; Torsæter, M.; Gawel, K.; Bjørge, R.; Gibaud, A.; Yang, Y.; Bruns, S.; Zheng, Y.; Sørensen, H.O.; Breiby, D.W. In-Situ X-ray Tomography Study of Cement Exposed to CO2 Saturated Brine. Environ. Sci. Technol. 2017, 51, 9344–9351. [Google Scholar] [CrossRef]
- Wasch, L.; Koenen, M. Injection of a CO2-Reactive Solution for Wellbore Annulus Leakage Remediation. Minerals 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Carey, J.W. Geochemistry of wellbore integrity in CO2 sequestration: Portland cement-steel-brine-CO2 interactions. Rev. Mineral. Geochem. 2013, 77, 505–539. [Google Scholar] [CrossRef]
- Cao, P.; Karpyn, Z.T.; Li, L. Self-healing of cement fractures under dynamic flow of CO2-rich brine. Water Resour. Res. 2015, 51, 4684–4701. [Google Scholar] [CrossRef]
- Vecchia, F.D.; Santos, V.H.J.M.; Schütz, M.K.; Ponzi, G.G.D.; Stepanha, A.S.d.e.; Malfatti, C.d.; da Costa, E.M. Wellbore integrity in a saline aquifer: Experimental steel-cement interface degradation under supercritical CO2 conditions representative of Brazil’s Parana basin. Int. J. Greenh. Gas Control 2020, 98, 103077. [Google Scholar] [CrossRef]
- Abdoulghafour, H.; Luquot, L.; Gouze, P. Characterization of the mechanisms controlling the permeability changes of fractured cements flowed through by CO2-rich brine. Environ. Sci. Technol. 2013, 47, 10332–10338. [Google Scholar] [CrossRef]
- Luquot, L.; Abdoulghafour, H.; Gouze, P. Hydro-dynamically controlled alteration of fractured Portland cements flowed by CO2-rich brine. Int. J. Greenh. Gas Control 2013, 16, 167–179. [Google Scholar] [CrossRef]
- Newell, D.L.; Carey, J.W. Experimental evaluation of wellbore integrity along the cement-rock boundary. Environ. Sci. Technol. 2012, 47, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wolterbeek, T.K.T.; Peach, C.J.; Spiers, C.J. Reaction and transport in wellbore interfaces under CO2 storage conditions: Experiments simulating debonded cement–casing interfaces. Int. J. Greenh. Gas Control 2013, 19, 519–529. [Google Scholar] [CrossRef]
- Jung, H.B.; Kabilan, S.; Carson, J.P.; Kuprat, A.P.; Um, W.; Martin, P.; Dahl, M.; Kafentzis, T.; Varga, T.; Stephens, S.; et al. Wellbore cement fracture evolution at the cement–basalt caprock interface during geologic carbon sequestration. Appl. Geochem. 2014, 47, 1–16. [Google Scholar] [CrossRef]
- Bachu, S.; Bennion, D.B. Experimental assessment of brine and/or CO2 leakage through well cements at reservoir conditions. Int. J. Greenh. Gas Control 2009, 3, 494–501. [Google Scholar] [CrossRef]
- Wolterbeek, T.K.T.; Ruckert, F.; van Moorsel, S.G.; Cornelissen, E.K. Reactive transport and permeability evolution in wellbore defects exposed to periodic pulses of CO2-rich water. Int. J. Greenh. Gas Control 2019, 91, 102835. [Google Scholar] [CrossRef]
- Nguyen, P.; Guthrie, G.D., Jr.; Carey, J.W. Experimental validation of self-sealing in wellbore cement fractures exposed to high-pressure, CO2-saturated solutions. Int. J. Greenh. Gas Control 2020, 100, 103112. [Google Scholar] [CrossRef]
- Mason, H.E.; Du Frane, W.L.; Walsh, S.D.C.; Dai, Z.; Charnvanichborikarn, S.; Carroll, S.A. Chemical and mechanical properties of wellbore cement altered by CO2-rich brine using a multianalytical approach. Environ. Sci. Technol. 2013, 47, 1745–1752. [Google Scholar] [CrossRef]
- Barlet-Gouédard, V.; Rimmele, G.; Porcherie, O.; Quisel, N.; Desroches, J. A solution against well cement degradation under CO2 geological storage environment. Int. J. Greenh. Gas Control 2009, 3, 206–216. [Google Scholar] [CrossRef]
- Jahanbakhsh, A.; Liu, Q.; Hadi Mosleh, M.; Agrawal, H.; Farooqui, N.M.; Buckman, J.; Recasens, M.; Maroto-Valer, M.; Korre, A.; Durucan, S. An Investigation into CO2–Brine–Cement–Reservoir Rock Interactions for Wellbore Integrity in CO2 Geological Storage. Energies 2021, 14, 5033. [Google Scholar] [CrossRef]
- Cao, P.; Karpyn, Z.T.; Li, L. Dynamic alterations in wellbore cement integrity due to geochemical reactions in CO2-rich environments. Water Resour. Res. 2013, 49, 4465–4475. [Google Scholar] [CrossRef]
- Brunet, J.-P.L.; Li, L.; Karpyn, Z.T.; Huerta, N.J. Fracture opening or self-sealing: Critical residence time as a unifying parameter for cement–CO2–brine interactions. Int. J. Greenh. Gas Control 2016, 47, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Iyer, J.; Walsh, S.D.C.; Hao, Y.; Carroll, S.A. Incorporating reaction-rate dependence in reaction-front models of wellbore-cement/carbonated-brine systems. Int. J. Greenh. Gas Control 2017, 59, 160–171. [Google Scholar] [CrossRef]
- Wolterbeek, T.K.T.; Raoof, A. Meter-Scale Reactive Transport Modeling of CO2-Rich Fluid Flow along Debonded Wellbore Casing-Cement Interfaces. Environ. Sci. Technol. 2018, 52, 3786–3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthrie, G.D.; Pawar, R.J.; Carey, J.W.; Karra, S.; Harp, D.R.; Viswanathan, H.S. The mechanisms, dynamics, and implications of self-sealing and CO2 resistance in wellbore cements. Int. J. Greenh. Gas Control 2018, 75, 162–179. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R.; Zhu, C.; Chou, I. An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO42−. Mar. Chem. 2006, 98, 131–139. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1482208687. [Google Scholar]
- Garba, M.D.; Petitt, I.; MacPhee, D.E. Sodium Silicate Cement Squeeze Best Practice. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Victoria Island, Lagos, 5–7 August 2014. [Google Scholar]
- Giannaros, P.; Kanellopoulos, A.; Al-Tabbaa, A. Sealing of cracks in cement using microencapsulated sodium silicate. Smart Mater. Struct. 2016, 25, 84005. [Google Scholar] [CrossRef]
- Dimas, D.; Giannopoulou, I.; Panias, D. Polymerization in sodium silicate solutions: A fundamental process in geopolymerization technology. J. Mater. Sci. 2009, 44, 3719–3730. [Google Scholar] [CrossRef]
- ISO 10426-2 Petroleum and Natural Gas Industries—Cements and Materials for Well Cementing—Part. 2: Testing of Well Cements (ANSI/API Recommended Practice 10B-2); International Organization for Standardization: Geneva, Switzerland, 2005.
- Sigma-Aldrich Product No. 338443 (Reagent Grade Sodium Silicate Solution). Available online: https://www.sigmaaldrich.com/catalog/product/sigald/338443 (accessed on 4 April 2020).
- Energy Safety Canada. Wellbore Remediation; An. Industry Recommended Practice (IRP) for the Canadian Oil and Gas. Industry; Energy Safety Canada: Calgary, AB, Canada, 2020. [Google Scholar]
- Guéguen, Y.; Palciauskas, V. Introduction to the Physics of Rocks; Princeton University Press: Princeton, NJ, USA, 1994; ISBN 0691034524. [Google Scholar]
- Wolterbeek, T.K.T.; Hangx, S.J.T. Supporting Data Set for Manuscript “Remediation of Annular Gas. Migration Along Cemented Wellbores Using Reactive Mineral. Fluids: Experimental Assessment of Sodium Bicarbonate And Sodium Silicate-Based Solutions”; Yoda Data Repository, Utrecht University: Utrecht, The Netherlands, 2021; Available online: https://public.yoda.uu.nl/UU01/K3B1IB.html (accessed on 30 July 2021). [CrossRef]
- Fleury, M.; Deschamps, H. Electrical conductivity and viscosity of aqueous NaCl solutions with dissolved CO2. J. Chem. Eng. Data 2008, 53, 2505–2509. [Google Scholar] [CrossRef]
- Islam, A.W.; Carlson, E.S. Viscosity models and effects of dissolved CO2. Energy Fuels 2012, 26, 5330–5336. [Google Scholar] [CrossRef]
- Mao, S.; Duan, Z. The Viscosity of Aqueous Alkali-Chloride Solutions up to 623 K, 1000 bar, and High Ionic Strength. Int. J. Thermophys. 2009, 30, 1510–1523. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W.; Yang, Q. The viscosity properties of sodium silicate solutions. J. Solut. Chem. 2008, 37, 73–83. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport. Phenomena, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Montgomery, C.T. Implications of cementing for well production and performance. In Well Cementing; Nelson, E.B., Guillot, D., Eds.; Schlumberger: Sugar Land, TX, USA, 2006; p. 773. ISBN 978-097885300-6. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Academic Press Limited: London, UK, 1992; ISBN 0-12-683900-X. [Google Scholar]
- Engkvist, I.; Albinsson, Y.; Johansson Engkvist, W. The Long-Term Stability of Cement: Leaching Tests; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 1996. [Google Scholar]
- Huerta, N.J.; Hesse, M.A.; Bryant, S.L.; Strazisar, B.R.; Lopano, C. Reactive transport of CO2-saturated water in a cement fracture: Application to wellbore leakage during geologic CO2 storage. Int. J. Greenh. Gas Control 2016, 44, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-S.; Young, D.; Nešić, S.; Gray, L.G.S. Wellbore integrity and corrosion of carbon steel in CO2 geologic storage environments: A literature review. Int. J. Greenh. Gas Control 2013, 16, S70–S77. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials—I: The crystallization pressure of large crystals. J. Cryst. Growth 2005, 282, 455–469. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Dzombak, D.A.; Lowry, G.V.; Thaulow, N. Degradation of well cement by CO2 under geologic sequestration conditions. Environ. Sci. Technol. 2007, 41, 4787–4792. [Google Scholar] [CrossRef] [PubMed]
- Liteanu, E.; Spiers, C.J. Fracture healing and transport properties of wellbore cement in the presence of supercritical CO2. Chem. Geol. 2011, 281, 195–210. [Google Scholar] [CrossRef]
- Duguid, A.; Radonjic, M.; Scherer, G.W. Degradation of cement at the reservoir/cement interface from exposure to carbonated brine. Int. J. Greenh. Gas Control 2011, 5, 1413–1428. [Google Scholar] [CrossRef]
- Duguid, A.; Radonjic, M.; Bruant, R.; Mandecki, T.; Scherer, G.; Celia, M. The Effect of CO2 Sequestration on Oil Well Cements. In Proceedings of the Greenhouse Gas Technologies Conference (GHGT–7), Vancouver, BC, Canada, 5–9 September 2004. [Google Scholar]
- Andersson, K.; Allard, B.; Bengtsson, M.; Magnusson, B. Chemical composition of cement pore solutions. Cem. Concr. Res. 1989, 19, 327–332. [Google Scholar] [CrossRef]
- Poyet, S.; Sellier, A.; Capra, B.; Foray, G.; Torrenti, J.-M.; Cognon, H.; Bourdarot, E. Chemical modelling of alkali silica reaction: Influence of the reactive aggregate size distribution. Mater. Struct. 2007, 40, 229–239. [Google Scholar] [CrossRef]
- Vollpracht, A.; Lothenbach, B.; Snellings, R.; Haufe, J. The pore solution of blended cements: A review. Mater. Struct. 2016, 49, 3341–3367. [Google Scholar] [CrossRef] [Green Version]
- Kouassi, S.S.; Tognonvi, M.T.; Soro, J.; Rossignol, S. Consolidation mechanism of materials obtained from sodium silicate solution and silica-based aggregates. J. Non-Cryst. Solids 2011, 357, 3013–3021. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Taylor, K.C. Evaluation of sodium silicate/urea gels used for water shut-off treatments. J. Pet. Sci. Eng. 2005, 48, 141–160. [Google Scholar] [CrossRef]
- Knoblich, B.; Gerber, T. Aggregation in SiO2 sols from sodium silicate solutions. J. Non-Cryst. Solids 2001, 283, 109–113. [Google Scholar] [CrossRef]
- Parcevaux, P.A.; Sault, P.H. Cement Shrinkage and Elasticity: A New Approach for A Good Zonal Isolation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 16–19 September 1984. [Google Scholar]
- Kawaguchi, T.; Hishikura, H.; Iura, J. Shrinkage behavior of silica gels during drying. J. Non-Cryst. Solids 1988, 100, 220–225. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Nugteren, H.W. Synthesis of ettringite: A way to deal with the acid wastewaters of aluminium anodising industry. Water Res. 2005, 39, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Cadorin, L.; Rubio, J. Sulphate ions removal from an aqueous solution: I. Co-precipitation with hydrolysed aluminum-bearing salts. Miner. Eng. 2010, 23, 1220–1226. [Google Scholar] [CrossRef]
- Ronquim, F.M.; Cotrim, M.E.B.; Guilhen, S.N.; Bernardo, A.; Seckler, M.M. Improved barium removal and supersaturation depletion in wastewater by precipitation with excess sulfate. J. Water Process. Eng. 2018, 23, 265–276. [Google Scholar] [CrossRef]
- Dou, W.; Zhou, Z.; Jiang, L.-M.; Jiang, A.; Huang, R.; Tian, X.; Zhang, W.; Chen, D. Sulfate removal from wastewater using ettringite precipitation: Magnesium ion inhibition and process optimization. J. Environ. Manag. 2017, 196, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Wolterbeek, T.K.T.; Cornelissen, E.K.; Nolan, S.; Todea, F.; Stam, W.; Roggeband, S.M.; Dam, L.; van Riet, E.J.; Ruckert, F.; Keultjes, W.J.G. Restoration of annular zonal isolation using localized casing expansion (LCE) technology: A proof of concept based on laboratory studies and field trial results. J. Pet. Sci. Eng. 2021, 197, 108103. [Google Scholar] [CrossRef]
- Green, C.; Evans, R.; Fry, B.; Wruck, W.S. New Technology Closes Micro-Annular Flow Paths in the Wellbore, Stopping Downhole Gas from Escaping to the Surface. In Proceedings of the SPE/AAPG/SEG Unconventional Resources Technology Conference, Houston, TX, USA, 26–28 July 2021. [Google Scholar]





| Properties | Sample BC-1 | Sample SIL-1 |
|---|---|---|
| [m] | 6 | 6 |
| [mm] | 8 | 8 |
| internal defect geometry | internal structure presumably similar to sample SIL-1 | micro-annulus plus short-ranged, crescent-shaped voids |
| [°C] | 50 | 50 |
| reactive flow experiment duration [h] | 91.3 | 300 |
| [MPa] | 0.3 | 0.6 |
| [MPa] | 0.6 | 6.0 |
| [mL h−1] | 1.6 | 13.2 |
| total volume of reactive fluid injected [mL] | 133 | 35 |
| [mPa s] | 0.55 | 0.55 |
| [m2] | 3.8 × 10−13 | 8.3 × 10−13 |
| [m2] | 9.0 × 10−14 | in range of 10−17–10−19 |
| [μm] | 17.3 | 22.1 |
| [mL] | 2.6 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolterbeek, T.K.T.; Hangx, S.J.T. Remediation of Annular Gas Migration along Cemented Wellbores Using Reactive Mineral Fluids: Experimental Assessment of Sodium Bicarbonate and Sodium Silicate-Based Solutions. Energies 2021, 14, 7507. https://doi.org/10.3390/en14227507
Wolterbeek TKT, Hangx SJT. Remediation of Annular Gas Migration along Cemented Wellbores Using Reactive Mineral Fluids: Experimental Assessment of Sodium Bicarbonate and Sodium Silicate-Based Solutions. Energies. 2021; 14(22):7507. https://doi.org/10.3390/en14227507
Chicago/Turabian StyleWolterbeek, Timotheus K. T., and Suzanne J. T. Hangx. 2021. "Remediation of Annular Gas Migration along Cemented Wellbores Using Reactive Mineral Fluids: Experimental Assessment of Sodium Bicarbonate and Sodium Silicate-Based Solutions" Energies 14, no. 22: 7507. https://doi.org/10.3390/en14227507






