Comparative Study on Quality of Fuel Pellets from Switchgrass Treated with Different White-Rot Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Fungal Growth and Inoculation Preparation
2.3. Solid-State Fermentation of Substrate
2.4. Experimental Design
2.5. Pelletization of the Switchgrass
2.6. Pellet Unit Density and Dimensional Stability
2.7. Pellet Tensile Strength
2.8. Moisture Absorption Isotherm of Pellets
2.9. Raw Material and Pellet Characterization
2.9.1. Ultimate Analysis
2.9.2. Higher Heating Value and Ash Content
2.9.3. Thermogravimetry Analysis (TGA)
2.9.4. Proximate Analysis
2.9.5. Chemical Composition
2.9.6. Microstructural Examination
3. Results and Discussion
3.1. Switchgrass Pellet Produced
3.2. Properties of the Switchgrass Pellets
3.3. Effects of Independent Variables and Their Interactions on the Switchgrass Pellet Quality
3.4. Optimization of the Fungal Pretreatment for Fuel Pellet Production
3.5. Moisture Absorption of the Switchgrass Pellets
3.6. Ultimate and Proximate Composition of the Switchgrass
3.7. Chemical Composition and Higher Heating Value (HHV)
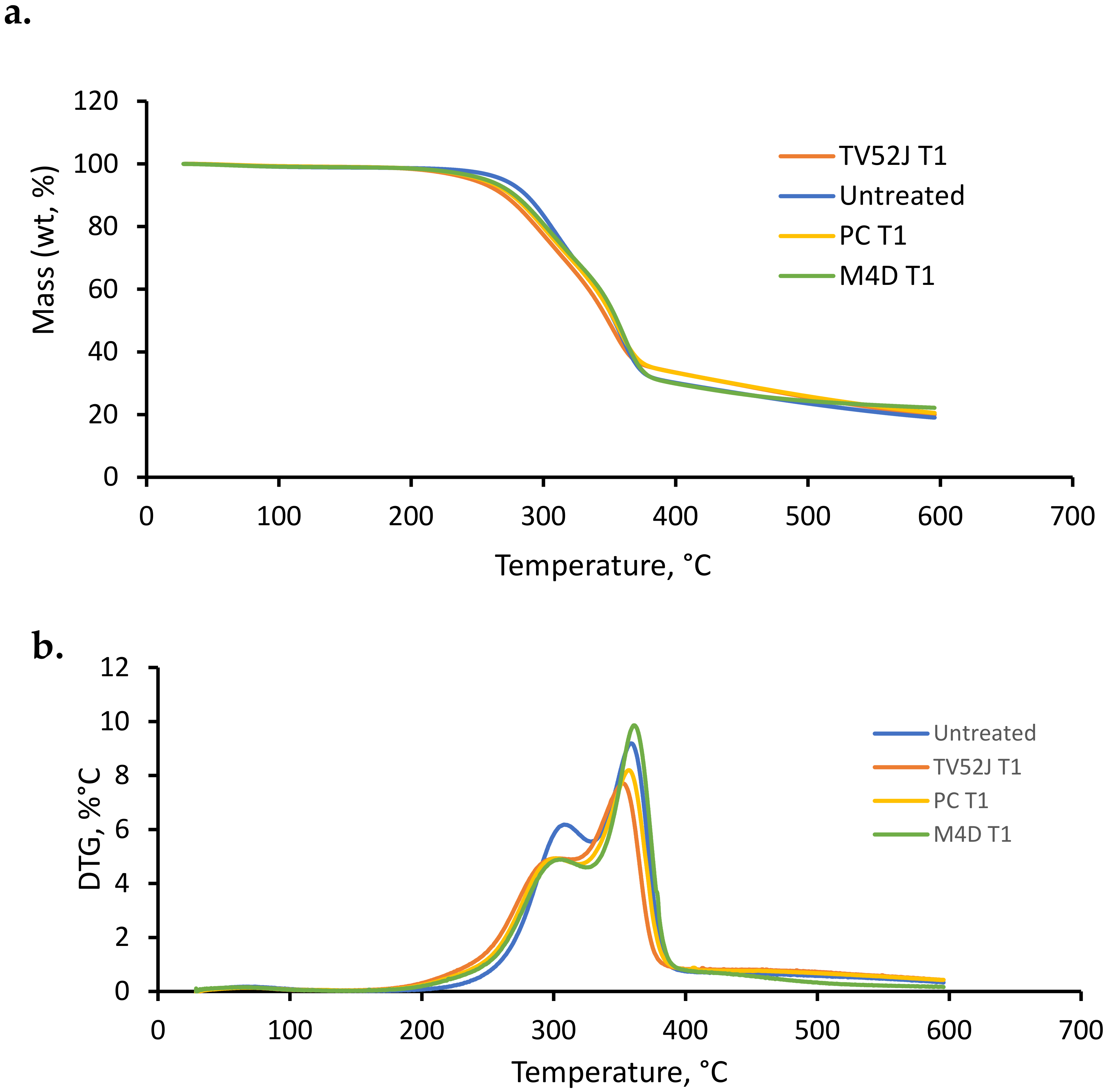
3.8. TGA/DTG Analysis
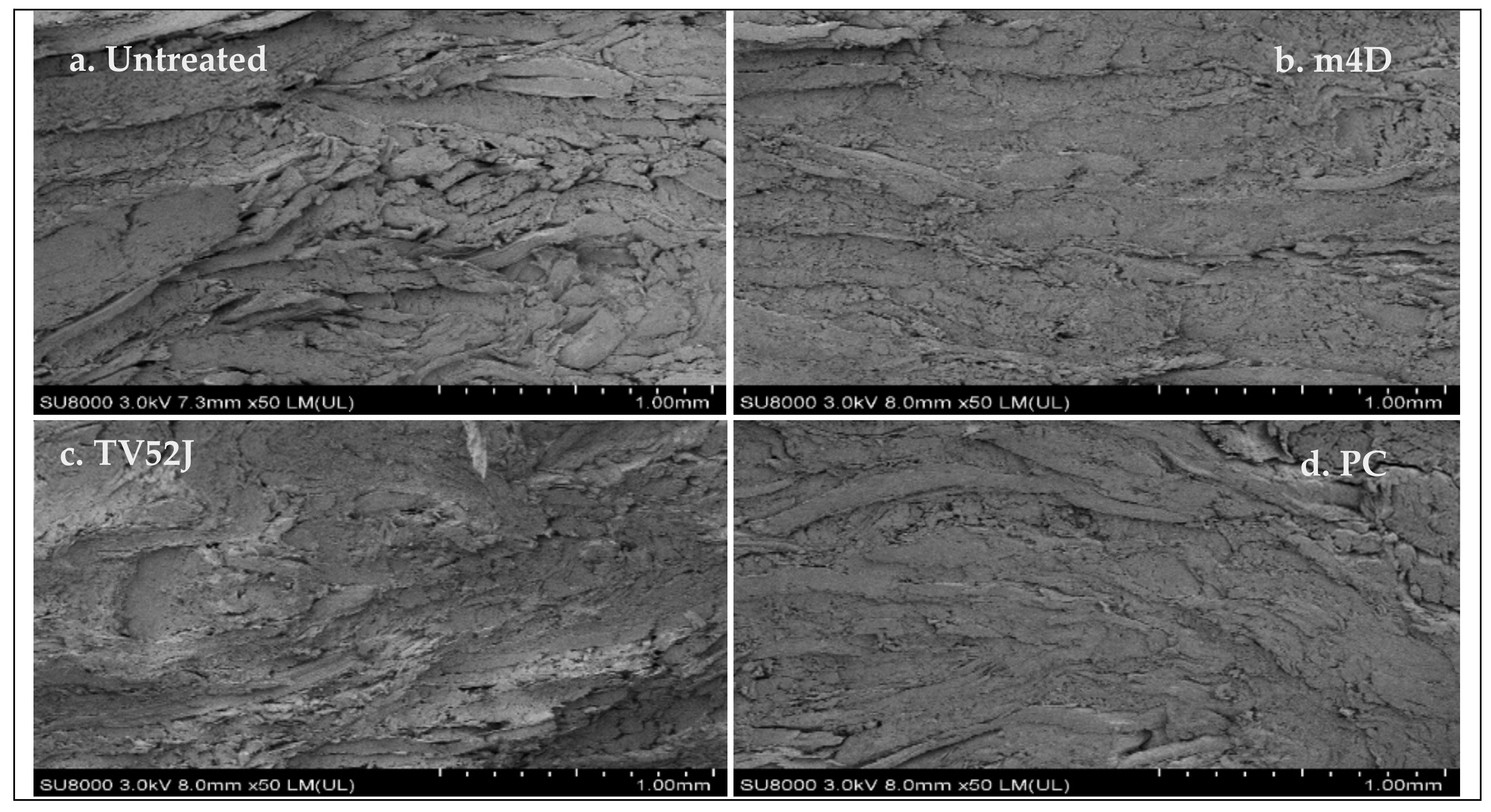
3.9. Microstructure Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilvari, H.; de Jong, W.; Schott, D.L. Quality Parameters Relevant for Densification of Bio-Materials: Measuring Methods and Affecting Factors—A Review. Biomass Bioenergy 2019, 120, 117–134. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Dalai, A. Densification of Agricultural Wastes and Forest Residues: A Review on Influential Parameters and Treatments. In Recent Advancements in Biofuels and Bioenergy Utilization; Nanda, S., Prakash, K.S., Mohanty, P., Eds.; Springer: Singapore, 2018; pp. 27–51. [Google Scholar] [CrossRef]
- Zamorano, M.; Popov, V.; Rodríguez, M.L.; García-Maraver, A. A Comparative Study of Quality Properties of Pelletized Agricultural and Forestry Lopping Residues. Renew. Energy 2011, 36, 3133–3140. [Google Scholar] [CrossRef]
- Lehtikangas, P. Quality Properties of Pelletised Sawdust, Logging Residues and Bark. Biomass Bioenergy 2001, 20, 351–360. [Google Scholar] [CrossRef]
- Picchio, R.; Latterini, F.; Venanzi, R.; Stefanoni, W.; Suardi, A.; Tocci, D.; Pari, L. Pellet Production from Woody and Non-Woody Feedstocks: A Review on Biomass Quality Evaluation. Energies 2020, 13, 2937. [Google Scholar] [CrossRef]
- Gilbert, P.; Ryu, C.; Sharifi, V.; Swithenbank, J. Effect of Process Parameters on Pelletisation of Herbaceous Crops. Fuel 2009, 88, 1491–1497. [Google Scholar] [CrossRef]
- Carone, M.T.; Pantaleo, A.; Pellerano, A. Influence of Process Parameters and Biomass Characteristics on the Durability of Pellets from the Pruning Residues of Olea europaea L. Biomass Bioenergy 2011, 35, 402–410. [Google Scholar] [CrossRef]
- Tilay, A.; Azargohar, R.; Drisdelle, M.; Dalai, A.; Kozinski, J. Canola Meal Moisture-Resistant Fuel Pellets: Study on the Effects of Process Variables and Additives on the Pellet Quality and Compression Characteristics. Ind. Crop. Prod. 2015, 63, 337–348. [Google Scholar] [CrossRef]
- Wongsiriamnuay, T.; Tippayawong, N. Effect of Densification Parameters on the Properties of Maize Residue Pellets. Biosyst. Eng. 2015, 139, 111–120. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Kang, K.; Bond, T.; Karunakaran, C.; Dalai, A.K.; Kozinski, J.A. Effects of Bio-Additives on the Physicochemical Properties and Mechanical Behavior of Canola Hull Fuel Pellets. Renew. Energy 2019, 132, 296–307. [Google Scholar] [CrossRef]
- Onu Olughu, O.; Tabil, L.G.; Dumonceaux, T. Effect of Ultrasonic Pretreatment on the Chemical Composition and Pellet Quality of Camelina Straw. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019. [Google Scholar] [CrossRef]
- Sokhansanj, S.; Mani, S.; Bi, X.; Zaini, P.; Tabil, L. Binderless Pelletization of Biomass. In Proceedings of the 2005 American Society of Agricultural and Biological Engineers (ASABE) Annual Meeting, Tampa, FL, USA, 17–20 July 2005. [Google Scholar] [CrossRef]
- Mupondwa, E.; Li, X.; Tabil, L.; Sokhansanj, S.; Adapa, P. Status of Canada’s Lignocellulosic Ethanol: Part I: Pretreatment Technologies. Renew. Sustain. Energy Rev. 2017, 72, 178–190. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Xiao, Z.; Liang, J.; Li, H.; Cao, L.; Wang, H.; Chen, X.; Zeng, G. A Comparative Study of Biomass Pellet and Biomass-Sludge Mixed Pellet: Energy Input and Pellet Properties. Energy Convers. Manag. 2016, 126, 509–515. [Google Scholar] [CrossRef]
- Lam, P.S.; Sokhansanj, S.; Bi, X.T.; Lim, C.J.; Larsson, S.H. Drying Characteristics and Equilibrium Moisture Content of Steam-Treated Douglas Fir (Pseudotsuga Menziesii L.). Bioresour. Technol. 2012, 116, 396–402. [Google Scholar] [CrossRef]
- Zhang, P.; Deines, T.; Nottingham, D.; Pei, Z.J.; Wang, D.; Wu, X. Ultrasonic Vibration-Assisted Pelleting of Biomass: A Designed Experimental Investigation on Pellet Quality and Sugar Yield. In Proceedings of the ASME 2010 International Manufacturing Science and Engineering Conference, Erie, PA, USA, 12–15 October 2010; pp. 137–145. [Google Scholar]
- Sarker, T.R.; Azargohar, R.; Dalai, A.K.; Venkatesh, M. Physicochemical and Fuel Characteristics of Torrefied Agricultural Residues for Sustainable Fuel Production. Energy Fuels 2020, 34, 14169–14181. [Google Scholar] [CrossRef]
- Singh, R.; Shukla, A.; Tiwari, S.; Srivastava, M. A Review on Delignification of Lignocellulosic Biomass for Enhancement of Ethanol Production Potential. Renew. Sustain. Energy Rev. 2014, 32, 713–728. [Google Scholar] [CrossRef]
- Laschi, A.; Marchi, E.; Gonz Alez-García, S. Environmental Performance of Wood Pellets’ Production through Life Cycle Analysis. Biosyst. Eng. 2015, 139, 111–120. [Google Scholar] [CrossRef]
- Canam, T.; Town, J.; Iroba, K.; Tabil, L.; Dumonceaux, T. Pretreatment of Lignocellulosic Biomass Using Microorganisms: Approaches, Advantages, and Limitations. In Sustainable Degradation of Lignocellulosic Biomass—Techniques, Applications and Commercialization; Silva, A.K.C., Da, S.S., Eds.; IntechOpen: Rijeka, Croatia, 2013; pp. 181–206. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Li, Y. Fungal Pretreatment of Lignocellulosic Biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Canam, T.; Town, J.R.; Tsang, A.; McAllister, T.A.; Dumonceaux, T.J. Biological Pretreatment with a Cellobiose Dehydrogenase-Deficient Strain of Trametes Versicolor Enhances the Biofuel Potential of Canola Straw. Bioresour. Technol. 2011, 102, 10020–10027. [Google Scholar] [CrossRef]
- Kalinoski, R.M.; Flores, H.D.; Thapa, S.; Tuegel, E.R.; Bilek, M.A.; Reyes-Mendez, E.Y.; West, M.J.; Dumonceaux, T.J.; Canam, T. Pretreatment of Hardwood and Miscanthus with Trametes Versicolor for Bioenergy Conversion and Densification Strategies. Appl. Biochem. Biotechnol. 2017, 183, 1401–1413. [Google Scholar] [CrossRef]
- Kalyani, D.; Lee, K.M.; Kim, T.S.; Li, J.; Dhiman, S.S.; Kang, Y.C.; Lee, J.K. Microbial Consortia for Saccharification of Woody Biomass and Ethanol Fermentation. Fuel 2013, 107, 815–822. [Google Scholar] [CrossRef]
- Guo, P.; Mochidzuki, K.; Cheng, W.; Zhou, M.; Gao, H.; Zheng, D.; Wang, X.; Cui, Z. Effects of Different Pretreatment Strategies on Corn Stalk Acidogenic Fermentation Using a Microbial Consortium. Bioresour. Technol. 2011, 102, 7526–7531. [Google Scholar] [CrossRef]
- Rouches, E.; Herpoël-Gimbert, I.; Steyer, J.P.; Carrere, H. Improvement of Anaerobic Degradation by White-Rot Fungi Pretreatment of Lignocellulosic Biomass: A Review. Renew. Sustain. Energy Rev. 2016, 59, 179–198. [Google Scholar] [CrossRef]
- Gao, W.; Tabil, L.G.; Liu, Q.; Zhao, R.; Zhang, M. Experimental Study on the Effect of Solid-State Fermentation on Pellet Density and Strength of Corn Stover. J. Biobased Mater. Bioenergy 2019, 13, 840–847. [Google Scholar] [CrossRef]
- Gao, W.; Tabil, L.G.; Dumonceaux, T.; Espinel Ríos, S.; Zhao, R. Optimization of Biological Pretreatment to Enhance the Quality of Wheat Straw Pellets. Biomass Bioenergy 2017, 97, 77–89. [Google Scholar] [CrossRef]
- Onu Olughu, O.; Tabil, L.G.; Dumonceaux, T. Ultrasonic Delignification and Microstructural Characterization of Switchgrass. Energies 2021, 14, 263. [Google Scholar] [CrossRef]
- Dumonceaux, T.; Bartholomew, K.; Valeanu, L.; Charles, T.; Archibald, F. Cellobiose Dehydrogenase Is Essential for Wood Invasion and Nonessential for Kraft Pulp Delignification by Trametes Versicolor. Enzym. Microb. Technol. 2001, 29, 478–489. [Google Scholar] [CrossRef]
- Schiling, C.; Wohler, M.; Yazdanpanah, F.; Bi, X.; Lau, A.; Lim, J.C.; Sokhansanj, S.; Pels, S.K. Development of a Novel Wood Pellet Durability Tester for Small Samples. In Proceedings of the 2015 World Sustainable Energy Days—Energy Efficiency & Biomass; World Sustainable Energy Days, Wels, Austria, 25–27 February 2015. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Tabil, L.G. Effect of Microwave-Chemical Pre-Treatment on Compression Characteristics of Biomass Grinds. Biosyst. Eng. 2011, 108, 36–45. [Google Scholar] [CrossRef]
- American Society of Agricultural Engineers. Standard Test for Moisture Content Measurement of Forages; ANSI/ASAE S358.3; American Society of Agricultural Engineers: St. Joseph, MI, USA, 2012. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 Analytical Procedure—Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2012.
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Evaluation of Compaction Equations Applied to Four Biomass Species. Can. Biosyst. Eng. Le Genie Biosyst. Can. 2004, 46, 55–61. [Google Scholar]
- Nottingham, D.; Pei, Z.J.; Zhang, M.; Deines, T. The Effects of Pelleting Time and Ultrasonic Power during Ultrasonic Vibration-Assisted Pelleting of Switchgrass. In Proceedings of the 2010 Industrial Engineering Research Conference, Reno, NV, USA, 26–28 November 2010; pp. 1–6. [Google Scholar]
- Gao, W.; Lei, Z.; Tabil, L.G.; Zhao, R. Biological Pretreatment by Solid-State Fermentation of Oat Straw to Enhance Physical Quality of Pellets. J. Chem. 2020, 2020, 3060475. [Google Scholar] [CrossRef]
- Liu, S.; Wu, S.; Pang, C.; Li, W.; Dong, R. Microbial Pretreatment of Corn Stovers by Solid-State Cultivation of Phanerochaete Chrysosporium for Biogas Production. Appl. Biochem. Biotechnol. 2013, 172, 1365–1376. [Google Scholar] [CrossRef]
- Noorfidza, H.; Muhammad, A. Effect of Particle Size on Mechanical Properties of Pellets Made from Biomass Blends. Procedia Eng. 2016, 148, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Reid, I.D. Solid-State Fermentations for Biological Delignification. Enzym. Microb. Technol. 1989, 11, 786–803. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Shah, A. Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Bals, B.; Rogers, C.; Jin, M.; Balan, V.; Dale, B. Evaluation of Ammonia Fibre Expansion (AFEX) Pretreatment for Enzymatic Hydrolysis of Switchgrass Harvested in Different Seasons and Locations. Biotechnol. Biofuels 2010, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Wang, Y.; Wen, Z. Alkali (NaOH) Pretreatment of Switchgrass by Radio Frequency-Based Dielectric Heating. Appl. Biochem. Biotechnol. 2008, 148, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Sokhansanj, S.; Lau, A.K.; Lim, J.; Bi, X.T. Moisture Adsorption Rate and Durability of Commercial Softwood Pellets in a Humid Environment. Biosyst. Eng. 2021, 203, 1–8. [Google Scholar] [CrossRef]
- Sarkar, M.; Kumar, A.; Tumuluru, J.S.; Patil, K.N.; Bellmer, D.D. Gasification Performance of Switchgrass Pretreated with Torrefaction and Densification. Appl. Energy 2014, 127, 194–201. [Google Scholar] [CrossRef]
- Via, B.K.; Adhikari, S.; Taylor, S. Modeling for Proximate Analysis and Heating Value of Torrefied Biomass with Vibration Spectroscopy. Bioresour. Technol. 2013, 133, 1–8. [Google Scholar] [CrossRef]
- Frodeson, S.; Henriksson, G.; Berghel, J. Pelletizing Pure Biomass Substances to Investigate the Mechanical Properties and Bonding Mechanisms. BioResources 2018, 13, 1202–1222. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Sykes, R.; Davis, M.F.; Charles Brummer, E.; Ragauskas, A.J. Chemical Profiles of Switchgrass. Bioresour. Technol. 2010, 101, 3253–3257. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, Q.; He, Z.; Wang, Z.; Wang, X.; Sun, J. Effect of Biodegradation on Thermogravimetric and Chemical Characteristics of Hardwood and Softwood by Brown-Rot Fungus. Bioresour. Technol. 2016, 211, 443–450. [Google Scholar] [CrossRef]
- Yub Harun, N.; Muhammad, A. Torrefaction of Agricultural and Forestry Biomass Using TGA-FTIR-MS. In Proceedings of the Sixth International Exergy, Energy and Environment Symposium, Rize, Turkey, 1–4 July 2013; pp. 317–325. [Google Scholar]
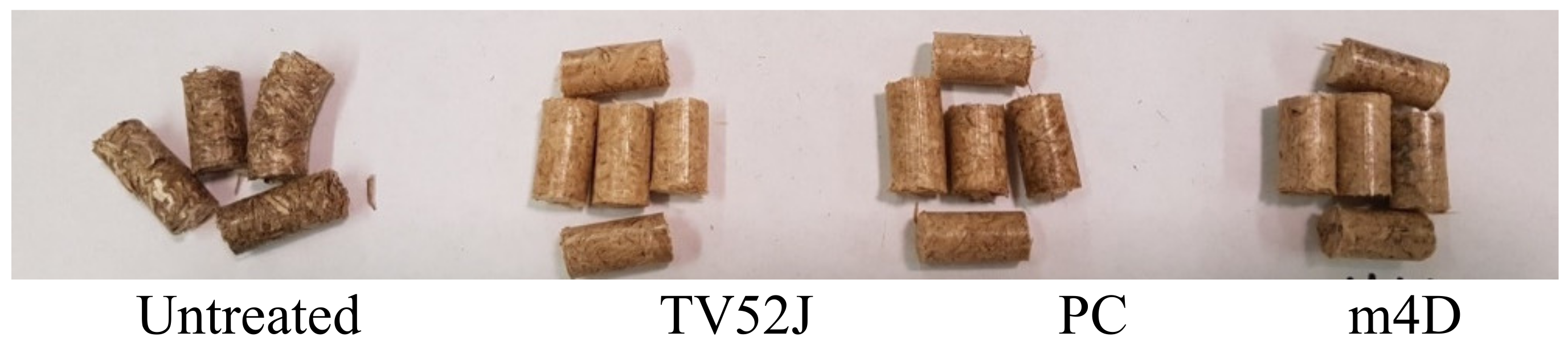
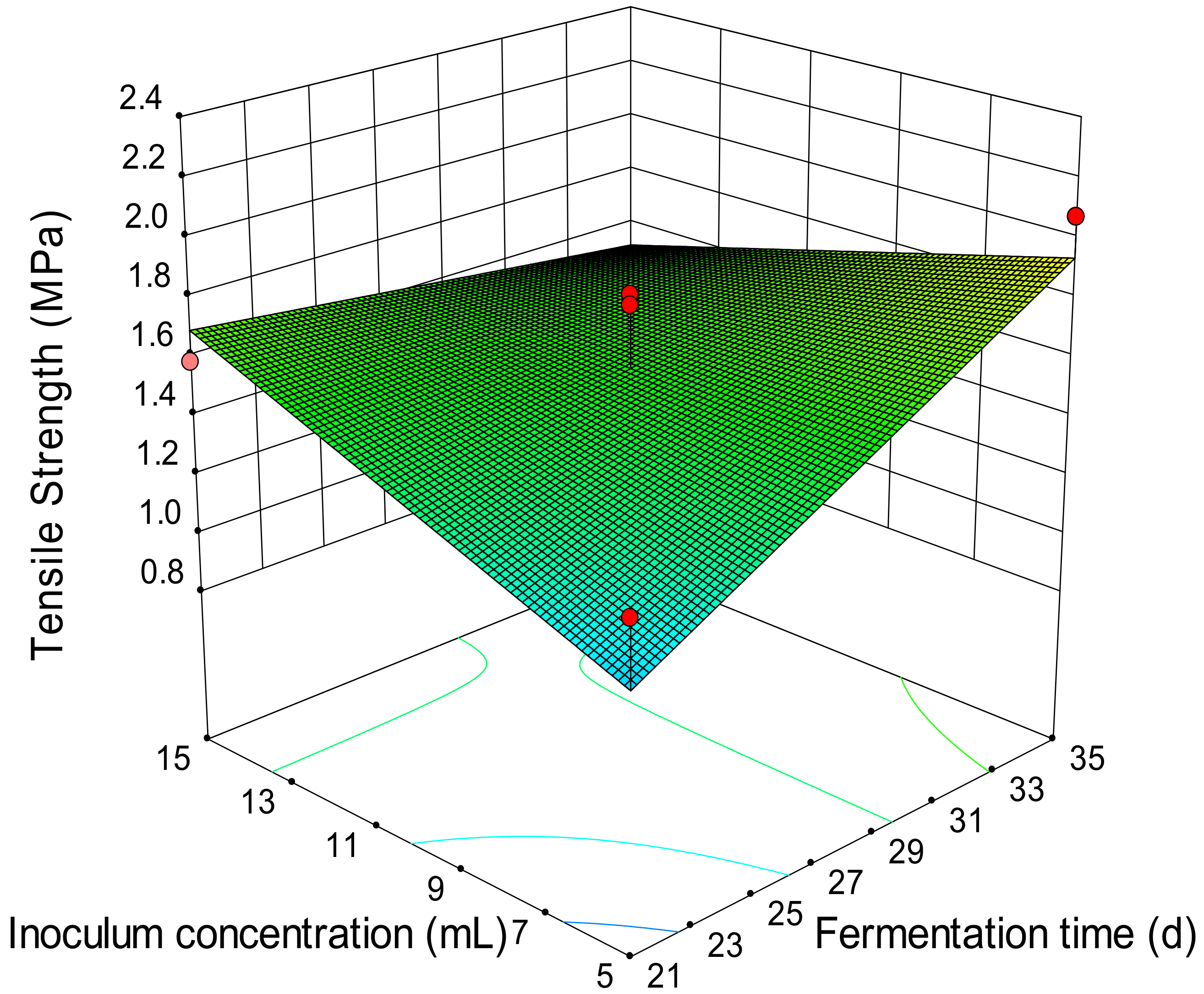
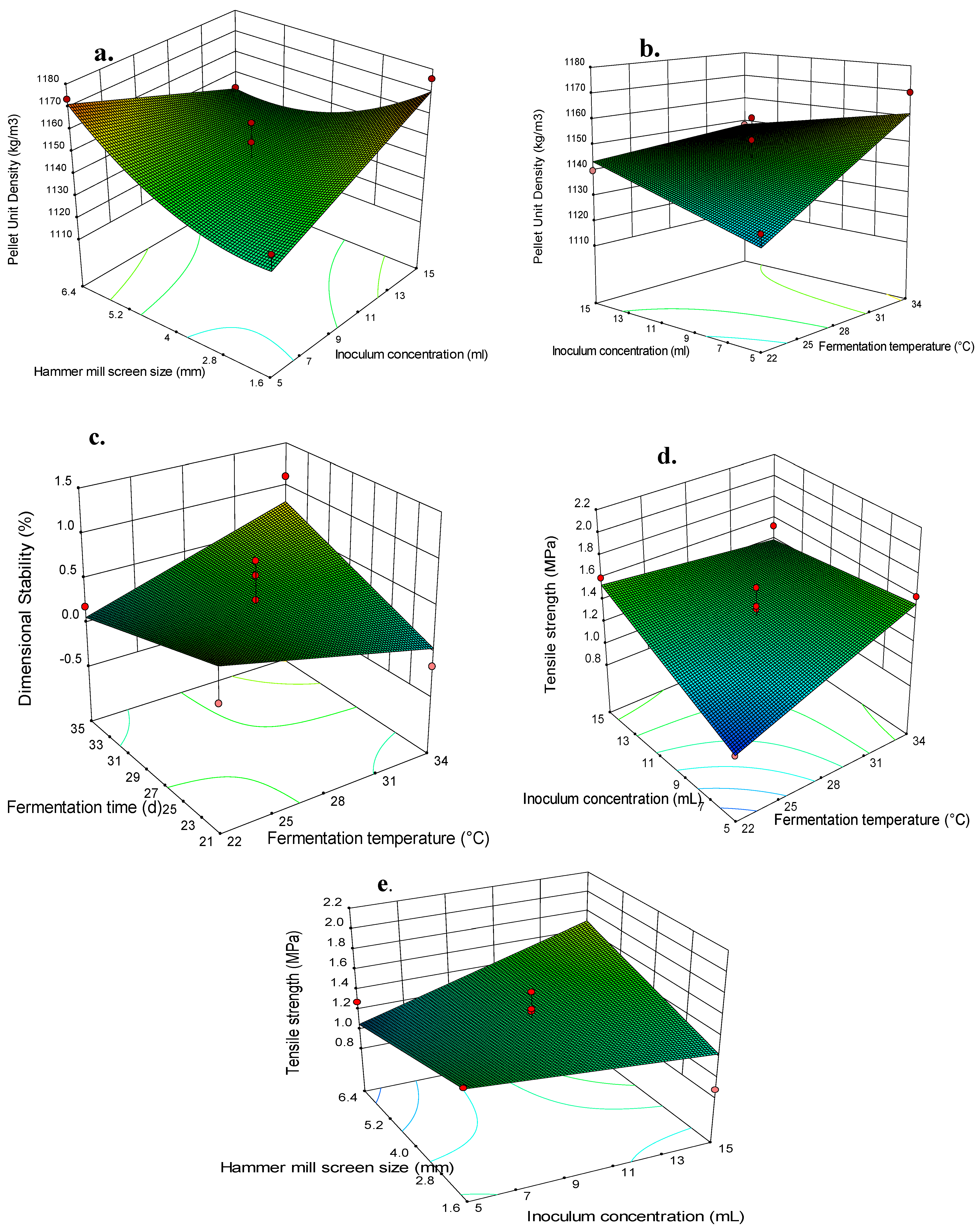
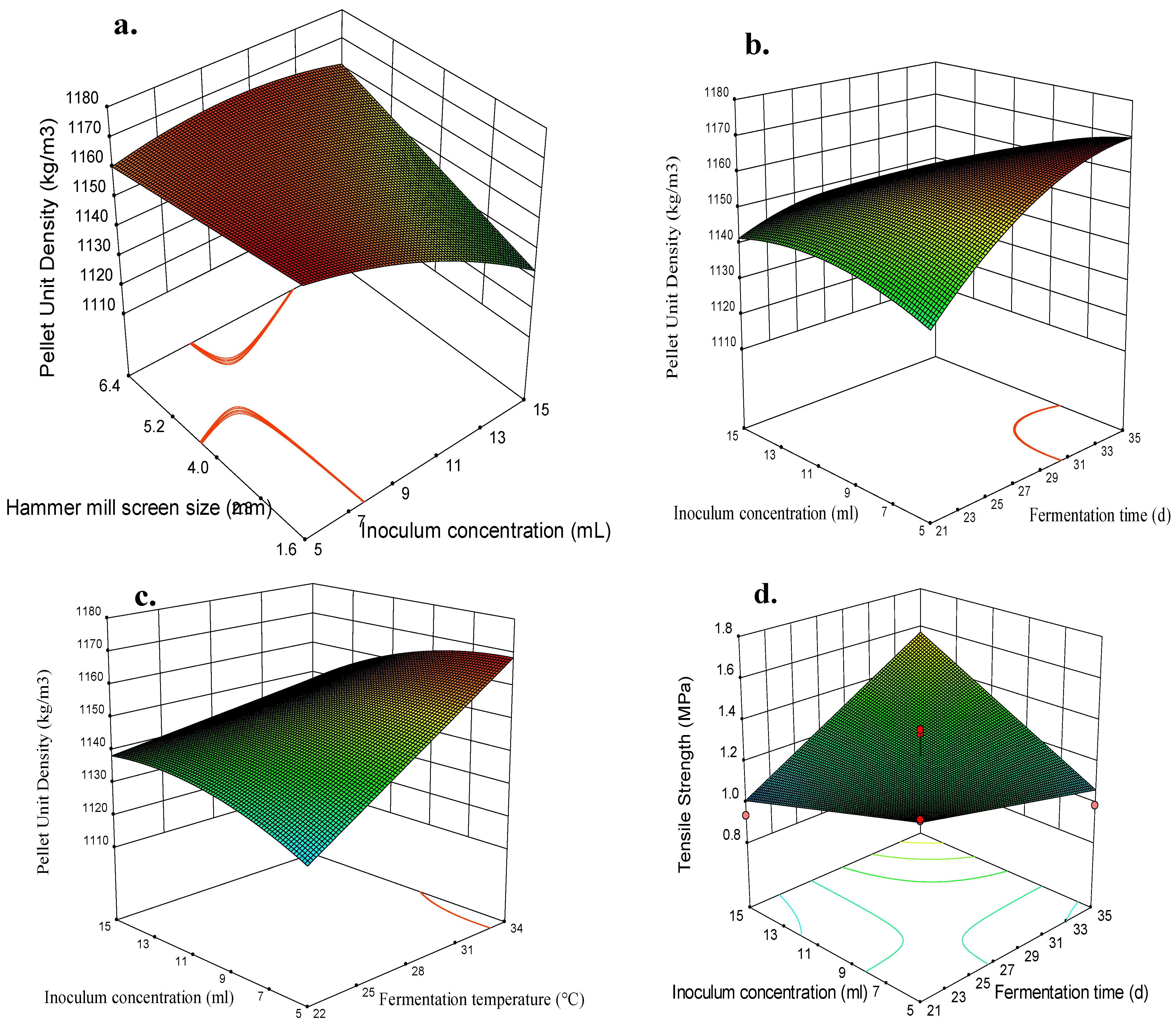

| Fungal Strain | Analysis of Variance (ANOVA) | |||||
|---|---|---|---|---|---|---|
| Sum of | Mean | F value | ρ value | |||
| Source | Squares | df | Square | |||
| Model | 0.71 | 4 | 0.18 | 6.87 | 0.0008 *** | |
| m4D | 0.42 | 1 | 0.42 | 16.38 | 0.0005 *** | |
| 0.09 | 1 | 0.091 | 3.55 | 0.0716 * | ||
| 0.15 | 1 | 0.150 | 5.83 | 0.0238 ** | ||
| Lack of Fit | 0.46 | 20 | 0.023 | 0.60 | 0.8051 | |
| Model | 1.33 | 6 | 0.22 | 5.91 | 0.0009 *** | |
| 0.16 | 1 | 0.16 | 4.36 | 0.0487 ** | ||
| TV52J | 0.68 | 1 | 0.68 | 18.05 | 0.0003 *** | |
| 0.16 | 1 | 0.16 | 4.35 | 0.0488 ** | ||
| 0.15 | 1 | 0.15 | 3.86 | 0.0622 * | ||
| 0.17 | 1 | 0.17 | 4.65 | 0.0423 ** | ||
| Lack of Fit | 0.74 | 18 | 0.04 | 1.83 | 0.2965 | |
| Model | 1.97 | 7 | 0.28 | 4.87 | 0.0022 *** | |
| 0.78 | 1 | 0.78 | 13.50 | 0.0014 *** | ||
| PC | 0.32 | 1 | 0.32 | 5.46 | 0.0295 ** | |
| 0.25 | 1 | 0.25 | 4.28 | 0.0511 * | ||
| 0.36 | 1 | 0.36 | 6.16 | 0.0216 ** | ||
| Lack of Fit | 1.02 | 17 | 0.060 | 1.22 | 0.4712 | |
| Fungal Strain | Analysis of Variance (ANOVA) | |||||
|---|---|---|---|---|---|---|
| Sum of | Mean | F-value | p-value | |||
| Source | Squares | df | Square | |||
| Model | 4485.54 | 10 | 448.55 | 7.57 | 0.0001 *** | |
| 2019.43 | 1 | 2019.43 | 34.06 | <0.0001 *** | ||
| m4D | 786.35 | 1 | 786.35 | 13.26 | 0.0019 *** | |
| 265.20 | 1 | 265.20 | 4.47 | 0.0486 ** | ||
| 306.25 | 1 | 306.25 | 5.17 | 0.0355 ** | ||
| 355.32 | 1 | 355.32 | 5.99 | 0.0248 ** | ||
| 399.13 | 1 | 399.13 | 6.73 | 0.0183 ** | ||
| Lack of Fit | 940.59 | 14 | 67.19 | 2.12 | 0.2436 | |
| Model | 4670.60 | 7 | 667.23 | 6.57 | 0.0003 *** | |
| 1345.78 | 1 | 1345.78 | 13.25 | 0.0015 *** | ||
| TV52J | 1174.14 | 1 | 1174.14 | 11.56 | 0.0027 *** | |
| 1027.84 | 1 | 1027.84 | 10.12 | 0.0045 *** | ||
| 771.01 | 1 | 771.01 | 7.59 | 0.0119 ** | ||
| Lack of Fit | 800.59 | 17 | 47.09 | 0.14 | 0.9985 | |
| Model | 30,946.79 | 4 | 7736.70 | 4.24 | 0.0097 *** | |
| PC | 22,356.88 | 1 | 22,356.88 | 12.27 | 0.0018 *** | |
| 5364.10 | 1 | 5364.10 | 2.94 | 0.0991 * | ||
| Lack of Fit | 32,577.3407 | 20 | 1628.87 | 0.58 | 0.8135 | |
| Fungal Strain | Analysis of Variance (ANOVA) | |||||
|---|---|---|---|---|---|---|
| Sum of | Mean | F-value | p-value | |||
| Source | Squares | df | Square | |||
| Model | 0.50 | 2 | 0.25 | 3.25 | 0.0550 * | |
| 0.26 | 1 | 0.26 | 3.32 | 0.0798 * | ||
| m4D | 0.24 | 1 | 0.24 | 3.17 | 0.0865 * | |
| Lack of Fit | 1.63 | 22 | 0.07 | 0.81 | 0.6748 | |
| Model | 1.09 | 7 | 0.16 | 2.63 | 0.0409 ** | |
| TV52J | 0.42 | 1 | 0.42 | 7.09 | 0.0146 ** | |
| 0.45 | 1 | 0.45 | 7.47 | 0.0125 ** | ||
| Lack of Fit | 0.90 | 17 | 0.05 | 0.59 | 0.8004 | |
| Model | 11.99 | 5 | 2.40 | 4.01 | 0.0092 *** | |
| PC | 2.84 | 1 | 2.84 | 4.75 | 0.0398 ** | |
| 6.50 | 1 | 6.50 | 10.87 | 0.0032 *** | ||
| 1.79 | 1 | 1.79 | 2.99 | 0.0972 * | ||
| Lack of Fit | 11.69 | 19 | 0.62 | 1.19 | 0.4821 | |
| Sample Treatment | Total Lignin (% Dry Weight) | Cellulose (% Dry Weight) | Hemicellulose (% Dry Weight) | Ash Content (% Dry Weight) | Higher Heating Value (MJ/kg) | Energy Loss (%) |
|---|---|---|---|---|---|---|
| Untreated | 26.75 | 36.30 | 15.10 | 2.37 ± 0.24 | 18.03 ± 0.019 | _ |
| M4D T1 | 23.50 | 49.20 | 24.20 | 3.44 ± 0.35 | 17.92 ± 0.051 | 0.6 |
| M4D P1 | 24.80 | 38.70 | 22.10 | 3.36 ± 0.28 | 17.94 ± 0.027 | 0.49 |
| PC P1 | 24.10 | 42.90 | 23.80 | 3.25 ± 0.71 | 17.67 ± 2.010 | 2.01 |
| PC T1 | 24.50 | 31.40 | 14.90 | 2.91 ± 0.83 | 17.71 ± 1.790 | 1.79 |
| TV52J P1 | 24.80 | 39.50 | 23.70 | 3.62 ± 0.32 | 17.75 ± 0.032 | 1.57 |
| TV52J T1 | 23.80 | 44.90 | 22.70 | 3.26 ± 0.51 | 17.82 ± 0.064 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onu Olughu, O.; Tabil, L.G.; Dumonceaux, T.; Mupondwa, E.; Cree, D. Comparative Study on Quality of Fuel Pellets from Switchgrass Treated with Different White-Rot Fungi. Energies 2021, 14, 7670. https://doi.org/10.3390/en14227670
Onu Olughu O, Tabil LG, Dumonceaux T, Mupondwa E, Cree D. Comparative Study on Quality of Fuel Pellets from Switchgrass Treated with Different White-Rot Fungi. Energies. 2021; 14(22):7670. https://doi.org/10.3390/en14227670
Chicago/Turabian StyleOnu Olughu, Onu, Lope G. Tabil, Tim Dumonceaux, Edmund Mupondwa, and Duncan Cree. 2021. "Comparative Study on Quality of Fuel Pellets from Switchgrass Treated with Different White-Rot Fungi" Energies 14, no. 22: 7670. https://doi.org/10.3390/en14227670








