Feasibility of Using Electrodes with Ultralow Pt Loading in Two-Chamber Microbial Electrolysis Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Set-Up
2.2. Reactor Operation and Measurements
2.3. Calculation
3. Results and Discussion
3.1. Effect of Electrode Type and Substrate Concentration on the Current Produced
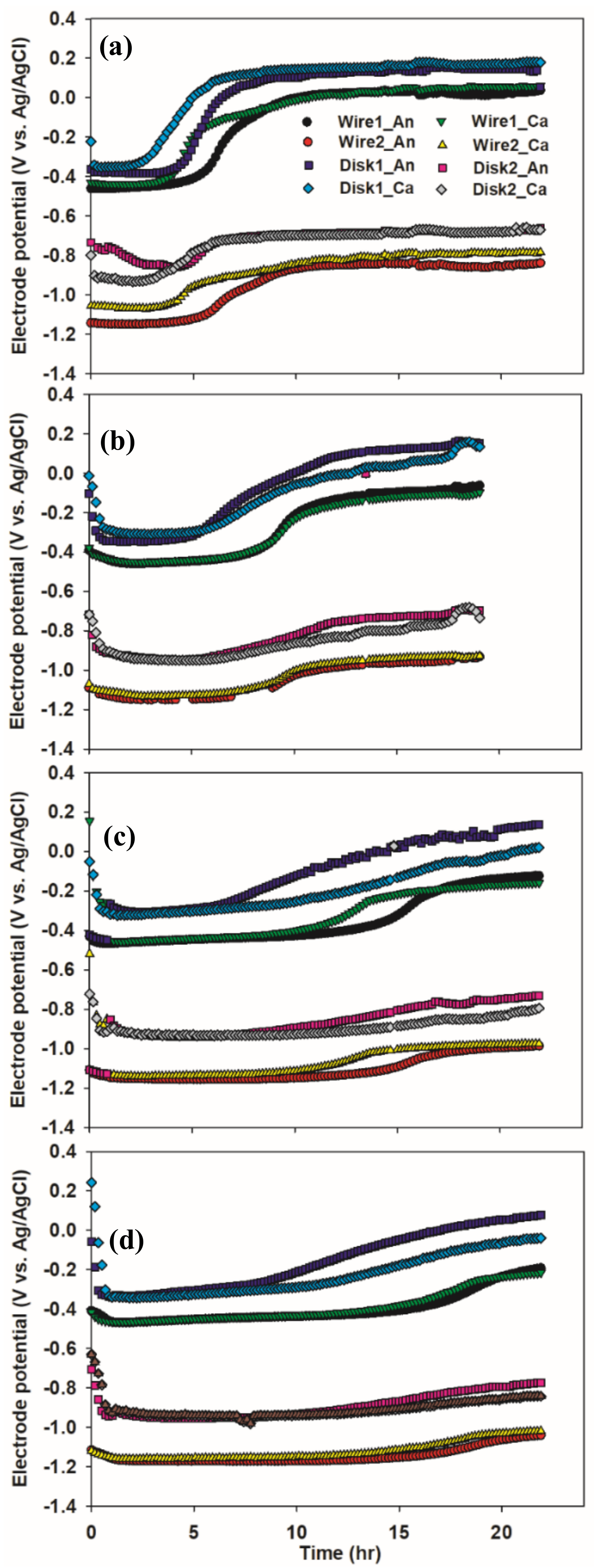
3.2. Effect of Cathode Type and Substrate Concentration on Electrode Potential, pH, and COD Removal
3.3. Hydrogen Production and Operational Efficiencies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, L.; Ren, Z.J. Microbial electrolysis cells for waste biorefinery: A state of the art review. Bioresour. Technol. 2016, 215, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.-L.; Bergel, A. Microbial electrolysis cell (MEC): Strengths, weaknesses and research needs from electrochemical engineering standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Yang, E.; Omar Mohamed, H.; Park, S.-G.; Obaid, M.; Al-Qaradawi, S.Y.; Castaño, P.; Chon, K.; Chae, K.-J. A review on self-sustainable microbial electrolysis cells for electro-biohydrogen production via coupling with carbon-neutral renewable energy technologies. Bioresour. Technol. 2021, 320, 124363. [Google Scholar] [CrossRef] [PubMed]
- Jwa, E.; Yun, Y.-M.; Kim, H.; Jeong, N.; Park, S.-C.; Nam, J.-Y. Domestic wastewater treatment in a tubular microbial electrolysis cell with a membrane electrode assembly. Int. J. Hydrog. Energy 2019, 44, 652–660. [Google Scholar] [CrossRef]
- Liang, D.-W.; Peng, S.-K.; Lu, S.-F.; Liu, Y.-Y.; Lan, F.; Xiang, Y. Enhancement of hydrogen production in a single chamber microbial electrolysis cell through anode arrangement optimization. Bioresour. Technol. 2011, 102, 10881–10885. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-Y.; Tokash, J.C.; Logan, B.E. Comparison of microbial electrolysis cells operated with added voltage or by setting the anode potential. Int. J. Hydrog. Energy 2011, 36, 10550–10556. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Cheng, K.Y.; Selvam, A.; Bose, A.; Wong, J.W.C. Bioelectrohydrogenesis and inhibition of methanogenic activity in microbial electrolysis cells—A review. Biotechnol. Adv. 2017, 35, 758–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.-Y.; Logan, B.E. Enhanced hydrogen generation using a saline catholyte in a two chamber microbial electrolysis cell. Int. J. Hydrog. Energy 2011, 36, 15105–15110. [Google Scholar] [CrossRef]
- Jayabalan, T.; Matheswaran, M.; Naina Mohammed, S. Biohydrogen production from sugar industry effluents using nickel based electrode materials in microbial electrolysis cell. Int. J. Hydrog. Energy 2019, 44, 17381–17388. [Google Scholar] [CrossRef]
- Call, D.F.; Merrill, M.D.; Logan, B.E. High Surface Area Stainless Steel Brushes as Cathodes in Microbial Electrolysis Cells. Environ. Sci. Technol. 2009, 43, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Wei, L.; Qiu, Z.; Wang, G.; Shen, J. Hydrogen production in single chamber microbial electrolysis cells with stainless steel fiber felt cathodes. J. Power Sources 2016, 301, 29–34. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Logan, B.E. Nickel powder blended activated carbon cathodes for hydrogen production in microbial electrolysis cells. Int. J. Hydrog. Energy 2019, 44, 13169–13174. [Google Scholar] [CrossRef]
- Rozenfeld, S.; Teller, H.; Schechter, M.; Farber, R.; Krichevski, O.; Schechter, A.; Cahan, R. Exfoliated molybdenum di-sulfide (MoS2) electrode for hydrogen production in microbial electrolysis cell. Bioelectrochemistry 2018, 123, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.; Bakonyi, P.; Buitrón, G. H2 production in membraneless bioelectrochemical cells with optimized architecture: The effect of cathode surface area and electrode distance. Chemosphere 2017, 171, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.; Lie, T.T.; Weber, C.C. Relative electrode size and organic load effects on the energy storage efficiency of microbial electrolysis cells. Bioresour. Technol. Rep. 2020, 11, 100518. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Zhu, Q.; Sun, J.; Qin, F.; Yu, L.; Chen, S.; Ren, Z. Water splitting by electrolysis at high current densities under 1.6 volts. Energy Environ. Sci. 2018, 11, 2858–2864. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Wain, A.J. The Butler-Volmer equation in electrochemical theory:Origins, value, and practical application. J. Electroanal. Chem. 2020, 872, 114145. [Google Scholar] [CrossRef]
- Xue, S.; Liu, Z.; Ma, C.; Cheng, H.-M.; Ren, W. A highly active and durable electrocatalyst for large current density hydrogen evolution reaction. Sci. Bull. 2020, 65, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]




| Substrate Concentration (g/L) | Disk Electrode (A/m3) | Wire Electrode (A/m3) |
|---|---|---|
| 0.4 | 132.5 ± 7.7 | 111.6 ± 7.6 |
| 0.6 | 131.9 ± 12.4 | 110.8 ± 7.8 |
| 0.8 | 119.2 ± 10.6 | 108.1 ± 2.1 |
| 1.0 | 128.4 ± 13.4 | 101.2 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jwa, E.; Kim, M.; Han, J.-H.; Jeong, N.; Kim, H.-C.; Yun, Y.-M.; Nam, J.-Y. Feasibility of Using Electrodes with Ultralow Pt Loading in Two-Chamber Microbial Electrolysis Cells. Energies 2021, 14, 7752. https://doi.org/10.3390/en14227752
Jwa E, Kim M, Han J-H, Jeong N, Kim H-C, Yun Y-M, Nam J-Y. Feasibility of Using Electrodes with Ultralow Pt Loading in Two-Chamber Microbial Electrolysis Cells. Energies. 2021; 14(22):7752. https://doi.org/10.3390/en14227752
Chicago/Turabian StyleJwa, Eunjin, Mijin Kim, Ji-Hyung Han, Namjo Jeong, Hyun-Chul Kim, Yeo-Myeong Yun, and Joo-Youn Nam. 2021. "Feasibility of Using Electrodes with Ultralow Pt Loading in Two-Chamber Microbial Electrolysis Cells" Energies 14, no. 22: 7752. https://doi.org/10.3390/en14227752






