Heat Flux Based Optimization of Combined Heat and Power Thermoelectric Heat Exchanger
Abstract
:1. Introduction
2. Materials and Methods
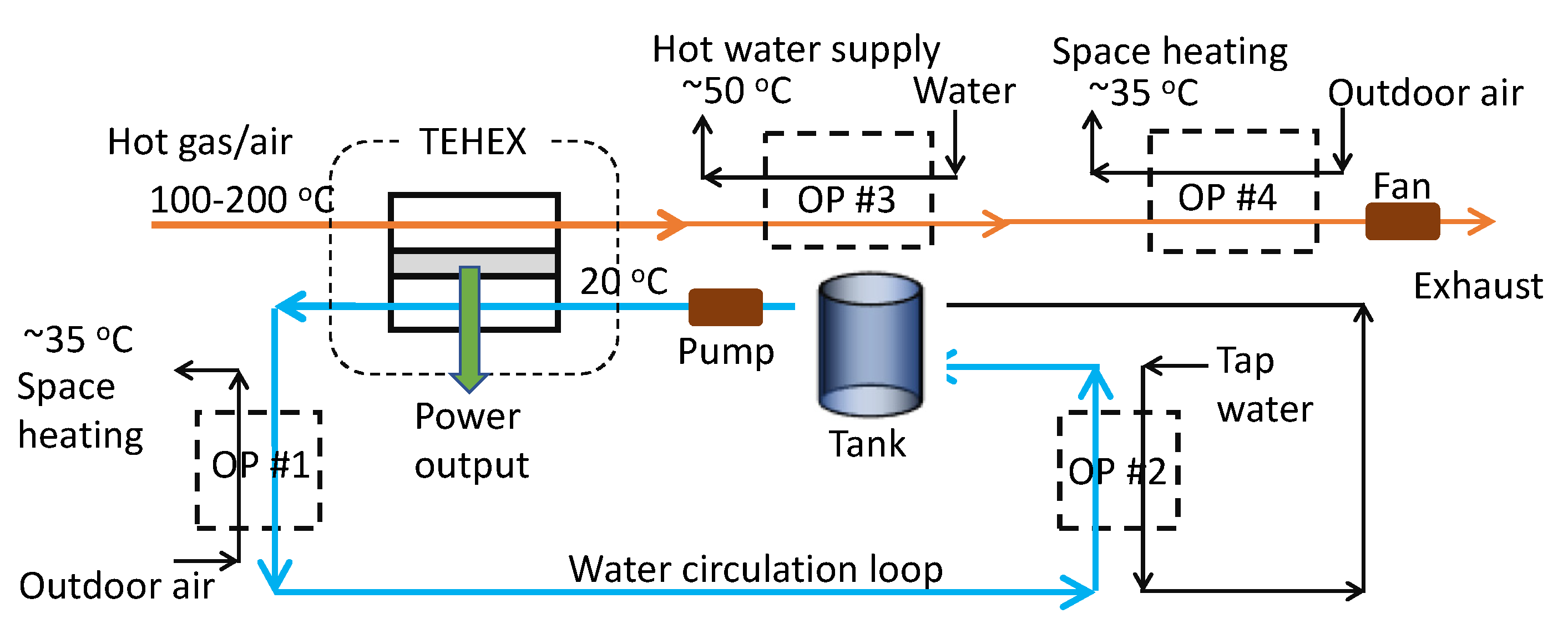
2.1. CHP System
2.2. Thermoelectric Integrated Subsystems
2.3. Working Fluids
2.4. Thermoelectric Materials
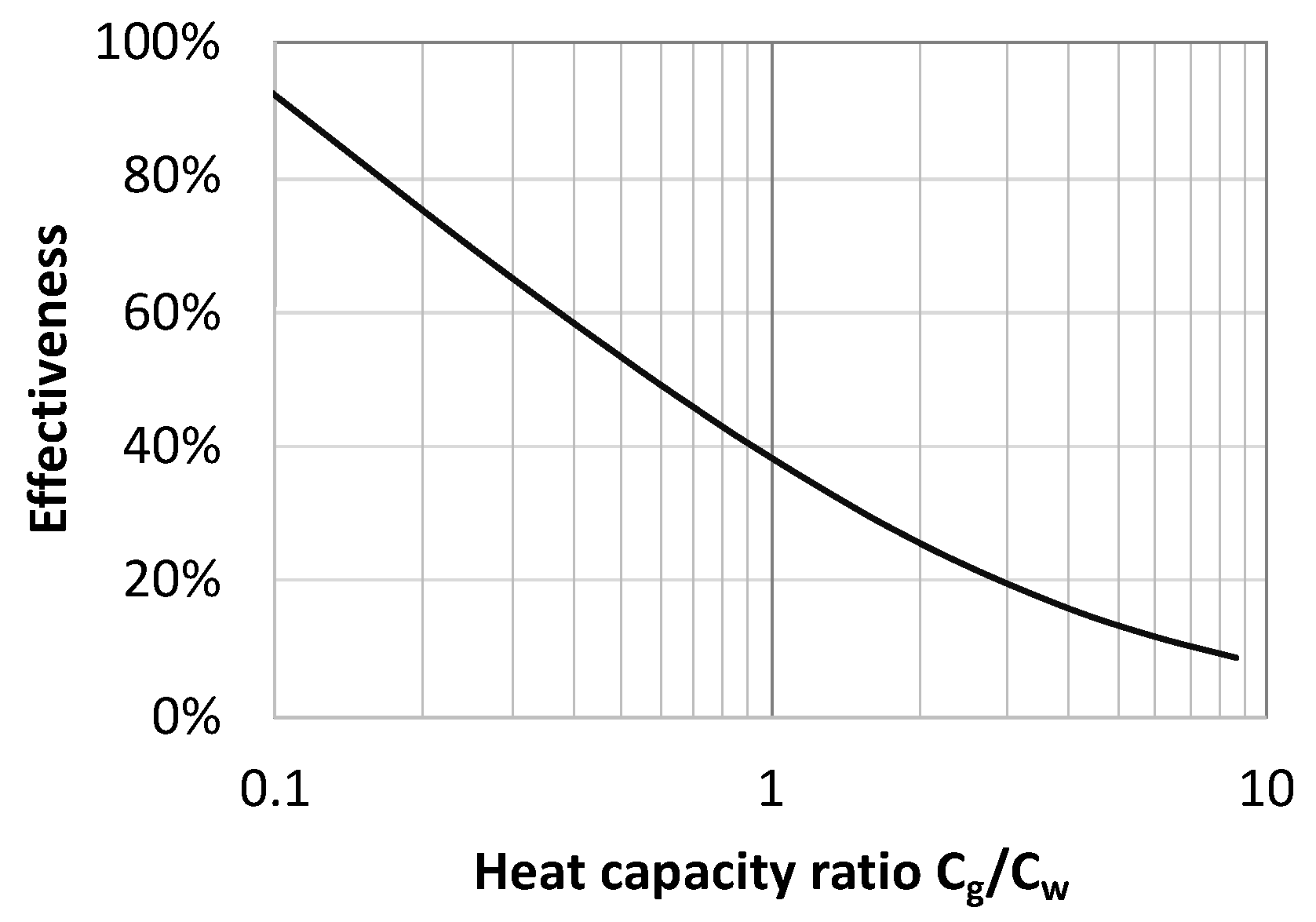
2.5. Effectiveness of Heat Exchanger
2.6. Thermoelectric Optimum Design
2.7. Uncertainty
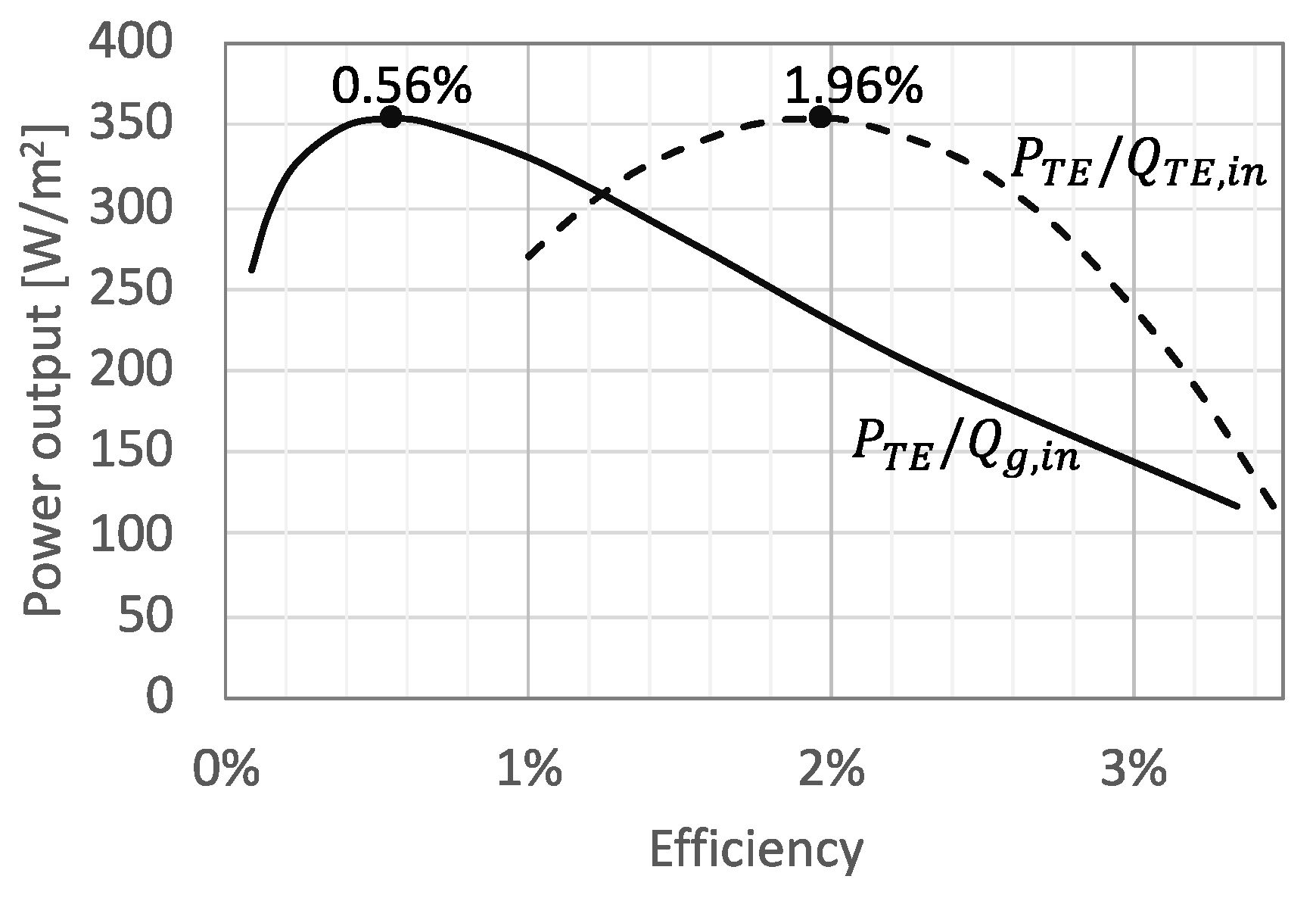
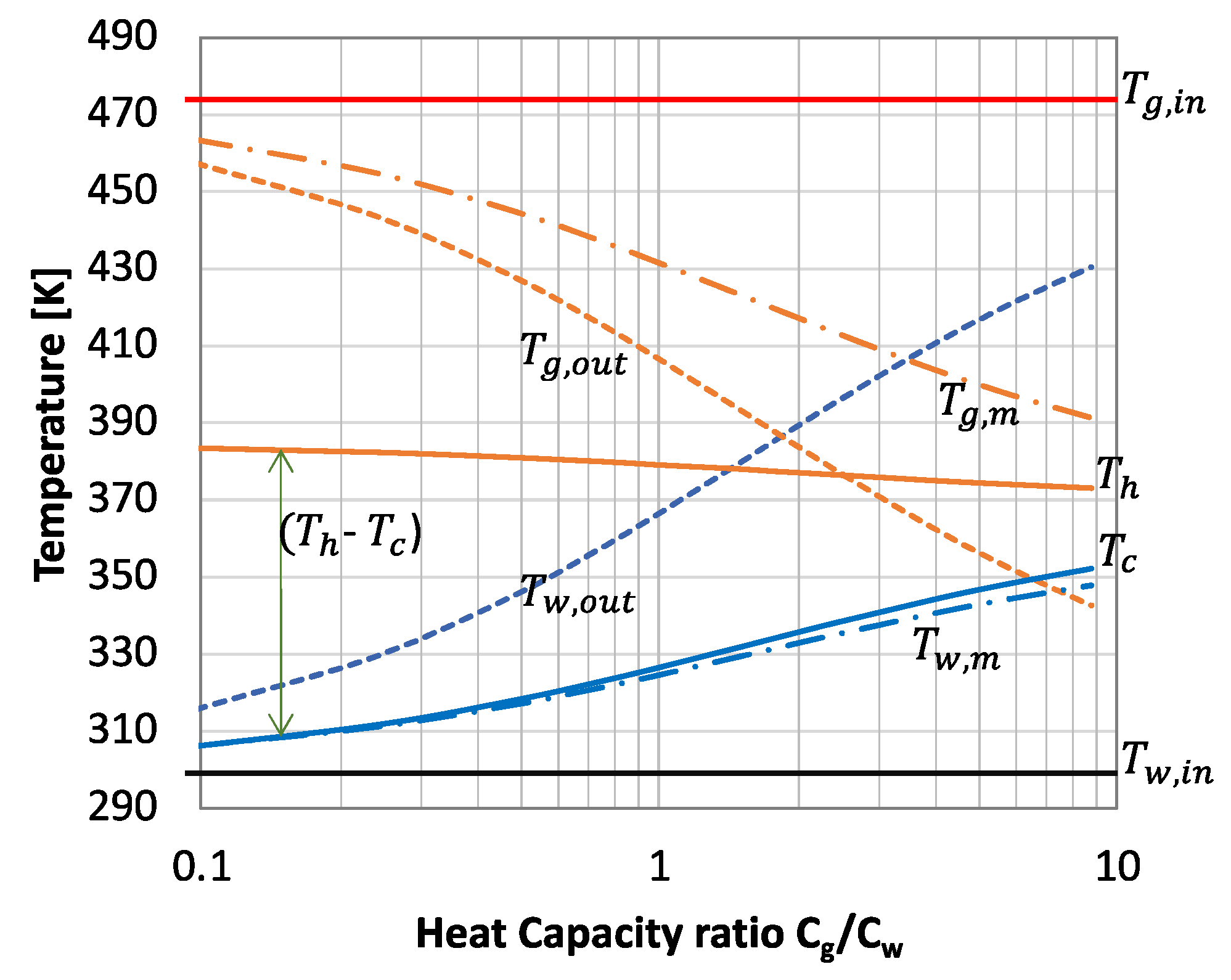
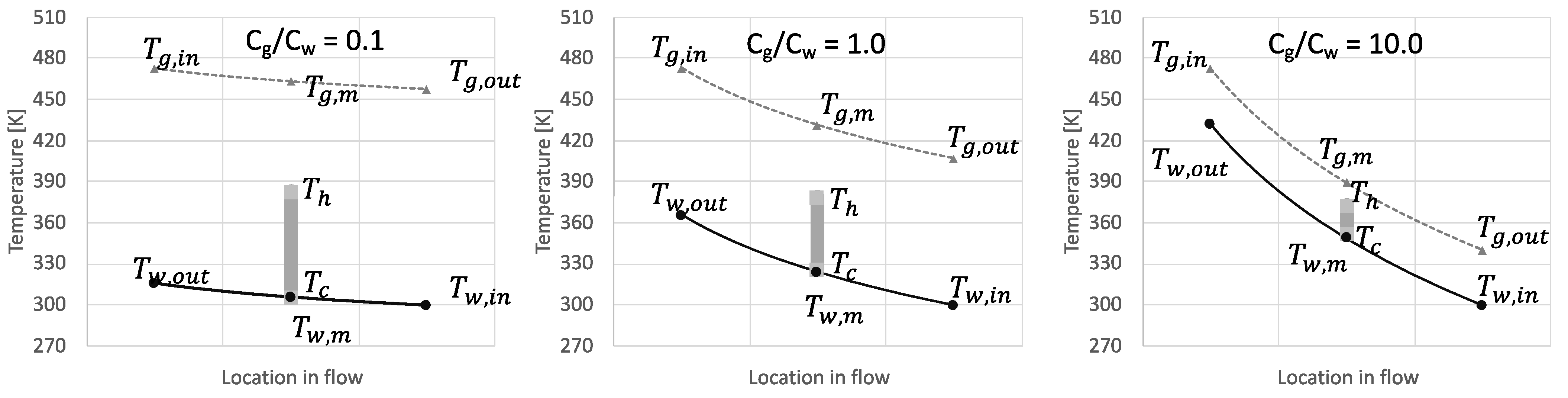
3. Results and Discussion
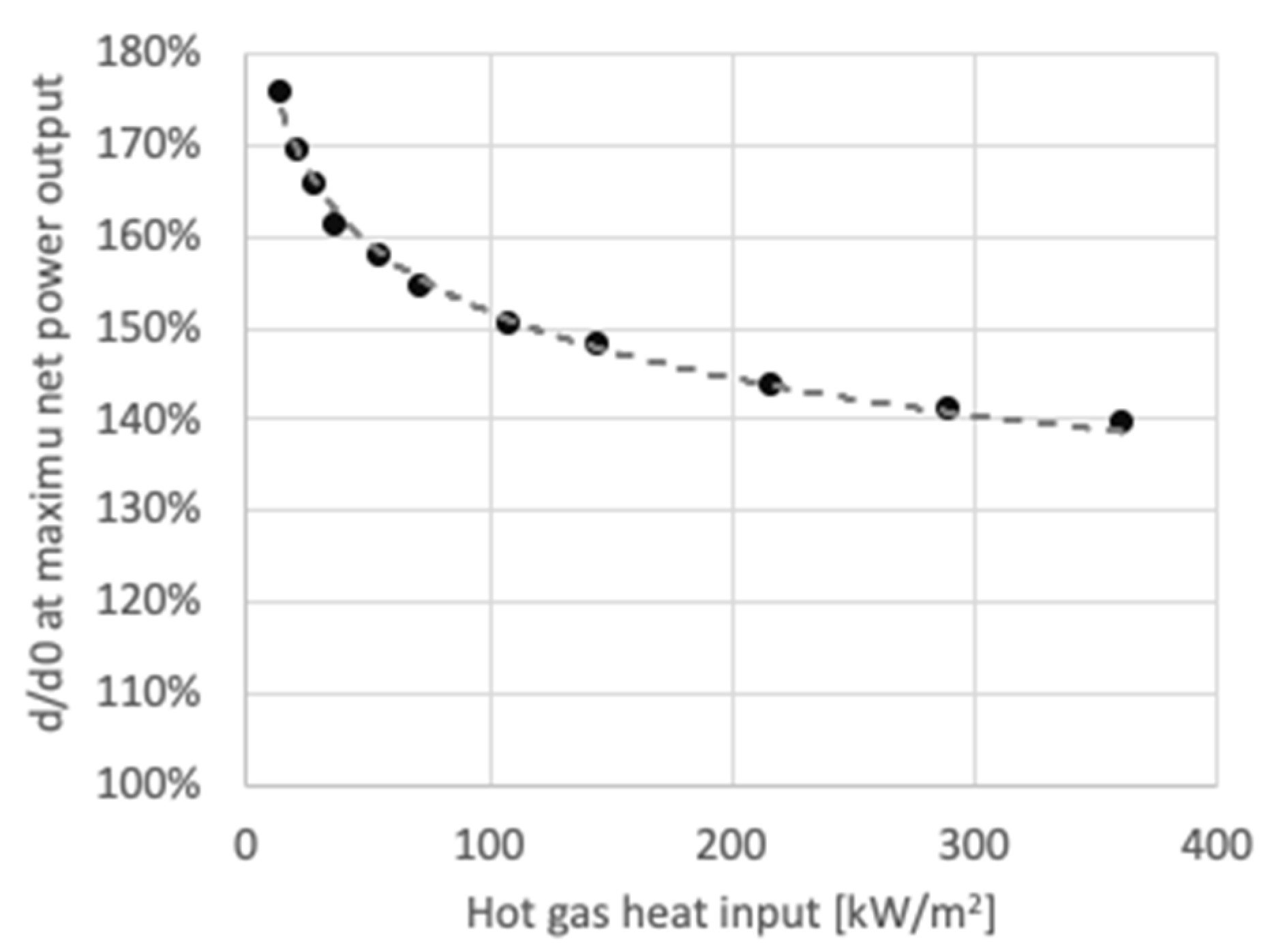
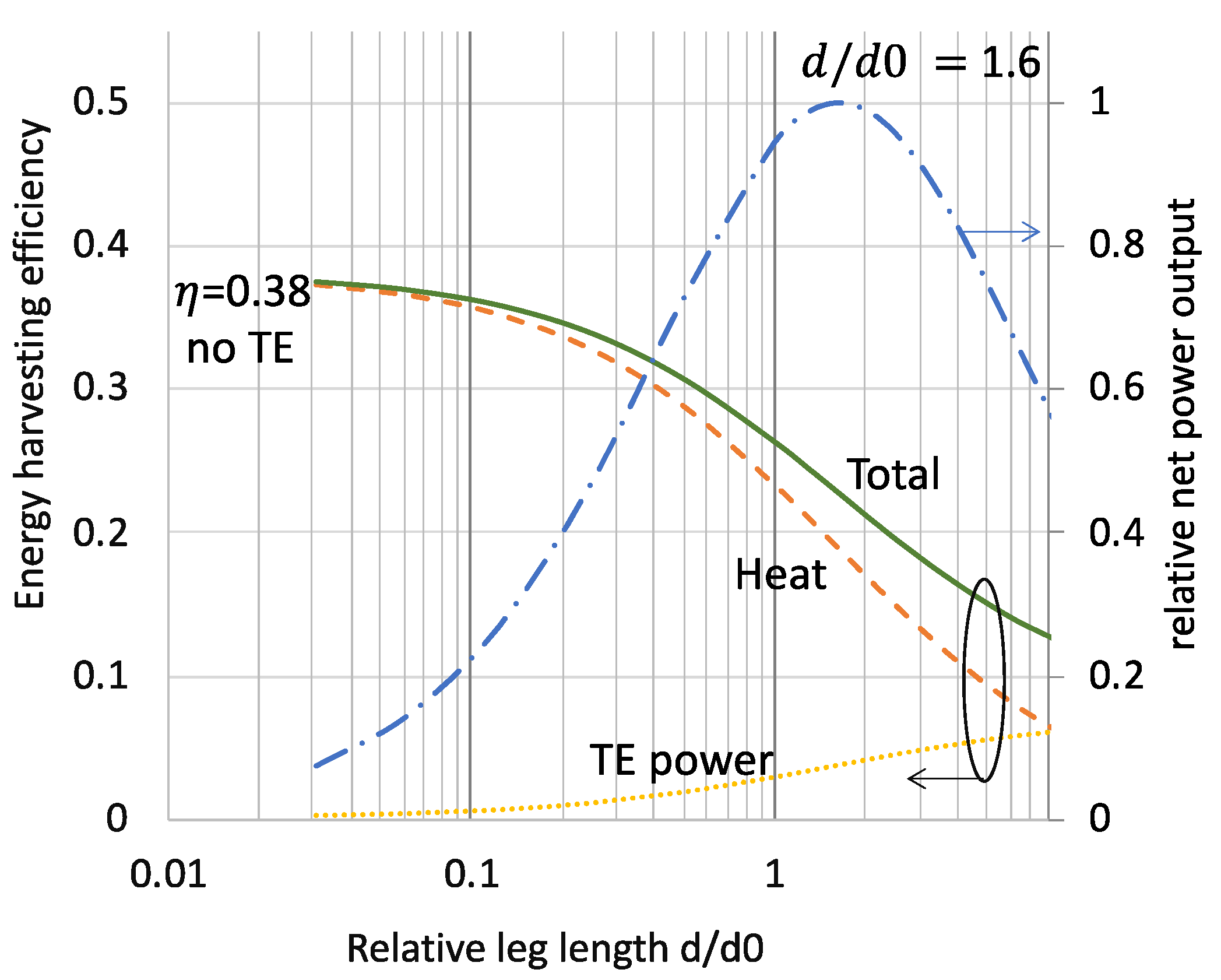
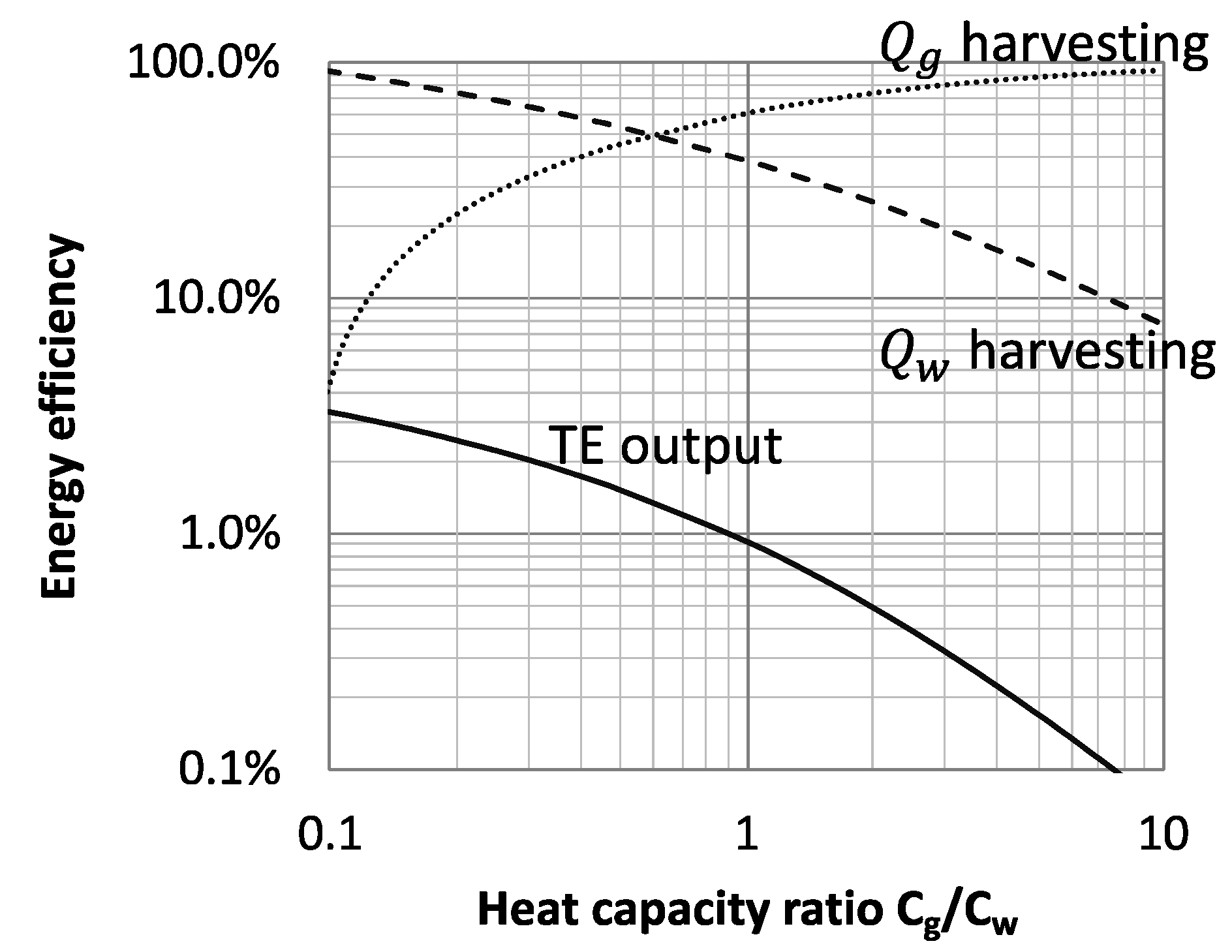
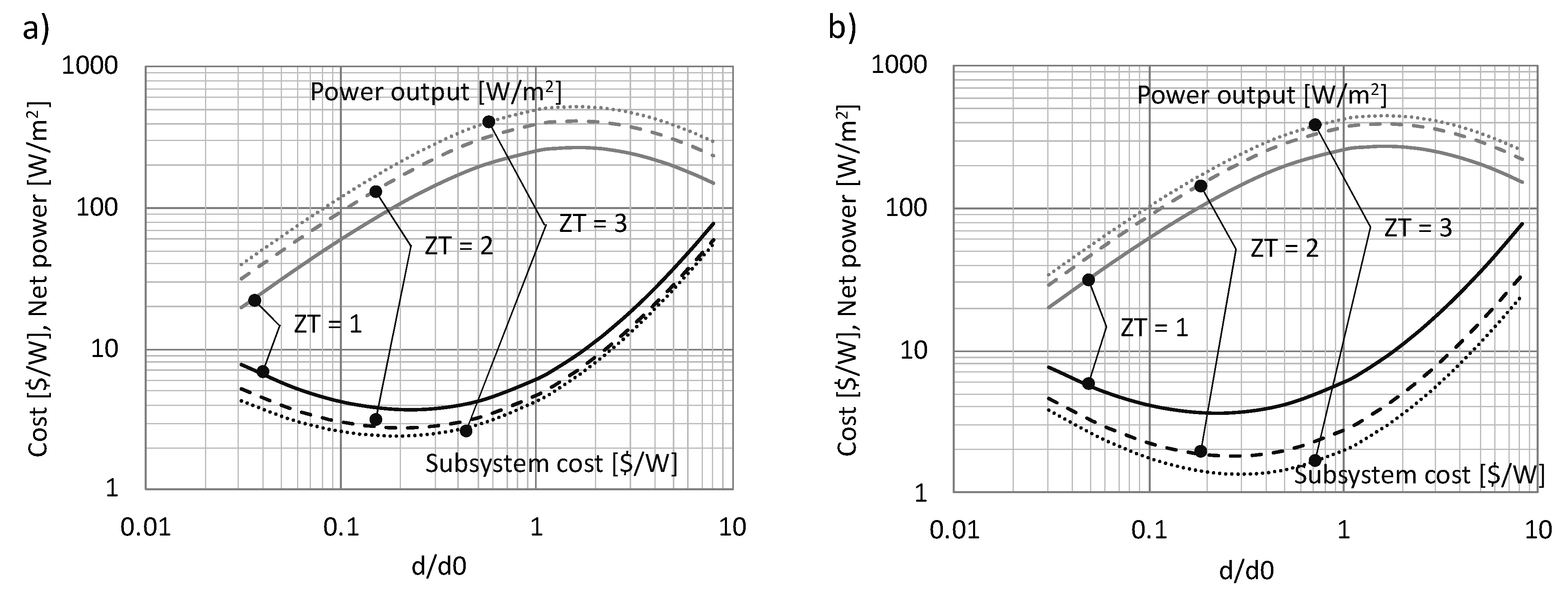
3.1. Performance Analysis
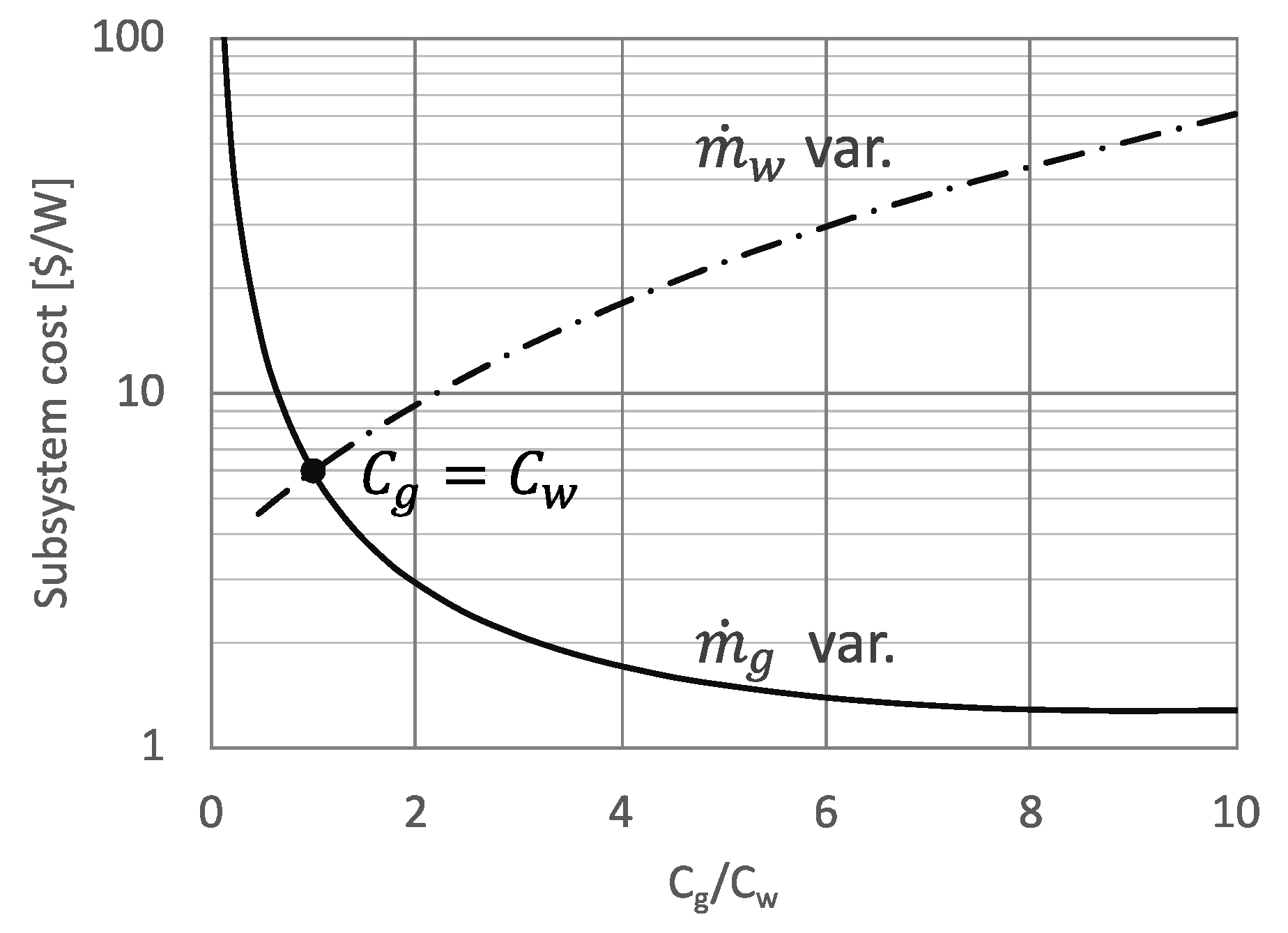
3.2. Cost Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHP | combined heat and power |
| heat capacity of hot gas flow | |
| heat capacity of cold water flow | |
| LMTD | logarithmic mean temperature difference |
| ORC | organic Rankine cycle |
| TE | thermoelectric |
| TEHEX | thermoelectric integrated heat exchanger |
| ZT | thermoelectric figure of merit |
References
- Lawrence Livermore National Laboratory, Energy Flow Chart, US 2020. 2021. Available online: https://flowcharts.llnl.gov/content/assets/images/charts/Energy/Energy_2020_United-States.png (accessed on 27 October 2021).
- IEA World Balance Chart. 2018. Available online: https://www.iea.org/sankey/#?c=World&s=Balance (accessed on 27 October 2021).
- Gundersen, T. An Introduction to the Concept of Exergy and Energy Quality, 4th ed.; Department of Energy and Process Engineering Norwegian University of Science and Technology: Trondheim, Norway, 2011. [Google Scholar]
- Yazawa, K.; Shakouri, A.; Hendricks, T.J. Thermoelectric heat recovery from glass melt processes. Energy 2017, 118, 1035–1043. [Google Scholar] [CrossRef]
- Kaşka, Ö. Energy and exergy analysis of an organic Rankine for power generation from waste heat recovery in steel industry. Energy Convers. Manag. 2014, 77, 108–117. [Google Scholar] [CrossRef]
- Nowicki, C.; Gosselin, L. An overview of opportunities for waste heat recovery and thermal integration in the primary aluminum industry. JOM 2012, 64, 990–996. [Google Scholar] [CrossRef]
- Johnson, I.; Choate, W.T.; Davidson, A. Waste Heat Recovery—Technology and Opportunities in US Industry; BCS, Inc.: Laurel, MD, USA, 2008. [Google Scholar]
- Bianchi, G.; Panayiotou, G.P.; Aresti, L.; Kalogirou, S.A.; Florides, G.A.; Tsamos, K.; Tassou, S.A.; Christodoulides, P. Estimating the waste heat recovery in the European Union Industry. Energy Ecol. Environ. 2019, 4, 211–221. [Google Scholar]
- Glaude, P.-A.; Fournet, R.; Bounaceur, R.; Molière, M. Adiabatic flame temperature from biofuels and fossil fuels and derived effect on NOx emissions. Fuel Process. Technol. 2010, 91, 229–235. [Google Scholar] [CrossRef]
- Camacho, J.; Singh, A.V.; Wang, W.; Shan, R.; Yapp, E.K.Y.; Chen, D.; Kraft, M.; Wang, H. Soot particle size distributions in premixed stretch-stabilized flat ethylene–oxygen–argon flames. Proc. Combust. Inst. 2017, 36, 1001–1009. [Google Scholar] [CrossRef]
- Yazawa, K.; Shakouri, A. Exergy analysis and entropy generation minimization of thermoelectric waste heat recovery for electronics. In Proceedings of the 2011 International Electronic Packaging Technical Conference and Exhibition, Portland, OR, USA, 6–8 July 2011; Volume 44618, pp. 741–747. [Google Scholar]
- Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar]
- Lecompte, S.; Huisseune, H.; van den Broek, M.; De Paepe, M. Methodical thermodynamic analysis and regression models of organic Rankine cycle architectures for waste heat recovery. Energy 2015, 87, 60–76. [Google Scholar] [CrossRef]
- Peris, B.; Navarro-Esbrí, J.; Molés, F.; Mota-Babiloni, A. Experimental study of an ORC (organic Rankine cycle) for low grade waste heat recovery in a ceramic industry. Energy 2015, 85, 534–542. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, J.; Gao, L. Parametric optimization and comparative study of organic Rankine cycle (ORC) for low grade waste heat recovery. Energy Convers. Manag. 2009, 50, 576–582. [Google Scholar] [CrossRef]
- Uusitalo, A.; Turunen-Saaresti, T.; Honkatukia, J.; Dhanasegaran, R. Experimental study of small scale and high expansion ratio ORC for recovering high temperature waste heat. Energy 2020, 208, 118321. [Google Scholar] [CrossRef]
- Yazawa, K.; Shakouri, A. Cost-efficiency trade-off and the design of thermoelectric power generators. Environ. Sci. Technol. 2011, 45, 7548–7553. [Google Scholar] [CrossRef] [PubMed]
- Bühler, F.; Petrović, S.; Karlsson, K.; Elmegaard, B. Industrial excess heat for district heating in Denmark. Applied Energy 2017, 205, 991–1001. [Google Scholar] [CrossRef] [Green Version]
- Rezania, A.; Yazawa, K.; Rosendahl, L.A.; Shakouri, A. Co-optimized design of microchannel heat exchangers and thermoelectric generators. Int. J. Therm. Sci. 2013, 72, 73–81. [Google Scholar] [CrossRef]
- El-Shibini, M.A.; Rakha, H.H. Maximum power point tracking technique. In Proceedings of the IEEE Electrotechnical Conference Integrating Research, Industry and Education in Energy and Communication Engineering, Lisbon, Portugal, 11–13 April 1989; pp. 21–24. [Google Scholar]
- Yazawa, K.; Shakouri, A. Optimization of power and efficiency of thermoelectric devices with asymmetric thermal contacts. J. Appl. Phys. 2012, 111, 024509. [Google Scholar] [CrossRef] [Green Version]
- Kay, J.M.; Nedderman, R.M. Fluid Mechanics and Transfer Processes; CUP Archive: Cambridge, UK, 1985. [Google Scholar]
- Incropera, F.P.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, 4th ed.; Wiley: New York, NY, USA, 2000; p. 493. [Google Scholar]
- 3M. 3M FluorinertTM Electronic Liquid FC72 Technical Data; 3M: St. Paul, MN, USA, 2019; Available online: https://multimedia.3m.com/mws/media/64892O/3m-fluorinert-electronic-liquid-fc72-en.pdf (accessed on 15 November 2021).
- Hendricks, T.J.; Yee, S.K.; LeBlanc, S. Cost scaling of a real-world exhaust waste heat recovery thermoelectric generator: A deeper dive. J. Electron. Mater. 2016, 45, 1751–1761. [Google Scholar] [CrossRef]
- Yazawa, K.; Bahk, J.-H.; Shakouri, A. Thermoelectric Energy Conversion Devices and Systems; World Scientific: Singapore, 2021. [Google Scholar]
- Kays, W.W.; London, A.L. Compact Heat Exchangers, 3rd ed.; Krieger: Florida, FL, USA, 1984; p. 18. [Google Scholar]
- Juhasz, A. A Mass Computation Model for Light Weight Brayton Cycle Regenerator Heat Exchangers. In Proceedings of the 8th Annual International Energy Conversion Engineering Conference, Nashville, TN, USA, 25–28 July 2010; p. 7087. [Google Scholar]
- EIA, Electric Power Monthly, US Total All Sector Average for August 2021. Available online: https://www.eia.gov/electricity/monthly/epm_table_grapher.php?t=epmt_5_6_a (accessed on 15 November 2021).





| Temperature | Density | Specific Heat | Thermal Conductivity | Viscosity | Performance Index | Relative Index | |
|---|---|---|---|---|---|---|---|
| °C | kg/m3 | J/(kg·K) | W/(m·K) | ×10−6 m2/s | |||
| Air | 20 | 1.166 | 1005 | 0.0257 | 15.6 | 3.16 × 108 | 1.00 |
| 160 | 0.789 | 1017 | 0.0359 | 30.8 | 1.49 × 108 | 0.47 | |
| Steam | 160 | 0.494 | 1984 | 0.0286 | 29.7 | 2.67 × 108 | 0.84 |
| Water | 20 | 998.2 | 4181 | 0.594 | 1.01 | 1.36 × 1010 | 43.0 |
| 40 | 992.3 | 4177 | 0.628 | 0.667 | 2.54 × 1010 | 80.6 | |
| 60 | 983.2 | 4186 | 0.653 | 0.481 | 4.18 × 1010 | 132.5 | |
| 80 | 971.8 | 4198 | 0.672 | 0.367 | 6.32 × 1010 | 200.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazawa, K.; Shakouri, A. Heat Flux Based Optimization of Combined Heat and Power Thermoelectric Heat Exchanger. Energies 2021, 14, 7791. https://doi.org/10.3390/en14227791
Yazawa K, Shakouri A. Heat Flux Based Optimization of Combined Heat and Power Thermoelectric Heat Exchanger. Energies. 2021; 14(22):7791. https://doi.org/10.3390/en14227791
Chicago/Turabian StyleYazawa, Kazuaki, and Ali Shakouri. 2021. "Heat Flux Based Optimization of Combined Heat and Power Thermoelectric Heat Exchanger" Energies 14, no. 22: 7791. https://doi.org/10.3390/en14227791





