First-Principles Study of Pt-Based Bifunctional Oxygen Evolution & Reduction Electrocatalyst: Interplay of Strain and Ligand Effects
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussions
3.1. Structural Stability of Pt3M/Pt Catalysts
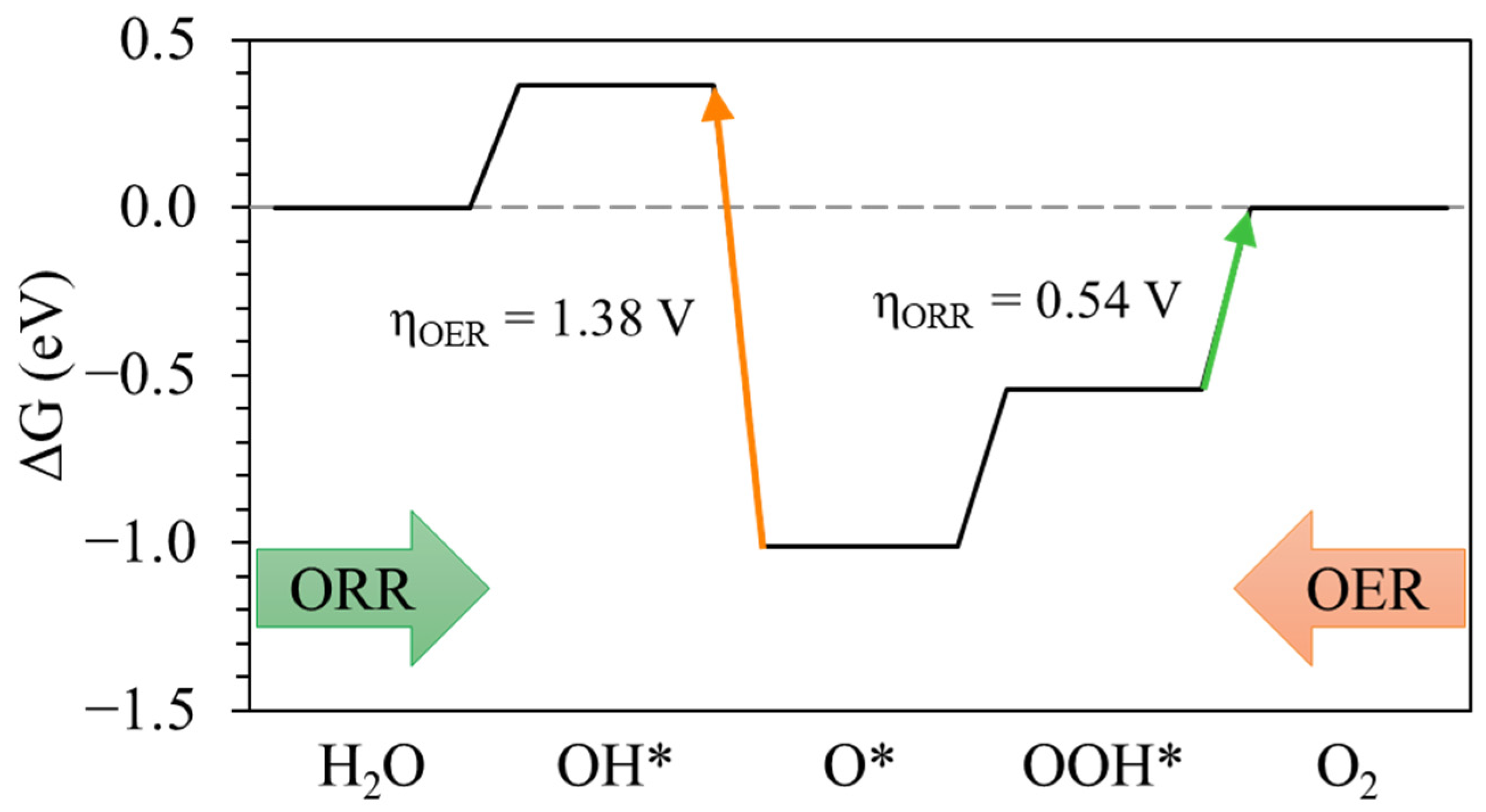
3.2. OER/ORR Mechanism and Reaction Energetics
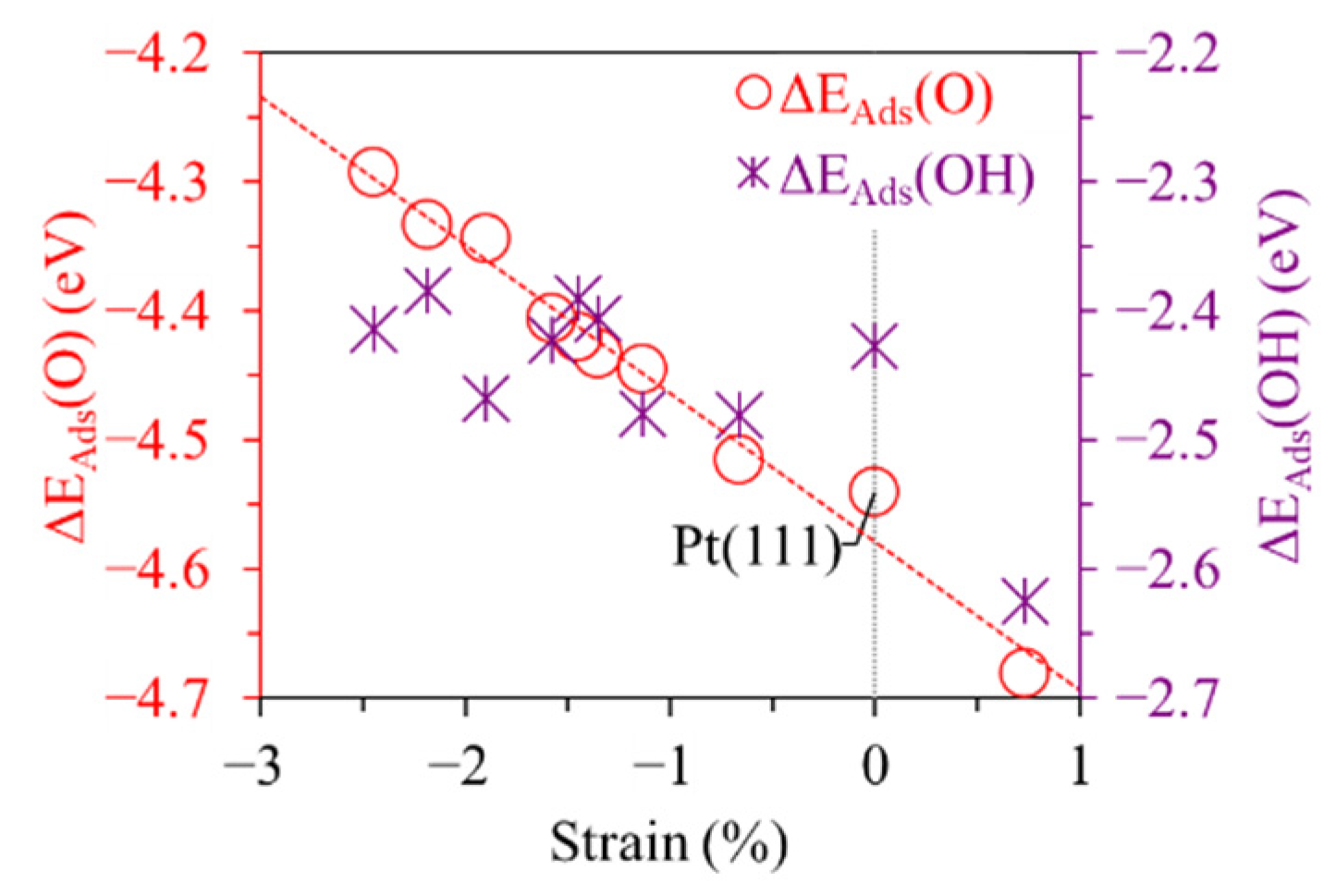
3.3. Origin of Enhanced OER/ORR Activity on Pt3M/Pt Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pivovar, B. Catalysts for fuel cell transportation and hydrogen related uses. Nat. Catal. 2019, 2, 562–565. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, 146. [Google Scholar] [CrossRef] [Green Version]
- Kan, D.; Wang, D.; Zhang, X.; Lian, R.; Xu, J.; Chen, G.; Wei, Y. Rational design of bifunctional ORR/OER catalysts based on Pt/Pd-doped Nb2CT2 MXene by first-principles calculations. J. Mater. Chem. A 2020, 8, 3097–3108. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lim, A.; Kim, J.M.; Lim, D.; Chae, K.H.; Cho, E.N.; Han, H.J.; Jeon, K.U.; Kim, M.; Lee, G.H.; et al. Highly efficient oxygen evolution reaction via facile bubble transport realized by three-dimensionally stack-printed catalysts. Nat. Commun. 2020, 11, 4921. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Shinagawa, T.; Nishimoto, T.; Takanabe, K. Recent advances in understanding oxygen evolution reaction mechanisms over iridium oxide. Inorg. Chem. Front. 2021, 8, 2900–2917. [Google Scholar] [CrossRef]
- Qu, H.-Y.; He, X.; Wang, Y.; Hou, S. Electrocatalysis for the Oxygen Evolution Reaction in Acidic Media: Progress and Challenges. Appl. Sci. 2021, 11, 4320. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.-X.; Liu, J.-N.; Ren, D.; Li, B.-Q.; Huang, J.-Q.; Zhang, Q. Quantitative kinetic analysis on oxygen reduction reaction: A perspective. Nano Mater. Sci. 2021, 3, 313–318. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal–Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Tian, X.T.; Lu, X.F.; Xia, B.Y.; Lou, X.W. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Rai, V.; Lee, K.P.; Safanama, D.; Adams, S.; Blackwood, D.J. Oxygen Reduction and Evolution Reaction (ORR and OER) Bifunctional Electrocatalyst Operating in a Wide pH Range for Cathodic Application in Li–Air Batteries. ACS Appl. Energy Mater. 2020, 3, 9417–9427. [Google Scholar] [CrossRef]
- Wu, X.; Tang, C.; Cheng, Y.; Min, X.; Jiang, S.P.; Wang, S. Bifunctional Catalysts for Reversible Oxygen Evolution Reaction and Oxygen Reduction Reaction. Chem. A Eur. J. 2020, 26, 3906–3929. [Google Scholar]
- Liu, Z.; Zhao, Z.; Peng, B.; Duan, X.; Huang, Y. Beyond Extended Surfaces: Understanding the Oxygen Reduction Reaction on Nanocatalysts. J. Am. Chem. Soc. 2020, 142, 17812–17827. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Nørskov, J.K. Ligand and ensemble effects in adsorption on alloy surfaces. Phys. Chem. Chem. Phys. 2001, 3, 3814–3818. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K.; et al. Alloying–realloying enabled high durability for Pt–Pd-3d-transition metal nanoparticle fuel cell catalysts. Nat. Commun. 2021, 12, 859. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Stumpf, R.; Lin, P.; Chou, M.Y. Catalytic effect of near-surface alloying on hydrogen interaction on the aluminum surface. Phys. Rev. B 2011, 83, 195419. [Google Scholar] [CrossRef] [Green Version]
- Zafeiratos, S.; Piccinin, S.; Teschner, D. Alloys in catalysis: Phase separation and surface segregation phenomena in response to the reactive environment. Catal. Sci. Technol. 2012, 2, 1787–1801. [Google Scholar] [CrossRef] [Green Version]
- Westsson, W.; Picken, S.; Koper, G. The effect of lattice strain on catalytic activity. Chem. Commun. 2019, 55, 1338–1341. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Jia, H.; Liu, Z.; Gao, P.; Zhao, J.; Luo, Z.; Yang, J.; Zeng, J. Understanding of Strain Effects in the Electrochemical Reduction of CO2: Using Pd Nanostructures as an Ideal Platform. Angew. Chem. 2017, 56, 3594–3598. [Google Scholar] [CrossRef] [PubMed]
- Bligaard, T.; Nørskov, J.K. Ligand effects in heterogeneous catalysis and electrochemistry. Electrochim. Acta 2007, 52, 5512–5516. [Google Scholar] [CrossRef]
- Li, S.; Tian, W.; Liu, Y. The ligand effect of atomically precise gold nanoclusters in tailoring catalytic properties. Nanoscale 2021, 13, 16847–16859. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 124, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Krupski, K.; Moors, M.; Jóźwik, P.; Kobiela, T.; Krupski, A. Structure Determination of Au on Pt(111) Surface: LEED, STM and DFT Study. Materials 2015, 8, 2935–2952. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Lee, S.; Han, J.; Yoon, S.P.; Nam, S.W.; Choi, S.H.; Lee, K.-Y.; Ham, H.C. Importance of Ligand Effect in Selective Hydrogen Formation via Formic Acid Decomposition on the Bimetallic Pd/Ag Catalyst from First-Principles. J. Phys. Chem. C 2014, 118, 22553–22560. [Google Scholar] [CrossRef]
- Lee, S.; Cho, J.; Jang, J.H.; Han, J.; Yoon, S.P.; Nam, S.W.; Lim, T.H.; Ham, H.C. Impact of d-Band Occupancy and Lattice Contraction on Selective Hydrogen Production from Formic Acid in the Bimetallic Pd3M (M = Early Transition 3d Metals) Catalysts. ACS Catal. 2016, 6, 134–142. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, W. Conjugate-gradient optimization method for orbital-free density functional calculations. J. Chem. Phys. 2004, 121, 2030–2036. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E.; Jepsen, O.; Anderson, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef] [PubMed]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved Grid-Based Algorithm for Bader Charge Allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Skúlason, E.; Tripkovic, V.; Björketun, M.E.; Gudmundsdóttir, S.; Karlberg, G.; Rossmeisl, J.; Bligaard, T.; Jónsson, H.; Nørskov, J.K. Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C 2010, 114, 18182. [Google Scholar] [CrossRef]
- NIST Standard Reference Database Number 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 21 November 2021).
- Hwang, C.-K.; Kim, J.M.; Hwang, S.; Kim, J.-H.; Sung, C.H.; Moon, B.-M.; Chae, K.H.; Singh, J.P.; Kim, S.-H.; Jang, S.S.; et al. Porous Strained Pt Nanostructured Thin-Film Electrocatalysts via Dealloying for PEM Fuel Cells. Adv. Mater. Interfaces 2020, 7, 1901326. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.W.; Chen, L.X.; Wen, Z.; Jiang, Q. Understanding electro-catalysis by using density functional theory. Phys. Chem. Chem. Phys. 2019, 21, 23782–23802. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Waghmare, U.; Lee, S.C. An improved d-band model of the catalytic activity of magnetic transition metal surfaces. Sci. Rep. 2016, 6, 35916. [Google Scholar] [CrossRef]



| α (Å) | ΔEBulk (eV/Atom) | ΔESeg (eV) | ΔESeg_O (eV) | |
|---|---|---|---|---|
| Pt3Sc | 4.00 | −1.05 | 1.05 | −0.02 |
| Pt3Ti | 3.94 | −0.84 | 1.20 | −0.21 |
| Pt3V | 3.92 | −0.43 | 0.96 | −0.99 |
| Pt3Cr | 3.91 | −0.25 | 0.86 | −0.28 |
| Pt3Mn | 3.92 | −0.39 | 0.89 | 0.15 |
| Pt3Fe | 3.91 | −0.20 | 0.89 | 0.11 |
| Pt3Co | 3.88 | −0.06 | 0.74 | 0.01 |
| Pt3Ni | 3.87 | −0.07 | 0.74 | 0.04 |
| Pt3Cu | 3.89 | −0.11 | 0.70 | 0.25 |
| ηOER (V) | ηOER (V) | ΔGGap (eV) | |
|---|---|---|---|
| Pt(111) | 1.38 | 0.54 | 1.92 |
| Pt3Sc/Pt | 1.46 | 0.74 | 2.20 |
| Pt3Ti/Pt | 1.35 | 0.59 | 1.94 |
| Pt3V/Pt | 1.29 | 0.52 | 1.81 |
| Pt3Cr/Pt | 1.28 | 0.50 | 1.79 |
| Pt3Mn/Pt | 1.32 | 0.59 | 1.91 |
| Pt3Fe/Pt | 1.31 | 0.54 | 1.85 |
| Pt3Co/Pt | 1.24 | 0.50 | 1.74 |
| Pt3Ni/Pt | 1.21 | 0.53 | 1.73 |
| Pt3Cu/Pt | 1.26 | 0.58 | 1.84 |
| Pt3Mn/Ptskin | 1.23 | 0.46 | 1.68 |
| Pt3Fe/Ptskin | 1.21 | 0.52 | 1.73 |
| Pt3Co/Ptskin | 1.15 | 0.47 | 1.63 |
| Pt3Ni/Ptskin | 1.12 | 0.47 | 1.59 |
| Pt3Cu/Ptskin | 1.11 | 0.50 | 1.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-h.; Kang, Y.; Ham, H.C. First-Principles Study of Pt-Based Bifunctional Oxygen Evolution & Reduction Electrocatalyst: Interplay of Strain and Ligand Effects. Energies 2021, 14, 7814. https://doi.org/10.3390/en14227814
Kim S-h, Kang Y, Ham HC. First-Principles Study of Pt-Based Bifunctional Oxygen Evolution & Reduction Electrocatalyst: Interplay of Strain and Ligand Effects. Energies. 2021; 14(22):7814. https://doi.org/10.3390/en14227814
Chicago/Turabian StyleKim, Seung-hoon, Yoonmook Kang, and Hyung Chul Ham. 2021. "First-Principles Study of Pt-Based Bifunctional Oxygen Evolution & Reduction Electrocatalyst: Interplay of Strain and Ligand Effects" Energies 14, no. 22: 7814. https://doi.org/10.3390/en14227814







