Sustainable Materials from Fish Industry Waste for Electrochemical Energy Systems
Abstract
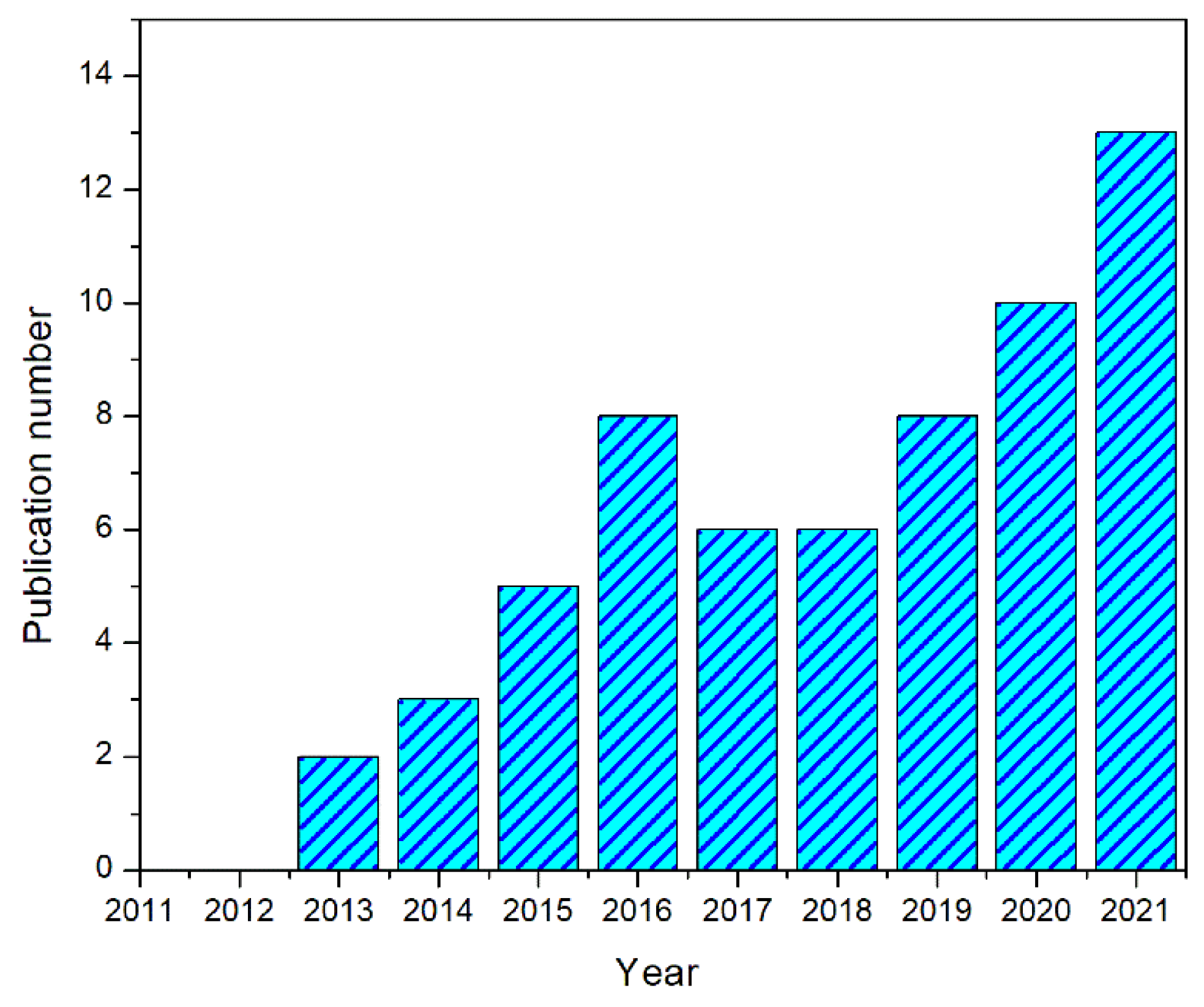
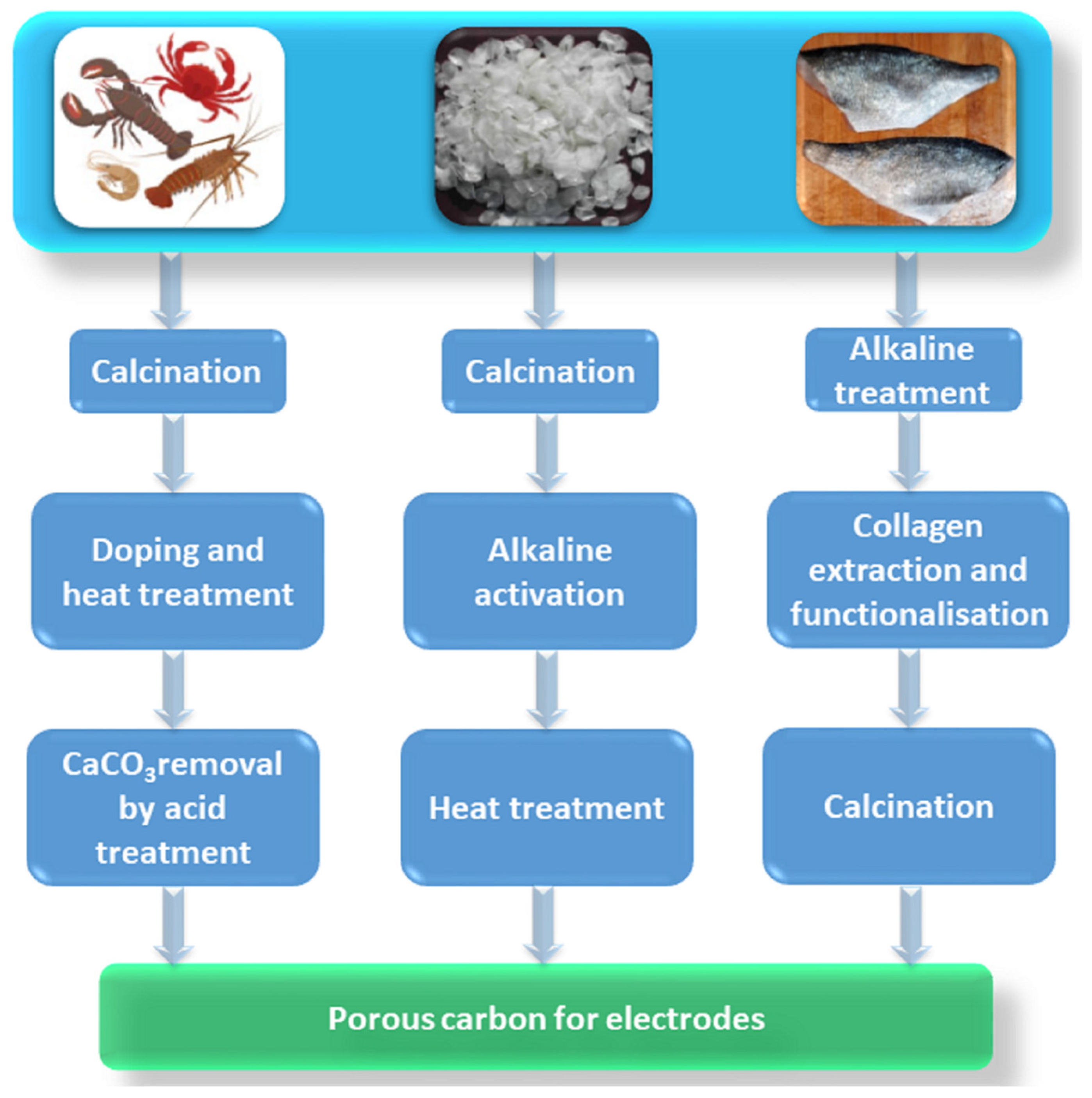
:1. Introduction
2. Applications in Lithium–Ion Batteries (LIBs)
| Fish Waste Source | Application | Current Density (mA/g) or C-Rate | Initial Discharge Capacity (mAh/g) | Reversible Specific Capacity (mAh/g) | Capacity Retention | References |
|---|---|---|---|---|---|---|
| Crab Shell | Si-encapsulated nanostructured anode | C/10-1C | 3060 @C/10 1580 @1C | 95% @200 cycles | [72] | |
| Crawfish shell | nanoCo3O4 doped anode | 100 | 1223 | 1060 | 98% @100 cycles | [89] |
| Prawn shells | anode | 50–1000 | 1735 | 950 @50 mA/g 300 @1000 mA/g | 84% @90 cycles | [87] |
| Prawn meat | anode | 50–1000 | 1132 | 420 @50 mA/g 100 @1000 mA/g | 40% @90 cycles | [87] |
| Prawn Shells | anode | 0.1 | 740 | 732 | 99% @150 cycles | [91] |
| Fish scales | N-doped nanoporous anode | 75 400 4000 | 541 418 214 | 509 390 179 | 94% @75 cycles 93% @75 cycles 84% @75 cycles | [22] |
| Collagen from Tilapia waste | nanoPd doped anode | 1C | 600 | 270 @1C | 100% @20 cycles | [60] |
| Crab Shell | anode | 50 | 1758 | 703 @50 mA/g | 83% @200 cycles | [92] |
| Commercial graphite-based anodes | 372 theoretical | 300–500 cycles | [93,94] |
3. Applications in Sodium-Ion Batteries (NIBs)
| Fish Waste Source | Application | Current Density (mA/g) or C-Rate | Initial Discharge Capacity (mAh/g) | Reversible Specific Capacity (mAh/g) | Capacity Retention | Ref. |
|---|---|---|---|---|---|---|
| Prawn Shells | Na-ion batteries anode | 100 | 370 | 325 @1C | 100% @200 cycles | [91] |
| Fish collagen (Tilapia) | nanoPd doped anode for Na-battery | 1C | 60 | NIB: 120 @1C | 40% @20 cycles | [60] |
| Crab Shell | anode | 50 mA/g | 1211 | 283 | 62% @300 cycles | [92] |
| Commercial graphite-based anodes | 25 | 250 | 184 @C/10 | 100 cycles | [104,105] |
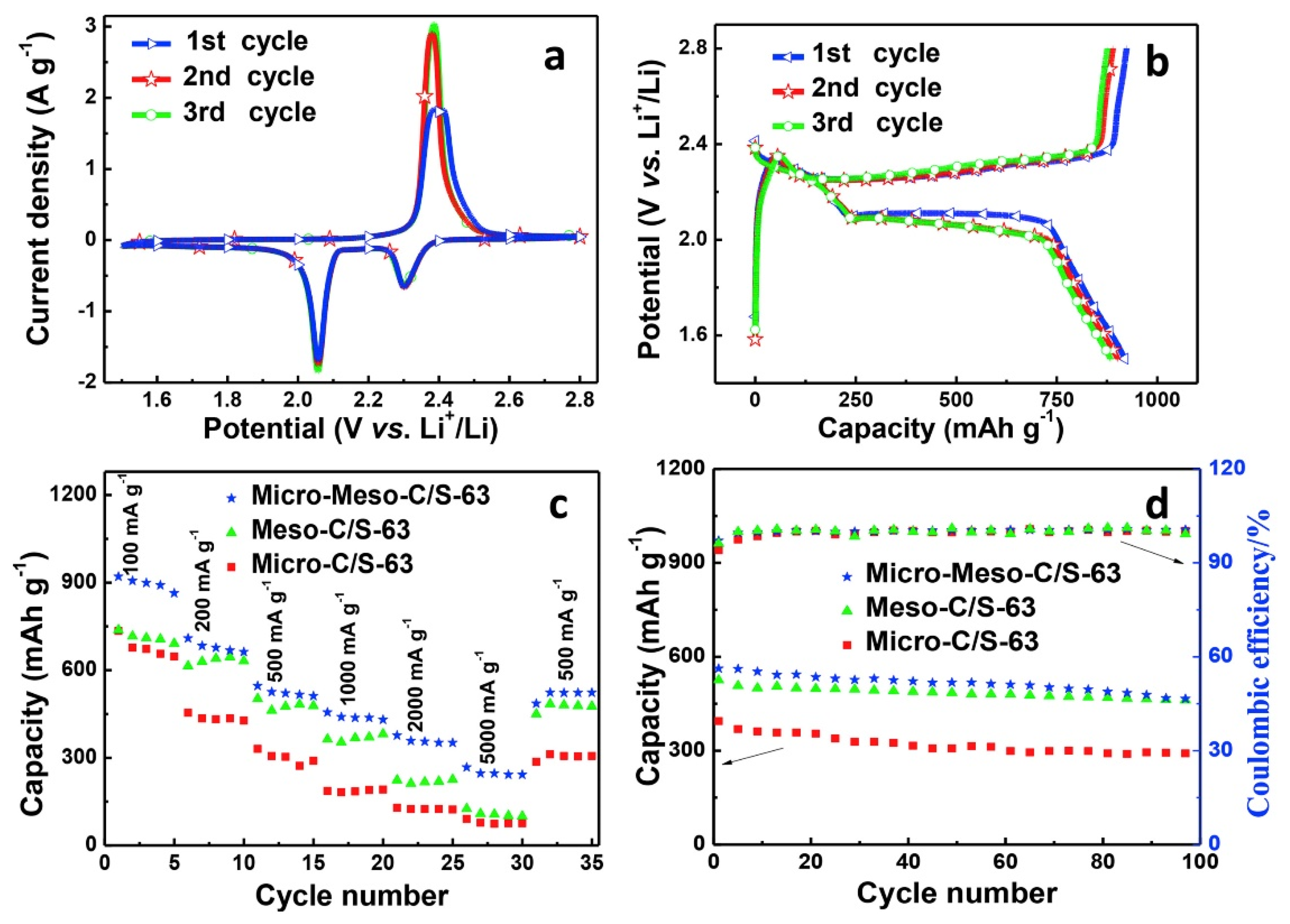
4. Applications in Lithium–Sulfur Batteries (LSBs)
| Fish Waste Source | Application | Current Density (mA/g) or C-Rate | Initial Discharge Capacity (mAh/g) | Reversible Specific Capacity (mAh/g) | Capacity Retention | References |
|---|---|---|---|---|---|---|
| Crab Shell | S-encapsulated nanostructured carbon cathode | 1673 | 1230 @C/5 | 1050 @C/10 690 @1C | 71% @100 cycles | [72] |
| Shrimp shell | N-doped micro-mesoporous carbon-sulfur cathode | 100–5000 | 920–290 | 90% @100 cycles @500 mA/g | [115] | |
| Fish Scales | Sulfur/carbon nanocomposite cathode | 1675 | 1039 | 1023 @1C | 97% @70 cycles | [116] |
| Fish Scale | Sulfur/carbon nanocomposite cathode | C/10 | 1484 | 878 @C/10 | 59% @50 cycles | [117] |
| Fish Scale | Sulfur/carbon nanocomposite cathode coated with porous carbon layer | C/10 | 1426 | 990 @1C | 80% @100 cycles @1C | [117] |
| Fish Scale | Sulfur/carbon nanocomposite cathode | C/10 | 1453 | 723 @C/10 | 99% @100 cycles | [71] |
| Crab shell | Ca(OH)2–carbon separator | C/2 | 1215 | 873 @C/2 | 72% @250 cycles | [118] |
| Fish bones | Porous carbon separator | C/5–2C | 1237 | 600 @1C | 55% 700 cycles | [119] |
| Carbon material | Commercial LSB | 1675 theoretical | [108,120] |
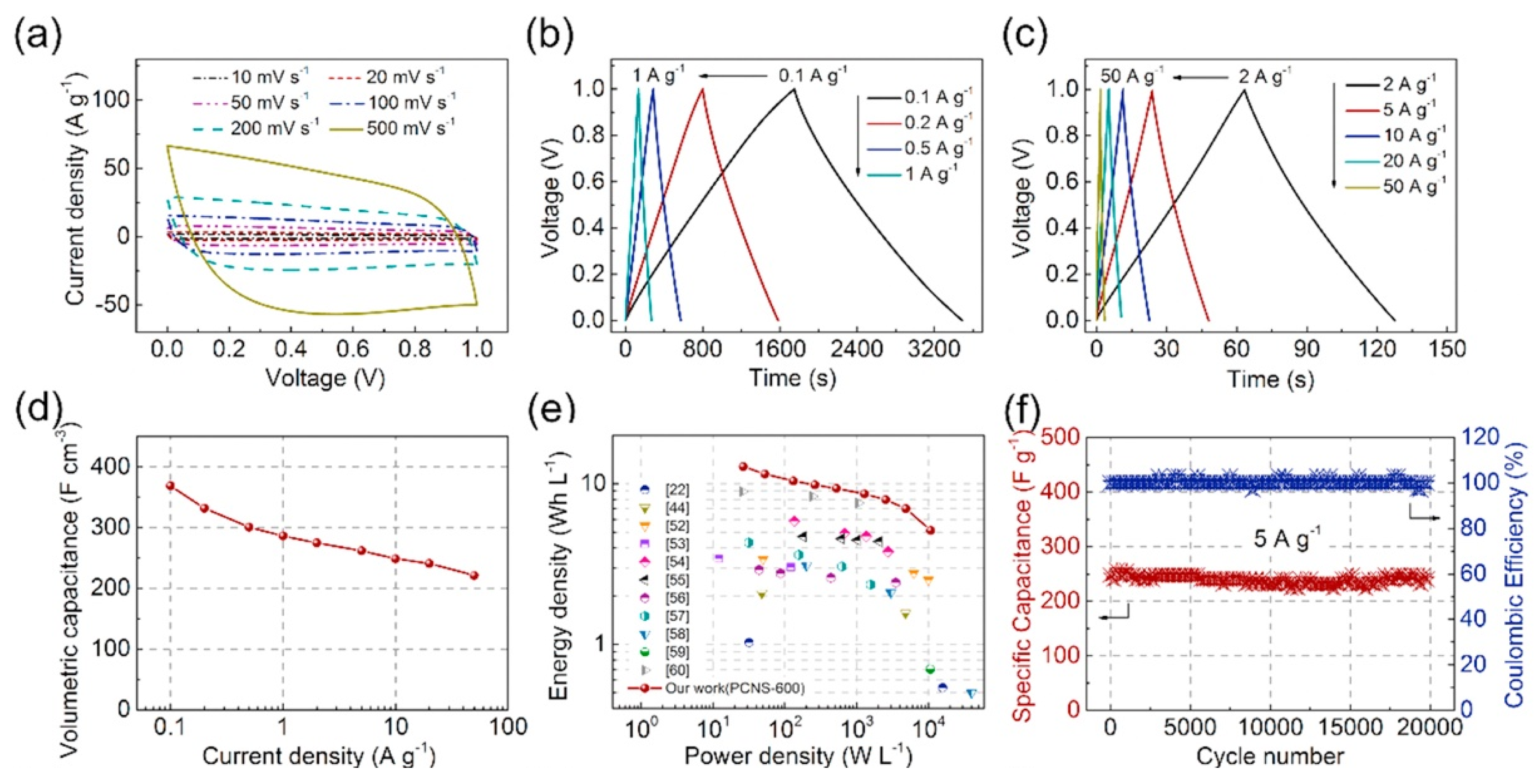
5. Applications in Supercapacitors
| Fish Waste Source | Obtained Material for Electrode | Surface Area (m2/g) | Current Density (A/g) | Reversible Specific Capacitance (F/g) | Capacity Retention | References |
|---|---|---|---|---|---|---|
| Crab Shell | Porous carbon | 1270 | - | 152 | 95% @1000 cycles | [86] |
| Fish scale | Porous carbon | 2273 | 40 | 130 | 77% | [69] |
| Prawn Shells | N-activated carbons | 1918 | 5 | 280 | 95% @5000 cycles @1 A/g | [134] |
| Fish Scales | P- and N- co-activated carbon | 1300 | 1–20 | 340 | 81% @10,000 cycles | [135] |
| Shaddock skin | Porous carbon with high graphitization degree | 2327 | 1–100 | 152 @1 A/g 132 @100 A/g | 97% @10,000 cycles @10 A/g | [138] |
| Gladius of Squid fish | N- and O- activated carbon | 1129 | 0.5–25 | 204 @0.5 A/g | 100% @25,000 cycles | [136] |
| Fish Scales | O-, N- and S- activated carbon | 962 | 0.1–100 | 519 @0.1 A/g 306 @1 A/g | 100% @20,000 cycles @5 A/g | [133] |
| Fish bones | N-S-P-O doped carbon | 1670 | 5 | 58 | 82% @20,000 cycles | [137] |
| Commercial activated carbon | 0.05 10 | 158 109 | [84] |
6. Protein Batteries Derived from Fish Industry Waste
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Yu, J.; Fu, N.; Zhao, J.; Liu, R.; Li, F.; Du, Y.; Yang, Z. High Specific Capacitance Electrode Material for Supercapacitors Based on Resin-Derived Nitrogen-Doped Porous Carbons. ACS Omega 2019, 4, 15904–15911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, L.S.; Vasudevan, M.; Perumal, V.; Ovinis, M.; Raja, P.B.; Edison, T.N.J.I. Bioresource-derived polymer composites for energy storage applications: Brief review. J. Environ. Chem. Eng. 2021, 9, 105832. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Larsson, S.H.; de Oliveira, H.P.; Thyrel, M.; Claudio Lima, E. Sustainable biomass activated carbons as electrodes for battery and supercapacitors—A mini-review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Ryu, H.; Yoon, H.J.; Kim, S.W. Hybrid Energy Harvesters: Toward Sustainable Energy Harvesting. Adv. Mater. 2019, 31, 1–19. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Mele, C.; Bilotta, A.; Bocchetta, P.; Bozzini, B. Characterization of the particulate anode of a laboratory flow Zn–air fuel cell. J. Appl. Electrochem. 2017, 47, 877–888. [Google Scholar] [CrossRef]
- Gao, M.; Shih, C.C.; Pan, S.Y.; Chueh, C.C.; Chen, W.C. Advances and challenges of green materials for electronics and energy storage applications: From design to end-of-life recovery. J. Mater. Chem. A 2018, 6, 20546–20563. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Gurría, P.; Ronzon, T.; Tamosiunas, S.; López, R.; García Condado, S.; Guillén, J.; Cazzaniga, N.E.; Jonsson, R.; Banja, M.; Fiore, G. Biomass Flows in the European Union; European Commission Joint Research Center: Seville, Spain, 2017. [Google Scholar]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [Green Version]
- Malmgren, A.; Riley, G. Biomass Power Generation; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; Volume 5, ISBN 9780080878737. [Google Scholar]
- Lionetto, F.; Esposito Corcione, C. An Overview of the Sorption Studies of Contaminants on Poly (Ethylene Terephthalate) Microplastics in the Marine Environment. J. Mar. Sci. Eng. 2021, 9, 445. [Google Scholar] [CrossRef]
- Caputo, A.C.; Palumbo, M.; Pelagagge, P.M.; Scacchia, F. Economics of biomass energy utilization in combustion and gasification plants: Effects of logistic variables. Biomass Bioenergy 2005, 28, 35–51. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Titirici, M.M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; Del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef]
- Selvamani, V.; Ravikumar, R.; Suryanarayanan, V.; Velayutham, D.; Gopukumar, S. Fish scale derived nitrogen doped hierarchical porous carbon—A high rate performing anode for lithium ion cell. Electrochim. Acta 2015, 182, 1–10. [Google Scholar] [CrossRef]
- Blankenship, T.S.; Balahmar, N.; Mokaya, R. Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nat. Commun. 2017, 8, 1633. [Google Scholar] [CrossRef] [Green Version]
- Senthil, C.; Vediappan, K.; Nanthagopal, M.; Seop Kang, H.; Santhoshkumar, P.; Gnanamuthu, R.; Lee, C.W. Thermochemical conversion of eggshell as biological waste and its application as a functional material for lithium-ion batteries. Chem. Eng. J. 2019, 372, 765–773. [Google Scholar] [CrossRef]
- Poochai, C.; Srikhaow, A.; Lohitkarn, J.; Kongthong, T.; Tuantranont, S.; Tuantranont, S.; Primpray, V.; Maeboonruan, N.; Wisitsoraat, A.; Sriprachuabwong, C. Waste coffee grounds derived nanoporous carbon incorporated with carbon nanotubes composites for electrochemical double-layer capacitors in organic electrolyte. J. Energy Storage 2021, 43, 103169. [Google Scholar] [CrossRef]
- Balahmar, N.; Mitchell, A.C.; Mokaya, R. Generalized Mechanochemical Synthesis of Biomass-Derived Sustainable Carbons for High Performance CO2 Storage. Adv. Energy Mater. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Sevilla, M.; Al-Jumialy, A.S.M.; Fuertes, A.B.; Mokaya, R. Optimization of the Pore Structure of Biomass-Based Carbons in Relation to Their Use for CO2 Capture under Low- and High-Pressure Regimes. ACS Appl. Mater. Interfaces 2018, 10, 1623–1633. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Wang, Y.; Zhang, H.; Zhou, H.; Wei, Y.; Tao, S.; Ma, T. Composite catalyst of rosin carbon/Fe3O4: Highly efficient counter electrode for dye-sensitized solar cells. Chem. Commun. 2014, 50, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Shi, Y.; Wu, D.; Liang, S.; Song, X.; An, Y.; Hao, C. Well-defined heteroatom-rich porous carbon electrocatalyst derived from biowaste for high-performance counter electrode in dye-sensitized solar cells. Electrochim. Acta 2018, 281, 646–653. [Google Scholar] [CrossRef]
- Li, L.; Sun, F.; Gao, J.; Wang, L.; Pi, X.; Zhao, G. Broadening the pore size of coal-based activated carbon: Via a washing-free chem-physical activation method for high-capacity dye adsorption. RSC Adv. 2018, 8, 14488–14499. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Zhang, H.; Sun, H.; Tadé, M.O.; Wang, S. One-step synthesis of flour-derived functional nanocarbons with hierarchical pores for versatile environmental applications. Chem. Eng. J. 2018, 347, 432–439. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Dissanayake, P.D.; Gao, B.; Liu, W.-J.; Lee, K.B.; Ok, Y.S. Review on upgrading organic waste to value-added carbon materials for energy and environmental applications. J. Environ. Manag. 2021, 296, 113128. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.D.; LaCoste, J.D.; Fetrow, C.J.; Fei, L.; Wei, S. Bio-derived nanomaterials for energy storage and conversion. Nano Sel. 2021, 2, 1682–1706. [Google Scholar] [CrossRef]
- Bhat, V.S.; Jayeoye, T.J.; Rujiralai, T.; Sirimahachai, U.; Chong, K.F.; Hegde, G. Influence of surface properties on electro-chemical supercapacitors utilizing Callerya atropurpurea pod derived porous nanocarbons: Structure property relationship between porous structures to energy storage devices. Nano Sel. 2020, 1, 226–243. [Google Scholar] [CrossRef]
- Zhao, J.; Cui, Y.; Zhang, J.; Wu, J.; Yue, Y.; Qian, G. Fabrication of a Sustainable Closed Loop for Waste-Derived Materials in Electrochemical Applications. Ind. Eng. Chem. Res. 2021, 60, 11637–11648. [Google Scholar] [CrossRef]
- Joseph, S.; Saianand, G.; Benzigar, M.R.; Ramadass, K.; Singh, G.; Gopalan, A.I.; Yang, J.H.; Mori, T.; Al-Muhtaseb, A.H.; Yi, J.; et al. Recent Advances in Functionalized Nanoporous Carbons Derived from Waste Resources and Their Applications in Energy and Environment. Adv. Sustain. Syst. 2021, 5, 1–30. [Google Scholar] [CrossRef]
- Upare, D.P.; Yoon, S.; Lee, C.W. Nano-structured porous carbon materials for catalysis and energy storage. Korean J. Chem. Eng. 2011, 28, 731–743. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.M. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed. 2008, 47, 373–376. [Google Scholar] [CrossRef]
- Kubo, S.; White, R.J.; Tauer, K.; Titirici, M.M. Flexible coral-like carbon nanoarchitectures via a dual block copolymer-latex templating approach. Chem. Mater. 2013, 25, 4781–4790. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Cui, G.; Chen, L. Biomass-derived materials for electrochemical energy storages. Prog. Polym. Sci. 2015, 43, 136–164. [Google Scholar] [CrossRef]
- Xu, G.; Han, J.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Li, H.; Zhang, X. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 17, 1668–1674. [Google Scholar] [CrossRef]
- Kigozi, M.; Kali, R.; Bello, A.; Padya, B.; Kalu-Uka, G.M.; Wasswa, J.; Jain, P.K.; Onwualu, P.A.; Dzade, N.Y. Modified activation process for supercapacitor electrode materials from african maize cob. Materials 2020, 13, 5412. [Google Scholar] [CrossRef]
- Januszewicz, K.; Cymann-Sachajdak, A.; Kazimierski, P.; Klein, M.; Łuczak, J.; Wilamowska-Zawłocka, M. Chestnut-derived activated carbon as a prospective material for energy storage. Materials 2020, 13, 4658. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019, 12, 1751–1779. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [Green Version]
- Vinodh, R.; Sasikumar, Y.; Kim, H.-J.; Atchudan, R.; Yi, M. Chitin and Chitosan Based Biopolymer Derived Electrode Materials for Supercapacitor Applications: A Critical Review. J. Ind. Eng. Chem. 2021, 104, 155–171. [Google Scholar] [CrossRef]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and chitosan based composites for energy and environmental applications: A Review. Waste Biomass Valoriz. 2020, 12, 4777–4804. [Google Scholar] [CrossRef]
- Ikram, R.; Mohamed Jan, B.; Abdul Qadir, M.; Sidek, A.; Stylianakis, M.M.; Kenanakis, G. Recent Advances in Chitin and Chitosan/Graphene-Based Bio-Nanocomposites for Energetic Applications. Polymers 2021, 13, 3266. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. Recent applications of biopolymers derived from fish industry waste in food packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Gautam, R.K.; Kumar, V.; Kakatkar, A.S.; Chatterjee, S. Synthesis of Biodegradable Films Using Gamma Irradiation from Fish Waste. Waste Biomass Valoriz. 2020, 12, 2247–2257. [Google Scholar] [CrossRef]
- Shahbandeh, M. Fish Production Worldwide 2002–2019. Available online: https://www.statista.com/statistics/264577/total-world-fish-production-since-2002/ (accessed on 4 November 2020).
- Qin, D.; Bi, S.; You, X.; Wang, M.; Cong, X.; Yuan, C.; Yu, M.; Cheng, X.; Chen, X.-G. Development and application of fish scale wastes as versatile natural biomaterials. Chem. Eng. J. 2022, 428, 131102. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Bharathiraja, B.; Rithika, J.; Dhanasree, S.; Ezhilarasi, V.; Lavanya, A.; Praveenkumar, R. Production of biofuels from fish wastes: An overview. Biofuels 2019, 10, 301–307. [Google Scholar] [CrossRef]
- Cadavid-Rodríguez, L.S.; Vargas-Muñoz, M.A.; Plácido, J. Biomethane from fish waste as a source of renewable energy for artisanal fishing communities. Sustain. Energy Technol. Assess. 2019, 34, 110–115. [Google Scholar] [CrossRef]
- Rai, A.K.; Swapna, H.C.; Bhaskar, N.; Halami, P.M.; Sachindra, N.M. Effect of fermentation ensilaging on recovery of oil from fresh water fish viscera. Enzyme Microb. Technol. 2010, 46, 9–13. [Google Scholar] [CrossRef]
- Odoom-Wubah, T.; Rubio, S.; Tirado, J.L.; Ortiz, G.F.; Akoi, B.J.; Huang, J.; Li, Q. Waste Pd/Fish-Collagen as anode for energy storage. Renew. Sustain. Energy Rev. 2020, 131, 9968. [Google Scholar] [CrossRef]
- Putro, S.P.; Sharani, J.; Adhy, S. Biomonitoring of the Application of Monoculture and Integrated Multi-Trophic Aquaculture (IMTA) Using Macrobenthic Structures at Tembelas Island, Kepulauan Riau Province, Indonesia. J. Mar. Sci. Eng. 2020, 8, 942. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, H.-J.; Moon, C.-H. Eutrophication Driven by Aquaculture Fish Farms Controls Phytoplankton and Dinoflagellate Cyst Abundance in the Southern Coastal Waters of Korea. J. Mar. Sci. Eng. 2021, 9, 362. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vázquez, J.A.; Perez-Martin, R.I. Sustainable Sources from Aquatic Organisms for Cosmeceuticals Ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Blanco, M.; Sotelo, C.G.; Pérez-Martín, R.I. New strategy to cope with common fishery policy landing obligation: Collagen extraction from skins and bones of undersized hake (Merluccius merluccius). Polymers 2019, 11, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Liu, D.; Wang, Y.-J.; Cui, L.; Ignaszak, A.; Yu, Y.; Zhang, J. Research advances in biomass-derived nanostructured carbons and their composite materials for electrochemical energy technologies. Prog. Mater. Sci. 2020, 118, 100770. [Google Scholar] [CrossRef]
- Ling, H.Y.; Chen, H.; Wu, Z.; Hencz, L.; Qian, S.; Liu, X.; Liu, T.; Zhang, S. Sustainable bio-derived materials for addressing critical problems of next-generation high-capacity lithium-ion batteries. Mater. Chem. Front. 2021, 5, 5932–5953. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Zhu, X.; Yang, B.; Liu, Q. Crab shell derived multi-hierarchical carbon materials as a typical recycling of waste for high performance supercapacitors. Carbon 2019, 141, 748–757. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liu, M.; Zan, Y.; Dou, M.; Liu, J.; Zhang, Z.; Huang, Y.; Wang, F. Porous carbons derived from collagen-enriched biomass: Tailored design, synthesis, and application in electrochemical energy storage and conversion. Adv. Funct. Mater. 2019, 29, 1905095. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Huang, Y.; Wang, W. A fish scale based hierarchical lamellar porous carbon material obtained using a natural template for high performance electrochemical capacitors. J. Mater. Chem. 2010, 20, 4773–4775. [Google Scholar] [CrossRef]
- Liu, J.; Poh, C.K.; Zhan, D.; Lai, L.; Lim, S.H.; Wang, L.; Liu, X.; Gopal Sahoo, N.; Li, C.; Shen, Z.; et al. Improved synthesis of graphene flakes from the multiple electrochemical exfoliation of graphite rod. Nano Energy 2013, 2, 377–386. [Google Scholar] [CrossRef]
- Gao, M.; Su, C.C.; He, M.; Glossmann, T.; Hintennach, A.; Feng, Z.; Huang, Y.; Zhang, Z. A high performance lithium-sulfur battery enabled by a fish-scale porous carbon/sulfur composite and symmetric fluorinated diethoxyethane electrolyte. J. Mater. Chem. A 2017, 5, 6725–6733. [Google Scholar] [CrossRef]
- Yao, H.; Zheng, G.; Li, W.; McDowell, M.T.; Seh, Z.; Liu, N.; Lu, Z.; Cui, Y. Crab shells as sustainable templates from nature for nanostructured battery electrodes. Nano Lett. 2013, 13, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Nai, J.; Sheng, O.; Yuan, H.; Zhang, W.; Tao, X.; Lou, X.W.D. Biomass-based materials for green lithium secondary batteries. Energy Environ. Sci. 2021, 14, 1326–1379. [Google Scholar] [CrossRef]
- Chen, Q.; Tan, X.; Liu, Y.; Liu, S.; Li, M.; Gu, Y.; Zhang, P.; Ye, S.; Yang, Z.; Yang, Y. Biomass-derived porous graphitic carbon materials for energy and environmental applications. J. Mater. Chem. A 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Pendashteh, A.; Orayech, B.; Ajuria, J.; Jáuregui, M.; Saurel, D. Exploring Vinyl Polymers as Soft Carbon Precursors for M-Ion (M = Na, Li) Batteries and Hybrid Capacitors. Energies 2020, 13, 4189. [Google Scholar] [CrossRef]
- Ali, M.U.; Zafar, A.; Nengroo, S.H.; Hussain, S.; Junaid Alvi, M.; Kim, H.-J. Towards a smarter battery management system for electric vehicle applications: A critical review of lithium-ion battery state of charge estimation. Energies 2019, 12, 446. [Google Scholar] [CrossRef] [Green Version]
- Bozzini, B.; Mele, C.; Veneziano, A.; Sodini, N.; Lanzafame, G.; Taurino, A.; Mancini, L. Morphological evolution of Zn-sponge electrodes monitored by in situ X-ray computed microtomography. ACS Appl. Energy Mater. 2020, 3, 4931–4940. [Google Scholar] [CrossRef]
- Lavagna, L.; Meligrana, G.; Gerbaldi, C.; Tagliaferro, A.; Bartoli, M. Graphene and lithium-based battery electrodes: A review of recent literature. Energies 2020, 13, 4867. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-P.; Choi, S.; Cho, S.; Song, W.-J.; Park, S. Fabrication of Carbon Nanofibers Decorated with Various Kinds of Metal Oxides for Battery Applications. Energies 2021, 14, 1353. [Google Scholar] [CrossRef]
- Verma, S.; Sinha-Ray, S.; Sinha-Ray, S. Electrospun CNF Supported Ceramics as Electrochemical Catalysts for Water Splitting and Fuel Cell: A Review. Polymers 2020, 12, 238. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wang, D.; Liu, J.; Qian, G.; Chen, Y.; Wang, Z. Hollow double-layer carbon nanocage confined Si nanoparticles for high performance lithium-ion batteries. Nanoscale Adv. 2020, 2, 3222–3230. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, D.; Liang, Z.; Zhao, Y.; Tian, W.; Ren, X.; Wang, J.; Li, X.; Gao, Y.; Wen, W. Facile renewable synthesis of nitrogen/oxygen co-doped graphene-like carbon nanocages as general lithium-ion and potassium-ion batteries anode. Carbon 2020, 167, 685–695. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Lu, M.; Zhang, J.; Li, T. Tuning morphology, defects and functional group types in hard carbon via phosphorus doped for rapid sodium storage. Carbon 2021, 183, 415–427. [Google Scholar] [CrossRef]
- Wang, H.; Hu, J.; Yang, Y.; Wu, Q.; Li, Y. Fabrication of high-performance lithium ion battery anode materials from polysilsesquioxane nanotubes. J. Alloys Compd. 2021, 859, 157801. [Google Scholar] [CrossRef]
- Liu, H.J.; Wang, X.M.; Cui, W.J.; Dou, Y.Q.; Zhao, D.Y.; Xia, Y.Y. Highly ordered mesoporous carbon nanofiber arrays from a crab shell biological template and its application in supercapacitors and fuel cells. J. Mater. Chem. 2010, 20, 4223–4230. [Google Scholar] [CrossRef]
- Lian, X.; Li, Q.; Zhao, Y.; Liu, S.; Liu, H.; Zhang, H. The electrochemical properties of porous carbon derived from the prawn as anode for lithium ion batteries. Int. J. Electrochem. Sci. 2018, 13, 2474–2482. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, B.; Lin, X.; Xie, Z. Biomass-derived hierarchical porous carbons: Boosting the energy density of supercapacitors via an ionothermal approach. J. Mater. Chem. A 2017, 5, 13009–13018. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Wang, X.; Chen, S.; Xu, F.; Zuo, L.; Wu, J.; Sun, L.; Li, Z.; Hou, H. Nitrogen-doped porous carbon/Co3O4 nanocomposites as anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 7117–7125. [Google Scholar] [CrossRef]
- Qadrdan, M.; Jenkins, N.; Wu, J. Smart grid and energy storage. In McEvoy’s Handbook of Photovoltaics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 915–928. [Google Scholar]
- Elizabeth, I.; Singh, B.P.; Trikha, S.; Gopukumar, S. Bio-derived hierarchically macro-meso-micro porous carbon anode for lithium/sodium ion batteries. J. Power Sources 2016, 329, 412–421. [Google Scholar] [CrossRef]
- Wang, X.-T.; Yu, H.-Y.; Liang, H.-J.; Gu, Z.-Y.; Nie, P.; Wang, H.; Guo, J.-Z.; Ang, E.H.; Wu, X.-L. Waste Utilization of Crab Shell: 3D Hierarchically Porous Carbon Towards High-Performance Na/Li Storage. New J. Chem. 2021, 45, 19439–19445. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material–fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Bresser, D.; Paillard, E.; Passerini, S. Advances in Batteries for Medium- and Large-Scale Energy Storage; Woodhead Publishing Series in Energy; Elservier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Wentker, M.; Greenwood, M.; Leker, J. A bottom-up approach to lithium-ion battery cost modeling with a focus on cathode active materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Lu, Y.; Chen, J. Advanced organic electrode materials for rechargeable sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1601792. [Google Scholar] [CrossRef]
- Darjazi, H.; Staffolani, A.; Sbrascini, L.; Bottoni, L.; Tossici, R.; Nobili, F. Sustainable Anodes for Lithium-and Sodium-Ion Batteries Based on Coffee Ground-Derived Hard Carbon and Green Binders. Energies 2020, 13, 6216. [Google Scholar] [CrossRef]
- Peters, J.F.; Peña Cruz, A.; Weil, M. Exploring the economic potential of sodium-ion batteries. Batteries 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. Energy Chem. 2019, 1, 100012. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Velez, V.; Ramos-Sánchez, G.; Lopez, B.; Lartundo-Rojas, L.; González, I.; Sierra, L. Synthesis of novel hard mesoporous carbons and their applications as anodes for Li and Na ion batteries. Carbon 2019, 147, 214–226. [Google Scholar] [CrossRef]
- del Saavedra Rios, C.M.; Simonin, L.; de Geyer, A.; Ghimbeu, C.M.; Dupont, C. Unraveling the Properties of Biomass-Derived Hard Carbons upon Thermal Treatment for a Practical Application in Na-Ion Batteries. Energies 2020, 13, 3513. [Google Scholar] [CrossRef]
- Luo, X.; Chen, S.; Hu, T.; Chen, Y.; Li, F. Renewable biomass-derived carbons for electrochemical capacitor applications. SusMat 2021, 1, 211–240. [Google Scholar] [CrossRef]
- Dahbi, M.; Nakano, T.; Yabuuchi, N.; Ishikawa, T.; Kubota, K.; Fukunishi, M.; Shibahara, S.; Son, J.-Y.; Cui, Y.-T.; Oji, H. Sodium carboxymethyl cellulose as a potential binder for hard-carbon negative electrodes in sodium-ion batteries. Electrochem. Commun. 2014, 44, 66–69. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhang, B.; Miao, L.; Cai, J.; Peng, L.; Huang, Y.; Jiang, J.; Huang, Y.; Zhang, L. Nitrogen-rich hard carbon as a highly durable anode for high-power potassium-ion batteries. Energy Storage Mater. 2017, 8, 161–168. [Google Scholar] [CrossRef]
- Pu, X.; Yang, G.; Yu, C. Liquid-type cathode enabled by 3D sponge-like carbon nanotubes for high energy density and long cycling life of Li-S batteries. Adv. Mater. 2014, 26, 7456–7461. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, Z.; Ning, X.; Liu, Z.; Yang, Z.; Zou, C.; Wang, S.; Chen, X.; Chen, Y.; Huang, S. Sulfur-Impregnated, Sandwich-Type, Hybrid Carbon Nanosheets with Hierarchical Porous Structure for High-Performance Lithium-Sulfur Batteries. Adv. Energy Mater. 2014, 4, 1301988. [Google Scholar] [CrossRef]
- Lin, Z.; Liang, C. Lithium–sulfur batteries: From liquid to solid cells. J. Mater. Chem. A 2015, 3, 936–958. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, T.; Liu, Y.; Nai, J.; Wang, Y.; Zhang, W.; Tao, X. A review of biomass materials for advanced lithium–sulfur batteries. Chem. Sci. 2019, 10, 7484–7495. [Google Scholar] [CrossRef] [Green Version]
- Xing, Z.; Tan, G.; Yuan, Y.; Wang, B.; Ma, L.; Xie, J.; Li, Z.; Wu, T.; Ren, Y.; Shahbazian-Yassar, R. Consolidating Lithiothermic-Ready Transition Metals for Li2S-Based Cathodes. Adv. Mater. 2020, 32, 2002403. [Google Scholar] [CrossRef]
- Ould Ely, T.; Kamzabek, D.; Chakraborty, D.; Doherty, M.F. Lithium–sulfur batteries: State of the art and future directions. ACS Appl. Energy Mater. 2018, 1, 1783–1814. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Liu, C.; Gao, Y.; Zhang, J.-M.; Wang, Y.-Q. Research progress of sulfur/carbon composite cathode materials and the corresponding safe electrolytes for advanced Li-S batteries. Nano 2020, 15, 2030002. [Google Scholar] [CrossRef]
- Eftekhari, A.; Kim, D.-W. Cathode materials for lithium–sulfur batteries: A practical perspective. J. Mater. Chem. A 2017, 5, 17734–17776. [Google Scholar] [CrossRef]
- Qu, J.; Lv, S.; Peng, X.; Tian, S.; Wang, J.; Gao, F. Nitrogen-doped porous “green carbon” derived from shrimp shell: Combined effects of pore sizes and nitrogen doping on the performance of lithium sulfur battery. J. Alloys Compd. 2016, 671, 17–23. [Google Scholar] [CrossRef]
- Zhao, S.; Li, C.; Wang, W.; Zhang, H.; Gao, M.; Xiong, X.; Wang, A.; Yuan, K.; Huang, Y.; Wang, F. A novel porous nanocomposite of sulfur/carbon obtained from fish scales for lithium-sulfur batteries. J. Mater. Chem. A 2013, 1, 3334–3339. [Google Scholar] [CrossRef]
- Gao, M.; Li, C.; Liu, N.; Chen, Y.; Wang, W.; Zhang, H.; Yu, Z.; Huang, Y. Inhibition on polysulfides dissolve during the discharge-charge by using fish-scale-based porous carbon for lithium-sulfur battery. Electrochim. Acta 2014, 149, 258–263. [Google Scholar] [CrossRef]
- Shao, H.; Huang, B.; Liu, N.; Wang, W.; Zhang, H.; Wang, A.; Wang, F.; Huang, Y. Modified separators coated with a Ca(OH)2—Carbon framework derived from crab shells for lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 16627–16634. [Google Scholar] [CrossRef]
- Ai, F.; Liu, N.; Wang, W.; Wang, A.; Wang, F.; Zhang, H.; Huang, Y. Heteroatoms-Doped Porous Carbon Derived from Tuna Bone for High Performance Li-S Batteries. Electrochim. Acta 2017, 258, 80–89. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Chi, Z.; Ke, F.; Yang, Y.; Wang, A.; Wang, W.; Miao, L. How Far Away Are Lithium-Sulfur Batteries from Commercialization? Front. Energy Res. 2019, 7, 123. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Häggström, F.; Delsing, J. Iot energy storage-a forecast. Energy Harvest. Syst. 2018, 5, 43–51. [Google Scholar] [CrossRef]
- Mele, C.; Catalano, M.; Taurino, A.; Bozzini, B. Electrochemical fabrication of nanoporous gold-supported manganese oxide nanowires based on electrodeposition from eutectic urea/choline chloride ionic liquid. Electrochim. Acta 2013, 87, 918–924. [Google Scholar] [CrossRef]
- Tobi, A.R.; Dennis, J.O. Activated carbon from composite of palm bio-waste as electrode material for solid-state electric double layer capacitor. J. Energy Storage 2021, 42, 103087. [Google Scholar] [CrossRef]
- Czupryński, A. Flame spraying of aluminum coatings reinforced with particles of carbonaceous materials as an alternative for laser cladding technologies. Materials 2019, 12, 3467. [Google Scholar] [CrossRef] [Green Version]
- Mensah-Darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor energy storage device using biowastes: A sustainable approach to green energy. Sustainability 2019, 11, 414. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Niu, J.; Zhang, Z.; Dou, M.; Li, Z.; Wang, F. Porous carbons with tailored heteroatom doping and well-defined porosity as high-performance electrodes for robust Na-ion capacitors. J. Power Sources 2019, 414, 68–75. [Google Scholar] [CrossRef]
- Long, C.; Jiang, L.; Wu, X.; Jiang, Y.; Yang, D.; Wang, C.; Wei, T.; Fan, Z. Facile synthesis of functionalized porous carbon with three-dimensional interconnected pore structure for high volumetric performance supercapacitors. Carbon 2015, 93, 412–420. [Google Scholar] [CrossRef]
- Hoffmann, V.; Jung, D.; Alhnidi, M.J.; Mackle, L.; Kruse, A. Bio-based carbon materials from potato waste as electrode materials in supercapacitors. Energies 2020, 13, 2406. [Google Scholar] [CrossRef]
- Lach, J.; Wróbel, K.; Wróbel, J.; Czerwiński, A. Applications of Carbon in Rechargeable Electrochemical Power Sources: A Review. Energies 2021, 14, 2649. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, B.; Halsted, J.K.; Subashchandrabose, R.; Stickle, W.F.; Ji, X. Direct fabrication of nanoporous graphene from graphene oxide by adding a gasification agent to a magnesiothermic reaction. Chem. Commun. 2015, 51, 1969–1971. [Google Scholar] [CrossRef]
- Sun, J.; Niu, J.; Liu, M.; Ji, J.; Dou, M.; Wang, F. Biomass-derived nitrogen-doped porous carbons with tailored hierarchical porosity and high specific surface area for high energy and power density supercapacitors. Appl. Surf. Sci. 2018, 427, 807–813. [Google Scholar] [CrossRef]
- Liu, M.; Niu, J.; Zhang, Z.; Dou, M.; Wang, F. Potassium compound-assistant synthesis of multi-heteroatom doped ultrathin porous carbon nanosheets for high performance supercapacitors. Nano Energy 2018, 51, 366–372. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.; Zhao, Z.; Wang, Z.; Qiu, J. Nitrogen-doped activated carbon derived from prawn shells for high-performance supercapacitors. Electrochim. Acta 2016, 190, 1134–1141. [Google Scholar] [CrossRef]
- Wang, J.; Shen, L.; Xu, Y.; Dou, H.; Zhang, X. Lamellar-structured biomass-derived phosphorus- and nitrogen-co-doped porous carbon for high-performance supercapacitors. New J. Chem. 2015, 39, 9497–9503. [Google Scholar] [CrossRef]
- Raj, C.J.; Rajesh, M.; Manikandan, R.; Yu, K.H.; Anusha, J.R.; Ahn, J.H.; Kim, D.W.; Park, S.Y.; Kim, B.C. High electrochemical capacitor performance of oxygen and nitrogen enriched activated carbon derived from the pyrolysis and activation of squid gladius chitin. J. Power Sources 2018, 386, 66–76. [Google Scholar] [CrossRef]
- Shan, B.; Cui, Y.; Liu, W.; Zhang, Y.; Liu, S.; Wang, H.; Sun, L.; Wang, Z.; Wu, R. Fibrous Bio-Carbon Foams: A New Material for Lithium-Ion Hybrid Supercapacitors with Ultrahigh Integrated Energy/Power Density and Ultralong Cycle Life. ACS Sustain. Chem. Eng. 2018, 6, 14989–15000. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Li, Z. Unusual interconnected graphitized carbon nanosheets as the electrode of high-rate ionic liquid-based supercapacitor. Carbon 2017, 119, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Sardar, A.; Khan, K.M.; Naz, M.Y.; Sulaiman, S.A.; Shukrullah, S. Construction of Rechargeable Protein Battery from Mixed-Waste Processing of Fish Scales and Chicken Feathers. Waste Biomass Valoriz. 2020, 11, 2129–2135. [Google Scholar] [CrossRef]
- Hussain, Z.; Bashir, N.; Ahmad, O.; Khan, K.M.; Karim, A.; Perveen, S.; Khan, M.; Rafiq, N. Preparation of a rechargeable battery using waste protein from the fish scales. J. Chem. Soc. Pak. 2015, 37, 824–829. [Google Scholar]
- Hussain, Z.; Khatak, K.; Sardar, A.; Khan, K.M.; Perveen, S. Sonic and microwaves assisted redox reactions of the hydrolysates of protein for the preparation of rechargeable battery. J. Chem. Soc. Pak. 2016, 38, 398–404. [Google Scholar]
- Battampara, P.; Ingale, D.; Guna, V.; Pradhan, U.U.; Reddy, N. Green Energy from Discarded Wool and Fish Scales. Waste Biomass Valoriz. 2021, 12, 6835–6845. [Google Scholar] [CrossRef]
- Guo, C.; Hu, R.; Liao, W.; Li, Z.; Sun, L.; Shi, D.; Li, Y.; Chen, C. Protein-enriched fish “biowaste” converted to three-dimensional porous carbon nano-network for advanced oxygen reduction electrocatalysis. Electrochim. Acta 2017, 236, 228–238. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef]
- Bozzini, B.; Amati, M.; Gregoratti, L.; Mele, C.; Abyaneh, M.K.; Prasciolu, M.; Kiskinova, M. In-situ photoelectron microspectroscopy during the operation of a single-chamber SOFC. Electrochem. Commun. 2012, 24, 104–107. [Google Scholar] [CrossRef]
- Liu, F.; Peng, H.; You, C.; Fu, Z.; Huang, P.; Song, H.; Liao, S. High-performance doped carbon catalyst derived from nori biomass with melamine promoter. Electrochim. Acta 2014, 138, 353–359. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Liu, S.; Zhang, X.; Wu, T.; Ge, X.; Zang, Y.; Zhao, H.; Wang, G. Shrimp-shell derived carbon nanodots as carbon and nitrogen sources to fabricate three-dimensional N-doped porous carbon electrocatalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2016, 18, 4095–4101. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Li, L.; Geng, K.; Wei, X.; Zhang, S. Recycling the biowaste to produce nitrogen and sulfur self-doped porous carbon as an efficient catalyst for oxygen reduction reaction. Nano Energy 2015, 16, 408–418. [Google Scholar] [CrossRef]
- Yuan, W.; Feng, Y.; Xie, A.; Zhang, X.; Huang, F.; Li, S.; Zhang, X.; Shen, Y. Nitrogen-doped nanoporous carbon derived from waste pomelo peel as a metal-free electrocatalyst for the oxygen reduction reaction. Nanoscale 2016, 8, 8704–8711. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lionetto, F.; Bagheri, S.; Mele, C. Sustainable Materials from Fish Industry Waste for Electrochemical Energy Systems. Energies 2021, 14, 7928. https://doi.org/10.3390/en14237928
Lionetto F, Bagheri S, Mele C. Sustainable Materials from Fish Industry Waste for Electrochemical Energy Systems. Energies. 2021; 14(23):7928. https://doi.org/10.3390/en14237928
Chicago/Turabian StyleLionetto, Francesca, Sonia Bagheri, and Claudio Mele. 2021. "Sustainable Materials from Fish Industry Waste for Electrochemical Energy Systems" Energies 14, no. 23: 7928. https://doi.org/10.3390/en14237928







