Selected Critical Raw Materials in Waste from Coal Gasification in Poland
Abstract
:1. Introduction
2. Samples and Research Methodology
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Higman, C.; van der Burgt, M. Gasification; Gulf Professional Pub./Elsevier Science: Amsterdam, The Netherlands, 2008; ISBN 9780080560908. [Google Scholar]
- Bielowicz, B.; Kasiński, J.R.J.R. The possibility of underground gasification of lignite from Polish deposits. Int. J. Coal Geol. 2014, 131, 304–318. [Google Scholar] [CrossRef]
- Produkcja Energii Elektrycznej w Polsce|Rynek Elektryczny. Available online: https://www.rynekelektryczny.pl/produkcja-energii-elektrycznej-w-polsce/ (accessed on 27 May 2021).
- Ministry of Energy. Polish Energy Policy until 2040 (Polityka Energetyczna Polski do 2040) PEP2040. 2018. Available online: https://www.gov.pl/web/klimat/polityka-energetyczna-polski (accessed on 4 October 2021).
- Radwanek-Bąk, B. Zasoby kopalin Polski w aspekcie oceny surowców krytycznych Unii Europejskiej. Gospod. Surowcami Miner.—Miner. Resour. Manag. 2011, 27, 5–19. [Google Scholar]
- Federal Register: Final List of Critical Minerals 2018. Available online: https://www.federalregister.gov/documents/2018/05/18/2018-10667/final-list-of-critical-minerals-2018 (accessed on 4 October 2021).
- 5-Year National Plan on Mineral Resources Approved. Available online: http://www.scio.gov.cn/32618/Document/1518844/1518844.htm (accessed on 4 October 2021).
- Australia’s Critical Minerals Strategy|Department of Industry, Science, Energy and Resources. Available online: https://www.industry.gov.au/data-and-publications/australias-critical-minerals-strategy (accessed on 4 October 2021).
- Tang, Y.; Guo, X.; Pan, X.; Finkelman, R.B.; Wang, Y.; Huan, B.; Wang, S. Changes and distribution of modes of occurrence of seventeen potentially-hazardous trace elements during entrained flow gasification of coals from Ningdong, China. Minerals 2018, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Niu, M.; Fu, Y.; Liu, S. Distribution and leachability of hazardous trace elements in Lurgi gasification ash from a Coal—to—SNG plant. J. Energy Inst. 2021, 98, 223–233. [Google Scholar] [CrossRef]
- Bielowicz, B. Ash characteristics and selected critical elements (Ga, Sc, V) in coal and ash in polish deposits. Resources 2020, 9, 115. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Jiang, J.; Hower, J.C.; Song, X.; Jiang, Y.; Wang, X.; Gornostaeva, T.; Li, X.; et al. Composition and modes of occurrence of minerals and elements in coal combustion products derived from high-Ge coals. Int. J. Coal Geol. 2014, 121, 79–97. [Google Scholar] [CrossRef]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilisation of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Blissett, R.S.S.; Smalley, N.; Rowson, N.A.A. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Querol, X.; JoséLuis, F.-T.; Angel, L.-T. Trace elements in coal and their behavior during combustion in a large power station. Fuel 1995, 74, 331–343. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dai, S.; Zou, J.; French, D.; Graham, I.T. Rare earth elements and yttrium in coal ash from the Luzhou power plant in Sichuan, Southwest China: Concentration, characterization and optimized extraction. Int. J. Coal Geol. 2019, 203, 1–14. [Google Scholar] [CrossRef]
- Fu, B.; Hower, J.C.; Zhang, W.; Luo, G.; Hu, H.; Yao, H. A review of rare earth elements and yttrium in coal ash: Content, modes of occurrences, combustion behavior, and extraction methods. Prog. Energy Combust. Sci. 2022, 88, 100954. [Google Scholar] [CrossRef]
- Guo, X.; Tang, Y.; Wang, Y.; Eble, C.F.; Finkelman, R.B.; Huan, B.; Pan, X. Potential utilization of coal gasification residues from entrained-flow gasification plants based on rare earth geochemical characteristics. J. Clean. Prod. 2021, 280, 124329. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Seredin, V.V. Metalliferous coals: Formation conditions and outlooks for development. Coal Resour. Russ. 2004, 6, 452–519. [Google Scholar]
- Seredin, V.V.; Finkelman, R.B. Metalliferous Coals: A Review of the Main Genetic and Geochemical Types; Elsevier: Amsterdam, The Netherlands, 2008; Volume 76, pp. 253–289. [Google Scholar]
- Dai, S.; Wang, X.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K.; Huang, W.; Li, T.; Li, X.; Liu, H.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 30–31, 72–99. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- ISO—ISO 589:2008—Hard Coal—Determination of Total Moisture. Available online: https://www.iso.org/standard/45370.html (accessed on 28 October 2021).
- ISO 1171. Solid Mineral Fuels—Determination of Ash. 2010. Available online: https://www.iso.org/standard/55944.html (accessed on 28 October 2021).
- ISO 562. Hard Coal and Coke—Determination of Volatile Matter. 2010. Available online: https://www.iso.org/standard/55943.html (accessed on 28 October 2021).
- ISO 1928. Coal and Coke—Determination of Gross Calorific Value. 2020. Available online: https://www.iso.org/standard/75883.html (accessed on 28 October 2021).
- ISO—ISO 29541:2010—Solid Mineral Fuels—Determination of Total Carbon, Hydrogen and Nitrogen Content—Instrumental Method. Available online: https://www.iso.org/standard/45546.html (accessed on 28 October 2021).
- ISO—ISO 19579:2006—Solid Mineral Fuels—Determination of Sulfur by IR Spectrometry. Available online: https://www.iso.org/standard/39113.html (accessed on 28 October 2021).
- ISO 7404-5. Methods for the Petrographic Analysis of Coals—Part 5: Method of Determining Microscopically the Reflectance of Vitrinite. 2009. Available online: https://www.iso.org/standard/42832.html (accessed on 28 October 2021).
- ISO 7404-3. Methods for the Petrographic Analysis of Coals—Part 3: Method of Determining Maceral Group Composition. 2009. Available online: https://www.iso.org/standard/42831.html (accessed on 28 October 2021).
- Chmielniak, T.; Ściążko, M.; Sobolewski, A.; Tomaszewicz, G.; Popowicz, J. Coal gasification with CO2 as Gasification agent—As a method for improving emission factors and process efficiency. Polityka Energetyczna 2014, 15, 125–138. [Google Scholar]
- Chmielniak, T.; Sobolewski, A.; Tomaszewicz, G. CO2-Enhanced coal gasification. Experience of the Institute for Chemical Processing of Coal Zgazowanie węgla przy wykorzystaniu CO2 jako czynnika zgazowującego. Doświadczenia IChPW. Przemysł Chemiczny 2015, 1, 16–22. [Google Scholar] [CrossRef]
- Błaszczuk, A.; Nowak, W.; Jagodzik, S. Bed-to-wall heat transfer in a supercritical circulating fluidised bed boiler. Chem. Process. Eng.—Inz. Chem. Proces. 2014, 35, 191–204. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a Promising Source of Critical Elements: Progress and Future Prospects; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 186, pp. 155–164. [Google Scholar]
- Eskenazy, G.M. On the geochemistry of indium in coal-forming process. Geochim. Cosmochim. Acta 1980, 44, 1023–1027. [Google Scholar] [CrossRef]
- Wei, Q.; Cui, C.; Dai, S. Organic-association of Ge in the coal-hosted ore deposits: An experimental and theoretical approach. Ore Geol. Rev. 2020, 117, 103291. [Google Scholar] [CrossRef]
- Dai, S.; Liu, J.; Ward, C.R.; Hower, J.C.; Xie, P.; Jiang, Y.; Hood, M.M.; O’Keefe, J.M.K.; Song, H. Petrological, geochemical, and mineralogical compositions of the low-Ge coals from the Shengli Coalfield, China: A comparative study with Ge-rich coals and a formation model for coal-hosted Ge ore deposit. Ore Geol. Rev. 2015, 71, 318–349. [Google Scholar] [CrossRef]
- Etschmann, B.; Liu, W.; Li, K.; Dai, S.; Reith, F.; Falconer, D.; Kerr, G.; Paterson, D.; Howard, D.; Kappen, P.; et al. Enrichment of germanium and associated arsenic and tungsten in coal and roll-front uranium deposits. Chem. Geol. 2017, 29–49. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; López-Soler, A.; Chimenos, J.M.; Fernández, A.I.; Burgos, S.; García Peña, F. Ge extraction from gasification fly ash. Fuel 2005, 84, 1384–1392. [Google Scholar] [CrossRef]
- Arroyo, F.; Font, O.; Chimenos, J.M.; Fernández-Pereira, C.; Querol, X.; Coca, P. IGCC fly ash valorisation. Optimisation of Ge and Ga recovery for an industrial application. Fuel Process. Technol. 2014, 124, 222–227. [Google Scholar] [CrossRef]
- Zhuang, X.; Querol, X.; Alastuey, A.; Juan, R.; Plana, F.; Lopez-Soler, A.; Du, G.; Martynov, V.V. Geochemistry and mineralogy of the Cretaceous Wulantuga high-germanium coal deposit in Shengli coal field, Inner Mongolia, Northeastern China. Int. J. Coal Geol. 2006, 66, 119–136. [Google Scholar] [CrossRef]
- Hernández-Expósito, A.; Chimenos, J.M.; Fernández, A.I.; Font, O.; Querol, X.; Coca, P.; García Peña, F. Ion flotation of germanium from fly ash aqueous leachates. Chem. Eng. J. 2006, 118, 69–75. [Google Scholar] [CrossRef]
- Arroyo, F.; Fernández-Pereira, C.; Olivares, J.; Coca, P. Hydrometallurgical recovery of germanium from coal gasification fly ash: Pilot plant scale evaluation. Ind. Eng. Chem. Res. 2009, 48, 3573–3579. [Google Scholar] [CrossRef]
- Yudovich, Y.E.; Ketris, M.P. Arsenic in coal: A review. Int. J. Coal Geol. 2005, 61, 141–196. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, S.; Nechaev, V.P.; Nechaeva, E.V.; Graham, I.T.; French, D.; Sun, J. Enrichment of critical elements (Nb-Ta-Zr-Hf-REE) within coal and host rocks from the Datanhao mine, Daqingshan Coalfield, northern China. Ore Geol. Rev. 2019, 111, 102951. [Google Scholar] [CrossRef]
- Hower, J.C.J.C.; Ruppert, L.F.L.F.; Eble, C.F.C.F. Lanthanide, yttrium, and zirconium anomalies in the Fire Clay coal bed, Eastern Kentucky. Int. J. Coal Geol. 1999, 39, 141–153. [Google Scholar] [CrossRef]
- Dai, S.; Liu, J.; Ward, C.R.; Hower, J.C.; French, D.; Jia, S.; Hood, M.M.; Garrison, T.M. Mineralogical and geochemical compositions of Late Permian coals and host rocks from the Guxu Coalfield, Sichuan Province, China, with emphasis on enrichment of rare metals. Int. J. Coal Geol. 2016, 166, 71–95. [Google Scholar] [CrossRef]
- Eskenazy, G.M. Zirconium and hafnium in Bulgarian coals. Fuel 1987, 66, 1652–1657. [Google Scholar] [CrossRef]
- Dai, S.; Yan, X.; Ward, C.R.; Hower, J.C.; Zhao, L.L.; Wang, X.; Zhao, L.L.; Ren, D.; Finkelman, R.B. Valuable elements in Chinese coals: A review. Int. Geol. Rev. 2018, 60, 590–620. [Google Scholar] [CrossRef]
- Cui, W.; Meng, Q.; Feng, Q.; Zhou, L.; Cui, Y.; Li, W. Occurrence and release of cadmium, chromium, and lead from stone coal combustion. Int. J. Coal Sci. Technol. 2019, 6, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Travar, I.; Kihl, A.; Kumpiene, J. Utilization of air pollution control residues for the stabilization/solidification of trace element contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 19101–19111. [Google Scholar] [CrossRef]
- Eskenazy, G.M. Geochemistry of arsenic and antimony in Bulgarian coals. Chem. Geol. 1995, 119, 239–254. [Google Scholar] [CrossRef]
- Narukawa, T.; Takatsu, A.; Chiba, K.; Riley, K.W.; French, D.H. Investigation on chemical species of arsenic, selenium and antimony in fly ash from coal fuel thermal power stations. J. Environ. Monit. JEM 2005, 7, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, C.; Li, Y.; Wang, J.; Liu, S. Li distribution and mode of occurrences in Li-bearing coal seam # 6 from the Guanbanwusu Mine, Inner Mongolia, northern China. Energy Explor. Exploit. 2012, 30, 109–130. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yang, J.; Zhao, C. Minimum mining grade of associated Li deposits in coal seams. Energy Explor. Exploit. 2012, 30, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Hou, X.; Zhang, J.; Li, S.; Wu, H.; Damø, A.J.; Li, H.; Wu, Q.; Xi, X. Distribution and occurrence of lithium in high-alumina-coal fly ash. Int. J. Coal Geol. 2018, 189, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Zhao, C.; Li, Y.; Zhang, Y. Review of Coal as a Promising Source of Lithium; Inderscience Enterprises Ltd.: Geneva, Switzerland, 2015; Volume 9, pp. 215–229. [Google Scholar]
- Norris, P.; Chen, C.W.; Pan, W.P. A technique for sequential leaching of coal and fly ash resulting in good recovery of trace elements. Anal. Chim. Acta 2010, 663, 39–42. [Google Scholar] [CrossRef]
- Occurrence of Rubidium and Cesium in Iqe Coal, Qinghai-Tibet Plateau: Evidence from Sequential Chemical Extraction Experiment—Cunliang Zhao, Bangjun Liu, Jialiang Ma, Shiming Liu, Maksim G Blokhin. 2017. Available online: https://journals.sagepub.com/doi/full/10.1177/0144598717690088 (accessed on 18 October 2021).
- Su, T.; Wang, J. Modeling batch leaching behavior of arsenic and selenium from bituminous coal fly ashes. Chemosphere 2011, 85, 1368–1374. [Google Scholar] [CrossRef]
- Li, X.; Dai, S.; Zhang, W.; Li, T.; Zheng, X.; Chen, W. Determination of As and Se in coal and coal combustion products using closed vessel microwave digestion and collision/reaction cell technology (CCT) of inductively coupled plasma mass spectrometry (ICP-MS). Int. J. Coal Geol. 2014, 124, 1–4. [Google Scholar] [CrossRef]
- Gerson, A.R.; Fan, R.; Qian, G.; Schumann, R.C.; Olin, P.; Howard, D.L.; Smart, R.S.C. Examination of multiple sources of selenium release from coal wastes and strategies for remediation. J. Hazard. Mater. 2022, 422, 126924. [Google Scholar] [CrossRef] [PubMed]
- Yudovich, Y.E.; Ketris, M.P. Selenium in coal: A review. Int. J. Coal Geol. 2006, 67, 112–126. [Google Scholar] [CrossRef]
- Deng, Z.G.; Wei, C.; Fan, G.; Li, M.T.; Li, C.X.; Li, X. Bin Extracting vanadium from stone-coal by oxygen pressure acid leaching and solvent extraction. Trans. Nonferrous Met. Soc. China 2010, 20, S1003–S6326. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Juan, R.; Casado, R.; Ruiz, C.R.; López-Soler, Á.; Coca, P.; Peña, F.G. Recovery of gallium and vanadium from gasification fly ash. J. Hazard. Mater. 2007, 139, 413–423. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Huggins, F.E.; Chimenos, J.M.; Fernández, A.I.; Burgos, S.; Peña, F.G. Speciation of major and selected trace elements in IGCC fly ash. Fuel 2005, 84, 1364–1371. [Google Scholar] [CrossRef]
- Li, T.; Wang, B.; Li, W.; Nie, J.; Song, Z.; Yang, W.; Ma, C.; Sun, L. Effect of occurrence modes of nickel and vanadium on gasification characteristics of petroleum coke. Fuel 2020, 263, 116686. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of Rare Earths: A Critical Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 51, pp. 1–22. [Google Scholar]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Abhilash; Meshram, P. Overview On Extraction and Separation of Rare Earth Elements from Red Mud: Focus on Scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Lim, Y.; Yu, J.; Chen, S.; Woo, S.W.; Yoon, S.; Bae, S.; Kim, H.S. Characterization of rare earth elements present in coal ash by sequential extraction. J. Hazard. Mater. 2021, 402. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Leake, M.; Allen, L.; Free, M.L.; Hu, X.; Kim, D.; Noble, A.; Luttrell, G.H. Efficient recovery of rare earth elements from coal based resources: A bioleaching approach. Mater. Today Chem. 2020, 16, 100246. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, W.; Ward, C.R.; Seredin, V.V.; Hower, J.C.; Li, X.; Song, W.; Wang, X.; Kang, H.; Zheng, L.; et al. Mineralogical and geochemical anomalies of late Permian coals from the Fusui Coalfield, Guangxi Province, southern China: Influences of terrigenous materials and hydrothermal fluids. Int. J. Coal Geol. 2013, 105, 60–84. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A. Mineralogy characterization and recovery of rare earth elements from the roof and floor materials of the Guxu coalfield. Fuel 2020, 270, 117533. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Zeng, Y.; Tan, F.; He, J. Geochemistry and origin of rare earth elements (REEs) in the Shengli River oil shale, northern Tibet, China. Geochemistry 2011, 71, 21–30. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Eterigho-Ikelegbe, O.; Harrar, H.; Bada, S. Rare earth elements from coal and coal discard—A review. Miner. Eng. 2021, 173, 107187. [Google Scholar] [CrossRef]
- Stuckman, M.Y.; Lopano, C.L.; Granite, E.J. Distribution and speciation of rare earth elements in coal combustion by-products via synchrotron microscopy and spectroscopy. Int. J. Coal Geol. 2018, 195, 125–138. [Google Scholar] [CrossRef]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Hood, M.M.; Taggart, R.K.; Smith, R.C.; Hsu-Kim, H.; Henke, K.R.; Graham, U.; Groppo, J.G.; Unrine, J.M.; Hower, J.C. Rare Earth Element Distribution in Fly Ash Derived from the Fire Clay Coal, Kentucky. Coal Combust. Gasif. Prod. 2017, 9, 22–33. [Google Scholar] [CrossRef]
- Xiao-quan, S.; Wen, W.; Bei, W. Determination of gallium in coal and coal fly ash by electrothermal atomic absorption spectrometry using slurry sampling and nickel chemical modification. J. Anal. At. Spectrom. 1992, 7, 761. [Google Scholar] [CrossRef]
- Goodarzi, F.; Huggins, F.E.; Sanei, H. Assessment of elements, speciation of As, Cr, Ni and emitted Hg for a Canadian power plant burning bituminous coal. Int. J. Coal Geol. 2008, 74, 1–12. [Google Scholar] [CrossRef]
- Nazari, E.; Rashchi, F.; Saba, M.; Mirazimi, S.M.J. Simultaneous recovery of vanadium and nickel from power plant fly-ash: Optimization of parameters using response surface methodology. Waste Manag. 2014, 34, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Kuboňová, L.; Langová, Š.; Nowak, B.; Winter, F. Thermal and hydrometallurgical recovery methods of heavy metals from municipal solid waste fly ash. Waste Manag. 2013, 33, 2322–2327. [Google Scholar] [CrossRef]
- Bielowicz, B. Selected harmful elements in polish lignite. Gospod. Surowcami Miner.—Miner. Resour. Manag. 2013, 29, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Riazi, M.R.; Gupta, R. Geochemistry of Coal: Occurrences and Environmental Impacts of Trace Elements. Coal Prod. Process. Technol. 2015, 65–92. [Google Scholar] [CrossRef]



| Parameter | Symbol | Norm | Unit | 1C | 2C | 3C |
|---|---|---|---|---|---|---|
| Moisture | M ad | ISO 589:2008 [26] | % | 5.30 | 3.6 | 50.3 |
| Ash | A db | ISO 1171:2010 [27] | % | 13.80 | 8.2 | 23.6 |
| Volatile matter | V daf | ISO 562:2010 [28] | % | 38.20 | 35.3 | 58.59 |
| Gross calorific value | GCV daf | ISO 1928:2020 [29] | MJ/kg | 31.50 | 33.2 | 18.1 |
| Carbon content | Ct daf | ISO 29541:2010 [30] | % | 79.93 | 81 | 62.2 |
| Hydrogen content | Ht daf | ISO 29541:2010 [30] | % | 5.14 | 4.5 | 3.74 |
| Nitrogen content | N daf | ISO 29541:2010 [30] | % | 1.30 | 1.2 | 0.52 |
| Total sulfur | St db | ISO 19579:2006 [31] | % | 1.39 | 0.42 | 0.84 |
| Random reflectance | R | ISO 7404-5:1994 [32] | % | 0.45 | 0.77 | 0.26 |
| Vitrinite/Huminite group | V | ISO 7404-3:2009 [33] | % | 51 | 60.96 | 81.72 |
| Liptinite group | L | ISO 7404-3:2009 [33] | % | 8 | 4.83 | 7.02 |
| Inertinite group | I | ISO 7404-3:2009 [33] | % | 36 | 28.97 | 3.57 |
| Mineral Matter | MM | ISO 7404-3:2009 [33] | % | 5 | 5.23 | 7.7 |
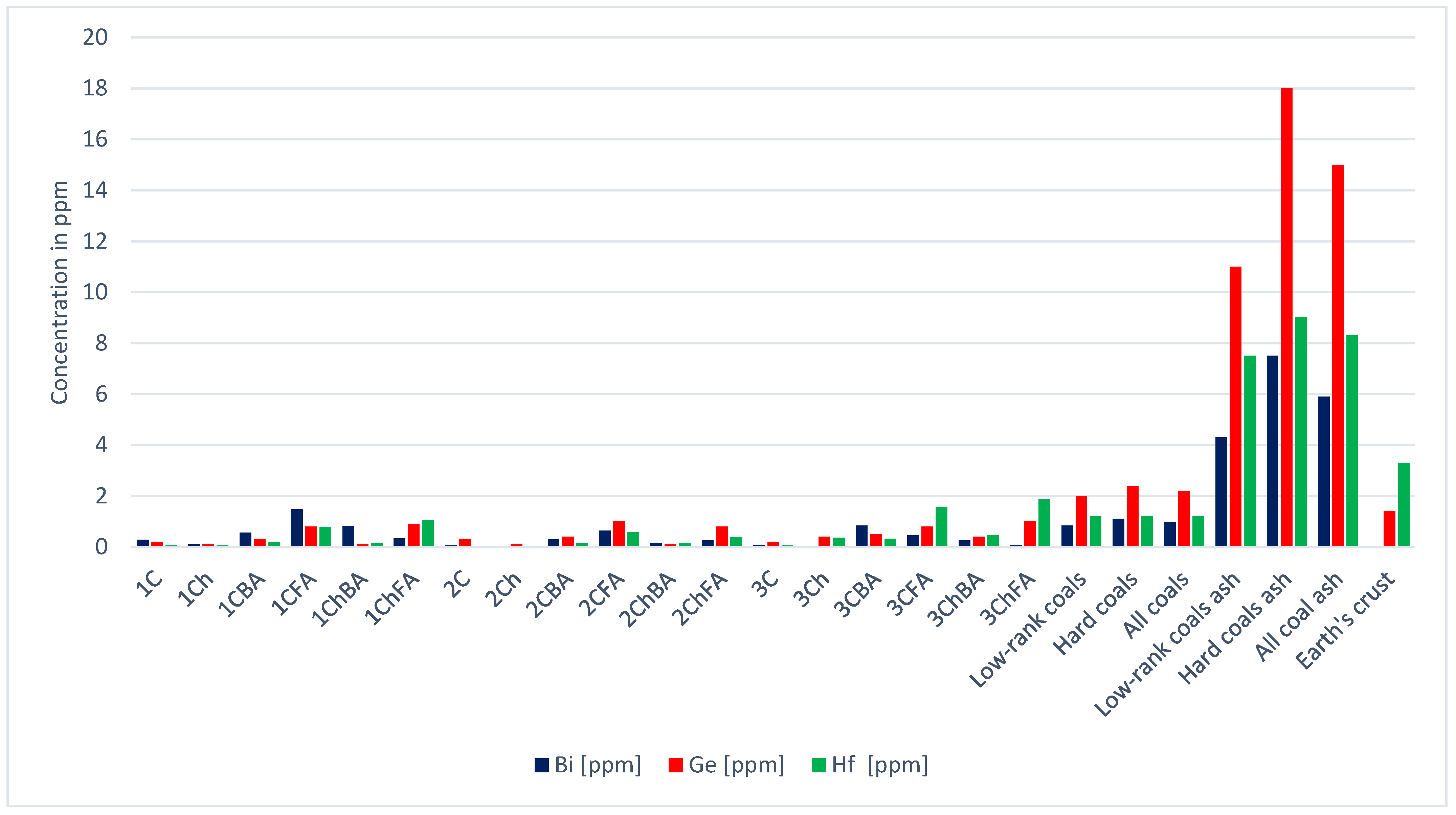
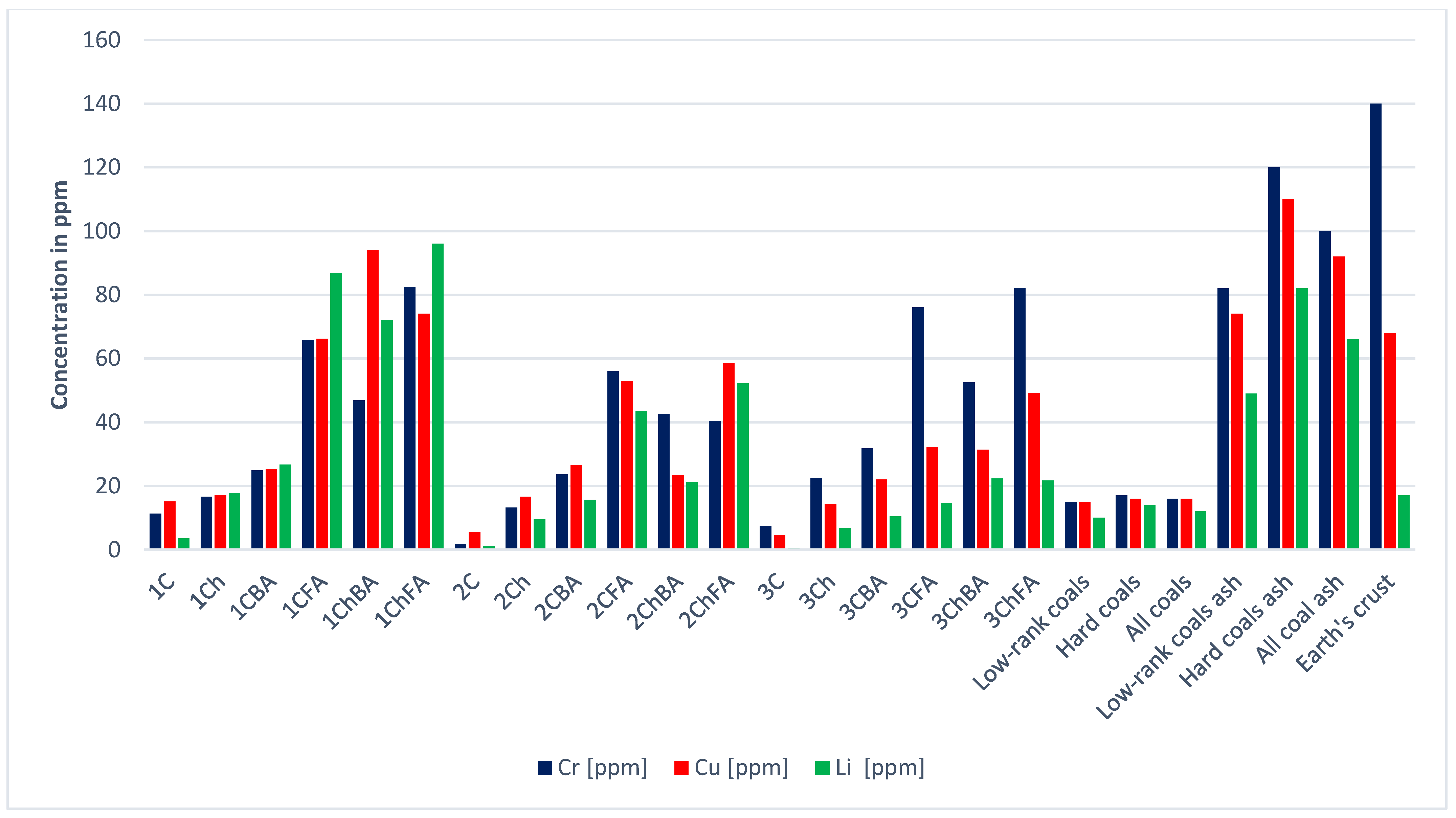
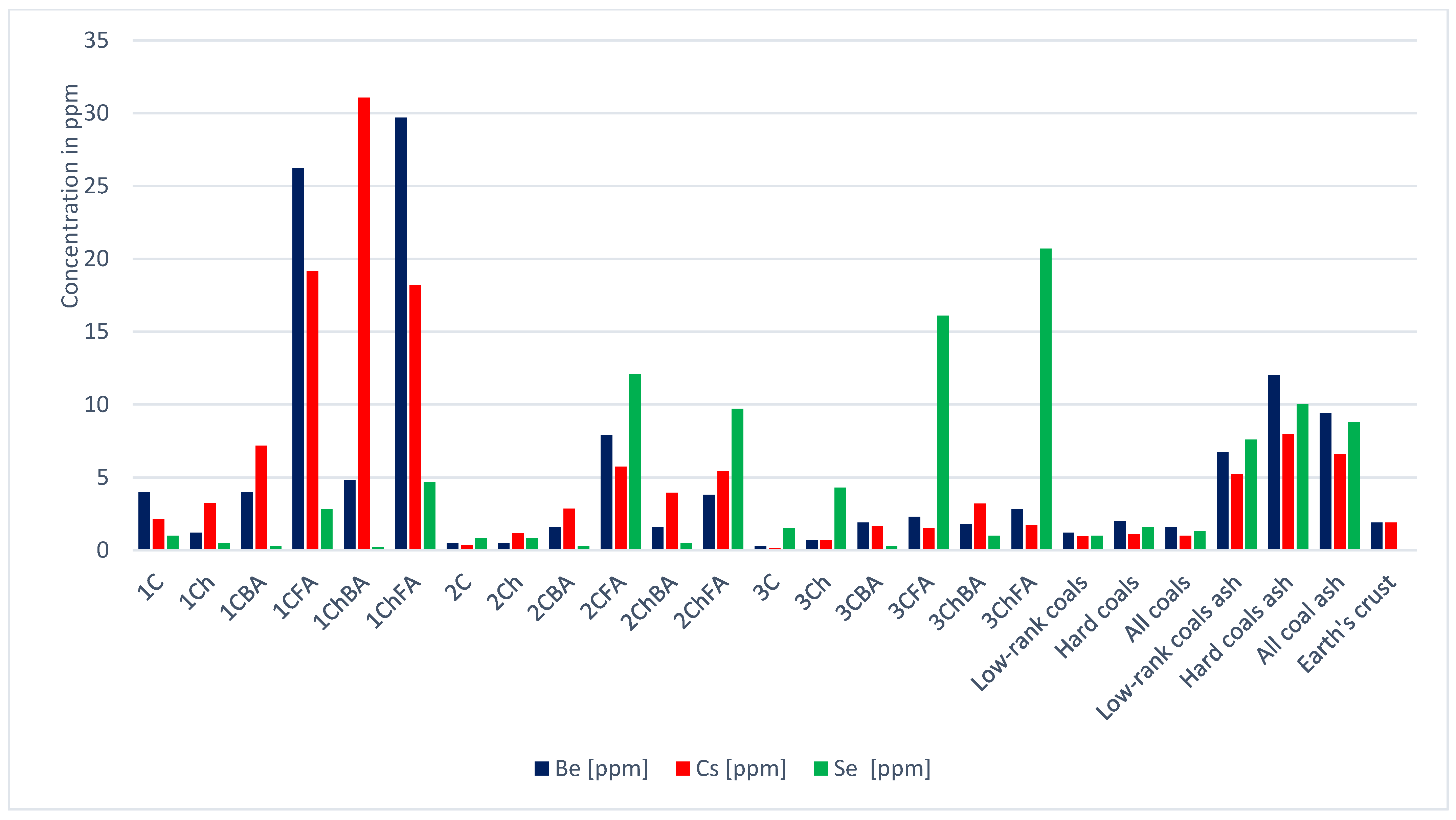
| Sample | Ag | As | Be | Bi | Co | Cr | Cs | Cu | Ga | Ge | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | |||||||||||
| 1C | 0.1 | 4.6 | 4.0 | 0.3 | 4.7 | 11.3 | 2.1 | 15.1 | 1.9 | 0.2 | |
| 1Ch | 0.1 | 1.3 | 1.2 | 0.1 | 3.4 | 16.6 | 3.2 | 17.1 | 2.0 | 0.1 | |
| 1CBA | 0.1 | 9.9 | 4.0 | 0.6 | 6.1 | 24.9 | 7.2 | 25.4 | 7.2 | 0.3 | |
| 1CFA | 0.3 | 18.6 | 26.2 | 1.5 | 25.5 | 65.7 | 19.2 | 66.2 | 22.1 | 0.8 | |
| 1ChBA | 0.3 | 24.0 | 4.8 | 0.8 | 11.7 | 46.9 | 31.1 | 94.0 | 25.3 | 0.1 | |
| 1ChFA | 0.4 | 13.3 | 29.7 | 0.3 | 31.0 | 82.4 | 18.2 | 74.0 | 26.2 | 0.9 | |
| 2C | 0.0 | 0.2 | 0.5 | 0.1 | 1.4 | 1.8 | 0.4 | 5.6 | 0.1 | 0.3 | |
| 2Ch | 0.0 | 0.8 | 0.5 | 0.1 | 2.2 | 13.2 | 1.2 | 16.6 | 1.4 | 0.1 | |
| 2CBA | 0.1 | 3.0 | 1.6 | 0.3 | 7.0 | 23.6 | 2.9 | 26.6 | 5.2 | 0.4 | |
| 2CFA | 0.2 | 7.6 | 7.9 | 0.6 | 15.3 | 56.0 | 5.7 | 52.8 | 9.6 | 1.0 | |
| 2ChBA | 0.1 | 1.7 | 1.6 | 0.2 | 6.9 | 42.6 | 4.0 | 23.3 | 6.4 | 0.1 | |
| 2ChFA | 0.2 | 2.6 | 3.8 | 0.3 | 15.0 | 40.4 | 5.4 | 58.5 | 7.9 | 0.8 | |
| 3C | 0.0 | 0.5 | 0.3 | 0.1 | 0.7 | 7.5 | 0.1 | 4.7 | 1.0 | 0.2 | |
| 3Ch | 0.1 | 1.2 | 0.7 | 0.0 | 1.7 | 22.5 | 0.7 | 14.3 | 4.1 | 0.4 | |
| 3CBA | 0.1 | 5.6 | 1.9 | 0.8 | 5.5 | 31.8 | 1.6 | 22.0 | 5.2 | 0.5 | |
| 3CFA | 0.1 | 6.1 | 2.3 | 0.5 | 5.7 | 76.1 | 1.5 | 32.2 | 15.6 | 0.8 | |
| 3ChBA | 0.2 | 4.7 | 1.8 | 0.3 | 7.3 | 52.5 | 3.2 | 31.3 | 10.3 | 0.4 | |
| 3ChFA | 0.2 | 9.9 | 2.8 | 0.1 | 8.4 | 82.1 | 1.7 | 49.3 | 17.8 | 1.0 | |
| Low-rank coals [21] | 0.1 | 7.6 | 1.2 | 0.8 | 4.2 | 15.0 | 1.0 | 15.0 | 5.5 | 2.0 | |
| Hard coals [21] | 0.1 | 9.0 | 2.0 | 1.1 | 6.0 | 17.0 | 1.1 | 16.0 | 6.0 | 2.4 | |
| All coals [21] | 0.1 | 8.3 | 1.6 | 1.0 | 5.1 | 16.0 | 1.0 | 16.0 | 5.8 | 2.2 | |
| Low-rank coal ash [21] | 0.6 | 48.0 | 6.7 | 4.3 | 26.0 | 82.0 | 5.2 | 74.0 | 29.0 | 11.0 | |
| Hard coal ash [21] | 0.6 | 46.0 | 12.0 | 7.5 | 37.0 | 120.0 | 8.0 | 110.0 | 36.0 | 18.0 | |
| Total; coal ash [21] | 0.6 | 47.0 | 9.4 | 5.9 | 32.0 | 100.0 | 6.6 | 92.0 | 33.0 | 15.0 | |
| Suggested cut-off grade [37] | 10 | - | 300 | - | - | - | 150 | - | 100 | 300 | |
| Earth’s crust | 0.1 | 2.1 | 1.9 | 0.0 | 30.0 | 140.0 | 1.9 | 68.0 | 19.0 | 1.4 | |
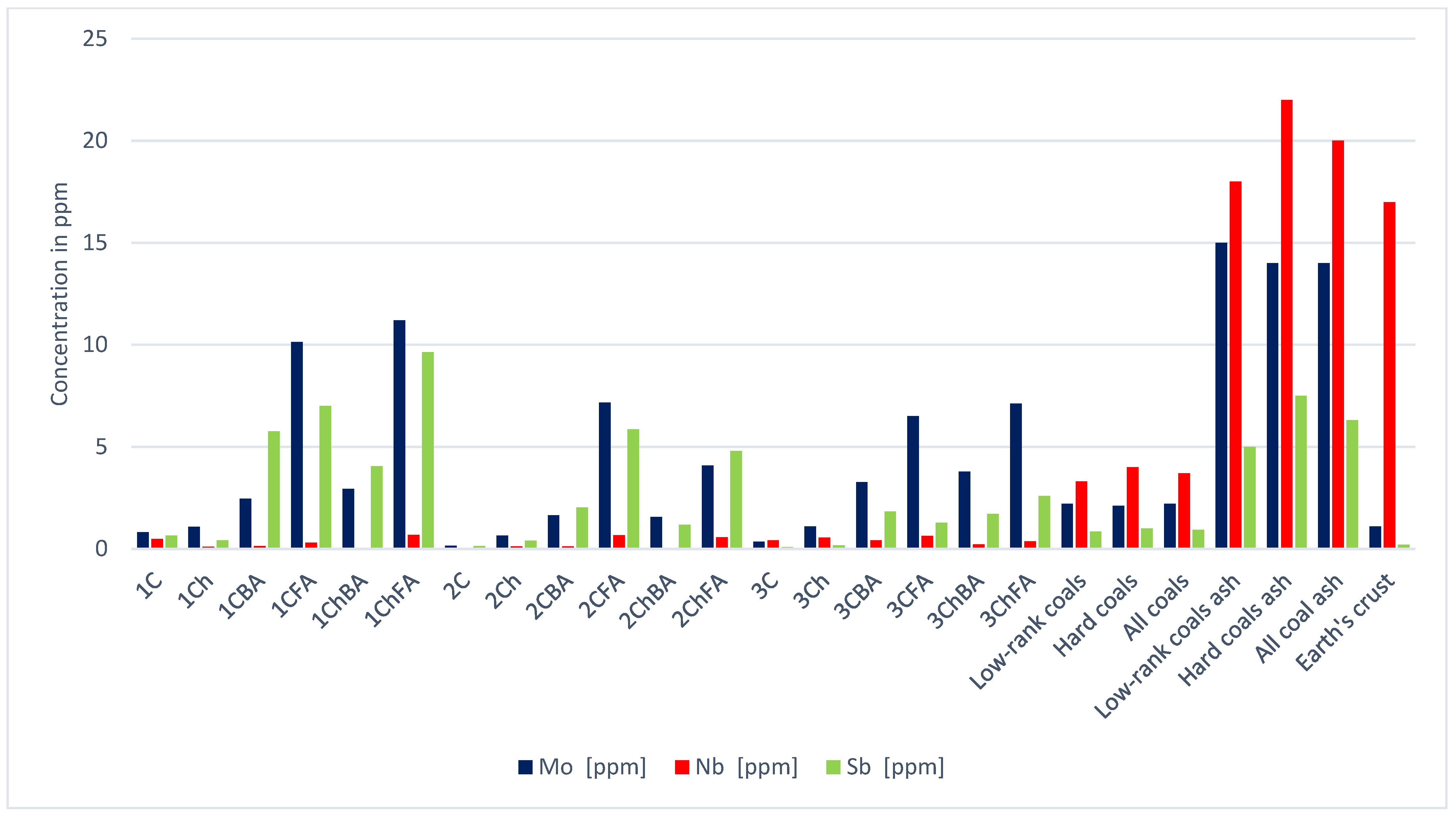
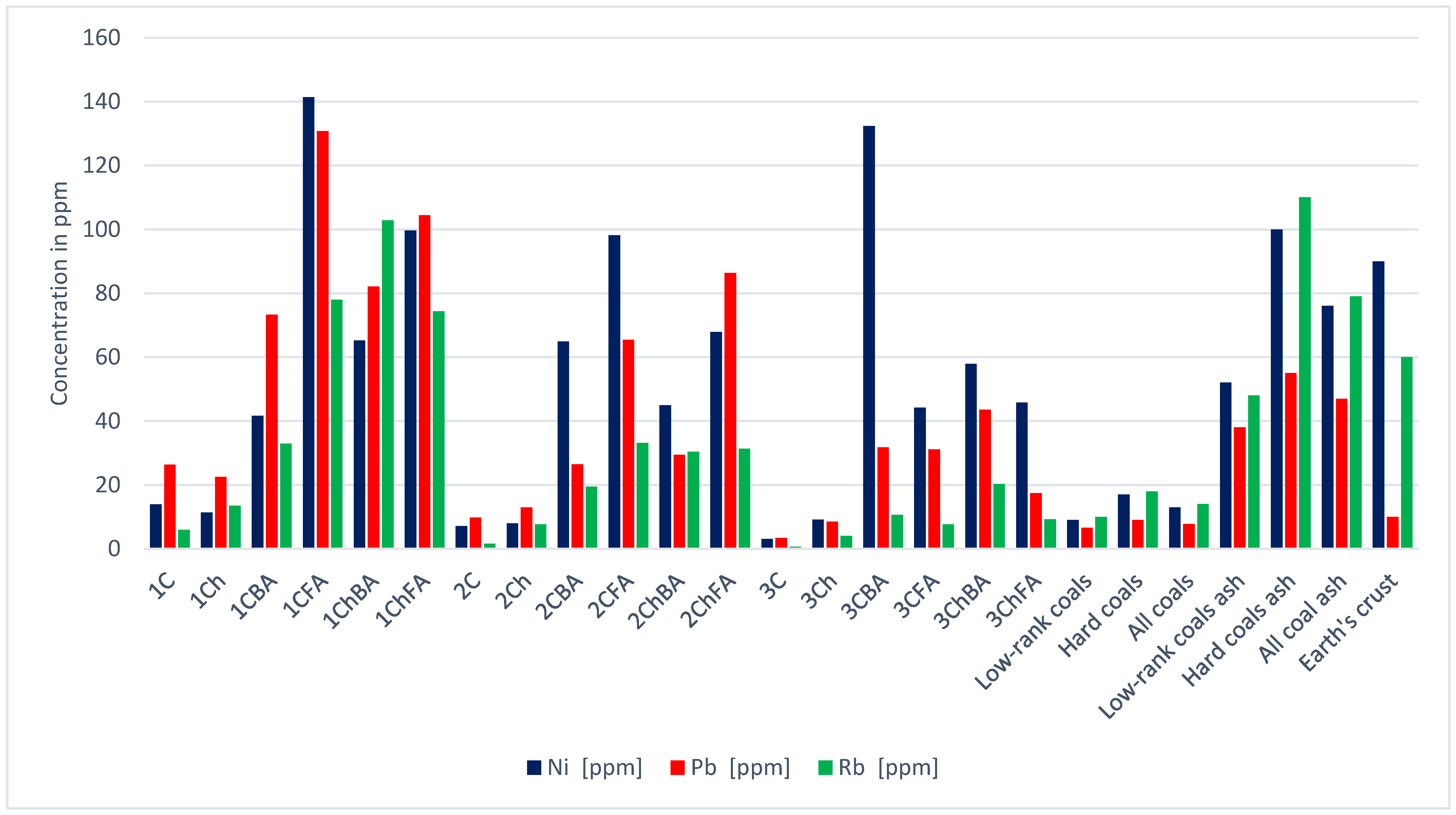
| Sample | Hf | In | Li | Mn | Mo | Nb | Ni | Pb | Rb | Sb | |
| Element | |||||||||||
| 1C | 0.1 | 0.0 | 3.6 | 100 | 0.8 | 0.5 | 13.9 | 26.3 | 6.0 | 0.6 | |
| 1Ch | 0.1 | 0.0 | 17.8 | 143 | 1.1 | 0.1 | 11.4 | 22.5 | 13.5 | 0.4 | |
| 1CBA | 0.2 | 0.1 | 26.7 | 205 | 2.5 | 0.1 | 41.6 | 73.3 | 32.9 | 5.8 | |
| 1CFA | 0.8 | 0.2 | 86.9 | 240 | 10.1 | 0.3 | 141.4 | 130.8 | 78.0 | 7.0 | |
| 1ChBA | 0.2 | 0.1 | 72.0 | 712 | 2.9 | 0.0 | 65.2 | 82.1 | 102.8 | 4.0 | |
| 1ChFA | 1.1 | 0.1 | 96.0 | 483 | 11.2 | 0.7 | 99.6 | 104.5 | 74.4 | 9.6 | |
| 2C | 0.0 | 0.0 | 1.1 | 273 | 0.2 | 0.1 | 7.1 | 9.8 | 1.6 | 0.1 | |
| 2Ch | 0.1 | 0.0 | 9.5 | 357 | 0.6 | 0.1 | 8.0 | 13.0 | 7.6 | 0.4 | |
| 2CBA | 0.2 | 0.0 | 15.7 | 1143 | 1.6 | 0.1 | 64.9 | 26.5 | 19.4 | 2.0 | |
| 2CFA | 0.6 | 0.1 | 43.5 | 986 | 7.2 | 0.7 | 98.1 | 65.4 | 33.1 | 5.9 | |
| 2ChBA | 0.2 | 0.0 | 21.2 | 1259 | 1.6 | 0.1 | 44.9 | 29.4 | 30.4 | 1.2 | |
| 2ChFA | 0.4 | 0.0 | 52.2 | 1015 | 4.1 | 0.6 | 67.9 | 86.4 | 31.3 | 4.8 | |
| 3C | 0.1 | 0.0 | 0.5 | 46 | 0.3 | 0.4 | 3.1 | 3.4 | 0.6 | 0.1 | |
| 3Ch | 0.4 | 0.0 | 6.7 | 165 | 1.1 | 0.5 | 9.1 | 8.5 | 4.0 | 0.2 | |
| 3CBA | 0.3 | 0.1 | 10.5 | 183 | 3.3 | 0.4 | 132.3 | 31.8 | 10.6 | 1.8 | |
| 3CFA | 1.6 | 0.1 | 14.6 | 264 | 6.5 | 0.6 | 44.2 | 31.2 | 7.6 | 1.3 | |
| 3ChBA | 0.5 | 0.1 | 22.4 | 670 | 3.8 | 0.2 | 57.9 | 43.6 | 20.3 | 1.7 | |
| 3ChFA | 1.9 | 0.1 | 21.7 | 564 | 7.1 | 0.4 | 45.8 | 17.5 | 9.2 | 2.6 | |
| Low-rank coals [21] | 1.2 | 0.0 | 10.0 | 100 | 2.2 | 3.3 | 9.0 | 6.6 | 10.0 | 0.8 | |
| Hard coals [21] | 1.2 | 0.0 | 14.0 | 71 | 2.1 | 4.0 | 17.0 | 9.0 | 18.0 | 1.0 | |
| All coals [21] | 1.2 | 0.0 | 12.0 | 86 | 2.2 | 3.7 | 13.0 | 7.8 | 14.0 | 0.9 | |
| Low-rank coal ash [21] | 7.5 | 0.1 | 49.0 | 550 | 15.0 | 18.0 | 52.0 | 38.0 | 48.0 | 5.0 | |
| Hard coal ash [21] | 9.0 | 0.2 | 82.0 | 430 | 14.0 | 22.0 | 100.0 | 55.0 | 110.0 | 7.5 | |
| Total; coal ash [21] | 8.3 | 0.2 | 66.0 | 490 | 14.0 | 20.0 | 76.0 | 47.0 | 79.0 | 6.3 | |
| Suggested cut-off grade [37] | - | - | - | - | 1000 | 300 | - | - | - | 1000 | |
| Earth’s crust | 3.3 | 0.2 | 17.0 | 1100 | 1.1 | 17.0 | 90.0 | 10.0 | 60.0 | 0.2 | |
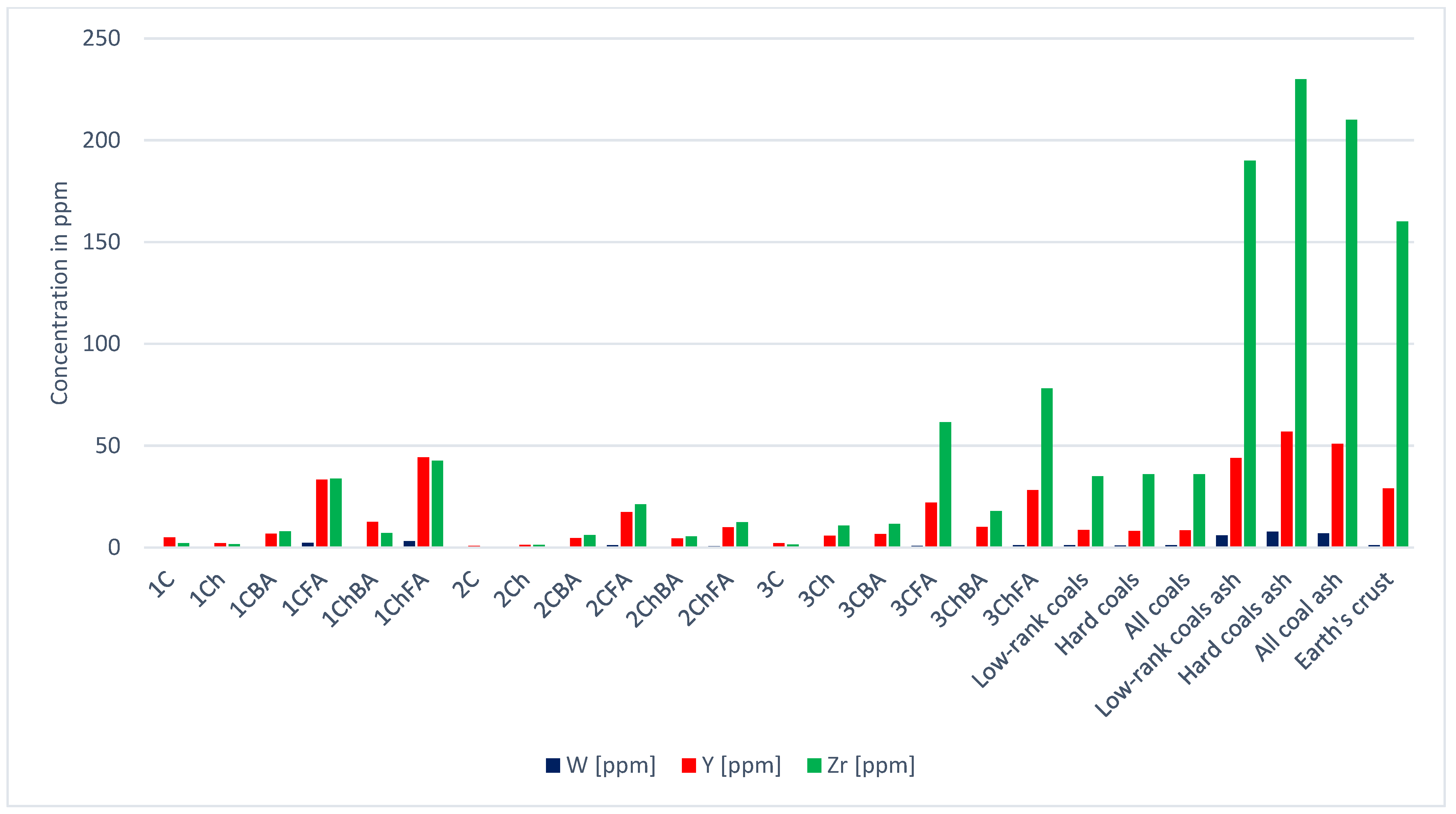
| Sample | Sc | Se | Sn | Sr | V | W | Y | Zn | Zr | REE | |
| Element | |||||||||||
| 1C | 2.1 | 1.0 | 0.7 | 27.7 | 23 | 0.4 | 4.9 | 100.2 | 2.2 | 25.8 | |
| 1Ch | 1.4 | 0.5 | 0.5 | 31.3 | 13 | 0.2 | 2.2 | 67.4 | 1.7 | 32.0 | |
| 1CBA | 3.9 | 0.3 | 3.7 | 42.6 | 34 | 0.4 | 6.8 | 99.2 | 8.0 | 98.7 | |
| 1CFA | 15.1 | 2.8 | 4.9 | 163.5 | 151 | 2.3 | 33.4 | 162.1 | 33.8 | 225.3 | |
| 1ChBA | 7.2 | 0.2 | 7.7 | 120.8 | 58 | 0.3 | 12.6 | 117.2 | 7.1 | 227.7 | |
| 1ChFA | 20.0 | 4.7 | 3.4 | 293.9 | 201 | 3.1 | 44.4 | 208.7 | 42.7 | 259.4 | |
| 2C | 0.4 | 0.8 | 0.1 | 24.8 | 2 | 0.1 | 0.8 | 12.8 | 0.5 | 5.9 | |
| 2Ch | 1.0 | 0.8 | 0.4 | 28.7 | 6 | 0.1 | 1.3 | 29.8 | 1.3 | 23.0 | |
| 2CBA | 3.3 | 0.3 | 1.5 | 75.0 | 24 | 0.2 | 4.7 | 66.7 | 6.2 | 62.0 | |
| 2CFA | 7.7 | 12.1 | 2.3 | 279.8 | 69 | 1.2 | 17.5 | 102.2 | 21.2 | 118.8 | |
| 2ChBA | 4.1 | 0.5 | 1.5 | 80.3 | 29 | 0.2 | 4.5 | 76.7 | 5.4 | 68.9 | |
| 2ChFA | 5.5 | 9.7 | 1.7 | 295.2 | 50 | 0.6 | 10.0 | 147.6 | 12.5 | 91.2 | |
| 3C | 1.5 | 1.5 | 0.3 | 247.2 | 9 | 0.1 | 2.2 | 13.2 | 1.5 | 17.9 | |
| 3Ch | 3.9 | 4.3 | 1.0 | 653.6 | 24 | 0.2 | 5.9 | 36.8 | 10.8 | 60.1 | |
| 3CBA | 3.2 | 0.3 | 2.2 | 192.9 | 23 | 0.5 | 6.7 | 91.2 | 11.6 | 59.9 | |
| 3CFA | 12.5 | 16.1 | 3.5 | 1874.6 | 88 | 0.9 | 22.1 | 51.9 | 61.6 | 197.3 | |
| 3ChBA | 7.1 | 1.0 | 2.3 | 448.8 | 51 | 0.4 | 10.1 | 113.4 | 18.0 | 112.4 | |
| 3ChFA | 16.0 | 20.7 | 4.9 | >2000.0 | 115 | 1.1 | 28.2 | 92.8 | 78.2 | 241.7 | |
| Low-rank coals [21] | 4.1 | 1.0 | 0.8 | 120.0 | 22 | 1.2 | 8.6 | 18.0 | 35.0 | 78.7 | |
| Hard coals [21] | 3.7 | 1.6 | 1.4 | 100.0 | 28 | 1.0 | 8.2 | 28.0 | 36.0 | 82.5 | |
| All coals [21] | 3.9 | 1.3 | 1.1 | 110.0 | 25 | 1.1 | 8.4 | 23.0 | 36.0 | 82.6 | |
| Low-rank coal ash [21] | 23.0 | 7.6 | 4.7 | 740.0 | 140 | 6.0 | 44.0 | 110.0 | 190.0 | 429.0 | |
| Hard coal ash [21] | 24.0 | 10.0 | 8.0 | 730.0 | 170 | 7.8 | 57.0 | 170.0 | 230.0 | 537.0 | |
| Total coal ash [21] | 23.0 | 8.8 | 6.4 | 740.0 | 155 | 6.9 | 51.0 | 140.0 | 210.0 | 485.0 | |
| Suggested cut-off grade [37] | - | 100 | 500 | - | - | 1000 | 1000 | 300 | - | 2000 | |
| Earth’s crust | 26.0 | 0.1 | 2.2 | 360.0 | 190 | 1.1 | 79.0 | 160.0 | 144.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielowicz, B. Selected Critical Raw Materials in Waste from Coal Gasification in Poland. Energies 2021, 14, 8071. https://doi.org/10.3390/en14238071
Bielowicz B. Selected Critical Raw Materials in Waste from Coal Gasification in Poland. Energies. 2021; 14(23):8071. https://doi.org/10.3390/en14238071
Chicago/Turabian StyleBielowicz, Barbara. 2021. "Selected Critical Raw Materials in Waste from Coal Gasification in Poland" Energies 14, no. 23: 8071. https://doi.org/10.3390/en14238071
APA StyleBielowicz, B. (2021). Selected Critical Raw Materials in Waste from Coal Gasification in Poland. Energies, 14(23), 8071. https://doi.org/10.3390/en14238071






