Asphaltene Inhibition and Flow Improvement of Crude Oil with a High Content of Asphaltene and Wax by Polymers Bearing Ultra-Long Side Chain
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
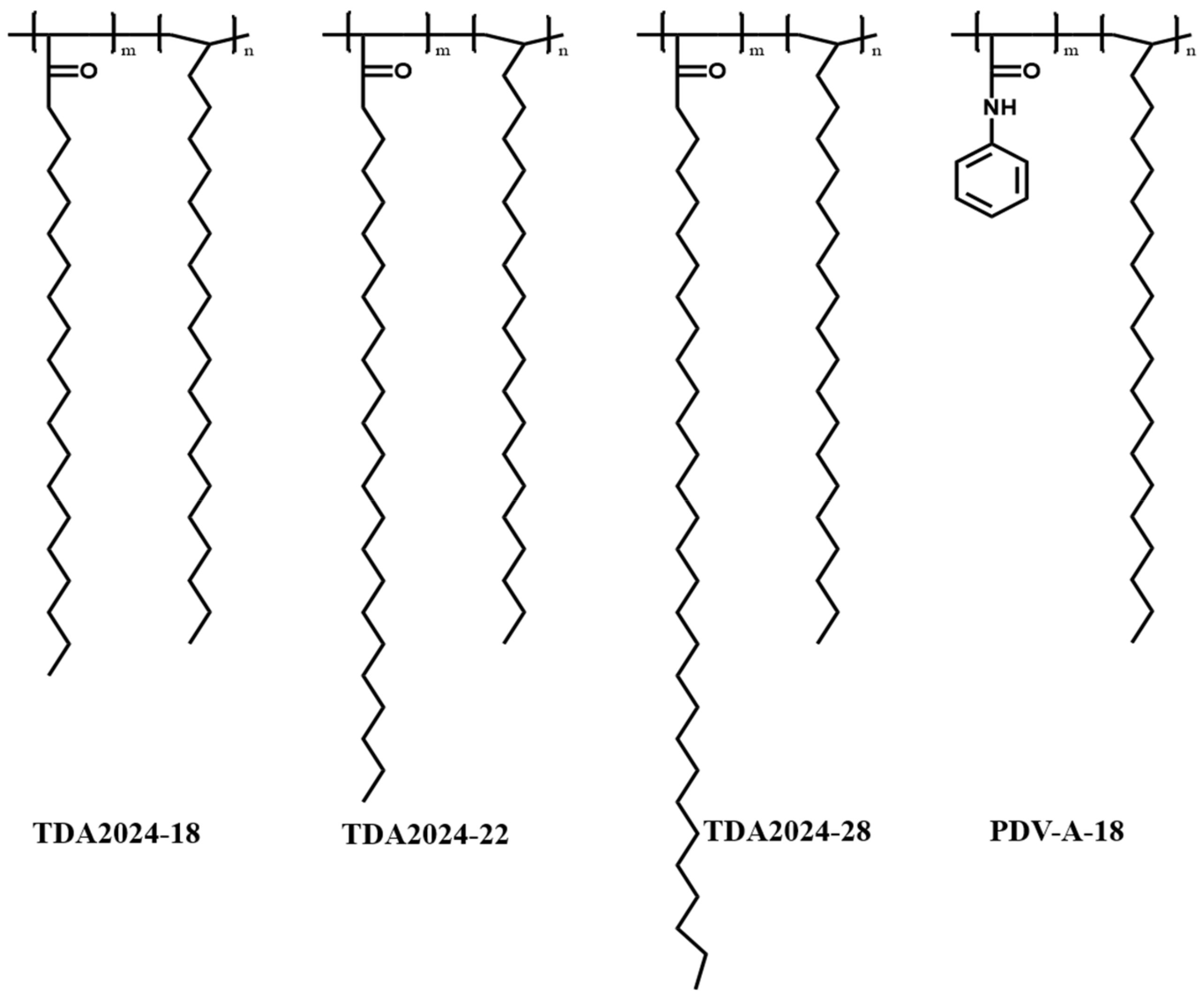
2.2. Preparation of α-Olefins/Ultra-Long Alkyl Acrylate Bipolymers
2.3. Methodology
2.4. Asphaltene
2.5. Precipitation Behaviors of Asphaltenes
2.6. Measurement of the Crude Oil
3. Results and Discussion
3.1. Chemical Structure of Synthesized Bipolymers
3.2. Characterization of Asphaltene
3.3. Effect of the Bipolymers on the Precipitation Behaviors of Asphaltene
3.4. Effect of Aliphatic Chain Length on the Size of Asphaltene Aggregates
3.5. Effect on the Crystallization Behaviors of Waxes in Crude Oil
3.6. Effect on the Rheology of Crude Oil
4. Conclusions
- Bipolymers of α-olefins and aliphatic acrylate with ultra-long aliphatic chain (18, 22 and 28) were synthesized, and their chemical structures were confirmed by FT-IR and 1H NMR.
- The chemical structure of asphaltene characterized by FTIR, 1H NMR, elemental analysis and TOFMS shows that the asphaltene has a relative high aromaticity, indicating that this asphaltene is apt to aggregate and precipitate.
- As revealed by UV–Vis spectra, with the increase in aliphatic chain length of bipolymers, the IPP of asphaltenes were increased. The sizes of asphaltene aggregates measured by DLS and observed using an optical microscope also showed similar results to IPP. Bipolymers with longer aliphatic chains can better suppress the precipitation of asphaltenes and disperse them.
- As revealed by DSC and polarized optical microscope, biopolymer s shorter aliphatic chains can inhibit the growth of wax crystals more effectively, since the size and precipitation amount of wax crystals in crude oil are smaller.
- From the rheological results, TDA2024-22 reduces the oil viscosity and thixotropic loop area most. So, a bipolymer with a medium aliphatic chain length (C22) can inhibit the asphaltenes and disperse the wax crystals simultaneously, thus significantly improving the flowability of crude oil; thus, it has better results than PDV-A-18 for containing phenyl pendants.
- The bipolymers with suitable aliphatic chain lengths are promising to enhance the flow of oil with a high wax and asphaltene content in recover and, transportation, and therefore they present great potential applications in the oil fields.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anita; Zaheer, W.; Douglas, L.; Sellers, D.G.; Banerjee, S. Asphaltene microencapsulation of bitumen as a means of solid-phase transport. Energy Fuels 2021, 35, 6576–6584. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, P.; Varfolomeev, M.A.; Yuan, C.D.; Rodionov, A.A. Integrative investigation of low-temperature oxidation characteristics and mechanisms of heavy crude oil. Ind. Eng. Chem. Res. 2019, 58, 14595–14602. [Google Scholar] [CrossRef]
- Makwashi, N.; Zhao, D.L.; Abdulkadir, M.; Ahmed, T.; Muhammad, I. Study on waxy crudes characterisation and chemical inhibitor assessment. J. Pet. Sci. Eng. 2021, 204, 108734. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Bian, R.; Cheng, J. A novel polyether for efficient demulsification of interfacially active asphaltenes stabilized water-in-oil emulsions. Energy Fuels 2020, 34, 3591–3600. [Google Scholar] [CrossRef]
- Zahra, S.; Ahmad, R.R.; Abdolhossein, H.S. A review on asphaltenes characterization by X-ray diffraction: Fundamentals, challenges, and tips. J. Mol. Struct. 2021, 1238, 130425. [Google Scholar]
- Atta, A.M.; Ezzat, A.O.; Abdullah, M.M. Effect of different families of hydrophobic anions of imadazolium ionic liquids on asphaltene dispersants in heavy crude oil. Energy Fuels 2017, 31, 8045–8053. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Liang, X. New amphiphilic macromolecule as viscosity reducer with both asphaltene dispersion and emulsifying capacity for offshore heavy oil. Energy Fuels 2021, 35, 1143–1151. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, R.; Yang, N.; Zhang, L.; Sun, Y.; Jian, C. Molecular mechanisms of suppressing asphaltene aggregation and flocculation by dodecylbenzenesulfonic acid probed by molecular dynamics simulations. Energy Fuels 2019, 33, 5067–5080. [Google Scholar] [CrossRef]
- Zhou, H.T.; Jia, H.; Liu, Y. Improvement of asphaltene dispersant evaluation method and synthesis of new high-efficiency dispersant. Oilfield Chem. 2014, 4, 585–588. [Google Scholar]
- Zhu, Q.; Lin, B.; Yan, Z.; Yao, Z.; Cao, K. Influences of molecular structure of poly (styrene-co-octadecyl maleimide) on stabilizing asphaltenes in crude oil. Energy Fuels 2020, 34, 3057–3064. [Google Scholar] [CrossRef]
- Cheng, R.; Zou, R.; He, L. Effect of aromatic pendants in a maleic anhydride-co-octadecene polymer on the precipitation of asphaltenes extracted from heavy crude oil. Energy Fuels 2021, 35, 10562–10574. [Google Scholar] [CrossRef]
- Ali, G.; Sohrab, Z.; Nima, R.; Ioannis, C. Effects of inhibitor concentration and thermodynamic conditions on n-octylphenol-asphaltene molecular behaviours. J. Mol. Liq. 2021, 24, 116897. [Google Scholar]
- Palermo, L.C.M.; Lucas, E.F. Asphaltene aggregation: Influence of composition of bipolymers based on styrene-stearyl methacrylate and styrene-stearyl cinnamate containing sulfate groups. Energy Fuels 2016, 30, 3941–3946. [Google Scholar] [CrossRef]
- Liu, G.; Yang, J.; Song, J. Inhibition of asphaltene precipitation in blended crude oil using novel oil-soluble maleimide polymers. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2460–2470. [Google Scholar] [CrossRef]
- Firoozinia, H.; Abad, K.F.H.; Varamesh, A. A comprehensive experimental evaluation of asphaltene dispersants for injection under reservoir conditions. Pet. Sci. 2016, 13, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Jing, G.; Sun, Z.; Tu, Z.; Bian, X.; Liang, Y. Influence of different vinyl acetate contents on the properties of the copolymer of ethylene and vinyl acetate/modified nano-SiO2 composite pour-point depressant. Energy Fuels 2017, 31, 5854–5859. [Google Scholar] [CrossRef]
- Zhao, Z.; Xue, Y.; Xu, G.; Zhou, J.; Lian, X.; Liu, P.; Chen, D.; Han, S.; Lin, H. Effect of the nano-hybrid pour point depressants on the cold flow properties of diesel fuel. Fuel 2017, 193, 65–71. [Google Scholar] [CrossRef]
- Yang, F.; Paso, K.; Norrman, J.; Li, C.; Oschmann, H.; Sjöblom, J. Hydrophilic nanoparticles facilitate wax inhibition. Energy Fuels 2015, 29, 1368–1374. [Google Scholar] [CrossRef]
- Castro, L.; Vazquez, F. Copolymers as Flow Improvers for Mexican Crude Oils. Energy Fuels 2008, 22, 4006–4011. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Yao, B.; Li, C.; Liu, D.; Sun, G. Synergistic effect of asphaltenes and octadecyl acrylate-maleic anhydride copolymers modified by aromatic pendants on the flow behavior of model waxy oils—ScienceDirect. Fuel 2020, 260, 116381. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Mu, Z.; Yang, F. Ethylene-vinyl acetate copolymer and resin-stabilized asphaltenes synergistically improve the flow behavior of model waxy oils. 1. Effect of wax content and the synergistic mechanism. Energy Fuels 2018, 32, 1567–1578. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Mu, Z.; Yang, F. Ethylene-vinyl acetate copolymer and resin-stabilized asphaltenes synergistically improve the flow behavior of model waxy oils. 2. Effect of asphaltene content. Energy Fuels 2018, 32, 5834–5845. [Google Scholar] [CrossRef]
- Yao, B.; Li, C.; Mu, Z.; Yang, F. Ethylene-Vinyl Acetate Copolymer (EVA) and Resin-Stabilized Asphaltenes Synergistically Improve the Flow Behavior of Model Waxy Oils. 3. Effect of Vinyl Acetate Content. Energy Fuels 2018, 32, 8374–8382. [Google Scholar] [CrossRef]
- Xu, J.; Zou, R.; Gai, D.C.; Theil, P.; Pickenbach, L.; Li, T.; Li, L. Effect of aromatic and aliphatic pendants in poly (maleic acid amide-co-vinyl acetate) on asphaltene precipitation in heavy oil. Ind. Eng. Chem. Res. 2018, 57, 10701–10708. [Google Scholar] [CrossRef]













| Output of Pipeline (BBL/D) | Wax Content (%) | Density (G/Cm3,15 °C) | Water Content (%) |
|---|---|---|---|
| 80,000–100,000 | 18.0 | 0.864 | 0.4 |
| Viscosity (mPa·s, 25 °C, 10 s−1) | WAT (°C) | pour point (°C) | asphaltene content (%) |
| 487 | 35.9 | 27.0 | 25.0 |
| Study Content | Asphaltene Precipitation Behaviors | Crystallization Behaviors of Waxes | Rheological Behaviors of Crude Oil |
|---|---|---|---|
| Method | UV–Vis spectroscopy | differential scanning calorimetry | rheometer |
| dynamic light scattering | polarized light microscope | ||
| optical microscope |
| Wavelength of Adsorption (cm−1) | Function Group |
|---|---|
| 3430 | -OH |
| 2920, 1600 | C=C stretching vibration on the benzene ring |
| 2850 | CH stretching vibration |
| 1460 | CH (asymmetric, in-plane) stretching vibration |
| 1380 | CH (symmetric, in-plane) |
| 1120, 1030 | CH (in-plane) |
| Asphaltene | Elemental Composition (%) | NH/NC | ||||
|---|---|---|---|---|---|---|
| C | H | O | N | S | ||
| 85.97 | 6.896 | 2.036 | 1.105 | 3.993 | 0.9626 | |
| Bipolymer | IPP (%) | Initial Precipitation Rate |
|---|---|---|
| None | 34.90 | −0.0224 |
| TDA 2024-18 | 41.62 | −0.0181 |
| TDA 2024-22 | 43.21 | −0.0303 |
| TDA 2024-28 | 44.17 | −0.0272 |
| PDV-A-18 | 43.05 | −0.0268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lu, S.; Niu, M.; Cheng, R.; Gong, Y.; Xu, J. Asphaltene Inhibition and Flow Improvement of Crude Oil with a High Content of Asphaltene and Wax by Polymers Bearing Ultra-Long Side Chain. Energies 2021, 14, 8243. https://doi.org/10.3390/en14248243
Li X, Lu S, Niu M, Cheng R, Gong Y, Xu J. Asphaltene Inhibition and Flow Improvement of Crude Oil with a High Content of Asphaltene and Wax by Polymers Bearing Ultra-Long Side Chain. Energies. 2021; 14(24):8243. https://doi.org/10.3390/en14248243
Chicago/Turabian StyleLi, Xinyuan, Shu Lu, Meifei Niu, Ruzhen Cheng, Yanjun Gong, and Jun Xu. 2021. "Asphaltene Inhibition and Flow Improvement of Crude Oil with a High Content of Asphaltene and Wax by Polymers Bearing Ultra-Long Side Chain" Energies 14, no. 24: 8243. https://doi.org/10.3390/en14248243
APA StyleLi, X., Lu, S., Niu, M., Cheng, R., Gong, Y., & Xu, J. (2021). Asphaltene Inhibition and Flow Improvement of Crude Oil with a High Content of Asphaltene and Wax by Polymers Bearing Ultra-Long Side Chain. Energies, 14(24), 8243. https://doi.org/10.3390/en14248243







