Numerical Simulation on Impacts of Thickness of Nafion Series Membranes and Relative Humidity on PEMFC Operated at 363 K and 373 K
Abstract
1. Introduction
2. Numerical Modeling
2.1. Model Description and Governing Equations
2.2. Model Assumption
- (i)
- The distributions of the inlet gas flow rate at the anode side and cathode side are uniform, respectively.
- (ii)
- The pressure of the outlet of the gas channel is the atmospheric pressure.
- (iii)
- No slip on the gas channel wall excluding the inlet and the outlet of the gas channel is considered.
- (iv)
- The cell voltage obtained by the power generation experiment is set at the cathode electrode and the earth ground is set at the anode electrode. The in-plane distribution of cell voltage at the cathode electrode is uniform.
- (v)
- Reactant gases are treated as an ideal gas and incompressible Newton fluid.
- (vi)
- H2O is treated as a vapor.
- (vii)
- The cell temperature is uniform and the outside boundary of the 3D model is set at Tini.
- (viii)
- The effective porosity and the permeability of the porous media are isotropic. The conductivity in the porous media is also isotropic.
3. Results and Discussion
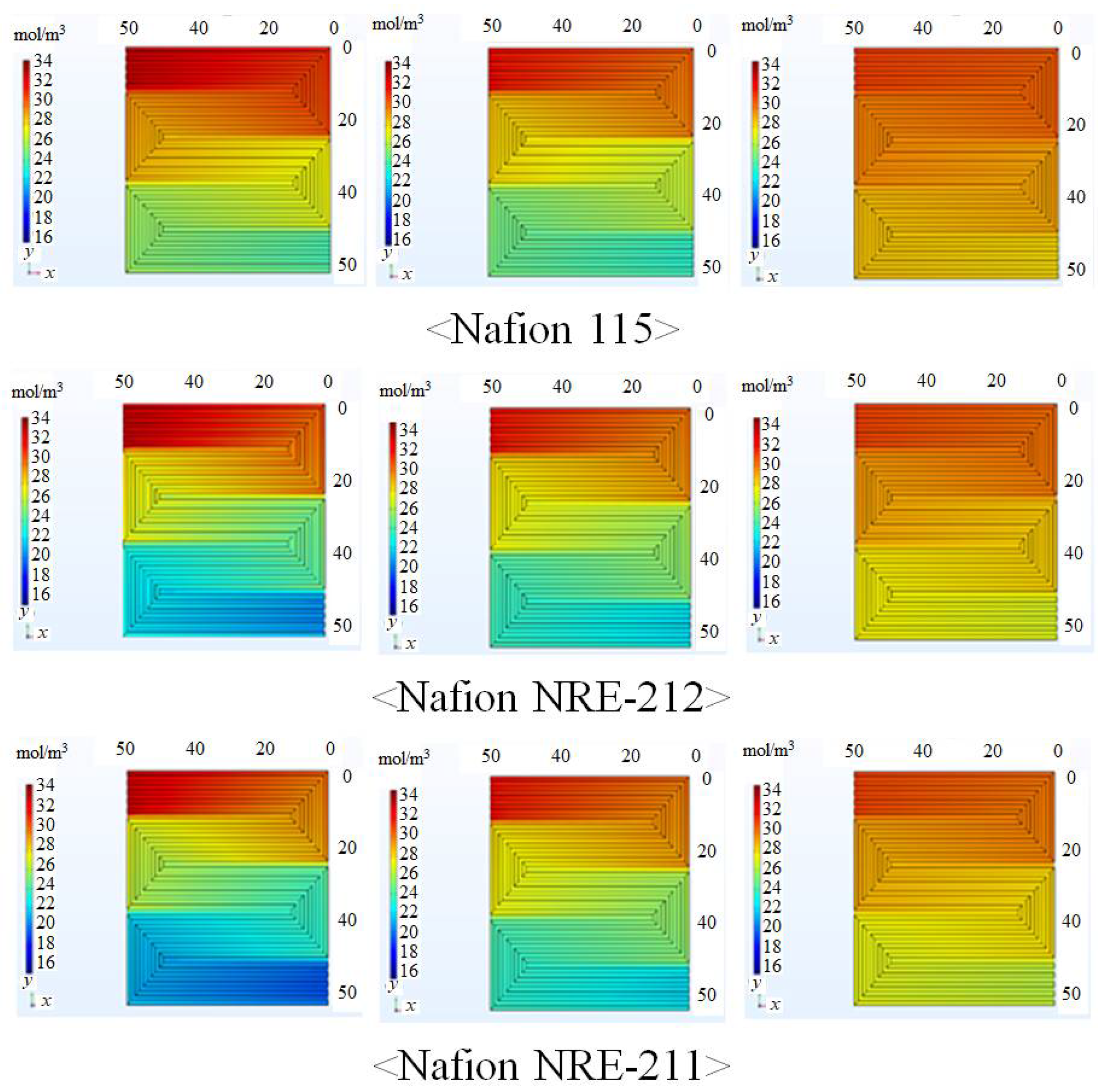
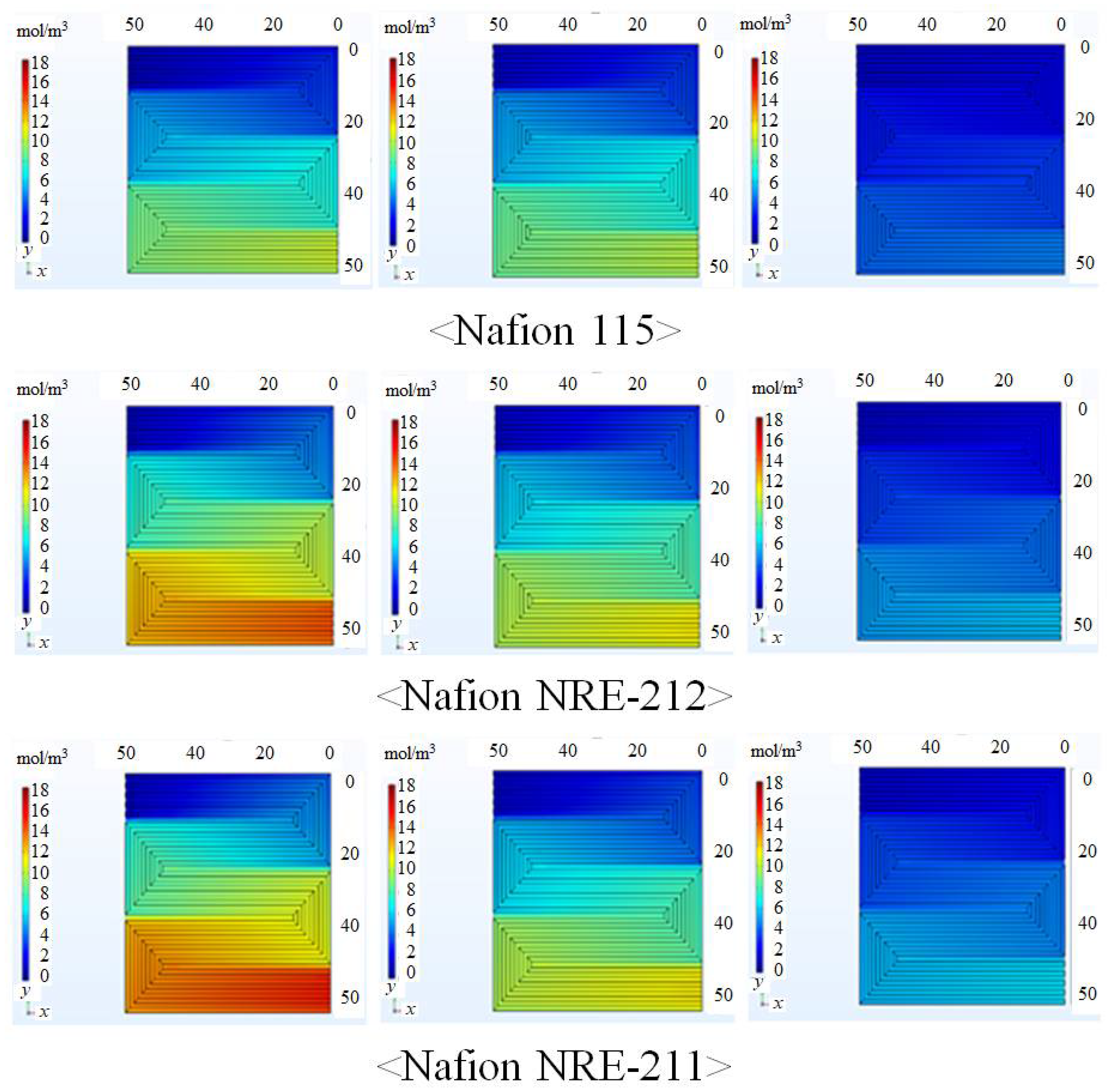
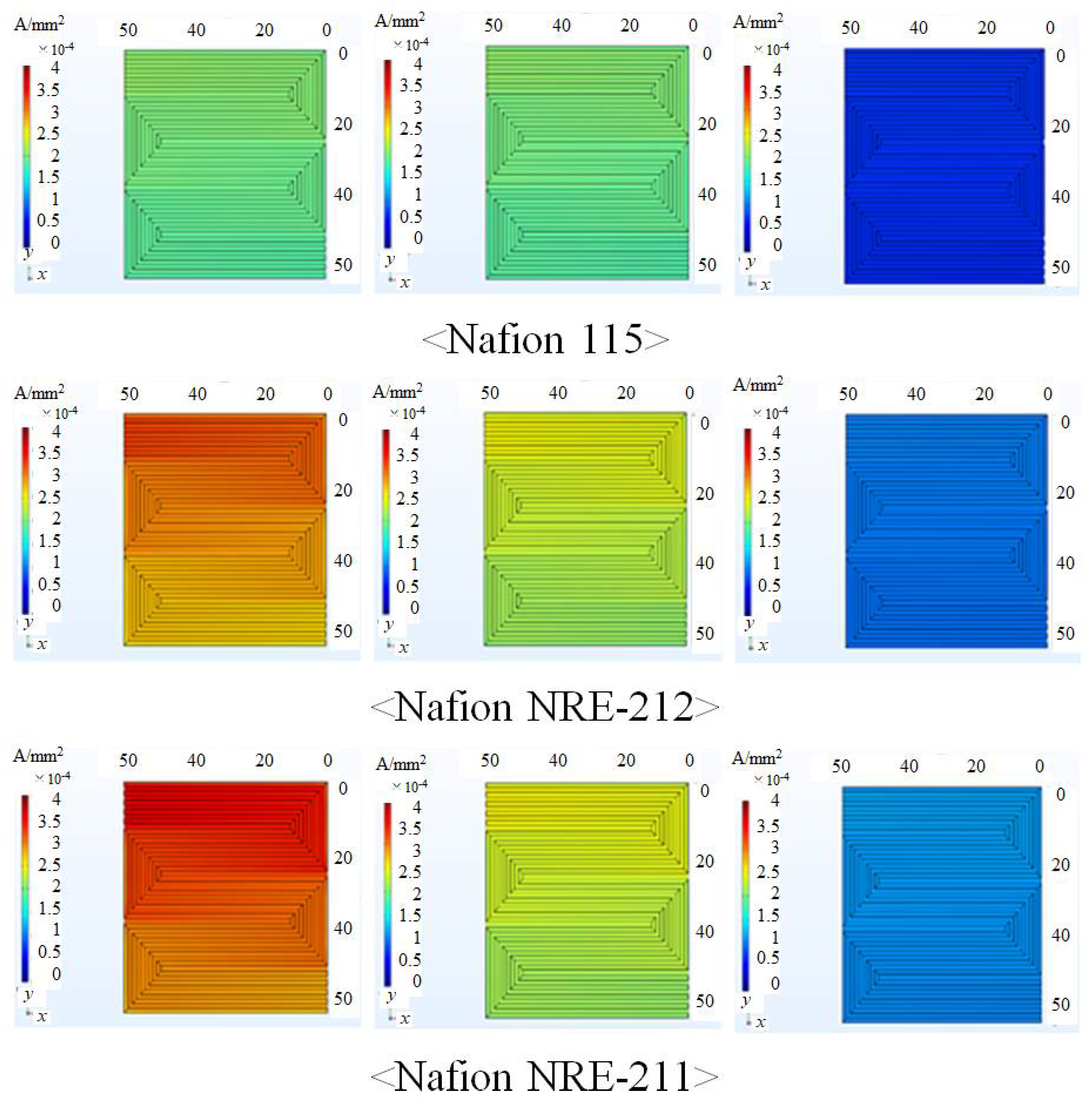
3.1. In-Plane Distribution of Mass and Current Density on the Interface between Nafion Membrane and Anode Catalyst Layer or the Interface between Nafion Membrane and Cathode Catalyst Layer
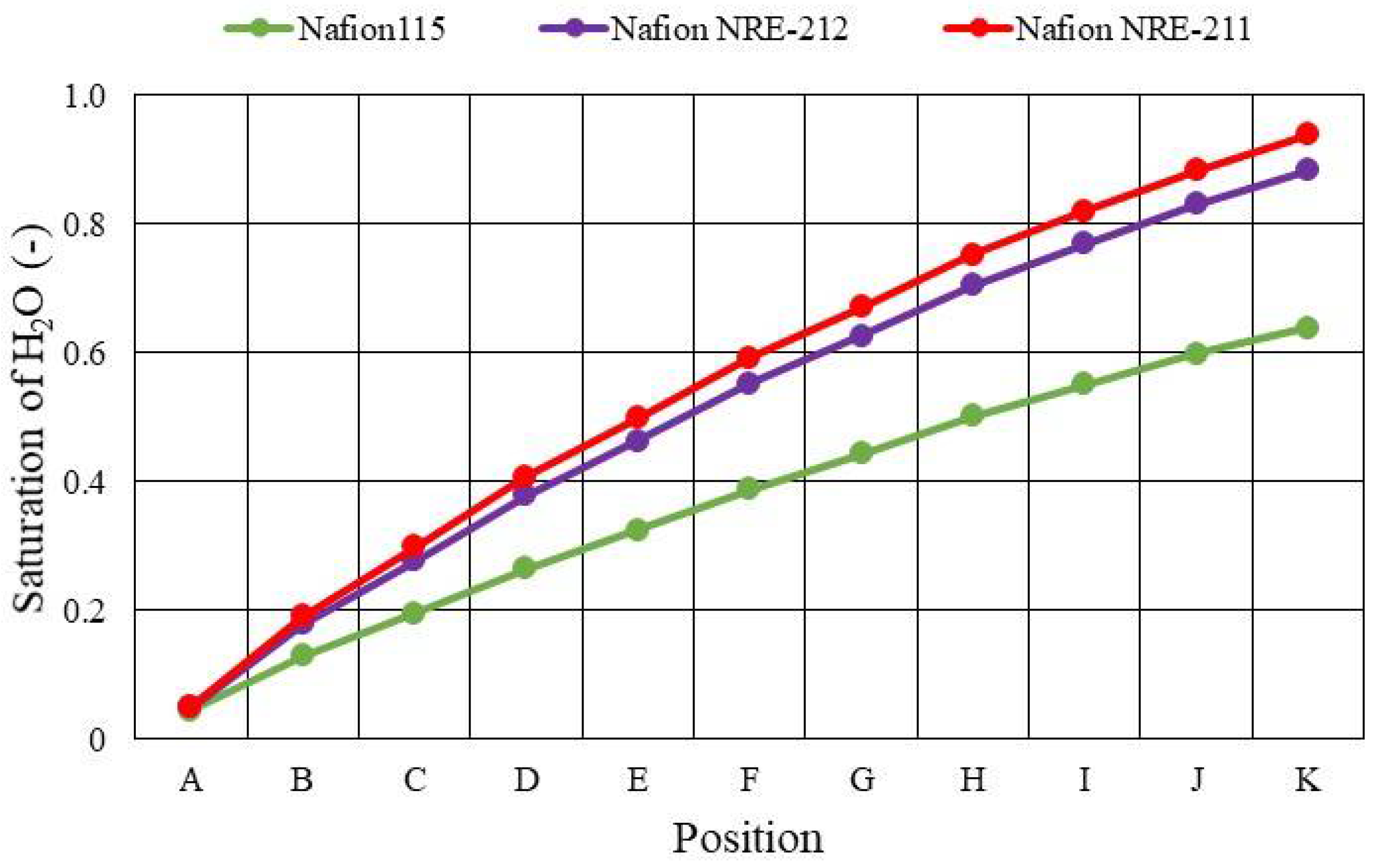
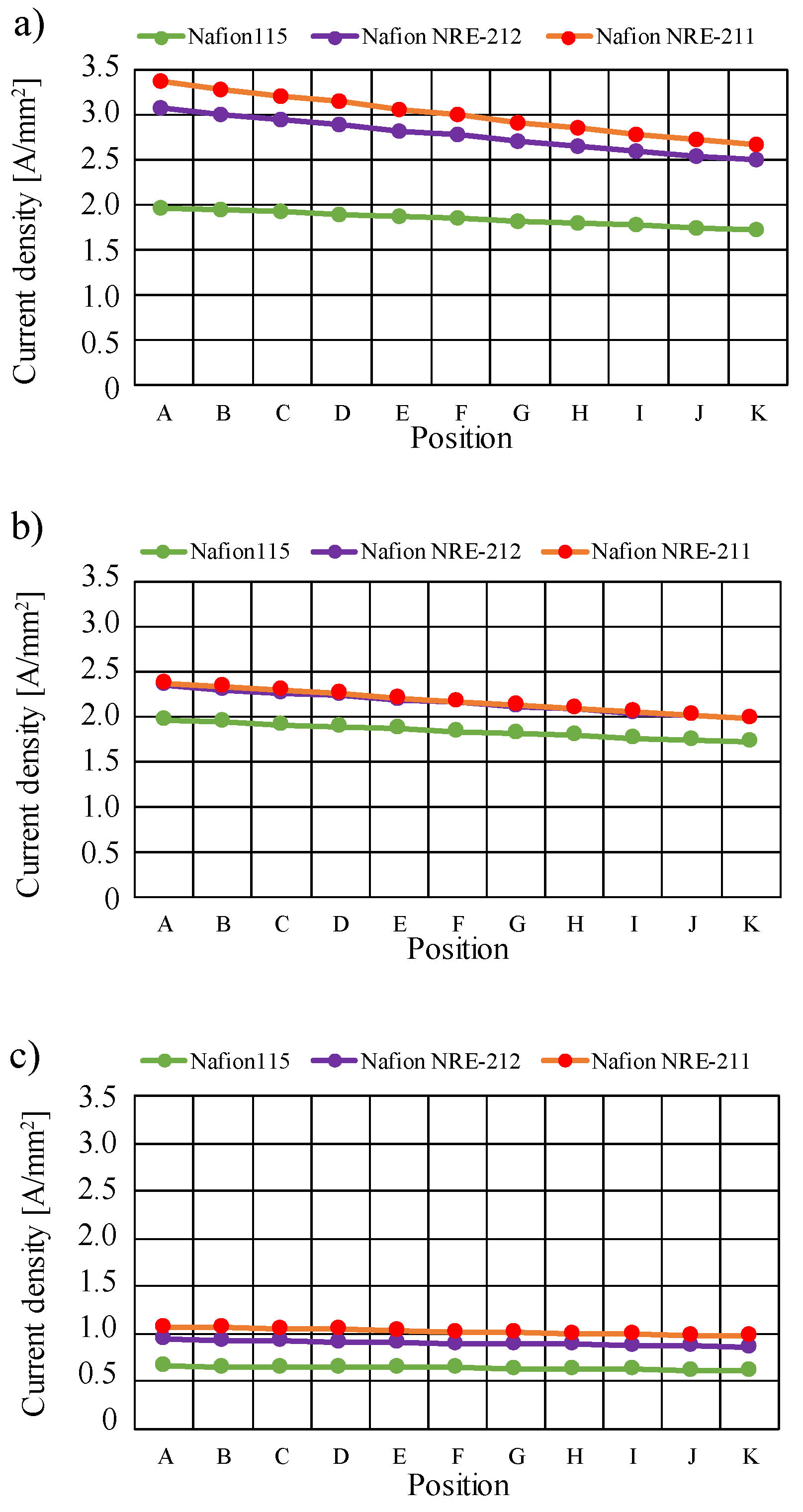
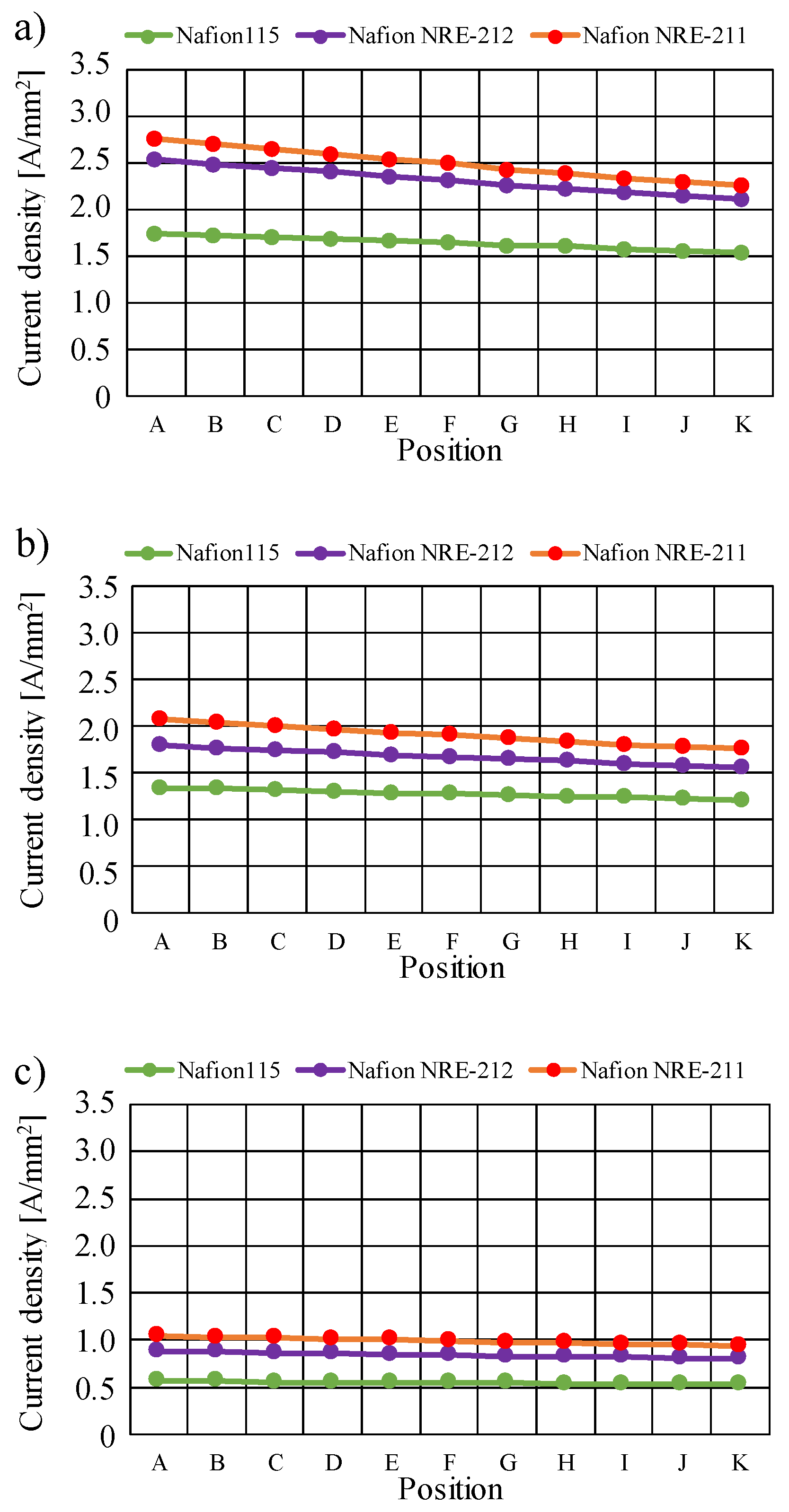
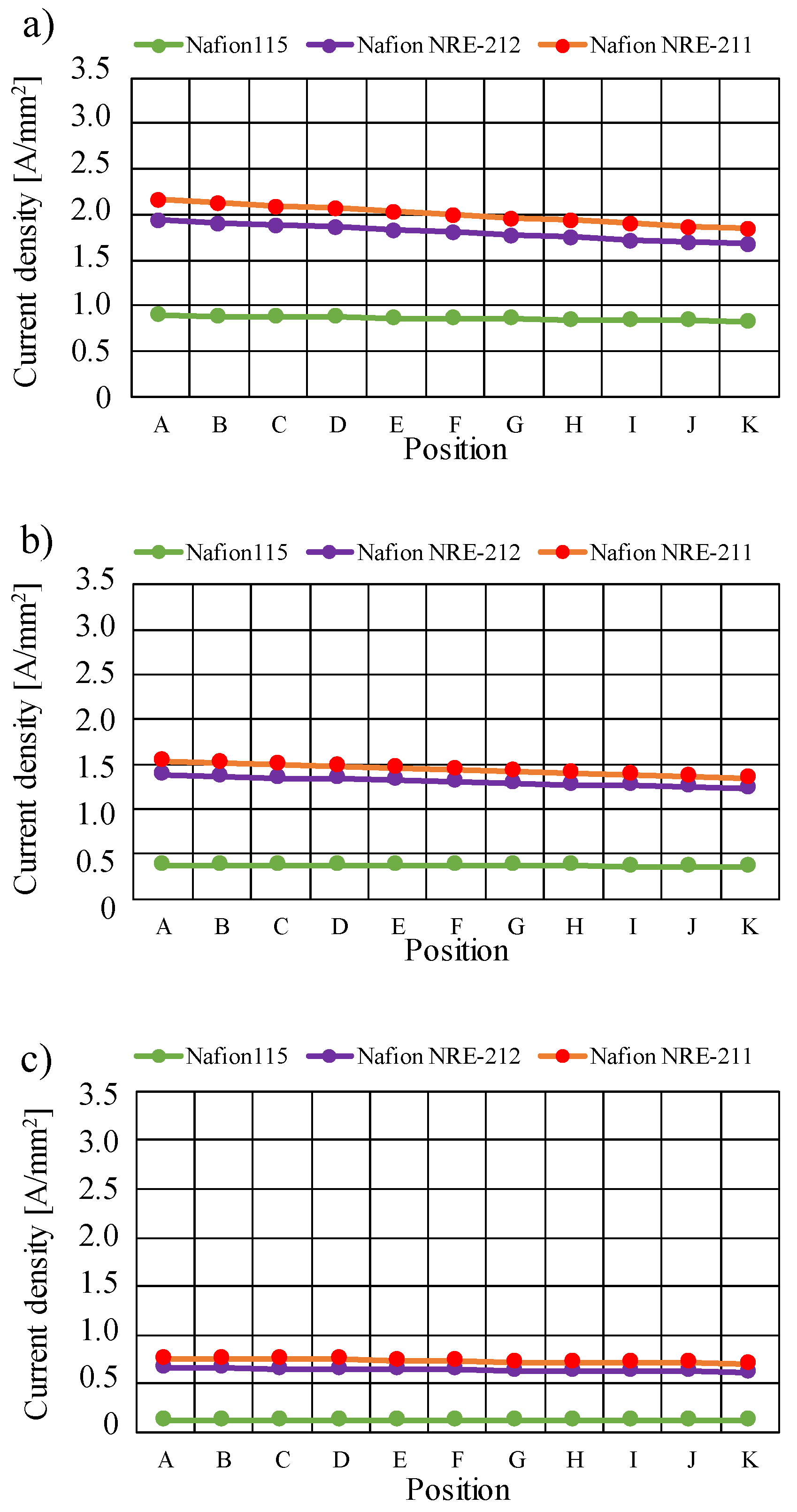
3.2. Quantitative Evaluation along with the Gas Flow through the Gas Channel on Mass and Current Density on the Interface between Nafion Membrane and Cathode Catalyst Layer
4. Conclusions
- (i).
- The molar concentration of H2 and O2 decreases along with the gas flow through the gas channel, irrespective of the Nafion membrane thickness and Tini.
- (ii).
- The O2 consumption in the fuel cell is the largest at Tini = 353 K, irrespective of Nafion membrane thickness.
- (iii).
- The molar concentration of H2O increases along with the gas flow through the gas channel, irrespective of the Nafion membrane thickness and Tini, which can be explained by the O2 reduction reaction at cathode.
- (iv).
- The current density decreases along with the gas flow through the gas channel, irrespective of Nafion membrane thickness and Tini. The current density is the largest at Tini = 353 K, irrespective of the Nafion membrane thickness.
- (v).
- The molar concentration of H2O increases when the relative humidity of the supply gas increases, irrespective of the Nafion membrane thickness and Tini. The molar concentration of H2O is the largest with A80%RH&C80% RH, while it is the smallest with A40%RH&C40%RH.
- (vi).
- The molar concentration of H2O generally decreases when the thickness of the Nafion membrane increases. The molar concentration of H2O for Nafion 115, whose thickness is 127 μm, is much smaller than that for the other thin Nafion membranes.
- (vii).
- It is revealed that the largest molar concentration of H2O is 15.1 mol/m3 near the outlet in the case of using Nafion NRE-211 at Tini = 353 K with A80%RH&C80%RH among the conditions investigated in this study.
- (viii).
- The current density is the highest at Tini = 353 K.
- (ix).
- The current density increases when the relative humidity of the supply gas increases, irrespective of the Nafion membrane thickness and Tini, which indicates that the power generation performance is enhanced with the increase in relative humidity due to the promotion of proton conductivity of the Nafion membrane.
- (x).
- The current density increases with the decrease in the Nafion membrane thickness since the H2O flux of the Nafion membrane as well as the conductivity of the Nafion membrane is promoted with the thinner Nafion membrane.
- (xi).
- It is revealed that the largest current density is 0.336 A/mm2 near the inlet in the case of using Nafion NRE-211 at Tini = 353 K with A80%RH&C80%RH among the conditions investigated in this study.
- (xii).
- This study reveals that the thinner Nafion membrane under well-humidified conditions is more desirable to obtain a higher power generation performance at higher temperatures, i.e., 363 K and 373 K. Thinner Nafion membranes can provide a uniform distribution of current density as well.
- (xiii).
- Since the current density at high temperatures of 363 K and 373 K, which are 0.237 A/mm2 and 0.107 A/mm2, respectively, is still low, even using Nafion NRE-211 with A80%RH&C80%RH, this study suggests the optimization of catalyst layer, MPL, and gas channel flow of gas separator in order to control the mass and heat transfer phenomena as well as to improve the electrochemical reaction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NEDO (New Energy and Industry Technology Development Organization). Available online: http://www.nedo.go.jp/cotent/100871973 (accessed on 1 November 2021). (In Japanese).
- Zhang, G.; Kandlikar, S.G.A. Critical Review of Cooling Technique in Proton Exchange Membrane Fuel Cell Stacks. Int. J. Hydrog. Energy 2012, 37, 2412–2429. [Google Scholar] [CrossRef]
- Agbossou, K.; Kolhe, M.; Hamelin, J.; Bose, T.K. Performance of a Stand-Alone Renewable Energy System Based on Energy Storage as Hydrogen. IEEE Trans. Energy Convers. 2004, 19, 633–640. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and Recent Development Polymer Electrolyte Membrane for Fuel Cells Operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef]
- Lee, C.Y.; Wng, F.; Kuo, Y.W.; Tsai, C.H.; Cheng, Y.T.; Cheng, C.K.; Lin, J.T. In-situ Measurement of High-temperature Proton Exchange Membrane Fuel Cell Stack Using Flexible Five-in-one Micro Sensor. Sensors 2016, 16, 1731. [Google Scholar] [CrossRef] [PubMed]
- Budak, Y.; Devrim, Y. Micro-cogeneration Application of a High-temperature PEM Fuel Cell Stack Operated with Polybenzimidazole Based Membranes. Int. J. Hydrog. Energy 2020, 45, 35198–35207. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, T.; Che, X.; Dong, J.; Liu, R.; Yang, J. New High-performance Bulky N-heterocyclic Group Functionalized Poly (Terphenyl Piperidinium) Membrane for HT-PEMFC Applications. J. Mem. Sci. 2022, 641, 119884. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, J.S.; Park, H.Y.; Park, H.S.; Lee, S.Y.; Song, K.H.; Kim, H.J. Improvement of Fuel Cell Performances through the Enhanced Dispersion of the PTFE Binder in Electrodes for Use in High Temperature Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrog. Energy 2020, 45, 32825–32833. [Google Scholar] [CrossRef]
- Xia, L.; Ni, M.; Xu, Q.; Xu, H.; Zheng, K. Optimization of Catalyst Layer Thickness for Achieving High Performance and Low Cost of High Temperature Proton Exchange Membrane Fuel Cell. Appl. Energy 2021, 291, 117012. [Google Scholar] [CrossRef]
- Huang, T.; Wang, W.; Yuan, Y.; Huang, J.; Chen, X.; Zhang, J.; Kong, X.; Zhang, Y.; Wan, Z. Optimization of High-temperature Proton Exchange Membrane Fuel Cell Flow Channel Based on Genetic Algorithm. Energy Rep. 2021, 7, 1374–1384. [Google Scholar] [CrossRef]
- Ghasabehi, M.; Shams, M.; Kanani, H. Multi-objective Optimization of Operating Conditions of an Enhanced Parallel Flow Filed Proton Exchange Membrane Fuel Cell. Energy Convers. Manag. 2021, 230, 113798. [Google Scholar] [CrossRef]
- Das, S.K.; Gibson, H.A. Three Dimensional Multi-Physics Modeling and Simulation for Assessment of Mass Transport Impact on the Performance of a High Temperature Polymer Electrolyte Membrane Fuel Cell. J. Power Sources 2021, 499, 161–188. [Google Scholar] [CrossRef]
- Xia, L.; Xu, Q.; He, Q.; Ni, M.; Seng, M. Numerical Study of High Temperature Proton Exchange Membrane Fuel Cell (HT-PEMFC) with a Focus on Rib Design. Int. J. Hydrog. Energy 2021, 46, 21098–21111. [Google Scholar] [CrossRef]
- Kanchan, B.K.; Randive, P.; Pati, S. Implications of Non-uniform Porosity Distribution in Gas Diffusion Layer on the Performance of a High Temperature PEM Fuel Cell. Int. J. Hydrog. Energy 2021, 46, 18571–18588. [Google Scholar] [CrossRef]
- Nishimura, A.; Kamiya, S.; Okado, T.; Sato, Y.; Hirota, M.; Kolhe, M.L. Heat and Mass Transfer Analysis in Single Cell of PEFC Using Different PEM and GDL at Higher Temperature. Int. J. Hydrog. Energy 2019, 44, 29631–29640. [Google Scholar] [CrossRef]
- Nishimura, A.; Okado, T.; Kojima, Y.; Hu, E. Impact of Microporous Layer on Heat and Mass Transfer in a Single Cell of Polymer Electrolyte Fuel Cell Using a Thin Polymer Electrolyte Membrane and a Thin Gas Diffusion Layer Operated at a High-temperature Range. ACS Omega 2021, 6, 14575–14584. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Kono, N.; Toyoda, K.; Kojima, Y.; Kolhe, M.L. Impact Analysis of MPL on a PEFC Cell’s Temperature Distribution with Thin PEM and GDL for Operating at Higher Temperature than Usual. J. Energy Power Eng. 2021, 15, 39–51. [Google Scholar] [CrossRef]
- Nishimura, A.; Fukuoka, H.; Yamamoto, K.; Okado, T.; Kojima, Y.; Hirota, M.; Kolhe, M.L. Numerical Analysis of Temperature Distributions in Single Cell of PEFC by Heat Transfer Model Considering Vapor Transfer. J. Energy Power Eng. 2020, 14, 1–15. [Google Scholar] [CrossRef]
- Miao, T.; Tongsh, C.; Wang, J.; Cheng, P.; Liang, J.; Wang, Z.; Chen, W.; Zhang, C.; Xi, F.; Du, Q. Current Density and Temperature Distribution Measurement and Homogeneity Analysis for a Large-area Proton Exchange Membrane Fuel Cell. Energy 2022, 239, 121922. [Google Scholar] [CrossRef]
- Han, C.; Jiang, T.; Shang, K.; Xu, B.; Chen, Z. Heat and Mass Transfer Performance of Proton Exchange Membrane Fuel Cells with Electrode of Anisotropic Thermal Conductivity. Int. J. Heat Mass Transf. 2022, 182, 121957. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottsfeld, S. Polymer Electrolyte Fuel Cell Models. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Penga, Z.; Tolj, I.; Barbir, F. Computational Fluid Dynamics Study of PEM Fuel Cell Performance. Int. J. Hydrog. Energy 2016, 41, 17585–17594. [Google Scholar] [CrossRef]
- Yablecki, J.; Bazylak, A. Determining the Effective Thermal Conductivity of Composed PEMFC GDLs through Thermal Resistance Modeling. J. Power Sources 2012, 217, 470–478. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Qu, Z.G.; Xu, H.T.; Talkhoncheh, F.K.; Liu, S.; Gao, Q. A Numerical Study on the Performance of PEMFC with Wedge-shaped Fins in the Cathode Channel. Int. J. Hydrog. Energy 2021, 46, 27700–27708. [Google Scholar] [CrossRef]
- Chen, H.; Guo, H.; Ye, F.; Ma, C.F. Improving Two-phase Mass Transportation under Non-Darcy Flow Effect in Orientated-type Flow Channels of Proton Exchange Membrane Fuel Cells. Int. J. Hydrog. Energy 2021, 46, 21600–21618. [Google Scholar] [CrossRef]
- Chen, H.; Guo, H.; Ye, F.; Ma, C.F. A Numerical Study of Oriented-type Flow Channels with Porous-blocked Baffles of Proton Exchange Membrane Fuel Cells. Int. J. Hydrog. Energy 2021, 46, 29443–29458. [Google Scholar] [CrossRef]
- Xia, L.; Ni, M.; He, Q.; Xu, Q.; Cheng, C. Optimization of Gas Diffusion Layer in High Temperature PEMFC with the Focuses on Thickness and Porosity. Appl. Energy 2021, 300, 117357. [Google Scholar] [CrossRef]
- Freunberger, S.A.; Reum, M.; Evertz, J.; Wokaun, A.; Buchi, F.M. Measuring the Current Distribution in PEFCs with Sub-Millimeter Resolution. J. Electrochem. Soc. 2006, 153, A2158–A2165. [Google Scholar] [CrossRef]
- Nishimura, A.; Sato, A.; Yoshimura, M.; Kamiya, S.; Hirota, M. Impact of Thickness of Polymer Electrolyte Membrane on Temperature Distribution in Single Cell of Polymer Electrolyte Fuel Cell Operated at High Temperature. J. Energy Power Eng. 2018, 12, 80–92. [Google Scholar] [CrossRef][Green Version]
- Nishimura, A.; Shibuya, K.; Morimoto, A.; Tanaka, S.; Hirota, M.; Nakamura, M.; Kojima, Y.; Narita, M.; Hu, E. Dominant Factor and Mechanism of Coupling Phenomena in Single Cell of Polymer Electrolyte Fuel Cell. Appl. Energy 2012, 1, 73–79. [Google Scholar] [CrossRef]
- Cooper, N.J.; Santamaria, A.D.; Becton, M.K.; Park, J.W. Neutron Radiography Measurements of In-situ PEMFC Liquid Water Saturation in 2 D & 3 D Morphology Gas Diffusion Layers. Int. J. Hydrog. Energy 2017, 42, 16269–16278. [Google Scholar]
- The Japan Society of Mechanical Engineers. JSME Heat Transfer Handbook, 1st ed.; The Japan Society of Mechanical Engineers, Maruzen: Tokyo, Japan, 1993; p. 387. [Google Scholar]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 1st ed.; McGraw-Hill: New York, USA, 1987; p. 591. [Google Scholar]
- Merck. Available online: http://www.sigmaaldrich.com/japan/materialscience/alternative/nafion.html (accessed on 5 November 2021).
- Senn, S.M.; Poulikakos, D. Polymer Electrolyte Fuel Cells with Porous Materials as Fluid Distributors and Comparisons with Traditional Channeled Systems. Trans. ASME 2004, 126, 410–418. [Google Scholar] [CrossRef]
- Kang, K.; Ju, H. Numerical Modeling and Analysis of Micro-porous Layer Effects in Polymer Electrolyte Fuel Cells. J. Power Sources 2006, 194, 763–773. [Google Scholar] [CrossRef]
- TORAY. Available online: http://www.torayca.com/en/lineup/composites/com_009_01.html (accessed on 5 November 2021).
- Takayama, T. Numerical Simulation of Transient Internal States of PEFC Cell and Stack Considering Control of Anode System. Res. Rep. Mizuho Res. Technol. 2018, 9, 1–14. [Google Scholar]
- Rostami, L.; Nejad, P.M.G.; Vatani, A. A Numerical Investigation of Serpentine Flow Channel with Different Bend Sizes in Polymer Electrolyte Membrane Fuel Cells. Energy 2016, 97, 400–410. [Google Scholar] [CrossRef]
- Xing, L.; Das, P.K.; Song, X.; Mamlouk, M.; Scott, K. Numerical Analysis of the Optimum Membrane/Ionomer Water Content of PEMFCs: The Interface of Nafion Ionomer Content and Cathode Relative Humidity. Appl. Energy 2015, 138, 242–257. [Google Scholar] [CrossRef]
- Jia, T.; Shen, S.; Zhao, J.; Jin, J.; Pan, B.; Duan, X.; Meng, C.; Che, Q. Ultrathin Membranes Formation via the Layer by Layer Self-assembly of Carbon Nanotubes-based Inorganics as High Temperature Proton Exchange Membranes. Int. J. Hydrog. Energy 2020, 45, 14517–14527. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Ramya, K.; Khalid, M.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Walvekar, R.; Kadhum, A.A.H. Additives in Proton Exchange Membranes for Low- and High-temperature Fuel Cell Applications: A Review. Int. J. Hydrog. Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Akitomo, F.; Sasabe, T.; Yoshida, T.; Naito, H.; Kawamura, K.; Hirai, S. Investigation of Effects of High Temperature and Pressure on a Polymer Electrolyte Fuel Cell with Polarization Analysis and X-ray Imaging of Liquid Water. J. Power Sources 2019, 431, 205–209. [Google Scholar] [CrossRef]
- Kim, D.H.; Min, C.M.; Lee, E.; Lee, J.S.; Pal, C. Effect of Vinylphosphonic Acid and Polymer Binders with Phosphate Groups on Performance of High-temperature Polymer Electrolyte Membrane Fuel Cell. Catal. Today 2020, 358, 333–337. [Google Scholar] [CrossRef]
- Fu, X.; Li, T.; Tang, L.; Deng, X.; Zhang, R.; Hu, S.; Zhao, F.; Li, X.; Liu, Q. Reticulated Polyaniline Nanowires as a Cathode Microporous Layer for High-temperature PEMFCs. Int. J. Hydrog. Energy 2021, 46, 8802–8809. [Google Scholar] [CrossRef]
- Abdin, Z.; Webb, C.J.; Gray, E.M. PEM Fuel Cell Model and Simulation in Matlab-Simulink Based on Physical Parameters. Energy 2016, 116, 1131–1144. [Google Scholar] [CrossRef]
- Mohanta, P.K.; Ripa, M.S.; Regnet, F.; Jorissen, L. Impact of Membrane Types and Catalyst Layers Composition on Performance of Polymer Electrolyte Membrane Fuel Cells. Chem. Open 2020, 9, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Rakhshanpouri, S.; Rowshanzamir, S. Water Transport through a PEM (Proton Exchange Membrane) Fuel Cell in a Seven-layer Model. Energy 2013, 50, 220–231. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Falcao, D.S.; Oliveira, V.B.; Pinto, A.M.F.R. Experimental Study on the Membrane Electrode Assembly of a Proton Exchange Membrane Fuel Cell: Effects of Microporous Layer, Membrane Thickness and Gas Diffusion Layer Hydrophobic Treatment. Electrochim. Acta 2017, 224, 337–345. [Google Scholar] [CrossRef]
- Xu, B.; Li, D.; Ma, Z.; Zheng, M.; Li, Y. Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications. Mathematics 2021, 9, 1792. [Google Scholar] [CrossRef]
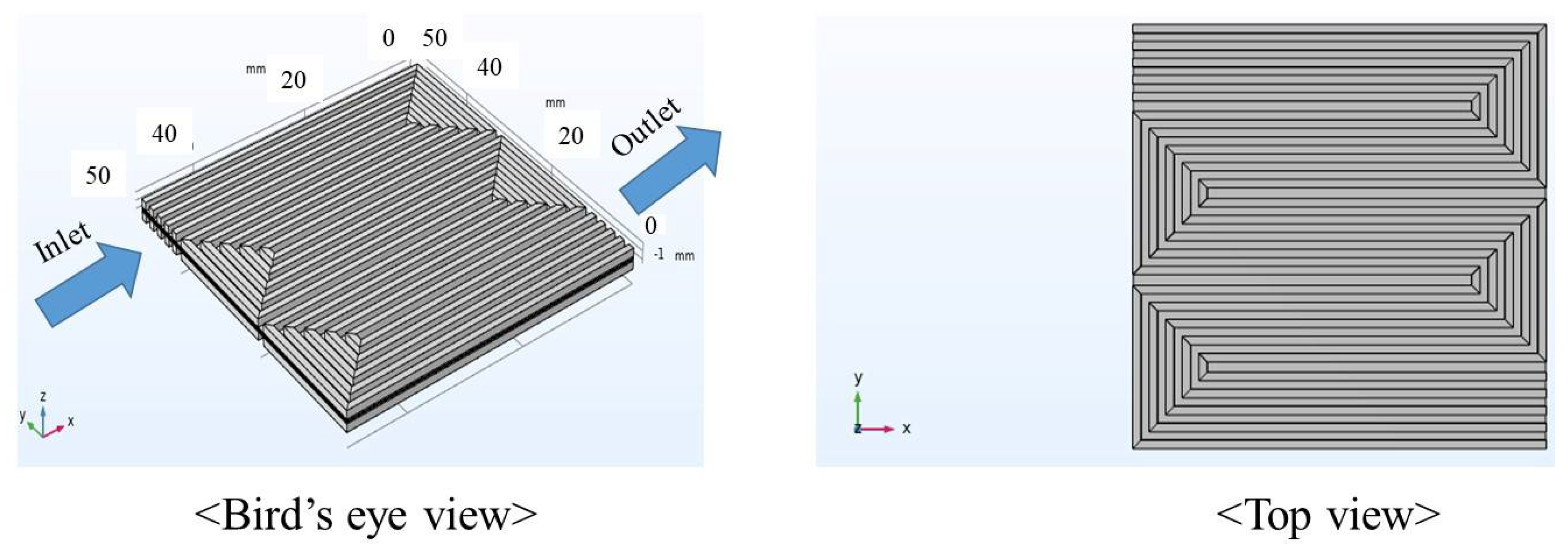

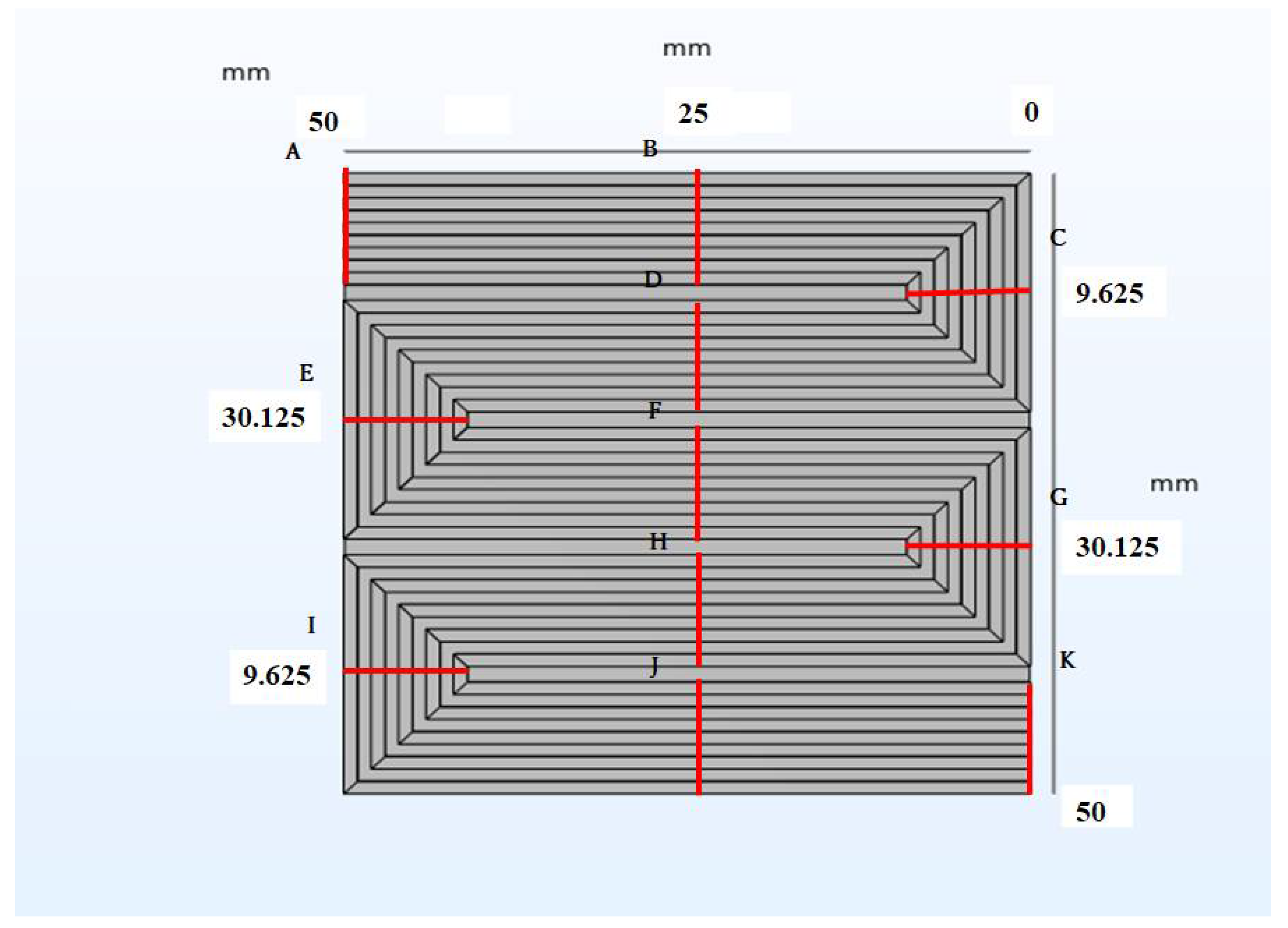


| Components | Size | Characteristics |
|---|---|---|
| PEM | 50.0 mm × 50.0 mm × 0.127 mm (for Nafion 115), 0.051 mm (for Nafion NRE-212) and 0.025 mm (for Nafion NRE-211) | Nafion 115, Nafion NRE-212, Nafion NRE-211 (manufactured by Du Pont Corp.) |
| Catalyst layer | 50.0 mm × 50.0 mm × 0.01 mm | Pt/C (Pt: 20 wt%) |
| MPL | 50.0 mm × 50.0 mm × 0.003 mm | PTFE + carbon black |
| GDL | 50.0 mm × 50.0 mm × 0.19 mm | TGP-H-060 (manufactured by Toray Corp.) |
| Gas separator | 50.0 mm × 50.0 mm × 2.00 mm (thickness of rib: 1.00 mm) (width of gas channel and rib: 1.0 mm, thickness of gas channel: 1.0 mm) | Carbon graphite, serpentine |
| Parameter Name | Value |
|---|---|
| Density of H2 (kg/m3) | 7.10 × 10−2 (353 K), 6.89 × 10−2 (363 K), 6.69 × 10−2 (373 K) [32] |
| Density of O2 (kg/m3) | 1.11 (353 K), 1.08 (363 K), 1.05 (373 K) [32] |
| Density of H2O (kg/m3) | 2.95 × 10−1 (353 K), 4.26 × 10−1 (363 K), 6.01 × 10−1 (373 K) [32] |
| Viscosity of H2 (Pa s) | 9.96 × 10−6 (353 K), 1.02 × 10−5 (363 K), 1.03 × 10−5 (373 K) [32] |
| Viscosity of O2 (Pa s) | 2.35 × 10−5 (353 K), 2.40 × 10−5 (363 K), 2.45 × 10−5 (373 K) [32] |
| Viscosity of H2O (Pa s) | 1.16 × 10−5 (353 K), 1.19 × 10−5 (363 K), 1.23 × 10−5 (373 K) [32] |
| Binary diffusion coefficient between H2 and H2O (m2/s) | 9.27 × 10−5 [33] |
| Binary diffusion coefficient between O2 and H2O (m2/s) | 3.57 × 10−5 [33] |
| Porosity of catalyst layer ( ) | 0.78 [17,22,29,30,31] |
| Permeability of catalyst layer (m2) | 8.69 × 10−12 [17,22,29,30,31] |
| Porosity of MPL ( ) | 0.60 [17,22,29,30,31] |
| Permeability of MPL (m2) | 1.00 × 10−13 [17,22,29,30,31] |
| Porosity of GDL ( ) | 0.78 [17,22,29,30,31] |
| Permeability of GDL (m2) | 8.69 × 10−12 [17,22,29,30,31] |
| Conductivity of Nafion series membrane (S/m) | 10 [34] |
| Conductivity of catalyst layer (S/m) | 53 [35] |
| Conductivity of MPL (S/m) | 1000 [36] |
| Conductivity of GDL (S/m) | 1250 [37] |
| Anode reference equilibrium potential (V) | 0 |
| Cathode reference equilibrium potential (V) | 1.229 |
| Anode reference exchange current density (A/m2) | 1000 [38] |
| Cathode reference exchange current density (A/m2) | 1 [38] |
| Anode charge transfer coefficient ( ) | 0.5 [39] |
| Cathode charge transfer coefficient ( ) | 0.5 [40] |
| Each Condition | Value | |
|---|---|---|
| The initial temperature of cell (Tini) (K) | 353, 363, 373 | |
| Cell voltage (V) | Experimental data are used [16,17] | |
| Supply gas condition | ||
| Anode | Cathode | |
| Gas type | H2 | O2 |
| Temperature of supply gas at inlet (K) | 353, 363, 373 | 353, 363, 373 |
| Relative humidity of supply gas (%RH) | 40, 80 | 40, 80 |
| Pressure of supply gas at inlet (absolute) (MPa) | 0.4 | 0.4 |
| Flow rate of supply gas at inlet [NL/min] (Stoichiometric ratio ( )) | 0.210 (1.5) | 0.105 (1.5) |
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 K | |||||||||||
| 115 | 0.721 | 2.07 | 3.14 | 4.27 | 5.22 | 6.25 | 7.12 | 8.05 | 8.85 | 9.61 | 10.3 |
| 212 | 0.793 | 2.87 | 4.46 | 6.10 | 7.46 | 8.88 | 10.1 | 11.3 | 12.4 | 13.3 | 14.2 |
| 211 | 0.813 | 3.08 | 4.81 | 6.57 | 8.02 | 9.53 | 10.8 | 12.1 | 13.2 | 14.2 | 15.1 |
| 363 K | |||||||||||
| 115 | 0.955 | 2.23 | 3.23 | 4.28 | 5.18 | 6.13 | 6.96 | 7.84 | 8.59 | 9.31 | 9.93 |
| 212 | 0.979 | 2.49 | 3.67 | 4.89 | 5.92 | 7.02 | 7.96 | 8.94 | 9.78 | 10.6 | 11.3 |
| 211 | 0.981 | 2.51 | 3.70 | 4.93 | 5.97 | 7.08 | 8.02 | 9.01 | 9.85 | 10.6 | 11.3 |
| 373 K | |||||||||||
| 115 | 1.17 | 1.58 | 1.91 | 2.27 | 2.58 | 2.93 | 3.23 | 3.56 | 3.85 | 4.13 | 4.38 |
| 212 | 1.19 | 1.77 | 2.24 | 2.74 | 3.17 | 3.65 | 4.06 | 4.52 | 4.91 | 5.29 | 5.63 |
| 211 | 1.20 | 1.86 | 2.39 | 2.95 | 3.45 | 3.98 | 4.45 | 4.95 | 5.40 | 5.82 | 6.20 |
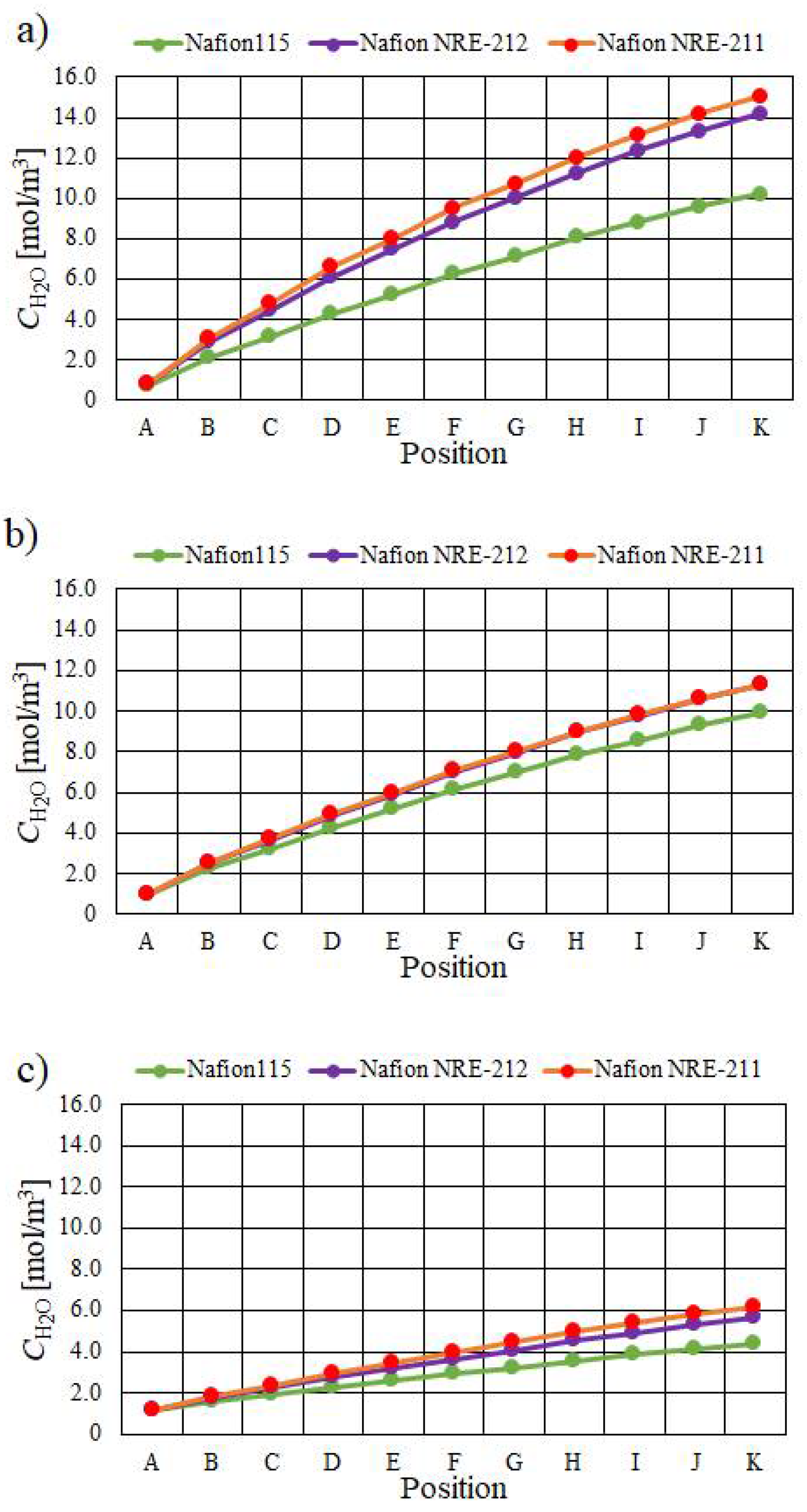
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 K | |||||||||||
| 115 | 0.425 | 1.68 | 2.68 | 3.73 | 4.62 | 5.59 | 6.41 | 7.30 | 8.05 | 8.77 | 9.40 |
| 212 | 0.477 | 2.28 | 3.68 | 5.13 | 6.35 | 7.63 | 8.71 | 9.85 | 10.8 | 11.7 | 12.5 |
| 211 | 0.492 | 2.44 | 3.96 | 5.52 | 6.81 | 8.17 | 9.31 | 10.5 | 11.5 | 12.5 | 13.3 |
| 363 K | |||||||||||
| 115 | 0.527 | 1.46 | 2.21 | 3.01 | 3.69 | 4.44 | 5.07 | 5.77 | 6.36 | 6.94 | 7.44 |
| 212 | 0.557 | 1.80 | 2.78 | 3.81 | 4.68 | 5.63 | 6.43 | 7.29 | 8.02 | 8.72 | 9.33 |
| 211 | 0.575 | 2.00 | 3.12 | 4.28 | 5.27 | 6.32 | 7.21 | 8.16 | 8.97 | 9.73 | 10.4 |
| 373 K | |||||||||||
| 115 | 0.644 | 1.02 | 1.33 | 1.66 | 1.95 | 2.28 | 2.56 | 2.87 | 3.14 | 3.40 | 3.64 |
| 212 | 0.664 | 1.25 | 1.72 | 2.23 | 2.67 | 3.16 | 3.57 | 4.03 | 4.43 | 4.82 | 5.16 |
| 211 | 0.675 | 1.37 | 1.92 | 2.52 | 3.15 | 3.60 | 4.08 | 4.61 | 5.07 | 5.51 | 5.91 |
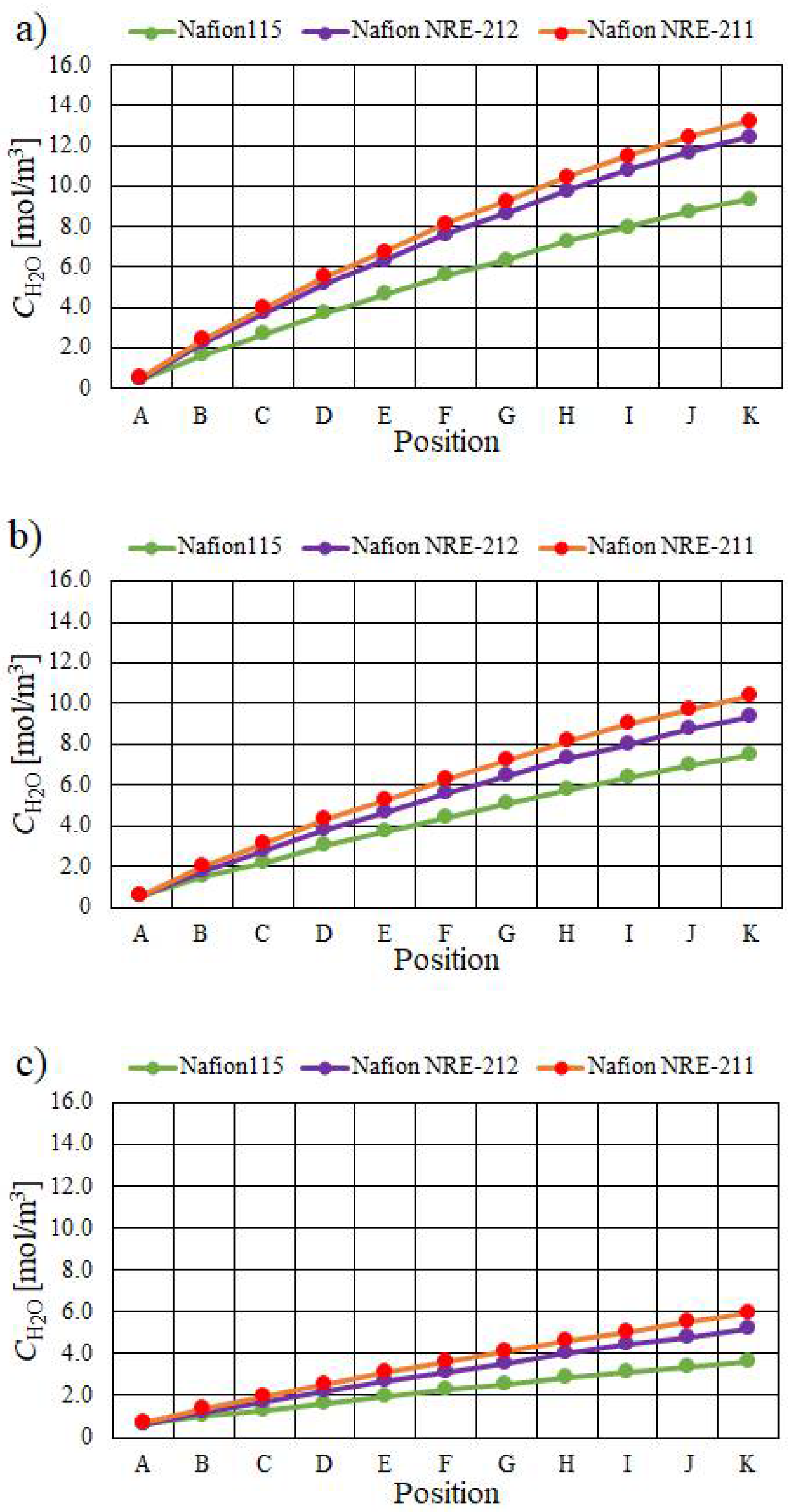
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 K | |||||||||||
| 115 | 0.706 | 1.91 | 2.86 | 3.87 | 4.73 | 5.67 | 6.46 | 7.31 | 8.05 | 8.74 | 9.36 |
| 212 | 0.758 | 2.49 | 3.83 | 5.23 | 6.40 | 7.64 | 8.69 | 9.79 | 10.7 | 11.6 | 12.4 |
| 211 | 0.773 | 2.65 | 4.10 | 5.60 | 6.85 | 8.17 | 9.27 | 10.4 | 11.4 | 12.3 | 13.1 |
| 363 K | |||||||||||
| 115 | 0.915 | 1.80 | 2.51 | 3.26 | 3.91 | 4.62 | 5.23 | 5.89 | 6.46 | 7.01 | 7.49 |
| 212 | 0.945 | 2.12 | 3.05 | 4.03 | 4.86 | 5.76 | 6.53 | 7.35 | 8.06 | 8.73 | 9.32 |
| 211 | 0.954 | 2.21 | 3.21 | 4.25 | 5.13 | 6.08 | 6.89 | 7.76 | 8.50 | 9.20 | 9.82 |
| 373 K | |||||||||||
| 115 | 1.16 | 1.41 | 1.62 | 1.85 | 2.05 | 2.27 | 2.46 | 2.68 | 2.87 | 3.05 | 3.22 |
| 212 | 1.18 | 1.70 | 2.11 | 2.55 | 2.94 | 3.37 | 3.74 | 4.15 | 4.50 | 4.85 | 5.15 |
| 211 | 1.19 | 1.80 | 2.28 | 2.81 | 3.26 | 3.76 | 4.19 | 4.66 | 5.07 | 5.46 | 5.82 |
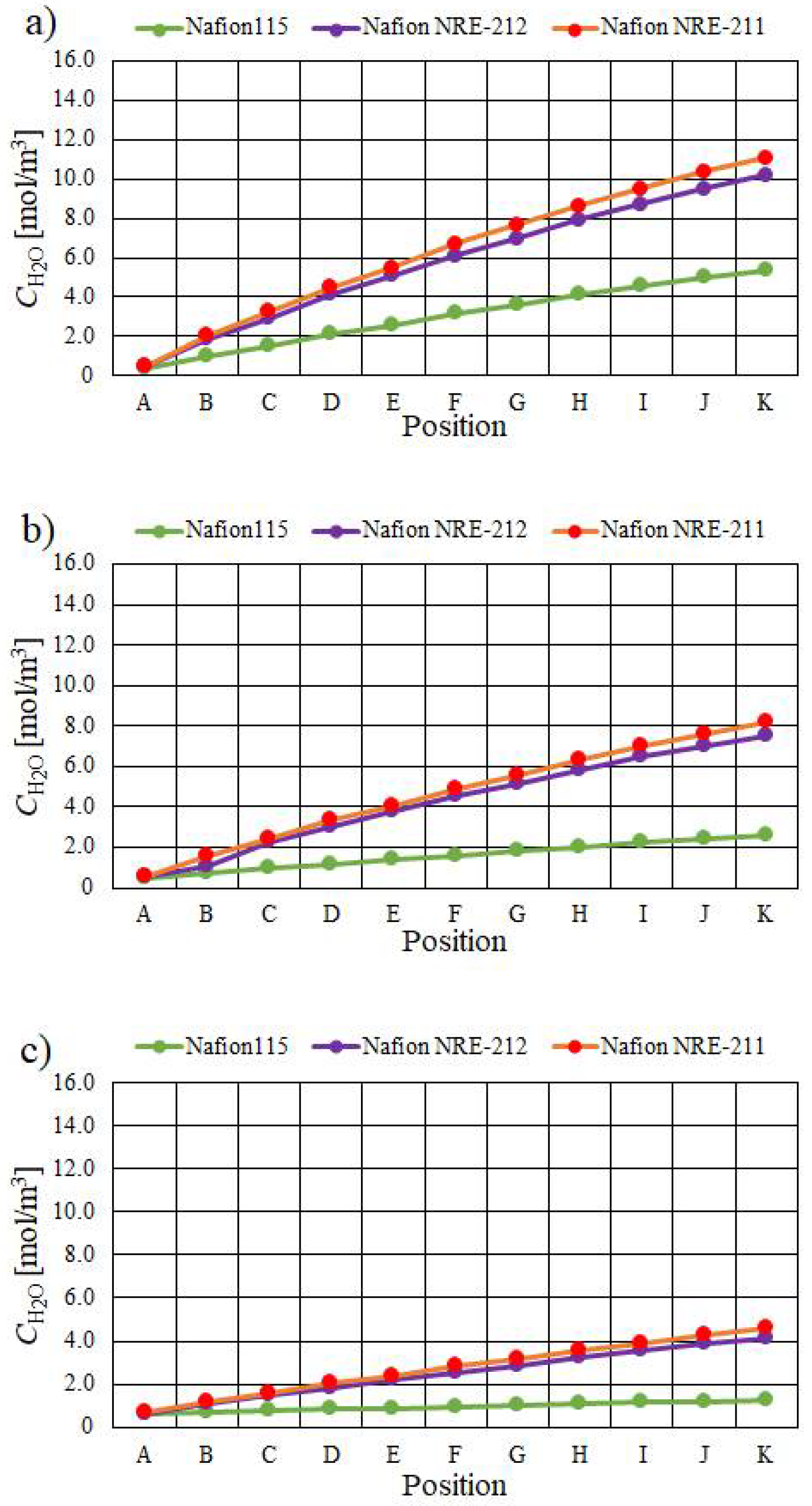
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 K | |||||||||||
| 115 | 0.368 | 1.01 | 1.54 | 2.11 | 2.59 | 3.13 | 3.60 | 4.11 | 4.56 | 4.99 | 5.37 |
| 212 | 0.438 | 1.83 | 2.93 | 4.09 | 5.06 | 6.12 | 7.00 | 7.96 | 8.77 | 9.53 | 10.2 |
| 211 | 0.453 | 2.00 | 3.22 | 4.50 | 5.56 | 6.71 | 7.67 | 8.70 | 9.57 | 10.4 | 11.1 |
| 363 K | |||||||||||
| 115 | 0.464 | 0.727 | 0.944 | 1.18 | 1.39 | 1.62 | 1.82 | 2.04 | 2.24 | 2.43 | 2.60 |
| 212 | 0.529 | 1.09 | 2.25 | 3.06 | 3.75 | 4.51 | 5.16 | 5.86 | 6.47 | 7.05 | 7.55 |
| 211 | 0.539 | 1.59 | 2.43 | 3.32 | 4.08 | 4.90 | 5.61 | 6.37 | 7.02 | 7.64 | 8.18 |
| 373 K | |||||||||||
| 115 | 0.615 | 0.693 | 0.757 | 0.828 | 0.890 | 0.960 | 1.02 | 1.09 | 1.15 | 1.21 | 1.26 |
| 212 | 0.650 | 1.09 | 1.45 | 1.84 | 2.18 | 2.56 | 2.88 | 3.24 | 3.55 | 3.86 | 4.13 |
| 211 | 0.657 | 1.16 | 1.57 | 2.01 | 2.40 | 2.82 | 3.19 | 3.60 | 3.90 | 4.29 | 4.59 |
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 K | |||||||||||
| 115 | 0.197 | 0.194 | 0.192 | 0.190 | 0.187 | 0.185 | 0.182 | 0.180 | 0.178 | 0.175 | 0.173 |
| 212 | 0.307 | 0.300 | 0.293 | 0.289 | 0.281 | 0.277 | 0.270 | 0.265 | 0.259 | 0.254 | 0.250 |
| 211 | 0.336 | 0.328 | 0.320 | 0.314 | 0.305 | 0.299 | 0.291 | 0.286 | 0.277 | 0.272 | 0.266 |
| 363 K | |||||||||||
| 115 | 0.197 | 0.193 | 0.191 | 0.189 | 0.186 | 0.184 | 0.181 | 0.179 | 0.176 | 0.174 | 0.172 |
| 212 | 0.234 | 0.230 | 0.226 | 0.223 | 0.218 | 0.215 | 0.211 | 0.208 | 0.204 | 0.200 | 0.198 |
| 211 | 0.237 | 0.232 | 0.228 | 0.225 | 0.220 | 0.217 | 0.212 | 0.209 | 0.205 | 0.201 | 0.198 |
| 373 K | |||||||||||
| 115 | 0.066 | 0.065 | 0.065 | 0.065 | 0.064 | 0.064 | 0.063 | 0.063 | 0.062 | 0.062 | 0.062 |
| 212 | 0.094 | 0.093 | 0.092 | 0.092 | 0.091 | 0.090 | 0.089 | 0.088 | 0.088 | 0.087 | 0.086 |
| 211 | 0.107 | 0.106 | 0.105 | 0.104 | 0.103 | 0.102 | 0.101 | 0.100 | 0.099 | 0.098 | 0.097 |
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 K | |||||||||||
| 115 | 0.175 | 0.172 | 0.170 | 0.168 | 0.166 | 0.164 | 0.162 | 0.160 | 0.158 | 0.156 | 0.154 |
| 212 | 0.253 | 0.248 | 0.244 | 0.240 | 0.235 | 0.231 | 0.226 | 0.223 | 0.218 | 0.214 | 0.211 |
| 211 | 0.276 | 0.270 | 0.246 | 0.260 | 0.253 | 0.249 | 0.243 | 0.239 | 0.233 | 0.229 | 0.225 |
| 363 K | |||||||||||
| 115 | 0.135 | 0.133 | 0.132 | 0.131 | 0.129 | 0.128 | 0.127 | 0.125 | 0.124 | 0.123 | 0.122 |
| 212 | 0.180 | 0.178 | 0.175 | 0.173 | 0.170 | 0.168 | 0.165 | 0.163 | 0.160 | 0.158 | 0.156 |
| 211 | 0.208 | 0.204 | 0.201 | 0.198 | 0.194 | 0.191 | 0.187 | 0.185 | 0.181 | 0.178 | 0.176 |
| 373 K | |||||||||||
| 115 | 0.057 | 0.056 | 0.056 | 0.055 | 0.055 | 0.055 | 0.054 | 0.054 | 0.054 | 0.053 | 0.053 |
| 212 | 0.088 | 0.087 | 0.086 | 0.086 | 0.085 | 0.084 | 0.083 | 0.083 | 0.082 | 0.081 | 0.081 |
| 211 | 0.104 | 0.103 | 0.102 | 0.101 | 0.100 | 0.099 | 0.098 | 0.097 | 0.096 | 0.095 | 0.094 |
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 K | |||||||||||
| 115 | 0.175 | 0.173 | 0.171 | 0.169 | 0.167 | 0.165 | 0.163 | 0.161 | 0.159 | 0.157 | 0.155 |
| 212 | 0.254 | 0.249 | 0.245 | 0.241 | 0.236 | 0.233 | 0.228 | 0.225 | 0.220 | 0.216 | 0.213 |
| 211 | 0.277 | 0.270 | 0.265 | 0.261 | 0.255 | 0.251 | 0.245 | 0.241 | 0.235 | 0.231 | 0.227 |
| 363 K | |||||||||||
| 115 | 0.136 | 0.134 | 0.133 | 0.132 | 0.130 | 0.132 | 0.128 | 0.127 | 0.125 | 0.124 | 0.123 |
| 212 | 0.181 | 0.178 | 0.176 | 0.174 | 0.171 | 0.170 | 0.167 | 0.165 | 0.162 | 0.160 | 0.159 |
| 211 | 0.195 | 0.192 | 0.189 | 0.187 | 0.183 | 0.181 | 0.178 | 0.176 | 0.173 | 0.170 | 0.168 |
| 373 K | |||||||||||
| 115 | 0.041 | 0.041 | 0.041 | 0.041 | 0.040 | 0.040 | 0.040 | 0.040 | 0.039 | 0.039 | 0.039 |
| 212 | 0.083 | 0.082 | 0.082 | 0.081 | 0.080 | 0.080 | 0.079 | 0.079 | 0.078 | 0.077 | 0.077 |
| 211 | 0.098 | 0.097 | 0.096 | 0.096 | 0.095 | 0.094 | 0.093 | 0.092 | 0.091 | 0.091 | 0.090 |
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 353 K | |||||||||||
| 115 | 0.089 | 0.088 | 0.088 | 0.087 | 0.086 | 0.086 | 0.085 | 0.085 | 0.084 | 0.083 | 0.083 |
| 212 | 0.195 | 0.191 | 0.189 | 0.186 | 0.183 | 0.181 | 0.178 | 0.176 | 0.173 | 0.170 | 0.168 |
| 211 | 0.218 | 0.214 | 0.210 | 0.207 | 0.203 | 0.200 | 0.196 | 0.194 | 0.190 | 0.190 | 0.184 |
| 363 K | |||||||||||
| 115 | 0.038 | 0.037 | 0.037 | 0.037 | 0.037 | 0.037 | 0.037 | 0.036 | 0.036 | 0.036 | 0.036 |
| 212 | 0.138 | 0.136 | 0.135 | 0.133 | 0.132 | 0.130 | 0.129 | 0.128 | 0.126 | 0.125 | 0.123 |
| 211 | 0.153 | 0.151 | 0.149 | 0.147 | 0.145 | 0.143 | 0.141 | 0.140 | 0.138 | 0.136 | 0.135 |
| 373 K | |||||||||||
| 115 | 0.012 | 0.012 | 0.012 | 0.011 | 0.011 | 0.011 | 0.011 | 0.011 | 0.011 | 0.011 | 0.011 |
| 212 | 0.066 | 0.066 | 0.065 | 0.065 | 0.064 | 0.064 | 0.064 | 0.063 | 0.062 | 0.062 | 0.062 |
| 211 | 0.076 | 0.075 | 0.075 | 0.074 | 0.074 | 0.073 | 0.072 | 0.072 | 0.072 | 0.071 | 0.070 |
| A80%RH&C80%RH | |||||||||
| Tini [K] | 353 | 363 | 373 | ||||||
| Nafion type | 115 | 212 | 211 | 115 | 212 | 211 | 115 | 212 | 211 |
| Voltage [V] | 0.581 | 0.631 | 0.636 | 0.601 | 0.611 | 0.606 | 0.461 | 0.501 | 0.516 |
| A80%RH&C40%RH | |||||||||
| Tini [K] | 353 | 363 | 373 | ||||||
| Nafion type | 115 | 212 | 211 | 115 | 212 | 211 | 115 | 212 | 211 |
| Voltage [V] | 0.561 | 0.601 | 0.606 | 0.541 | 0.571 | 0.586 | 0.441 | 0.481 | 0.511 |
| A40%RH&C80%RH | |||||||||
| Tini [K] | 353 | 363 | 373 | ||||||
| Nafion type | 115 | 212 | 211 | 115 | 212 | 211 | 115 | 212 | 211 |
| Voltage [V] | 0.561 | 0.601 | 0.606 | 0.541 | 0.571 | 0.576 | 0.401 | 0.481 | 0.501 |
| A40%RH&C40%RH | |||||||||
| Tini [K] | 353 | 363 | 373 | ||||||
| Nafion type | 115 | 212 | 211 | 115 | 212 | 211 | 115 | 212 | 211 |
| Voltage [V] | 0.461 | 0.561 | 0.571 | 0.371 | 0.531 | 0.541 | 0.291 | 0.451 | 0.466 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Toyoda, K.; Kojima, Y.; Ito, S.; Hu, E. Numerical Simulation on Impacts of Thickness of Nafion Series Membranes and Relative Humidity on PEMFC Operated at 363 K and 373 K. Energies 2021, 14, 8256. https://doi.org/10.3390/en14248256
Nishimura A, Toyoda K, Kojima Y, Ito S, Hu E. Numerical Simulation on Impacts of Thickness of Nafion Series Membranes and Relative Humidity on PEMFC Operated at 363 K and 373 K. Energies. 2021; 14(24):8256. https://doi.org/10.3390/en14248256
Chicago/Turabian StyleNishimura, Akira, Kyohei Toyoda, Yuya Kojima, Syogo Ito, and Eric Hu. 2021. "Numerical Simulation on Impacts of Thickness of Nafion Series Membranes and Relative Humidity on PEMFC Operated at 363 K and 373 K" Energies 14, no. 24: 8256. https://doi.org/10.3390/en14248256
APA StyleNishimura, A., Toyoda, K., Kojima, Y., Ito, S., & Hu, E. (2021). Numerical Simulation on Impacts of Thickness of Nafion Series Membranes and Relative Humidity on PEMFC Operated at 363 K and 373 K. Energies, 14(24), 8256. https://doi.org/10.3390/en14248256








