Characterization of Pd60Cu40 Composite Membrane Prepared by a Reverse Build-Up Method for Hydrogen Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reverse Build-Up Method
2.2. Sample Characterization
3. Results and Discussion
3.1. Sample Characterization
3.2. Evaluation of H2 Permeation Measurement
3.3. Analysis of the Membrane after H2 Permeation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Pre-exponential factor at 300–320 °C (-) |
| Au | Gold |
| Ar | Argon |
| Cu | Copper |
| Ea | Activation energy (kJ mol−1) |
| H2 | Hydrogen |
| He | Helium |
| JH2 | H2 permeation flux on the secondary side of the test chamber (mol m−2 s−1) |
| L | Pd60Cu40 layer thickness (µm) |
| n | H2 pressure exponent (-) (0.50 ≤ n ≤ 1.0) |
| N2 | Nitrogen |
| Ni | Nickel |
| PH2,feed | Partial pressure of H2 on the primary side of the chamber (Pa) |
| PH2,perm | Partial pressure of H2 on the secondary side of the chamber (Pa) |
| Pd | Palladium |
| PdAg | Palladium-silver |
| PdCu | Palladium-copper |
| R | Universal gas constant (J mol−1 K−1) |
| R2 | Coefficient of determination (-) |
| Ru | Ruthenium |
| S | H2-permeable membrane area (m2) |
| T | Absolute temperature (K) |
| αH2/He | H2/He selectivity (-) |
| φH2 | H2 permeability coefficient (mol m−1 s−1 Pa-n) (0.50 ≤ n ≤ 1.0) |
| φHe | He permeability coefficient (mol m−1 s−1 Pa-n) (n = 1.0) |
| φH2/L | H2 permeance (mol m−2 s−1 Pa-n) (0.50 ≤ n ≤ 1.0) |
References
- Itoh, N.; Kikuchi, Y.; Furusawa, T.; Sato, T. Tube-wall catalytic membrane reactor for hydrogen production by low-temperature ammonia decomposition. Int. J. Hydrogen Energy 2021, 46, 20257–20265. [Google Scholar] [CrossRef]
- Anzelmo, B.; Wilcox, J.; Liguori, S. Natural gas steam reforming reaction at low temperature and pressure conditions for hydrogen production via Pd/PSS membrane reactor. J. Memb. Sci. 2017, 522, 343–350. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Chaniago, Y.D.; Ali, W.; Saulat, H.; Lee, M. Membrane-assisted removal of hydrogen and nitrogen from synthetic natural gas for energy-efficient liquefaction. Energies 2020, 13, 5023. [Google Scholar] [CrossRef]
- Endo, N.; Dezawa, N.; Komo, Y.; Maeda, T. One-step electroplating of palladium–copper alloy layers on a vanadium membrane for hydrogen separation: Quick, easy, and low-cost preparation. Int. J. Hydrogen Energy 2021, 46, 32570–32576. [Google Scholar] [CrossRef]
- Yun, S.; Ted Oyama, S. Correlations in palladium membranes for hydrogen separation: A review. J. Memb. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Kian, K.; Woodall, C.M.; Wilcox, J.; Liguori, S. Performance of Pd-based membranes and effects of various gas mixtures on H2 permeation. Environments 2018, 5, 128. [Google Scholar] [CrossRef] [Green Version]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A. Review of supported pd-based membranes preparation by electroless plating for ultra-pure hydrogen production. Membranes 2018, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paglieri, S.N.; Way, J.D. Innovations in palladium membrane research. Sep. Purif. Methods 2002, 31, 1–169. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Höllein, V.; Daub, K. Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium. J. Mol. Catal. A Chem. 2001, 173, 135–184. [Google Scholar] [CrossRef]
- Tong, J.; Su, C.; Kuraoka, K.; Suda, H.; Matsumura, Y. Preparation of thin Pd membrane on CeO2-modified porous metal by a combined method of electroless plating and chemical vapor deposition. J. Memb. Sci. 2006, 269, 101–108. [Google Scholar] [CrossRef]
- Gryaznov, V.M. Hydrogen Permeable Palladium Membrane Catalysts. Platin. Met. Rev. 1986, 30, 68–72. [Google Scholar]
- Castro-Dominguez, B.; Mardilovich, I.P.; Ma, L.C.; Ma, R.; Dixon, A.G.; Kazantzis, N.K.; Ma, Y.H. Integration of methane steam reforming and water gas shift reaction in a Pd/Au/Pd-based catalytic membrane reactor for process intensification. Membranes 2016, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallucci, F.; Medrano, J.A.; Fernandez, E.; Melendez, J.; Van Sint Annaland, M.; Pacheco-Tanaka, D.A. Advances on high temperature Pd-based membranes and membrane reactors for hydrogen purifcation and production. J. Membr. Sci. Res. 2017, 3, 142–156. [Google Scholar] [CrossRef]
- Basile, A. Hydrogen production using Pd-based membrane reactors for fuel cells. Top. Catal. 2008, 51, 107–122. [Google Scholar] [CrossRef]
- Baloyi, L.N.; North, B.C.; Langmi, H.W.; Bladergroen, B.J.; Ojumu, T.V. The production of hydrogen through the use of a 77 wt% Pd 23 wt% Ag membrane water gas shift reactor. S. Afr. J. Chem. Eng. 2016, 22, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Miller, H.A.; Ruggeri, J.; Marchionni, A.; Bellini, M.; Pagliaro, M.V.; Bartoli, C.; Pucci, A.; Passaglia, E.; Vizza, F. Improving the energy efficiency of direct formate fuel cells with a Pd/C-CeO2 anode catalyst and anion exchange ionomer in the catalyst layer. Energies 2018, 11, 369. [Google Scholar] [CrossRef] [Green Version]
- Byun, M.Y.; Kim, J.S.; Baek, J.H.; Park, D.W.; Lee, M.S. Liquid-phase hydrogenation of maleic acid over Pd/Al2O3 catalysts prepared via deposition–precipitation method. Energies 2019, 12, 284. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.B.; Hidajat, K.; Kawi, S. Ultra thin Pd membrane on α-Al2O3 hollow fiber by electroless plating: High permeance and selectivity. J. Memb. Sci. 2006, 284, 110–119. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Goodman, D.W. CO oxidation over AuPd(100) from ultrahigh vacuum to near-atmospheric pressures: The critical role of contiguous Pd atoms. J. Am. Chem. Soc. 2009, 131, 5734–5735. [Google Scholar] [CrossRef]
- Sonwane, C.G.; Wilcox, J.; Yi, H.M. Solubility of hydrogen in PdAg and PdAu binary alloys using density functional theory. J. Phys. Chem. B 2006, 110, 24549–24558. [Google Scholar] [CrossRef]
- Melendez, J.; Fernandez, E.; Gallucci, F.; van Sint Annaland, M.; Arias, P.L.; Pacheco Tanaka, D.A. Preparation and characterization of ceramic supported ultra-thin (~1 µm) Pd-Ag membranes. J. Memb. Sci. 2017, 528, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, H.; Yakabe, H.; Yasuda, I.; Peters, T.; Bredesen, R. Inhibition effect of CO on hydrogen permeability of Pd-Ag membrane applied in a microchannel module configuration. Int. J. Hydrogen Energy 2014, 39, 17201–17209. [Google Scholar] [CrossRef]
- Endo, N.; Furukawa, Y.; Goshome, K.; Yaegashi, S.; Mashiko, K.; Tetsuhiko, M. Characterization of mechanical strength and hydrogen permeability of a PdCu alloy film prepared by one-step electroplating for hydrogen separation and membrane reactors. Int. J. Hydrogen Energy 2019, 44, 8290–8297. [Google Scholar] [CrossRef]
- OBrien, C.P.; Miller, J.B.; Morreale, B.D.; Gellman, A.J. The kinetics of H 2-D 2 exchange over Pd, Cu, and PdCu surfaces. J. Phys. Chem. C 2011, 115, 24221–24230. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Speight, J.; Book, D. Effects of low Ag additions on the hydrogen permeability of Pd-Cu-Ag hydrogen separation membranes. J. Memb. Sci. 2014, 451, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Bellini, S.; de Nooijer, N.C.A.; Sun, Y.; Pacheco Tanaka, D.A.; Tang, C.; Li, H.; Gallucci, F.; Caravella, A. Hydrogen permeation and stability in ultra-thin Pd–Ru supported membranes. Int. J. Hydrogen Energy 2020, 45, 7455–7467. [Google Scholar] [CrossRef]
- Ryi, S.K.; Li, A.; Lim, C.J.; Grace, J.R. Novel non-alloy Ru/Pd composite membrane fabricated by electroless plating for hydrogen separation. Int. J. Hydrogen Energy 2011, 36, 9335–9340. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Speight, J.D.; Book, D. A novel Pd-Cu-Zr hydrogen separation membrane with a high tolerance to sulphur poisoning. Chem. Commun. 2015, 51, 15842–15845. [Google Scholar] [CrossRef]
- Yoshida, K.; Morigami, H. Thermal properties of diamond/copper composite material. Microelectron. Reliab. 2004, 44, 303–308. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Steinberger-Wilckens, R. X-ray diffraction study on the effects of hydrogen on Pd60Cu40 wt% foil membranes. J. Memb. Sci. 2018, 545, 266–274. [Google Scholar] [CrossRef]
- Yun, S.; Ko, J.H.; Oyama, S.T. Ultrathin palladium membranes prepared by a novel electric field assisted activation. J. Memb. Sci. 2011, 369, 482–489. [Google Scholar] [CrossRef]
- Yang, J.Y.; Nishimura, C.; Komaki, M. Hydrogen Permeation of Pd60Cu40/V-15Ni Composite Membrane under Mixing Gases of H2+H2S. Trans. Mater. Res. Soc. Jpn. 2007, 978, 975–978. [Google Scholar] [CrossRef]
- Chen, W.H.; Escalante, J.; Chi, Y.H.; Lin, Y.L. Hydrogen permeation enhancement in a Pd membrane tube system under various vacuum degrees. Int. J. Hydrogen Energy 2020, 45, 7401–7411. [Google Scholar] [CrossRef]
- Acha, E.; Requies, J.; Barrio, V.L.; Cambra, J.F.; Güemez, M.B.; Arias, P.L.; van Delft, Y.C. PdCu membrane applied to hydrogen production from methane. J. Memb. Sci. 2012, 415–416, 66–74. [Google Scholar] [CrossRef]
- Jayaraman, V.; Lin, Y.S.; Pakala, M.; Lin, R.Y.; Wei, L.; Yu, J.; Hu, X.; Huang, Y. Fabrication of ultrathin metallic membranes on ceramic supports by sputter deposition. J. Memb. Sci. 1995, 99, 89–100. [Google Scholar] [CrossRef]
- Wei, L.; Yu, J.; Hu, X.; Huang, Y. Fabrication of H2-permeable palladium membranes based on pencil-coated porous stainless steel substrate. Int. J. Hydrogen Energy 2012, 37, 13007–13012. [Google Scholar] [CrossRef]
- Kato, Y.; Yaegashi, S.; Watanabe, T.; Hatsuda, H.; Ryu, J. Metal Composited Hydrogen Permeation Membrane and Its Production Method. Japan Patent 6014920, 23 February 2017. [Google Scholar]
- Kato, Y.; Inoue, K.; Urasaki, M.; Tanaka, S.; Ninomiya, H.; Minagawa, T.; Ryu, J. Hydrogen permeability of composite hydrogen permeation membrane using a reverse buildup method. Heat Transf. Eng. 2013, 34, 917–924. [Google Scholar] [CrossRef]
- Tong, J.; Su, L.; Haraya, K.; Suda, H. Thin and defect-free Pd-based composite membrane without any interlayer and substrate penetration by a combined organic and inorganic process. Chem. Commun. 2006, 10, 1142–1144. [Google Scholar] [CrossRef]
- Shinoda, Y.; Takeuchi, M.; Dezawa, N.; Komo, Y.; Harada, T.; Takasu, H.; Kato, Y. Development of a H2-permeable Pd60Cu40-based composite membrane using a reverse build-up method. Int. J. Hydrogen Energy 2021, 46, 36291–36300. [Google Scholar] [CrossRef]
- Yaegashi, S.; Watanabe, T.; Kato, Y.; Hatsuda, H.; Ryu, J. Metal Composited Hydrogen Permeation Membrane and Its Production Method. Japan Patent 6056023, 26 May 2016. [Google Scholar]
- Zhang, X.; Xiong, G.; Yang, W. A modified electroless plating technique for thin dense palladium composite membranes with enhanced stability. J. Memb. Sci. 2008, 314, 226–237. [Google Scholar] [CrossRef]
- Dunbar, Z.W. Hydrogen purification of synthetic water gas shift gases using microstructured palladium membranes. J. Power Sources 2015, 297, 525–533. [Google Scholar] [CrossRef]
- Ryi, S.K.; Park, J.S.; Kim, S.H.; Cho, S.H.; Kim, D.W.; Um, K.Y. Characterization of Pd-Cu-Ni ternary alloy membrane prepared by magnetron sputtering and Cu-reflow on porous nickel support for hydrogen separation. Sep. Purif. Technol. 2006, 50, 82–91. [Google Scholar] [CrossRef]
- Dunbar, Z.W.; Lee, I.C. Effects of elevated temperatures and contaminated hydrogen gas mixtures on novel ultrathin palladium composite membranes. Int. J. Hydrogen Energy 2017, 42, 29310–29319. [Google Scholar] [CrossRef]
- McCool, B.; Xomeritakis, G.; Lin, Y.S. Composition control and hydrogen permeation characteristics of sputter deposited palladium-silver membranes. J. Memb. Sci. 1999, 161, 67–76. [Google Scholar] [CrossRef]
- Zhao, H.B.; Pflanz, K.; Gu, J.H.; Li, A.W.; Stroh, N.; Brunner, H.; Xiong, G.X. Preparation of palladium composite membranes by modified electroless plating procedure. J. Memb. Sci. 1998, 142, 147–157. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Lin, Y.S. CVD Synthesis and Gas Permeation Properties of Thin Palladium/Alumina Membranes. AIChE J. 1998, 44, 174–183. [Google Scholar] [CrossRef]
- Jun, C.S.; Lee, K.H. Palladium and palladium alloy composite membranes prepared by metal-organic chemical vapor deposition method (cold-wall). J. Memb. Sci. 2000, 176, 121–130. [Google Scholar] [CrossRef]
- Itoh, N.; Akiha, T.; Sato, T. Preparation of thin palladium composite membrane tube by a CVD technique and its hydrogen permselectivity. Catal. Today 2005, 104, 231–237. [Google Scholar] [CrossRef]
- Mejdell, A.L.; Jøndahl, M.; Peters, T.A.; Bredesen, R.; Venvik, H.J. Experimental investigation of a microchannel membrane configuration with a 1.4 μm Pd/Ag23 wt.% membrane-Effects of flow and pressure. J. Memb. Sci. 2009, 327, 6–10. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. Ultra-thin (<1 μm) internally-coated Pd-Ag alloy hollow fiber membrane with superior thermal stability and durability for high temperature H2 separation. J. Memb. Sci. 2014, 452, 127–142. [Google Scholar] [CrossRef]
- Li, Z.Y.; Maeda, H.; Kusakabe, K.; Morooka, S.; Anzai, H.; Akiyama, S. Preparation of palladium-silver alloy membranes for hydrogen separation by the spray pyrolysis method. J. Memb. Sci. 1993, 78, 247–254. [Google Scholar] [CrossRef]
- Fernandez, E.; Sanchez-Garcia, J.A.; Melendez, J.; Spallina, V.; van Sint Annaland, M.; Gallucci, F.; Pacheco Tanaka, D.A.; Prema, R. Development of highly permeable ultra-thin Pd-based supported membranes. Chem. Eng. J. 2016, 305, 149–155. [Google Scholar] [CrossRef]
- Roa, F.; Way, J.D. Influence of Alloy Composition and Membrane Fabrication on the Pressure Dependence of the Hydrogen Flux of Palladium-Copper Membranes. Ind. Eng. Chem. Res. 2003, 42, 5827–5835. [Google Scholar] [CrossRef]
- Seshimo, M.; Hirai, T.; Rahman, M.M.; Ozawa, M.; Sone, M.; Sakurai, M.; Higo, Y.; Kameyama, H. Functionally graded Pd/γ-alumina composite membrane fabricated by electroless plating with emulsion of supercritical CO2. J. Memb. Sci. 2009, 342, 321–326. [Google Scholar] [CrossRef]
- McKinley, D.L. Metal Alloy for Hydrogen Separation and Purification. U.S. Patent 3,350,845, 7 November 1967. [Google Scholar]
- McKinley, D.L. Method for Hydrogen Separation and Purification. U.S. Patent 3,439,474, 22 April 1969. [Google Scholar]
- Ma, Y.H.; Akis, B.C.; Ayturk, M.E.; Guazzone, F.; Engwall, E.E.; Mardilovich, I.P. Characterization of intermetallic diffusion barrier and alloy formation for Pd/Cu and Pd/Ag porous stainless steel composite membranes. Ind. Eng. Chem. Res. 2004, 43, 2936–2945. [Google Scholar] [CrossRef]
- Samingprai, S.; Tantayanon, S.; Ma, Y.H. Chromium oxide intermetallic diffusion barrier for palladium membrane supported on porous stainless steel. J. Memb. Sci. 2010, 347, 8–16. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, H.; Rui, Z.; Liu, P.; Li, Y.; Lin, Y.S. High-temperature stability of palladium membranes on porous metal supports with different intermediate layers. Ind. Eng. Chem. Res. 2009, 48, 1880–1886. [Google Scholar] [CrossRef]


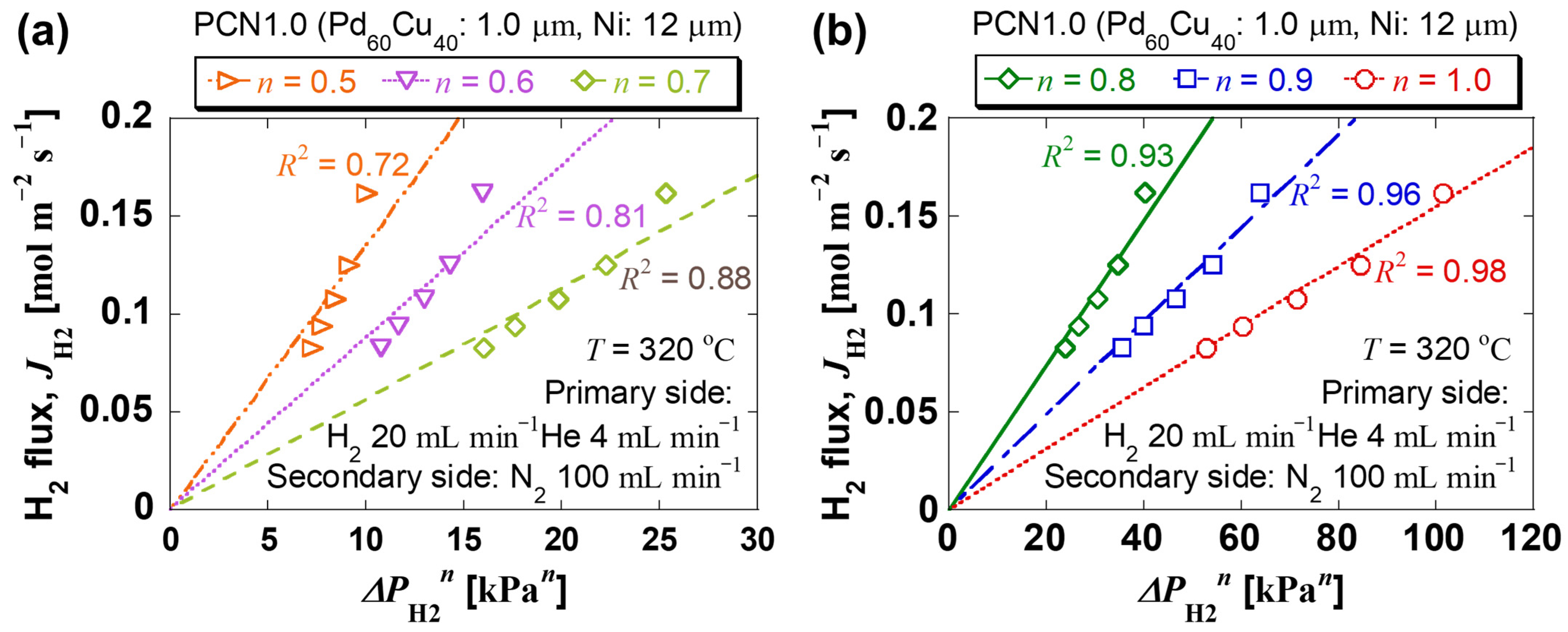
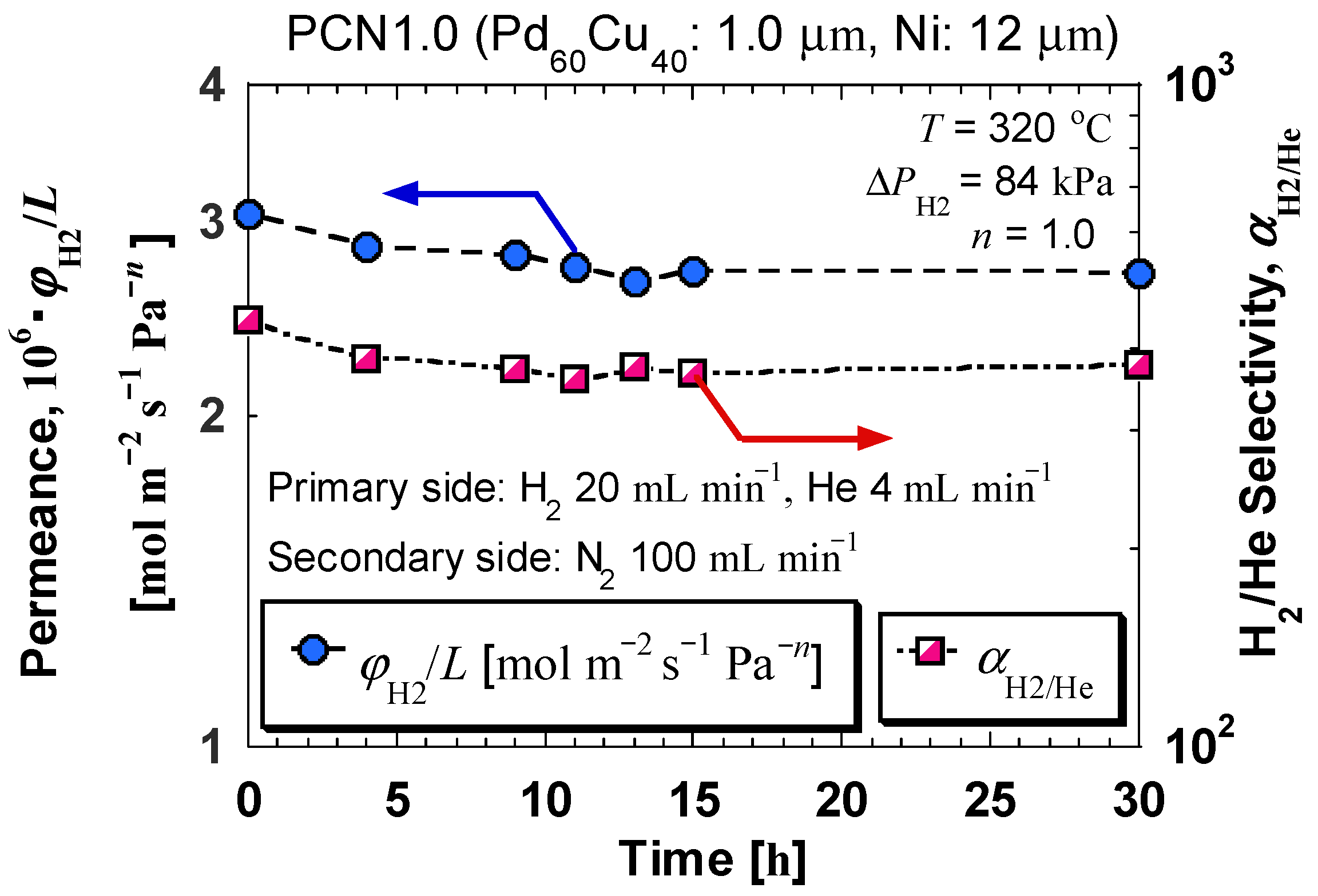
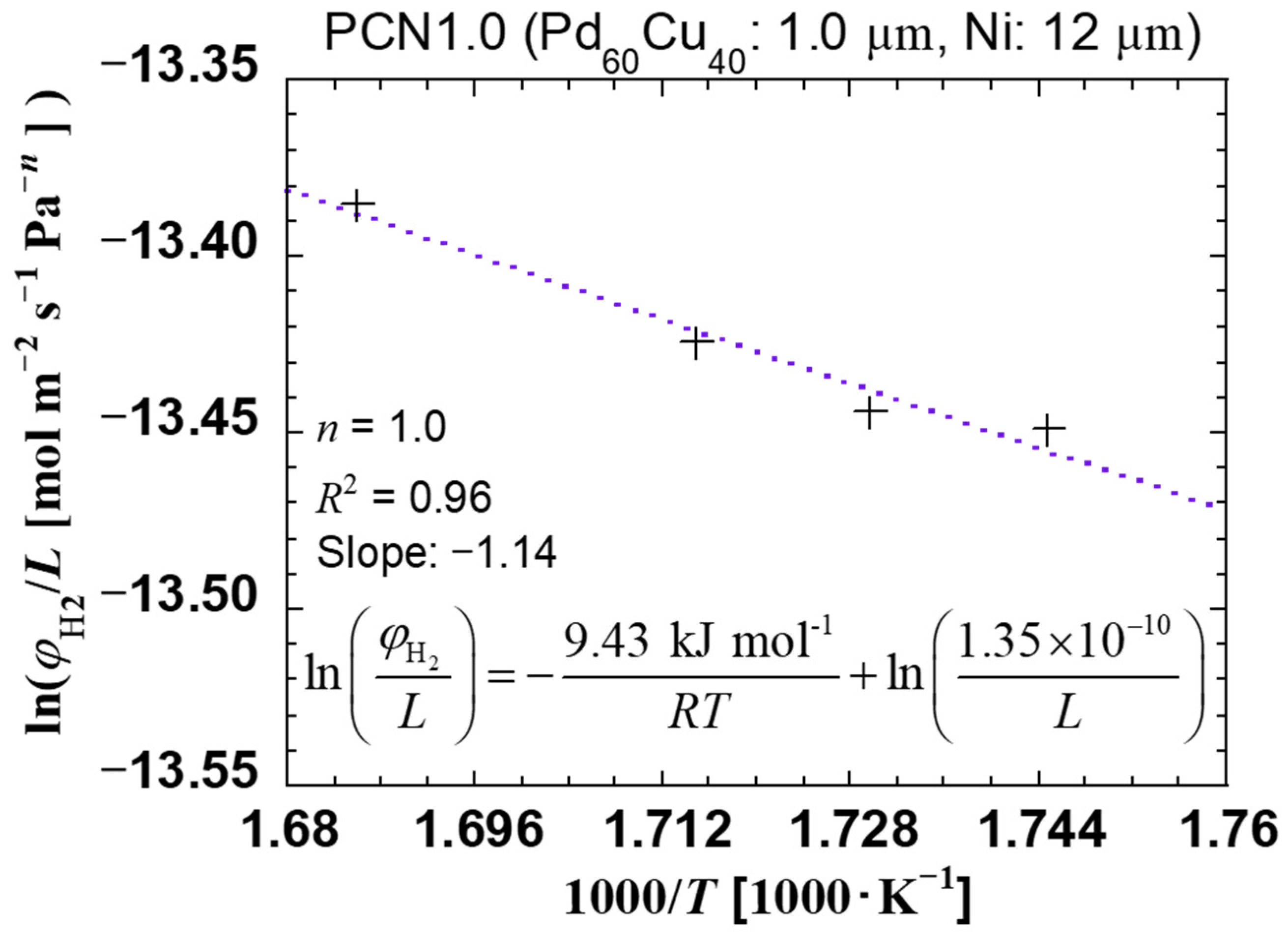
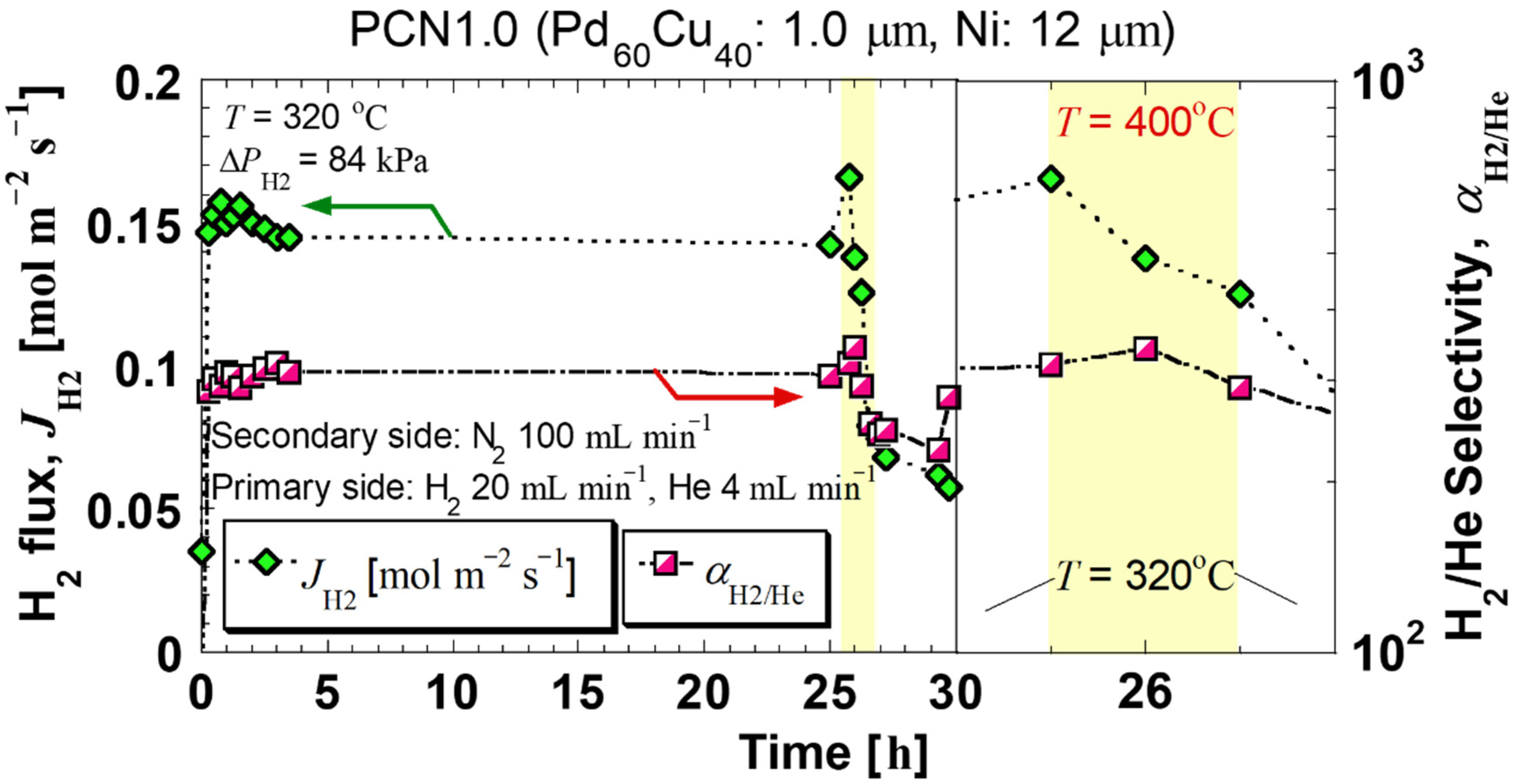
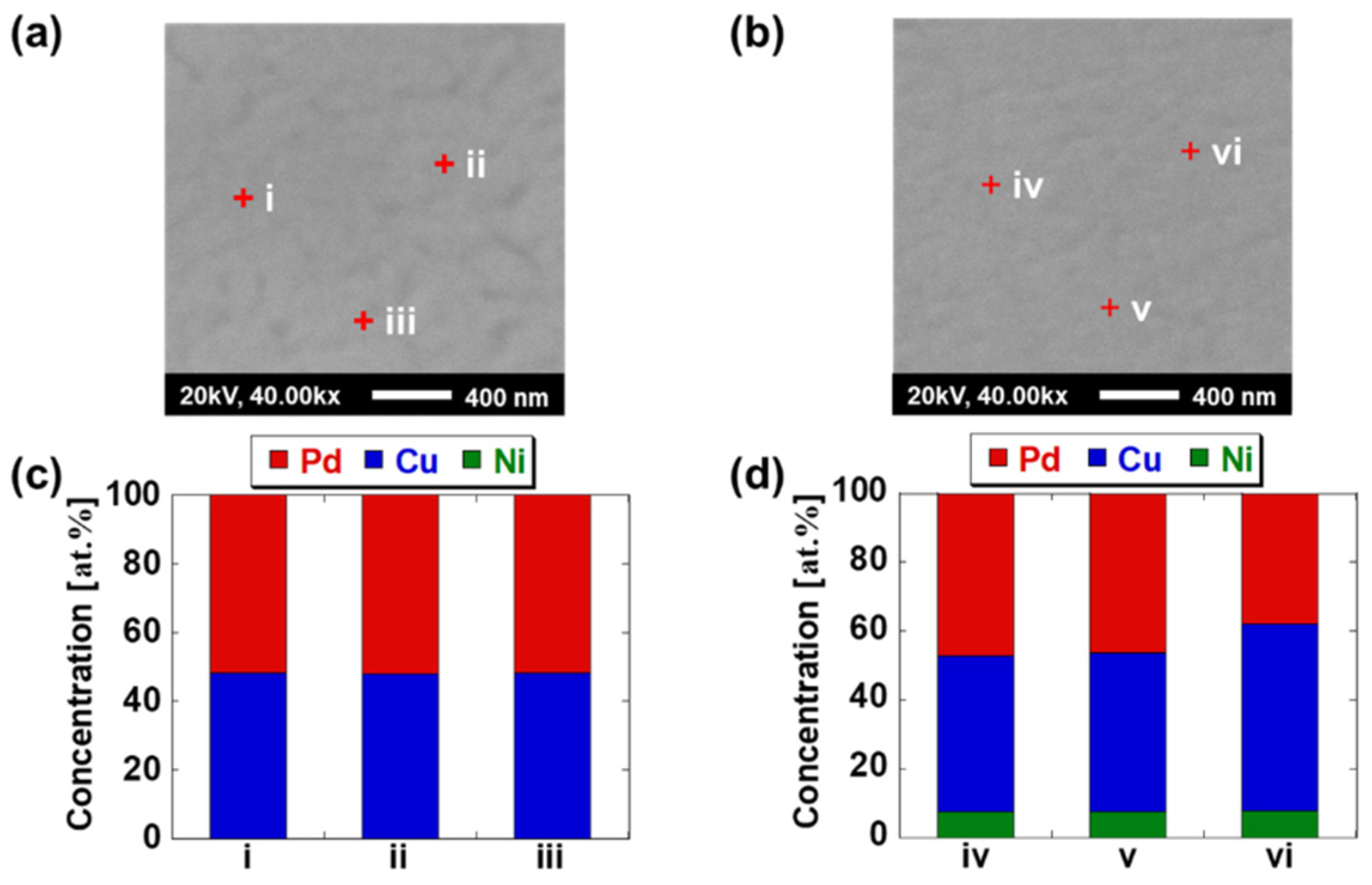
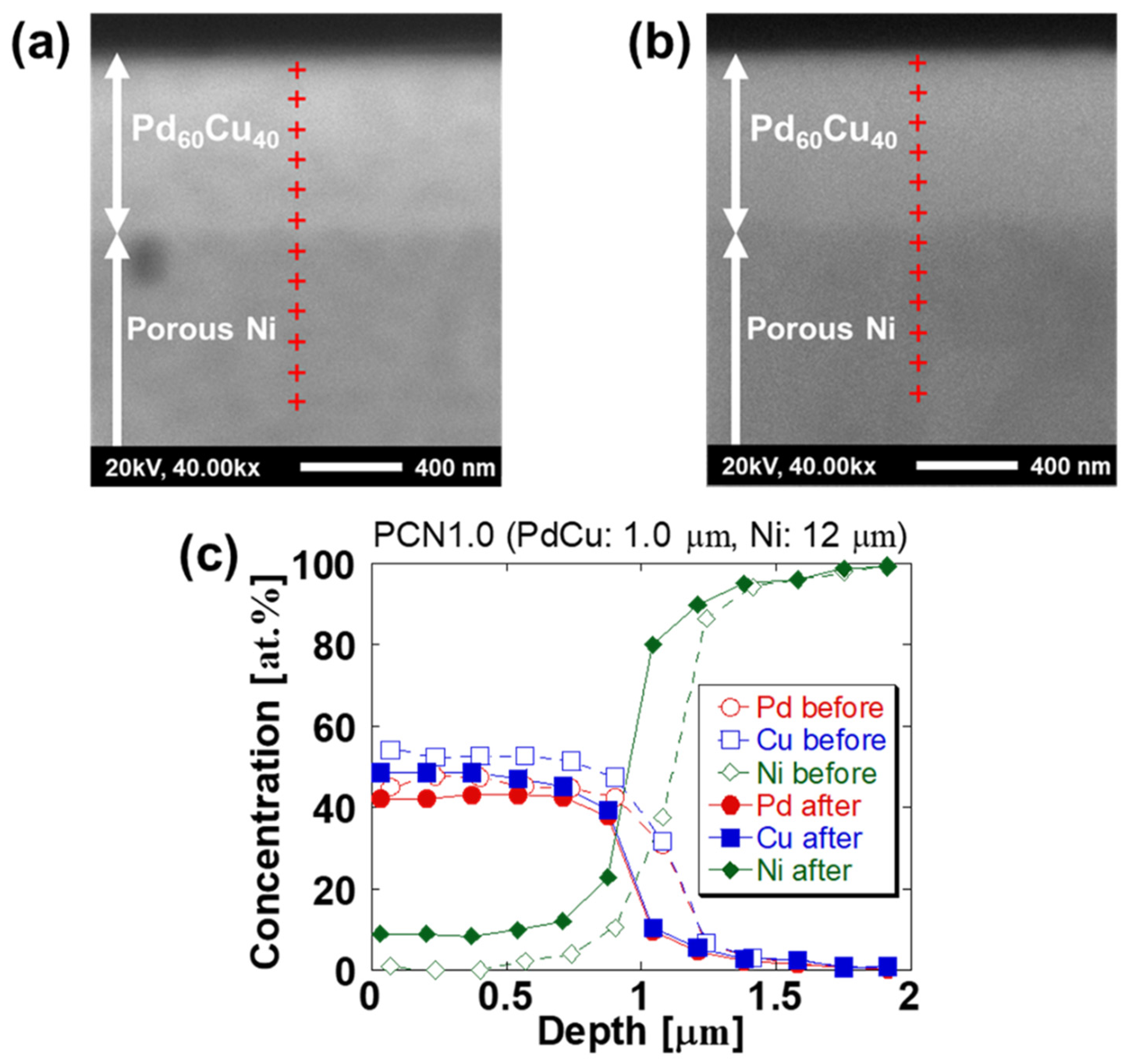
| Sample Specifications | PCN1.0 |
|---|---|
| wt.% Pd:Cu | 60:40 |
| Thickness of the PdCu alloy layer (µm) | 1.0 |
| Thickness of porous Ni support layer (µm) | 12 |
| Total membrane thickness (µm) | 13 |
| Effective diameter of the membrane sample (mm) | 5.6 |
| Effective membrane surface area of the membrane sample (mm2) | 24.6 |
| Concentrations | Analyte Elements | |
|---|---|---|
| Pd | Cu | |
| Analyzed concentration (mg L−1) | 28.51 | 18.89 |
| Calculated atomic ratio (at.%) | 47.06 | 52.94 |
| Calculated weight ratio (wt.%) | 60.15 | 39.85 |
| Sputtering target weight ratio (wt.%) | 60 | 40 |
| Selective Layer | Thickness of Selective Layer | Thickness of Supported Layer | T | ΔP | H2 Permeability Coefficient | H2 Permeance | H2/N2 Selectivity | n | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Materials | µm | µm | °C | kPa | mol m−1 s−1 Pa-n | mol m−2 s−1 Pa-n | |||
| Pd/γ-Al2O3 with Pd | 1 | Alumina | 450 | 100 | 1.06 × 10−5 | 23 | 1 | [48] | |
| Pd/γ-Al2O3 with Pd | 1 | Alumina | 400 | 75 | 6.7 × 10−7 | 23 | 1 | ||
| Pd/γ-Al2O3 | 1 | Alumina | 300 | 2 × 10−7 | >200 (H2/He) | 1 | [49] | ||
| Pd | 1 | Alumina | 450 | 68 | 2.06 × 10−6 | 780 | 1 | [50] | |
| Pd-Ni alloy | 1 | Alumina | 450 | 68 | 2.06 × 10−6 | 317 | 1 | ||
| PdNi0.2-0.3/Ni powder | 1 | Stainless steel | 450 | 68 | 2.21 × 10−6 | >400 | 1 | ||
| PdNi0.2-0.3 (at.%) | 1–2 | PSS/Ni | 450 | 5.90 × 10−9 | 0.5 | ||||
| Pd99.7Nb0.3 (at.%) | 1–2 | PSS/Ni | 450 | 2.8 × 10−9 | 0.5 | ||||
| Pd | 1 | Alumina | 350 | 100 | 3.3 × 10−6 | 1 | [51] | ||
| Pd | 1 | Ni microstructured support grid | 350 | 100 | 3.6 × 10−6 | 1 | [46] | ||
| Pd77Ag23 (wt.%) | 1.4 | microchannel | 300 | 1.70 × 10−10 | 5700–390 | 0.5 | [52] | ||
| Pd77Ag23 (wt.%) | 1.2 | YSZ-doped Al2O3 hollow fiber | 450 | 100 | 5.81 × 10−12 | 1583 | 1 | [53] | |
| Pd | 1.2 | YSZ-doped Al2O6 hollow fiber | 450 | 100 | 3.55 × 10−12 | 2600 | 1 | ||
| Pd | 1.3 | hollow-fiber α-alumina | 460 | 105 | 8.0–40.0 × 10−7 | 160–5500 | 1 | [54] | |
| PdAg | 1 | ZrO2 | 400 | 100 | 8.00 × 10−6 | 500 | 1 | [55] | |
| Pd60Cu40 (wt.%) | 1.5 | Al2O3 | 350 | 100 | 5.7 × 10−9 | 3.81 × 10−3 | 93 | 0.5 | [56] |
| Pd | 1 | anodized alumina | 400 | 101 | 7.40 × 10−8 | 200 | 1 | [57] | |
| Pd60Cu40 (wt.%) | 1.0 | Porous Ni | 320 | 84 | 2.70 × 10−12 | 2.70 × 10−6 | 377 (H2/He) | 1 | This study |
| Pd60Cu40 (wt.%) | 1.0 | Porous Ni | 320 | 84 | 7.83 × 10−10 | 7.83 × 10−4 | 377 (H2/He) | 0.5 | This study |
| T = 320 °C, ΔPH2 = 84 kPa n = 1.0 | This Study PCN3.7 | This Study PCN2.2 | This Study PCN1.0 | Reference Pd60Cu40 [58,59] |
|---|---|---|---|---|
| Layer thickness (µm) | Pd60Cu40: 3.7 Porous Ni: 13 | Pd60Cu40: 2.2 Porous Ni: 15 | Pd60Cu40: 1.0 Porous Ni: 12 | Pd60Cu40: 25.4 |
| H2 permeation flux, JH2 (mol m−2 s−1) | 2.22 × 10−1 | 2.24 × 10−1 | 2.27 × 10−1 | 1.93 × 10−1 |
| H2 permeability coefficient, φH2 (mol m−1 s−1 Pa−n) | 9.65 × 10−12 | 5.87 × 10−12 | 2.70 × 10−12 | 5.84 × 10−11 |
| H2 permeance, φH2/L (mol m−2 s−1 Pa−n) | 2.64 × 10−6 | 2.67 × 10−6 | 2.70 × 10−6 | 2.29 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinoda, Y.; Takeuchi, M.; Mizukami, H.; Dezawa, N.; Komo, Y.; Harada, T.; Takasu, H.; Kato, Y. Characterization of Pd60Cu40 Composite Membrane Prepared by a Reverse Build-Up Method for Hydrogen Purification. Energies 2021, 14, 8262. https://doi.org/10.3390/en14248262
Shinoda Y, Takeuchi M, Mizukami H, Dezawa N, Komo Y, Harada T, Takasu H, Kato Y. Characterization of Pd60Cu40 Composite Membrane Prepared by a Reverse Build-Up Method for Hydrogen Purification. Energies. 2021; 14(24):8262. https://doi.org/10.3390/en14248262
Chicago/Turabian StyleShinoda, Yasunari, Masakazu Takeuchi, Hikaru Mizukami, Norikazu Dezawa, Yasuhiro Komo, Takuya Harada, Hiroki Takasu, and Yukitaka Kato. 2021. "Characterization of Pd60Cu40 Composite Membrane Prepared by a Reverse Build-Up Method for Hydrogen Purification" Energies 14, no. 24: 8262. https://doi.org/10.3390/en14248262
APA StyleShinoda, Y., Takeuchi, M., Mizukami, H., Dezawa, N., Komo, Y., Harada, T., Takasu, H., & Kato, Y. (2021). Characterization of Pd60Cu40 Composite Membrane Prepared by a Reverse Build-Up Method for Hydrogen Purification. Energies, 14(24), 8262. https://doi.org/10.3390/en14248262







